Introduction
There continues a shortage of donor lungs despite years of research into improving marginal lungs prior to transplantation. We revisit a case reported in 2001 where marginal lungs were successfully transplanted with the help of extracorporeal membrane oxygenation (ECMO) in the immediate postoperative period.[1] While ex vivo lung perfusion (EVLP) holds promise for improving marginal lung utilization, this case highlights an alternative approach in which lungs are allowed to rehabilitate in vivo.
Case
The recipient was a 55-year old woman with end-stage respiratory failure from pulmonary fibrosis. Her pulmonary function was poor with a forced expiratory volume in 1 second (FEV1) of 0.71L (33% predicted), a forced vital capacity (FVC) of 0.85L (30% predicted) and a baseline oxygen requirement of 5L per minute. Based on these data, her survival without transplant was estimated to be 3 months. Upon notification of the availability of a suitable donor and arrival at the hospital for the expected transplant she was profoundly dyspneic on 5L of oxygen and thought to have further advanced in her respiratory failure. The donor lungs were from a young individual with brain death who developed abrupt and severe pulmonary edema after the decision was granted for organ donation. The PO2/FiO2 ratio at the time of procurement was 133, down from 414 earlier in the day. In light of worsening chest radiograph, negative drug screen, normal blood pressure and absence of fluid administration, the origin of the pulmonary edema was thought to be neurogenic. The high risk nature of the donor lungs were discussed in detail including the virtual certainty of the need for ECMO after transplantation, ultimately the decision was made to proceed with transplantation.[1]
After uneventful implantation of both lungs, as anticipated she could not be separated from cardiopulmonary bypass because of persistent hypoxemia and right atrium to pulmonary artery ECMO support was initiated as described previously. [1] After two days, the patient was weaned from ECMO circuit and decannulated. Early after transplantation she had excellent pulmonary function with FEV1 1.95L (96% predicted) and FVC 2.37L (87% predicted). She was followed for 14 years and did exceedingly well during that timeframe remaining stable on tactolimus and prednisone with only one transplant related hospital readmission. She led a normal life, going back to work full time and traveling around the world. Her husband reports she played with her grandkids, she gardened, took care of their home and yard and had a load of hobbies. Her pulmonary function remained normal with a FEV1 1.24L (67% predicted) and FVC 1.79L (73% normal) 14 years after transplant. Unfortunately towards the end of her life at age 69 she developed neurocognitive decline, became quite frail, and eventually succumbed to non-transplant related causes at home with her husband.
Comment
Despite severe neurogenic pulmonary edema in these donor lungs, we demonstrated good long-term lung function after brief period of post-operative ECMO support.[1] Considering the fact that lung transplant outcomes are the worst of all organs, transplant teams are hesitant to procure and transplant lungs without clear evidence of high quality.[2] Additionally, there are ethical issues to consider when obtaining recipient consent for marginal lungs that may require prolonged postoperative support and ECMO. However, animal studies have shown that marginal lungs can be successfully transplanted and that any subsequent ischemia-reperfusion injury is manageable.[3–5] It is important to recognize different types of pulmonary edema with different presentation and clinical course. Neurogenic pulmonary edema tends to be rapid onset leading to non-Diffuse Alveolar Damage Acute Respiratory Distress Syndrome (non-DAD ARDS) compared to other causes like pneumonia or aspiration causing ARDS with DAD.
Increasing procurement rates and improving the utilization of marginal lungs may help alleviate the current donor lung shortage. EVLP is gaining clinical acceptance as a platform for lung assessment, rehabilitation, and prolonged transport prior to transplant. In this particular case, the use of ECMO postoperatively provided support for the patient while allowing the lungs to recover in vivo under controlled conditions. This use of ECMO to support the transplant recipient and allow for pulmonary function improvement may provide an additional alternative approach to increase marginal lung utilization. Currently, rates of primary graft dysfunction and chronic rejection continue to influence transplant teams to be conservative when evaluating donor lungs. However, with improved perioperative use of EVLP and ECMO, imperfect donor characteristics may not preclude procurement and transplant. Becoming comfortable with the idea of rehabilitating marginal donor lungs, with EVLP or ECMO as described here, may help maximize the number of lungs transplanted and decrease wait list mortality.
Figure 1.
Yearly Post-Transplant FEV1
Acknowledgments
Special thanks to Heidi Flanagan RN and Mark Robbins MD for their assistance with compiling data and updating the record. Also, image management at UVA for locating and converting a 20 year old chest radiograph.
Funding: This work was supported by the National Institutes of Health T32 HL07849.
Abbreviations
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- ECMO
extracorporeal membrane oxygenation
- EVLP
ex vivo lung perfusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None to disclose
Central Message: Appropriate use of post-transplant ECMO may provide a therapeutic option to improve utilization of marginal donor lungs.
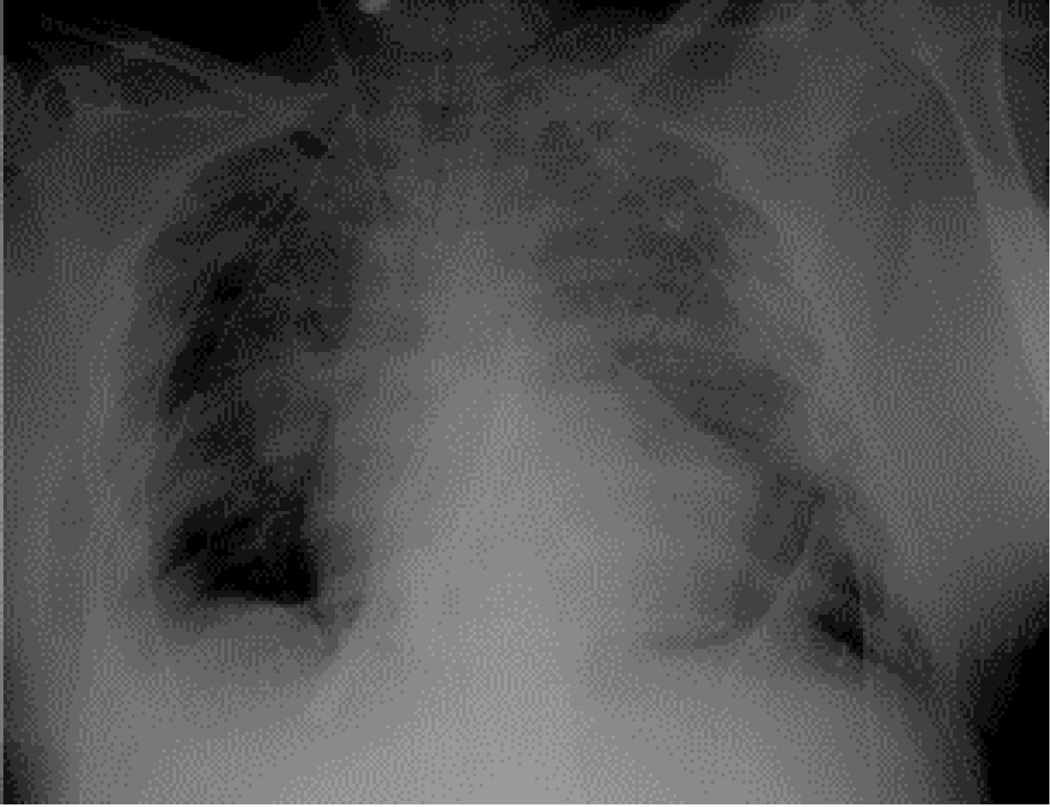
Central Picture Legend: Initial postoperative chest radiographs with ECMO cannulas inplace.
References
- 1.Fiser SM, et al. Donor lung salvage after neurogenic pulmonary edema with the use of post-transplant extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2001;122(6):1257–1258. doi: 10.1067/mtc.2001.116464. [DOI] [PubMed] [Google Scholar]
- 2.Ailawadi G, et al. Does reperfusion injury still cause significant mortality after lung transplantation? J Thorac Cardiovasc Surg. 2009;137(3):688–694. doi: 10.1016/j.jtcvs.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Yeung JC, et al. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques--non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin. 2009;19(2):261–274. doi: 10.1016/j.thorsurg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Cypel M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Wagner CE, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg. 2016;151(2):538–546. doi: 10.1016/j.jtcvs.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]