Abstract
Morphological changes in the size and shape of the nucleus are highly prevalent in cancer, but the underlying molecular mechanisms and the functional relevance remain poorly understood. Nuclear envelope proteins, which can modulate nuclear shape and organization, have emerged as key components in a variety of signalling pathways long implicated in tumourigenesis and metastasis. The expression of nuclear envelope proteins is altered in many cancers, and changes in levels of nuclear envelope proteins lamins A and C are associated with poor prognosis in multiple human cancers. In this review we highlight the role of the nuclear envelope in different processes important for tumour initiation and cancer progression, with a focus on lamins A and C. Lamin A/C controls many cellular processes with key roles in cancer, including cell invasion, stemness, genomic stability, signal transduction, transcriptional regulation, and resistance to mechanical stress. In addition, we discuss potential mechanisms mediating the changes in lamin levels observed in many cancers. A better understanding of cause-and-effect relationships between lamin expression and tumour progression could reveal important mechanisms for coordinated regulation of oncogenic processes, and indicate therapeutic vulnerabilities that could be exploited for improved patient outcome.
Keywords: Lamins, cancer, signal transduction, cell migration, cell mechanics, gene regulation
Introduction
It has long been recognized that cancer cells exhibit characteristic changes in the size and shape of their nuclei, and these features serve as important biomarkers in the diagnosis and prognosis of cancer patients (de Las Heras and Schirmer, 2014). The broad prevalence of these changes is particularly intriguing, since nuclear abnormalities are common across a vast spectrum of cancer types, regardless of tissue source, mutational spectrum, and signalling dependencies. The frequency of nuclear alterations would thus suggest that changes in nuclear structure may be critically linked to the transformation process; however, the factors driving these nuclear abnormalities, and the associated functional consequences, are still incompletely understood.
Nuclear morphology could be affected by cancer-associated changes in DNA content (aneuploidy) or higher order chromatin organization (Bustin and Misteli, 2016; Reddy and Feinberg, 2013). The composition of the nuclear envelope (NE) that surrounds the DNA can further influence chromatin architecture and mediate changes in nuclear structure (Pombo and Dillon, 2015). In cancer, morphological abnormalities can occur in the NE itself, appearing as irregular folding, deep grooves, and cytoplasmic inclusions, which is in contrast with the generally smooth NE outline of most normal cells (Fischer, 2014). Notably, altered NE morphology is a crucial part of pathologists’ assessment of tumour grade, and correlates with prognosis (Bussolati et al., 2014; Bussolati et al., 2008). Some studies even indicate that NE irregularities may be a direct result of oncogene activation, lack of tumour suppressor function, or genomic instability (Boyd et al., 1991; Fischer, 2014; Fischer et al., 1998). Taken together, these findings suggest that changes in the structure and composition of the NE may be regulated events occurring early in the transformation process, and could thus be directly linked to tumourigenesis.
The NE includes the inner and outer nuclear membranes (INM and ONM, respectively) and associated membrane proteins, the underlying network of intermediate filaments, termed the nuclear lamina, and nuclear pore complexes spanning the NE (Hetzer, 2010) (Fig. 1). The lamina consists mainly of A-type lamins (A and C) and B-type lamins (B1 and B2). Lamins A and C, as well as less abundant isoforms, are alternatively spliced from the LMNA gene, whereas lamins B1 and B2 are encoded by the LMNB1 and LMNB2 genes, respectively. Lamins are type V intermediate filaments composed of a N-terminal head domain, a rod domain, and a long C-terminal tail containing an immunoglobulin-like domain (Ho and Lammerding, 2012). Lamins form homodimers through coiled-coil interactions. Homodimers organize head-to-tail into polymers, and polymers further assemble in anti-parallel fashion into non-polar filaments (Gruenbaum and Medalia, 2015). Intriguingly, mutations in the LMNA gene cause a wide range of diseases, collectively termed laminopathies, that include Hutchinson-Gilford progeria syndrome (HGPS), dilated cardiomyopathy, and Emery-Dreifuss muscular dystrophy (EDMD) (Schreiber and Kennedy, 2013). Research aimed at understanding treatment options and disease progression of laminopathies has driven the characterization of many cellular functions for A-type lamins, revealing roles in pathways also known to contribute to tumour progression, such as proliferation and genomic instability. In addition to these signalling pathways, the NE also provides physical strength and stability to protect the nucleus (Isermann and Lammerding, 2013; Lammerding et al., 2006). The structural support generated by lamins is particularly important in cell types subjected to greater mechanical stress, such as in skeletal and cardiac tissues (Isermann and Lammerding, 2013; Swift et al., 2013; Zuela et al., 2016; Zwerger et al., 2013). Thus, alterations in the NE could impact both the physical and biochemical properties of cells during tumour initiation and progression.
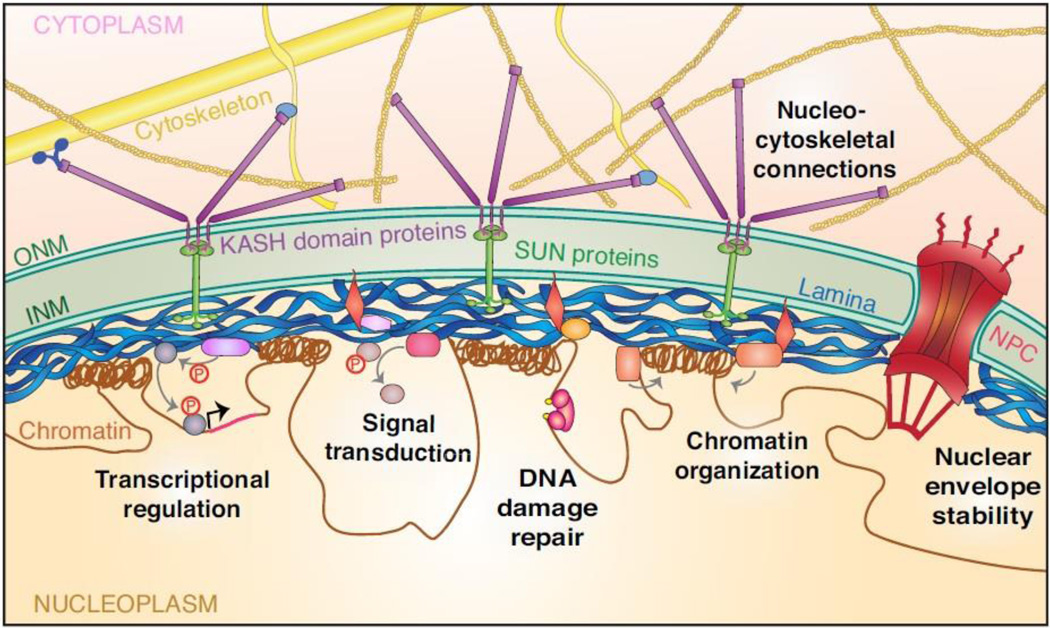
Figure 1. Overview of nuclear envelope organization and lamin A/C functions.
The nuclear envelope (NE) consists of the outer nuclear membrane (ONM), inner nuclear membrane (INM), nuclear pore complexes (NPC), nuclear lamina, and additional proteins bound to the lamina and INM. The lamina is a meshwork of intermediate filaments beneath the INM composed of A-type and B-type lamins. Lamins can also localize to the nuclear interior (not depicted here). Lamin A/C performs many cellular functions, including providing physical stiffness to protect the nucleus and tethering chromatin into transcriptionally repressed regions at the nuclear periphery. The lamina and associated proteins can also form complexes with signalling molecules to influence nuclear accumulation, stability, and substrate engagement. The nucleus is connected to the cytoskeleton through the linker of nucleoskeleton and cytoskeleton (LINC) complex. The LINC complex is composed of Sad1 and UNC-84 (SUN) domain proteins in the INM that bind to Klarsicht, ANC-1, and Syne homology (KASH) domain proteins that span the ONM and connect to the cytoskeleton, facilitating nuclear positioning and mechanotransduction signalling.
Strengthening the potential connection between NE proteins and tumourigenic processes, altered NE protein expression has been detected in many human cancers and can have prognostic significance (reviewed in Denais and Lammerding (2014) and Chow et al. (2012)). For example, decreased expression of lamin A/C is found in many prostate, breast, colon, ovarian, and gastric cancers and is associated with worse prognosis (Belt et al., 2011; Gong et al., 2015; Matsumoto et al., 2015; Saarinen et al., 2015; Wu et al., 2009). Conversely, studies on colorectal and prostate tumours have identified an association between increased expression of lamin A/C and disease progression (Kong et al., 2012; Willis et al., 2008). These studies indicate that the role of A-type lamins in cancer is likely context-dependent, and further studies are required to determine how lamins function in cancer progression in different cell types, mutational backgrounds, and stages of disease. Furthermore, despite the widely documented changes in NE composition in cancer, few studies have been able to directly connect factors initiating alterations in the NE to functional consequences in malignant cell behaviours. Thus, there is a need to understand the cause-and-effect relationships between changes in NE structure and composition and the tumourigenic process. Although many NE proteins are altered in cancer and likely contribute to tumourigenesis and cancer progression, this review focuses mainly on how A-type lamins and their interaction partners could act as a key point of dysregulation in cancer, and promote growth and metastasis through effects on cell structure, signalling, genomic stability, and gene expression.
The nuclear envelope in migration, invasion, and metastasis
Invasive behaviour is one of the hallmarks of cancerous cells (Hanahan and Weinberg, 2011). Cancer cell invasion contributes to the spread of tumour growth to additional tissues, termed metastasis, which is the main cause of morbidity and mortality in most cancers (Talmadge and Fidler, 2010). During in vivo migration, cells navigate through spaces in extracellular matrix (ECM) and cellular environments as small as 2 µm in diameter, which is substantially smaller than the size of the nucleus (Stoitzner et al., 2002; Weigelin et al., 2012). Cells can navigate these tight spaces by remodeling ECM fibers to modify their environment, including through ECM degradation by secreted matrix metalloproteases (MMPs), which are upregulated in many cancers and associated with poor prognosis (Coussens et al., 2002). Alternatively, cells can deform to squeeze through the available space (McGregor et al., 2016). This versatility may have contributed to the failure of MMP inhibitors to improve patient outcome in clinical trials (Coussens et al., 2002). While the dynamic cytoplasm deforms easily to fit through micron-scale spaces, the nucleus is the largest and stiffest cellular organelle, and nuclear deformation can impose a rate-limiting step on migration in 3D environments (McGregor et al., 2016; Wolf et al., 2013). Accordingly, cells migrating in 3D collagen matrices show extensive nuclear deformation when MMP activity is inhibited, and sufficiently small pore sizes can lead to complete stalling of cell movement as the nucleus becomes ‘trapped’ in the dense network (Wolf et al., 2013). The deformability of the nucleus is largely determined by expression of A-type lamins (Lammerding et al., 2006; Lammerding et al., 2004; Pajerowski et al., 2007; Schape et al., 2009), although changes in B-type lamin expression can also impact nuclear elasticity (Ferrera et al., 2014; Swift et al., 2013). As a result, cells with decreased lamin A/C levels migrate significantly faster through narrow constrictions in microfluidics devices and transwell plates with 3 µm pore sizes (Davidson et al., 2014; Harada et al., 2014; Shin et al., 2013). Consistent with these findings, cells in the periphery of a xenograft tumour model showed more nuclear deformation and lower lamin A expression relative to cells in the tumour core, suggesting that subpopulations of cells with lower lamin A were more efficient at invasion in vivo (Harada et al., 2014). The importance of lamin A/C levels in in vivo cell migration is further exemplified in neutrophils, where maintaining nuclear deformability is critical for passage through narrow capillaries less than one-fifth of the cell diameter (Doerschuk et al., 1993). A-type lamins are normally downregulated during neutrophil differentiation (granulopoiesis), and ectopic expression of lamin A in neutrophil-type cells impairs their ability to pass through micron-scale constrictions (Rowat et al., 2013). These studies demonstrate the importance of lamin A/C-dependent nuclear stiffness in normal physiology and cancer cell invasion.
Interestingly, decreased lamin A/C expression may facilitate migration in confined space, but also renders cells more susceptible to physical stress. This is particularly relevant for cancer cells subjected to changing physical conditions, such as increased hydrostatic pressure within the tumour, mechanical stress associated with migration through tight interstitial spaces, and fluid shear stress in the circulatory system. Increased nuclear fragility and NE rupture has been reported in lamin A/C-deficient and lamin mutant models for laminopathies (Broers et al., 2004; De Vos et al., 2011; Lammerding et al., 2004; Vargas et al., 2012). The NE has a critical role in protecting the genome, and loss of NE integrity could damage DNA and potentially lead to cell death (Denais et al., 2016; Raab et al., 2016). Cells expressing less lamin A/C have a general increased sensitivity to mechanical stress, as cells with reduced lamin A/C levels exhibit more cell death in response to mechanical strain (Lammerding et al., 2004), fluid shear stress (Mitchell et al., 2015), and migration through confined spaces (Harada et al., 2014; Raab et al., 2016). However, other studies did not report increased cell death from confined migration (Davidson et al., 2014; Denais et al., 2016; Liu et al., 2015; Rowat et al., 2013), indicating that different cell types or experimental conditions may impact the specific sensitivity to mechanical stress in confined spaces. Recent work from our lab and others has shed additional light on the potential consequences of nuclear deformation in confined migration. Migration through tight spaces in vitro and in vivo resulted in rupture of the NE in a broad panel of cell types, and the incidence of NE rupture increased dramatically with smaller pore sizes and depletion of lamins (Denais et al., 2016; Harada et al., 2014; Raab et al., 2016). NE rupture was characterized by uncontrolled exchange of material between the nucleus and cytoplasm, chromatin herniation into the cytoplasm, and DNA damage (Denais et al., 2016; Raab et al., 2016). Strikingly, the majority of cells survive these NE rupture events, but survival requires rapid ESCRT (endosomal sorting complex required for transport)-dependent NE repair and intact DNA damage repair machinery (Denais et al., 2016; Raab et al., 2016). In cancer cells, migration-induced DNA damage could further contribute to genomic instability and thereby promote cancer progression. However, nuclear rupture could also be a unique vulnerability of metastasizing cancer cells, such that targeting NE repair and DNA damage repair in these cells could have therapeutic value.
In addition to protecting the nuclear content, lamins and associated LINC complex proteins also play important roles in organizing cytoskeletal structure, dynamics, and polarity, which are important for cell migration. Interrupting nucleo-cytoskeletal coupling by disruption of the LINC-complex or lack of A-type lamins results in altered organization of actin, tubulin, and vimentin networks and impaired cell polarization and 2D cell migration, although effects on migration vary across different studies (Broers et al., 2004; Davidson et al., 2014; Houben et al., 2009; Lee et al., 2007; Lombardi et al., 2011; Razafsky et al., 2014). Increased levels of lamin A can also promote migration of colorectal cancer cells through increased expression of T-plastin, which downregulates E-cadherin, a key cell-cell adhesion molecule frequently absent in cancer (Willis et al., 2008).
Taken together, these studies indicate that lamin A/C expression may be a double-edged sword: low levels promote cell invasion through increased nuclear deformability, while high levels protect against mechanical forces, such as increased interstitial pressure within the tumour, and some minimum function of lamins is required for proper cell polarization and cytoskeletal organization. In addition, cell viability in response to strain or after rupture may depend on other functions of lamin A/C that impact signalling pathways or DNA damage repair (discussed below). Thus, the effect of A-type lamin levels on migration and metastasis is likely context-dependent, favoring high or low expression in different cancer types or stages of disease.
Lamin A/C and cancer stem cells
A-type lamin expression also has important effects in the context of pluripotent stem cells and differentiation. The association of changes in lamin expression with the progression from a stem cell to a differentiated state was first described during mouse development. Lamin A/C levels are absent in embryonic stem cells, and mice lacking A-type lamins undergo seemingly normal embryonic development (Constantinescu et al., 2006; Stewart and Burke, 1987; Sullivan et al., 1999). However, A-type lamins are required for postnatal growth, and A-type lamin expression increases with differentiation, not appearing in some tissues until after birth (Rober et al., 1989; Sullivan et al., 1999). In adult mouse tissues, cell types that exhibit more stem cell or proliferative properties also retain low lamin A/C levels, which is in contrast to the higher lamin A/C expression detected in most somatic cells and tissues (Broers et al., 1997; Rober et al., 1990; Swift et al., 2013; Takamori et al., 2007). The inverse association between lamin A/C levels and stem cell characteristics is further illustrated in in vitro differentiation and stem cell induction assays. Somatic cells treated to induce pluripotency show loss of lamin A/C expression, demonstrating that lamin levels are not static but scale reversibly with the degree of differentiation (Alberio et al., 2005; Bru et al., 2008). Conversely, somatic cells expressing higher levels of lamin A show decreased efficiency in reprogramming to induced pluripotent stem cells (iPSCs), and experimental manipulation to increase or decrease lamin A levels results in reduced or enhanced induction of iPSCs, respectively, suggesting that levels of lamin A in somatic cell populations reflect varying degrees of plasticity (Zuo et al., 2012). In addition, overexpression of lamin A in normal human fibroblasts results in decreased replicative lifespan (Candelario et al., 2008). Furthermore, overexpression of lamin A/C impairs adipogenic conversion of pre-adipocytes (Boguslavsky et al., 2006), possibly due to the requirement to remodel the nuclear lamina during adipogenesis (Verstraeten et al., 2011). These studies show that lamin A/C expression is not just a marker of differentiation, but also actively contributes to lineage commitment and loss of phenotypic plasticity.
The function of lamin A/C in differentiation can be attributed in part to the ability of lamin A/C to interact with chromatin and influence the structural organization of the nucleus. Interactions between chromatin and lamins change during differentiation and play an important role in lineage determination (Lund et al., 2013; Peric-Hupkes et al., 2010). Increased NE shape fluctuations are an identifying feature of mesenchymal stem cells, and lack of lamin A/C has been shown to increase both nuclear deformations and chromatin dynamics (Bronshtein et al., 2015; Lee et al., 2014; Makhija et al., 2016). Hundreds of genomic regions termed lamina-associated domains (LADs) interact with the lamina and typically exhibit transcriptional repression (Guelen et al., 2008). Artificial tethering to the lamina can downregulate expression of targeted genes, and directing lamin A to promoter regions results in transcriptional repression (Finlan et al., 2008; Lee et al., 2009; Reddy et al., 2008). Transcriptional silencing of chromatin tethered to the lamina is mediated at least in part by epigenetic modifications associated with heterochromatin (reviewed in Gruenbaum and Foisner (2015)), and many proteins involved in epigenetic silencing interact with NE proteins (Guarda et al., 2009; Somech et al., 2005; Zhong et al., 2005). Accordingly, correct expression levels of A-type lamins are required for normal chromatin organization and control of gene expression during differentiation (Frock et al., 2006; Solovei et al., 2013). Increased lamin A/C levels in differentiated cells could thus solidify phenotypic reprogramming by locking in tissue-specific patterns of heterochromatin at the nuclear periphery; conversely, lamin A/C deficiency is associated with loss of heterochromatin markers (Gonzalo, 2014). It is interesting to consider these mechanisms in the context of epigenetic alterations associated with tumourigenesis (Esteller, 2008; Galiova et al., 2008; Scaffidi and Misteli, 2005).
The involvement of lamin A/C in cellular differentiation and self-renewal is of particular importance when considering the function of lamins in cancer. The cancer stem cell (CSC) model describes a subset of cells in a tumour that have the ability to continually self-renew and to differentiate into a heterogeneous population (Medema, 2013). The phenotypic flexibility and renewal potential from these CSCs, also called tumour-initiating cells, can mediate tumour growth, therapeutic resistance, and completion of the metastatic cascade (Vermeulen et al., 2012), motivating research efforts to define, identify, and treat the stem cell-like population in human cancers. In addition to regulating chromatin organization, the role of lamin A/C in determining cellular plasticity may also involve reprogramming of signalling pathways known to be important for stemness, such as Wnt/β-catenin, TGF-β, and Notch, which are discussed further in sections below. These pathways are already implicated in stem cell phenotypes in cancer, but studies have yet to address the potential involvement of lamin A/C in the CSC phenotype promoted by these signalling events. Nonetheless, Nardella et al. (2015) demonstrated that depletion of A-type lamins in a neuroblastoma cell line resulted in a cell subpopulation with enhanced stem cell characteristics. The authors suggest that this phenotype was mediated by altered expression of microRNA-101, which results in upregulation of the MYC proto-oncogene, and reveals an inverse correlation between expression of lamin A/C and MYC in human neuroblastoma biopsies (Nardella et al., 2015). Based on our emerging understanding of lamins and their role in stemness and differentiation, the decreased levels of lamin A/C in human cancers may both reflect and promote CSC qualities for proliferation and differentiation, which can determine tumour growth, metastasis, and response to therapeutics.
As some tumours exhibit increased expression of A-type lamins, it will be interesting to determine whether these tumours still contain a subpopulation of CSCs with lower expression of lamin A/C. Since increased lamin levels could also potentially promote stemness though regulation of signalling and transcription, it will be relevant to determine whether high, rather than low, levels of A-type lamins typify stem cell populations in a particular cancer tissue of origin. Thus, high expression of lamins in such cancers could reflect activation of a stem cell program specific to that tissue. This appears to be the case in colorectal cancer, where the colon stem cell niche stains strongly for lamin A, and increased A-type lamin expression has been associated with aggressive cell behaviour and poor outcome (Willis et al., 2008). In contrast, lamin A/C expression is absent from gastrointestinal (GI) tract stem cells, and a mouse model of GI-specific Lmna deletion showed enhanced polyp growth in response to oncogenic stimulus (Wang et al., 2015). These studies highlight the multi-faceted role of lamins in cancers, and demonstrate that the cell type of origin could impact the functional consequences of lamin A/C expression.
A-type lamins in DNA damage and genome stability
The role of the nuclear lamina in physically protecting the nucleus, tethering chromatin, and regulating protein complexes, including components of the DNA damage repair machinery, provides a central position for lamins in the maintenance of genome integrity. Genomic instability is a hallmark feature of most cancers and drives tumour evolution and progression. Maintaining genome integrity has a key role in the suppression of cancer, which is evident in both the tumour suppressor function of DNA damage checkpoint proteins and the existence of hereditary cancers arising from mutations in DNA repair genes (Negrini et al., 2010).
The importance of lamins in maintaining genomic stability is apparent in studies of laminopathy disease progression (reviewed in Gonzalo (2014)). Lamin A/C-mutant and -deficient models exhibit misshapen nuclei, loss of heterochromatin markers, accumulation of unrepaired DNA damage, and chromosomal abnormalities (Gonzalo, 2014; Hutchison, 2011; Lammerding et al., 2004). There are diverse mechanisms through which lamins can contribute to genomic stability. Lamin A/C stabilizes the interaction of telomere repeat-binding protein 2 (TRF2) with telomeres, which influences length and protection of telomeric DNA at the ends of chromosomes (Wood et al., 2014). Loss of lamin A/C alters positioning of telomeres in the nucleus and results in telomere shortening, which promotes genomic instability (Gonzalez-Suarez et al., 2009; Wood et al., 2014). However, telomere shortening is also considered tumour-suppressive, since telomere maintenance is required for continued DNA replication and cell immortalization, and is often mediated in cancer by activation of the telomerase enzyme (Harley, 2008). TRF2 is a potential oncogene upregulated in some cancers, and TRF2 overexpression can maintain telomere length independently of telomerase through facilitating telomere recombination events, which also foster chromosomal instability (Blanco et al., 2007). It is tempting to speculate that lamin A/C may be required in TRF2-dependent tumours for TRF2 to function in telomere maintenance. Therefore, both increased and decreased lamin A/C expression could potentially contribute to genomic instability through regulation of TRF2. Decreased expression of lamin A/C may also subject cells to increased DNA damage due to enhanced susceptibility to mechanical stress, increased nuclear deformation, and more frequent NE rupture events (Denais et al., 2016; Raab et al., 2016). Furthermore, loss of lamin A/C impacts chromatin organization, gene silencing, and gene positioning, and may therefore increase DNA translocations, which occur more often in transcriptionally active regions and between chromosomes in close proximity (Chiarle et al., 2011; Parada et al., 2004). Cells with loss or mutation of lamin A/C also show separation of chromatin into nuclear blebs or fragments, which could further promote chromosomal instability (Denais et al., 2016; Hatch et al., 2013; Lammerding et al., 2006; Vigouroux et al., 2001). Interestingly, treatment of laminopathic cells with the N-acetyltransferase (NAT) 10 inhibitor, remodelin, restores nuclear shape and decreases DNA damage, supporting the idea that genomic instability could be a direct consequence of altered lamin A/C levels and aberrant nuclear structure in cancer (Larrieu et al., 2014).
Altered lamin levels can also impact the ability of cells to repair DNA damage, potentially accelerating mutagenesis and enhancing tumour initiation and progression. Disruption of lamin A/C increases sensitivity of cells to DNA damaging agents and enhances activation of p53 signalling and p53-dependent senescence (Liu et al., 2005; Singh et al., 2013; Varela et al., 2005). Because p53 is frequently mutated in cancers and therefore unable to suppress growth in response to unrepaired DNA damage, genomic instability caused by altered lamin A/C levels could contribute to tumour progression rather than senescence in cancer cells (Muller and Vousden, 2014). Lamin A/C expression may further impact DNA repair responses through regulation of p53-binding protein-1 (53BP1), a DNA repair protein and potential tumour suppressor (Ward et al., 2003). Mouse embryonic fibroblasts (MEFs) and human fibroblasts both show decreased 53BP1 stability upon lamin A/C deficiency, leading to defects in DNA repair (Gibbs-Seymour et al., 2015; Gonzalez-Suarez et al., 2009; Redwood et al., 2011). Furthermore, 53BP1 interacts with lamin A/C, and depletion of lamin A/C decreases recruitment of remaining 53BP1 to sites of DNA damage and decreases cell viability in response to DNA damage (Gibbs-Seymour et al., 2015). This suggests that loss of lamin A/C can promote genomic instability through effects on both activity and protein levels of 53BP1.
Lamin A/C also interacts with proteins involved in chromatin remodeling, such as HDAC1 and RBBP4, and altered epigenetic modifications of chromatin can diminish recruitment of components of the DNA repair machinery and contribute to the accumulation of DNA damage (Krishnan et al., 2011; Pegoraro et al., 2009). Interestingly, depletion of A-type lamins also decreases levels of heat shock protein 90 (HSP90), which is a molecular chaperone implicated in DNA damage repair (Harada et al., 2014). However, as a chaperone protein facilitating protein homeostasis, HSP90 also has pro-tumourigenic effects on survival and growth of cancer cells expressing mutated and oncogenic proteins (Trepel et al., 2010). Thus, lower levels of lamin A/C may be detrimental to the growth of tumours driven by HSP90 client proteins, which may explain the increased lamin A/C levels observed in some cancers. In considering the role of lamin A/C-related signalling networks in cancer therapy, it will be important to determine whether tumours with low lamin A/C levels show enhanced sensitivity to treatment with either DNA damaging agents or inhibitors of DNA repair pathways.
Lamin A/C interplay with oncogenes and tumour suppressors
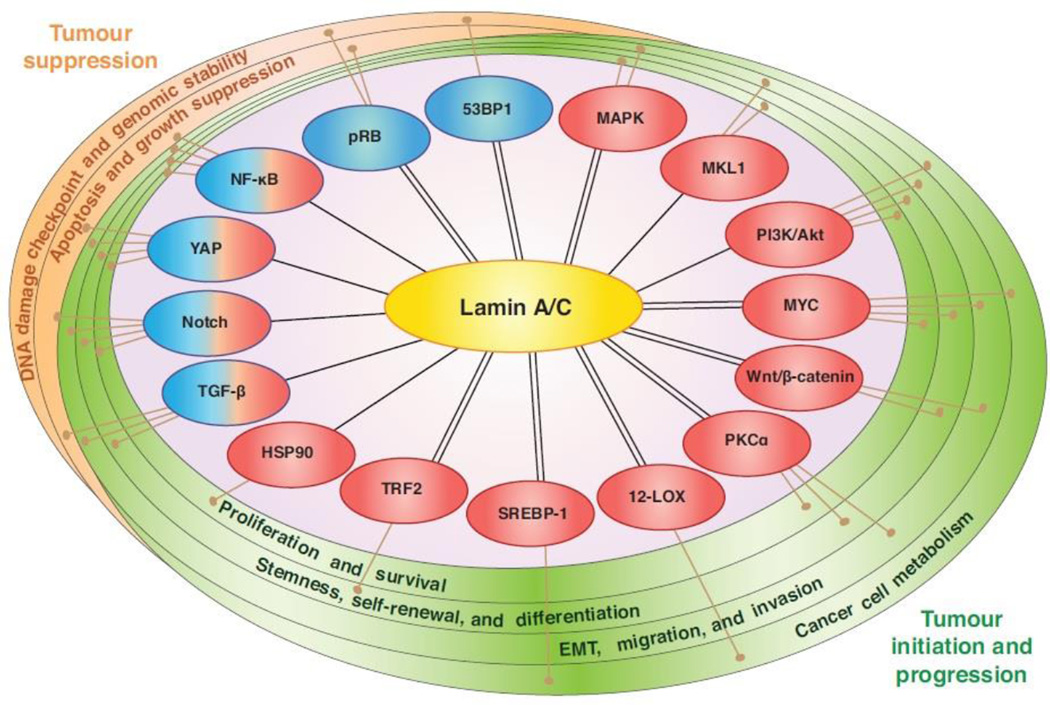
Recent studies suggest that lamins and other NE proteins can serve as a hub and modulator for numerous important signalling pathways, such as growth factor-induced kinase signalling and the transcriptional regulation of cell cycle progression (Andres and Gonzalez, 2009). Importantly, these pathways also contribute to many of the hallmarks of cancer, including self-sustained proliferation, insensitivity to anti-growth signals, evasion of apoptosis, limitless self-renewal potential, and invasion and metastasis (Fig. 2). We briefly highlight the role of lamins in some of the most relevant pathways below.
Figure 2. Connections between lamin A/C and pathways known to suppress or promote cancer formation and progression.
Lamin A/C participates in many pathways characterized to have oncogenic (red) or tumour suppressor (blue) functions in cancer. Lamin A/C expression influences protein levels or downstream activity to modulate signal transduction, and in many cases interactions between lamin A/C and signalling proteins have also been detected (double lines). It is important to note that many of these functions have been identified in lamin A/C mutant or deletion models for laminopathies, and an understanding of lamin A/C-related signalling in the context of cancer demands further study.
pRB/E2F
Inactivation of the retinoblastoma protein (pRB) is a widespread feature of human cancers (Johnson et al., 2016). The tumour suppressor function of pRB is attributed to its role in regulating cell proliferation. pRB binds to the E2F transcription factor, resulting in repression of genes involved in cell cycle progression and DNA replication. The cyclin-dependent kinase (CDK)-mediated phosphorylation of pRB causes release of E2F, increased E2F transcriptional activity, and G1-S cell cycle progression (Giacinti and Giordano, 2006). Recent studies indicate that lamins A/C and the lamina-associated polypeptide 2α (LAP2α) exert an additional layer of control on pRB. Lamin A/C tethers pRB either through direct interaction or in complex with LAP2α, promoting accumulation of hypophosphorylated pRB and delay of cell cycle entry (Dorner et al., 2006; Markiewicz et al., 2002; Van Berlo et al., 2005). Furthermore, in the absence of lamin A/C, pRB is mislocalized and targeted for proteasomal degradation, suggesting that depletion of lamin A/C could promote increased proliferation through loss of the pRB checkpoint (Johnson et al., 2004). Accordingly, lamin A/C-deficient MEFs exhibit increased cell cycle progression, and depletion of lamin A/C in a human lung cancer cell line increased in vivo tumour growth rate (Harada et al., 2014; Johnson et al., 2004). In contrast, depleting lamin A/C in primary human diploid fibroblasts results in G1 arrest, demonstrating cell type-dependent effects of lamin A/C levels on proliferation (Pekovic et al., 2007). The pRB/E2F and p53 tumour suppressor pathways are interconnected, suggesting that mutation of p53 in cancer cells may account for some of the context-dependent effects on proliferation observed with loss of lamin A/C (Muller and Vousden, 2014; Polager and Ginsberg, 2009). Additional NE components may also influence the role of LAP2α and lamin A/C in pRB regulation, as lack of the INM protein emerin has been associated with diminished ability of pRB to repress transcription (Melcon et al., 2006). Finally, it is worth noting that multiple studies in cell lines and tissues support an inverse relationship between lamin A/C levels and proliferation, which is consistent with the association of increased lamin A/C with differentiation (Broers et al., 1997; Ivorra et al., 2006; Nitta et al., 2006; Van Berlo et al., 2005). Thus, dysregulation of pRB and increased cell proliferation could be a key consequence of lamin A/C alterations in cancer, particularly in cells lacking functional p53.
Wnt/β-catenin
The Wnt proto-oncogene is a secreted factor that binds to the Frizzled receptor to promote stabilization of β-catenin, which is followed by β-catenin nuclear translocation and regulation of transcription (reviewed in Clevers and Nusse, 2012). The Wnt/β-catenin pathway is implicated in promoting CSCs and epithelial-mesenchymal transition (EMT) in cancer, which has driven the development of therapies targeting Wnt pathway (Takebe et al., 2015). Recent studies indicate that several NE proteins interact with β-catenin and can modulate Wnt/β-catenin signalling. Emerin interacts with β-catenin and represses its activity through negative regulation of nuclear accumulation (Markiewicz et al., 2006). Loss of emerin results in β-catenin nuclear localization and hyperactivation (Markiewicz et al., 2006; Stubenvoll et al., 2015). Lamin A directly interacts with emerin and is required for its NE localization, and lamin A/C has also been identified in complex with β-catenin (Bermeo et al., 2015; Vaughan et al., 2001). However, lamin A/C expression increases nuclear translocation and activity of β-catenin, and lamin A/C mutations inhibit Wnt/β-catenin activity in mouse models of the segmental aging disease HGPS (Bermeo et al., 2015; Espada et al., 2008; Hernandez et al., 2010). The KASH domain protein, Nesprin-2, also interacts with emerin and β-catenin, and depletion of Nesprin-2 reduces β-catenin nuclear translocation and activity, demonstrating an additional layer of complexity in NE-mediated regulation of β-catenin (Neumann et al., 2010). Moreover, the Wnt/β-catenin pathway also modulates expression of NE proteins, as depletion of β-catenin decreases emerin gene expression and increases lamin A/C expression (Tilgner et al., 2009). These studies demonstrate complex, bi-directional interplay between β-catenin and the NE and stimulate further investigation to determine whether alterations in NE-mediated regulation of β-catenin also occur in tumourigenesis, and whether such changes could contribute to maintenance of the CSC population and EMT.
MAPK
Mitogen activated protein kinase (MAPK) signalling kinases phosphorylate both cytoplasmic and nuclear targets to modulate diverse cellular processes related to malignant transformation (Dhillon et al., 2007). Dysregulation of MAPK signalling is a common feature in cancer, often occurring downstream of growth factor signalling or through mutation (Dhillon et al., 2007). Interestingly, loss of lamin A/C or emerin, as well as expression of lamin A/C mutations associated with muscular dystrophy and dilated cardiomyopathy, result in hyperactivation of multiple MAPK signalling branches, namely extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38α MAPK (Emerson et al., 2009; Muchir et al., 2007; Muchir et al., 2012a; Muchir et al., 2012b; Muchir et al., 2009). The molecular mechanism by which lamin A/C and emerin can modulate MAPK signalling remains incompletely understood. A-type lamins interact directly with ERK1/2 and c-fos, resulting in ERK1/2-dependent release of the c-fos transcription factor from lamin A/C and activation of AP1-mediated transcription and proliferation (Gonzalez et al., 2008). Competitive ERK1/2 binding to lamin A may also regulate cell cycle progression through displacement and inactivation of pRB (Rodriguez et al., 2010). Interestingly, lamin A was also found to interact with protein kinase C (PKC)α, which is a serine-threonine kinase implicated in MAPK activation and cancer progression (Haughian et al., 2009; Martelli et al., 2002; Mauro et al., 2002). Translating these findings to signalling in the context of cancer cells could be an important step in determining the role of lamin A/C alterations in tumour progression. Inhibitors of the MAPK pathway are in clinical trials for cancer treatment, raising the question of whether levels of A-type lamins could have prognostic value for response to MAPK inhibition (Roberts and Der, 2007).
PI3K/Akt
The phosphoinositide 3-kinase (PI3K)/Akt signalling network, which controls cancer cell proliferation, metabolism, and invasion, is the most frequently altered pathway in human cancers, making it a focus for targeted therapeutics (Fruman and Rommel, 2014). Increased PI3K/Akt activity can result from alterations in GTPase or tyrosine kinase signalling inputs, activating PI3K/Akt mutations, or loss of negative regulators, such as the PTEN lipid phosphatase (Engelman, 2009). However, it remains unclear what role lamins play in Akt-mediated cellular responses, particularly in the context of cancer. Interestingly, the heart and muscle of lamin A/C-deficient mice show hyperactivation of mammalian target of rapamycin complex 1 (mTORC1), which is a downstream target of Akt (Ramos et al., 2012). In contrast, increased levels of lamin A/C were found to positively regulate the PI3K/Akt pathway in prostate cancer, where lamin A/C expression has been associated with higher risk tumours (Kong et al., 2012; Skvortsov et al., 2011). Low grade prostate cancers showed very little lamin A/C, suggesting that the pro-tumourigenic function of lamin A/C may arise in cooperation with other changes associated with high grade tumours, rather than as a general feature of prostate cancer.
TGF-β
Transforming growth factor-β (TGF-β) ligands bind to type I and type II receptors to activate Smad-dependent and Smad-independent signalling cascades (Moses and Barcellos-Hoff, 2011). Phosphorylation of Smads results in dimerization and translocation into the nucleus, where they act as co-activators and co-repressors interacting with an extensive collection of transcription factors (Ten Dijke et al., 2002). The outcome of TGF-β signalling is dependent on the transcription factors present and on activation of parallel signalling pathways (Gomis et al., 2006). This allows the TGF-β pathway to assume diverse, context-dependent roles in tissue development and homeostasis, acting as a mediator of EMT, ECM remodeling, apoptosis, and cell cycle arrest (Wu and Hill, 2009). Despite having tumour suppressive effects at the initiation stage of tumourigenesis, TGF-β can drive the progression and metastasis of established tumours and has been pursued as a therapeutic target (Papageorgis, 2015; Siegel et al., 2003; Tang et al., 2003).
Increasing findings suggest that A-type lamins and other NE proteins may play a part in determining the biological outcomes of TGF-β stimulation. Regulation of pRB is a primary mechanism through which TGF-β inhibits proliferation, and the absence of pRB can switch TGF-β signalling effects from growth-inhibitory to tumour-promoting. Dysregulation of pRB in lamin A/C-deficient MEFs diminishes the ability of TGF-β1 to decrease proliferation, and lamin A/C expression is required for TGF-β-stimulated dephosphorylation of pRB through regulation of protein phosphatase 2A (PP2A) (Van Berlo et al., 2005). Interestingly, lamin A/C levels also impact the kinetics of TGF-β1-induced Smad phosphorylation, with more rapid, intense, and shorter duration phosphorylation of Smad2/3 occurring in the absence of lamin A/C (Van Berlo et al., 2005). Lamin A/C may further co-ordinate responses to TGF-β signalling through regulation of other lamina-associated proteins, such as MAN1 and Nesprin-2. MAN1, an integral INM protein, binds directly to Smad2 and Smad3, possibly acting as a competitive inhibitor of formation of the transcriptionally active complex (Lin et al., 2005). Overexpression of MAN1 is sufficient to inhibit induction of both transcriptional activity and proliferation arrest downstream of TGF-β stimulation (Lin et al., 2005). In contrast, Nesprin-2 mediates TGF-β-induced nuclear translocation of Smad2/3 and c-fos (Rashmi et al., 2012). Therefore, the specific composition of the NE can promote or inhibit aspects of the TGF-β signalling program. Interestingly, increasing lamin A expression decreases secretion of TGF-β, demonstrating both upstream and downstream roles for lamins in TGF-β signalling (Evangelisti et al., 2015). Additionally, TGF-β1 stimulation was found to reduce the levels of lamin A/C in endothelial cells, and to promote proliferation and migration (Qi et al., 2011). Given the wide variety of phenotypes elicited by TGF-β, it will be interesting to determine whether changes in lamin levels can control the switch between the growth-suppressive and tumour-promoting effects of TGF-β in different stages or types of cancer.
MKL1/SRF
Megakaryoblastic leukemia protein-1 (MKL1, also termed MRTF-A or MAL) is a transcription coactivator of serum response factor (SRF) that regulates expression of genes involved in cell motility, growth, and differentiation (Pipes et al., 2006; Scharenberg et al., 2010). MKL1/SRF signalling has key functions in tumour progression, such as mediating TGF-β-induced EMT and promoting cell migration and metastasis (Kircher et al., 2015; Medjkane et al., 2009; Morita et al., 2007). MKL1 is localized in the cytoplasm through binding to G-actin, but mitogenic or mechanical stimulation results in actin polymerization and translocation of MKL1 to the nucleus, where it serves a co-factor for SRF to activate expression of numerous proteins involved in cytoskeletal organization, contractility, and adhesion, including integrins, vinculin, and actin (Olson and Nordheim, 2010). MKL1 is also regulated by nuclear actin dynamics, and loss of lamin A/C or emerin from the NE impairs nuclear translocation and signalling of MKL1 due to the role of emerin in controlling nuclear actin polymerization (Baarlink et al., 2013; Ho et al., 2013; Holaska et al., 2004). Consequently, the loss of nuclear accumulation of MKL1 and diminished MKL1/SRF signalling further alters cytoskeletal actin dynamics in lamin A/C- and emerin-deficient cells (Ho et al., 2013). Decreased MKL1/SRF transcriptional activity due to the absence of lamin A/C would be predicted to reduce invasive capabilities of tumour cells, suggesting that this pathway may provide selective pressure to retain a critical amount of lamin A/C, and select against LMNA gene deletions and dominant mutations.
Additional pathways
In addition to the above pathways, lamin A/C has been shown to influence NF-κB transcriptional activity (Lammerding et al., 2004), which can have tumour promoting and tumour suppressive effects (Perkins, 2004). Lamin A/C has also been found to influence the YAP transcriptional coactivator (Bertrand et al., 2014; Swift et al., 2013), which is a major downstream effector of the Hippo pathway and increasingly implicated in tumour progression (Moroishi et al., 2015). Furthermore, lamin A/C mutations responsible for HGPS disturb the Notch pathway (Scaffidi and Misteli, 2008), which is another signalling pathway that can have both oncogenic and tumour suppressor functions (Lobry et al., 2011). A-type lamins were also found to interact with the pro-tumourigenic transcription factors, c-Fos and MYC, and the sterol regulatory element-binding protein 1 (SREBP-1) transcription factor, which is a master regulator of pro-tumourigenic lipid metabolism (Guo et al., 2014; Ivorra et al., 2006; Lloyd et al., 2002; Myant et al., 2015). Interestingly, lamins may also impact tumourigenesis by modulating fatty acid metabolism. Lamin A was identified in a yeast two-hybrid screen for interactors of the pro-tumourigenic enzyme 12-lipoxygenase (12-LOX) (Tang et al., 2000). LOX enzymes are implicated in carcinogenesis by mediating conversion of polyunsaturated fatty acids into metabolites involved in modulating diverse signalling pathways (Shureiqi and Lippman, 2001). 12-LOX has been implicated in promoting survival, growth, and metastasis in many human cancers (Agarwal et al., 2009; Dilly et al., 2013; Honn et al., 1994; Kerjaschki et al., 2011; Klampfl et al., 2012). Further study is required to determine whether the regulation of these pathways by lamin A/C contributes to tumourigenesis and metastasis.
Mechanisms controlling lamin A/C levels in cancer
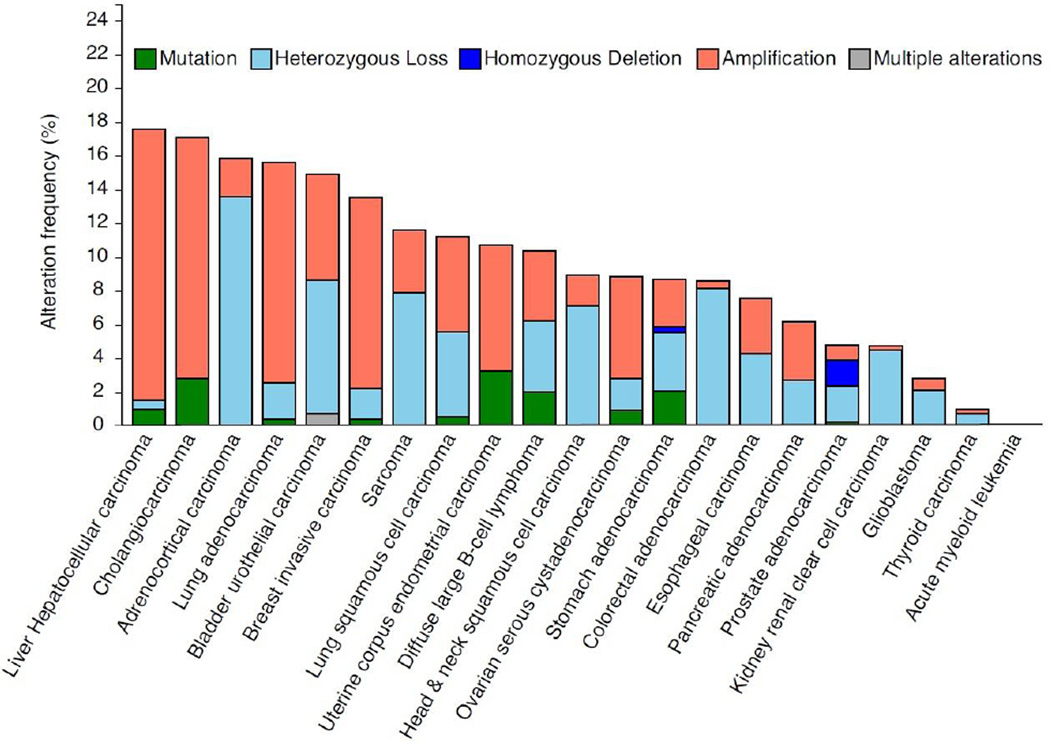
Mutations in the LMNA gene are responsible for the various laminopathies, but genomic analysis of human tumours suggests that LMNA mutations, deletions, or copy-number variations are not a common feature of most cancers (Fig. 3). However, it is interesting to note the recent identification of a translocation between the tropomyosin-receptor kinase (TRK) and LMNA genes resulting in a fusion product, LMNA-NTRK1, in metastatic colorectal cancer and congenital infantile fibrosarcoma (Sartore-Bianchi et al., 2016; Wong et al., 2016). In both cases, most of the LMNA gene (exons 1–10 or 1–11) is fused to an intact NTRK1 kinase domain, and tumours responded to tyrosine kinase inhibition. However, the mechanistic role of the LMNA portion of the fusion protein in affecting kinase activation, localization, or substrate selection requires further study. Despite the relatively infrequent genomic alterations, changes in lamin A/C expression are common in many cancers at the protein and transcript level, suggesting the involvement of epigenetic, transcriptional, and/or post-translational mechanisms of regulation.
Figure 3. Frequency of mutations and copy-number alterations in the LMNA gene in human cancers.
The results shown here are based upon data generated by the TCGA Research Network (http://cancergenome.nih.gov/) and analyzed via cBioPortal (http://www.cbioportal.org/) (Cerami et al., 2012; Gao et al., 2013) on April 10, 2016. These results should therefore not be viewed as original data, but as reference to existing publicly available resources. In the analysis, shallow loss determined by copy-numberanalysis is referred to here as “heterozygous deletion” and deep loss is referred to as “homozygous deletion”. Copy number variations make up the majority of genomic changes in the LMNA gene in cancer, whereas mutations are rare. It remains to be determined whether LMNA genomic alterations correlate closely with protein levels in cancer, and which transcriptional and/or post-translational mechanisms are important contributors to altered lamin A/C expression in cancer.
LMNA gene expression
Transcript levels of lamin A/C are governed by epigenetic modifications, transcription factor activity, and microRNAs. The retinoic acid receptor transcription factors have been shown to act in regulation of lamin A/C (Okumura et al., 2004; Swift et al., 2013), and CpG island promoter hypermethylation is responsible for decreased lamin A/C expression in hematologic cancers and associated with poor prognosis in lymphoma (Agrelo et al., 2005). However, LMNA promoter hypermethylation was not detected in other cancer types, suggesting alternative mechanisms for altered lamin A/C levels in these cases (Agrelo et al., 2005; Lee et al., 2012). One such mechanism could be the microRNA miR-9, which represses lamin A, but not lamin C, in the brain (Jung et al., 2012). Altered miR-9 expression occurs in cancer, and miR-9 has multiple targets that may contribute to tumourigenesis (Cekaite et al., 2012; Lu et al., 2014; Ma et al., 2007). For example, expression of the MYC proto-oncogene in breast cancer cells increases miR-9 expression, which in turn promotes invasion and metastasis through downregulation of the cell-cell adhesion protein, E-cadherin (Ma et al., 2010). Therefore, regulation of lamin A/C by miR-9 may be coordinated as part of a larger metastasis-related signalling network. Akt signalling has also been shown to control LMNA gene expression, demonstrating that multiple signalling programs could contribute to transcriptional regulation of lamin A/C (Bertacchini et al., 2013).
Although lamins A and C are both expressed from the LMNA gene, regulation of splicing can control the relative expression levels of these two proteins. Alternative splicing of the LMNA gene contributes to the large variation in tissue-specific differences between expression of lamin A and C (Aljada et al., 2016). Importantly, relative to normal tissues, the ratio of lamin C to lamin A mRNA was found to be elevated in cancers from breast, colon, liver, lung, ovary, thyroid, and prostate (Aljada et al., 2016). Most commonly used antibodies cannot distinguish between lamin A and C, which are identical for the N-terminal 566 amino acids. Nonetheless, differentiating between lamin A and lamin C will be important when determining the relationship between lamin expression and prognosis, as these isoforms may have distinct effects on cancer progression. For example, in epithelial ovarian cancer, downregulation of lamin A, but not lamin C, is associated with metastasis and decreased disease-free survival (Gong et al., 2015). These results reveal that regulation of LMNA splicing may play an important role in fine-tuning the properties of the nucleus in different tissues, and may also provide an important point of dysregulation in cancer.
Post-translational modifications of lamins A/C
The assembly, function, and stability of lamin A/C is also regulated by post-translational modifications. The best characterized lamin post-translational modification is phosphorylation. CDK1- and/or PKC-mediated phosphorylation of lamins disrupts head-to-tail polymer formation promoting lamina disassembly during mitosis (Collas, 1999; Peter et al., 1990). There are 61 known lamin A/C phosphorylation sites, with Ser-22, Ser-392, Ser-404, and Ser-406 being key residues for mitotic lamin depolymerization (Simon and Wilson, 2013). Recent studies found that phosphorylation can also modulate lamin assembly and function during interphase (Buxboim et al., 2014; Kochin et al., 2014). Changes in interphase lamin A/C phosphorylation and subsequent degradation in response to substrate stiffness are thought to regulate the stiffness-dependent levels of lamin A/C (Buxboim et al., 2014; Swift et al., 2013). Stiffer matrix and myosin-IIA activity decreases levels of lamin A Ser22 phosphorylation, while cyclin-dependent kinase (CDK)-mediated increases in Ser22 phosphorylation occurred upon reduction of stiffness and nuclear tension (Buxboim et al., 2014).
Intriguingly, lamin A is also a nuclear substrate for Akt phosphorylation (Barati et al., 2006; Cenni et al., 2008). Akt-mediated Ser-404 phosphorylation targets prelamin A for degradation, and Akt-mediated degradation of lamin A is important for normal epidermal differentiation (Bertacchini et al., 2013; Cenni et al., 2008; Naeem et al., 2015). Since dysregulated PI3K/Akt signalling is frequently associated with tumourigenesis, this suggests a potential mechanism for downregulation of lamin A/C in cancer.
Other post-translational modifications that could impact lamin A/C assembly and function are ubiquitination, and acetylation, and the covalent addition of small ubiquitin-like modifier (SUMO) proteins (Simon et al., 2013; Simon and Wilson, 2013; Zhang and Sarge, 2008). Furthermore, the addition of β-O-linked N-acetylglucosamine, termed O-GlcNAcylation, to lamins has long been recognized, but the impact of this modification on lamin functions remains incompletely understood (Ferraro et al., 1989; Simon and Wilson, 2013). An additional mechanism for post-translational modification of lamins is the oxidation of cysteine residues in the tail domain of lamin A, resulting in disulphide bridges in response to oxidative stress (Pekovic et al., 2011). Thus, there are many possible avenues for post-translationally tuning lamin A/C stability and organization, but a functional understanding of these modifications and their consequences is still lacking.
Lamins and the mechanical tumour microenvironment
It is increasingly becoming apparent that the mechanical tissue microenvironment is playing a crucial role in tumourigenesis and tumour progression (Pickup et al., 2014; Wei and Yang, 2016). The tumour microenvironment is composed of various cell types, ECM proteins, blood vessels, lymphatics, and soluble factors that together create a niche that can support or hinder tumour progression (Quail and Joyce, 2013). During tumourigenesis, remodeling the cellular and ECM architecture in the tissue alters the function of tumour and stromal cells, which can conversely further remodel the microenvironment (Yu et al., 2011). In particular, ECM composition and rigidity modulate signalling pathways associated with tumour progression, such as ERK, TGFβ, and PI3K, thereby affecting EMT and metastasis (Chaudhuri et al., 2014; Leight et al., 2012; Levental et al., 2009; Paszek et al., 2005; Pickup et al., 2014; Wei and Yang, 2016). Increased stiffness in the stroma surrounding the tumour was shown to accompany tumour progression in a mouse mammary tumour model, and increasing stiffness in vitro is sufficient to convert mammary epithelial cells to an invasive, malignant phenotype (Chaudhuri et al., 2014; Levental et al., 2009; Paszek et al., 2005).
Recent studies suggest that lamins could play important roles both upstream and downstream of this mechanosensitive process (Irianto et al., 2016). Since lamins and emerin play a central role in the mechanoregulation of gene expression, changes in lamin levels could influence how cells interpret and respond to changes in their mechanical environment. (Guilluy et al., 2014; Ho et al., 2013; Lammerding et al., 2005; Lammerding et al., 2004). Conversely, a proteomic analysis of soft and stiff tissues revealed that lamin A/C increases with tissue stiffness, whereas B-type lamins exhibited fairly constant abundance, and that the increase in lamin A/C may contribute to lineage specification in differentiation (Swift et al., 2013). Furthermore, xenograft tumours of U251 glioblastoma cells exhibit higher lamin A/C levels when grown in the stiffer subcutaneous flank compared to the brain, suggesting that A-type lamin levels can adjust to tissue stiffness in vivo (Swift et al., 2013). However, it cannot be excluded that lamin A/C levels could be affected by other differences in the properties of these microenvironments, such as levels of tissue-specific growth factors, local metabolite concentrations, or other signaling pathways. In an in vitro model of scar tissue, lamin A lev-els in mesenchymal stem cells increased with increasing matrixrigidity, further supporting regulation of lamin A/C levels as a func-tion of the mechanical environment (Dingal et al., 2015). In addition to modulating total levels of lamin A/C, the physical properties of the microenvironment can also affect intranuclear lamina organization. For example, with increased substrate stiffness and cell spreading, a lamin A/C conformational epitope in the Ig-domain becomes masked in the basal portion of the NE (Ihalainen et al., 2015), suggesting structural rearrangements within the lamina that could further impact interactions of lamins with chromatin and other binding partners. These studies demonstrate that organization and levels of lamin A/C are both dynamically regulated in response to the physical microenvironment and can modulate cellular mechanotransduction signalling.
Altered lamin A/C levels could in turn influence the microenvironment, as increased collagen deposition and fibrosis are features of multiple laminopathies, and loss of lamin A/C increases collagen production in MEFs (Arimura et al., 2005; Hernandez et al., 2010; Van Berlo et al., 2005; van Tintelen et al., 2007). This suggests that lamin A/C levels could respond to cancer-associated changes in the microenvironment to influence not only nuclear structure, but also feedback to remodel the tissue structure and microenvironment. Further research is required to understand how changes in lamin A/C contribute to ECM alterations in cancer, and whether the mechanosensitive scaling of lamin A/C remains generally intact in cancer cells and contributes to tumour pathology.
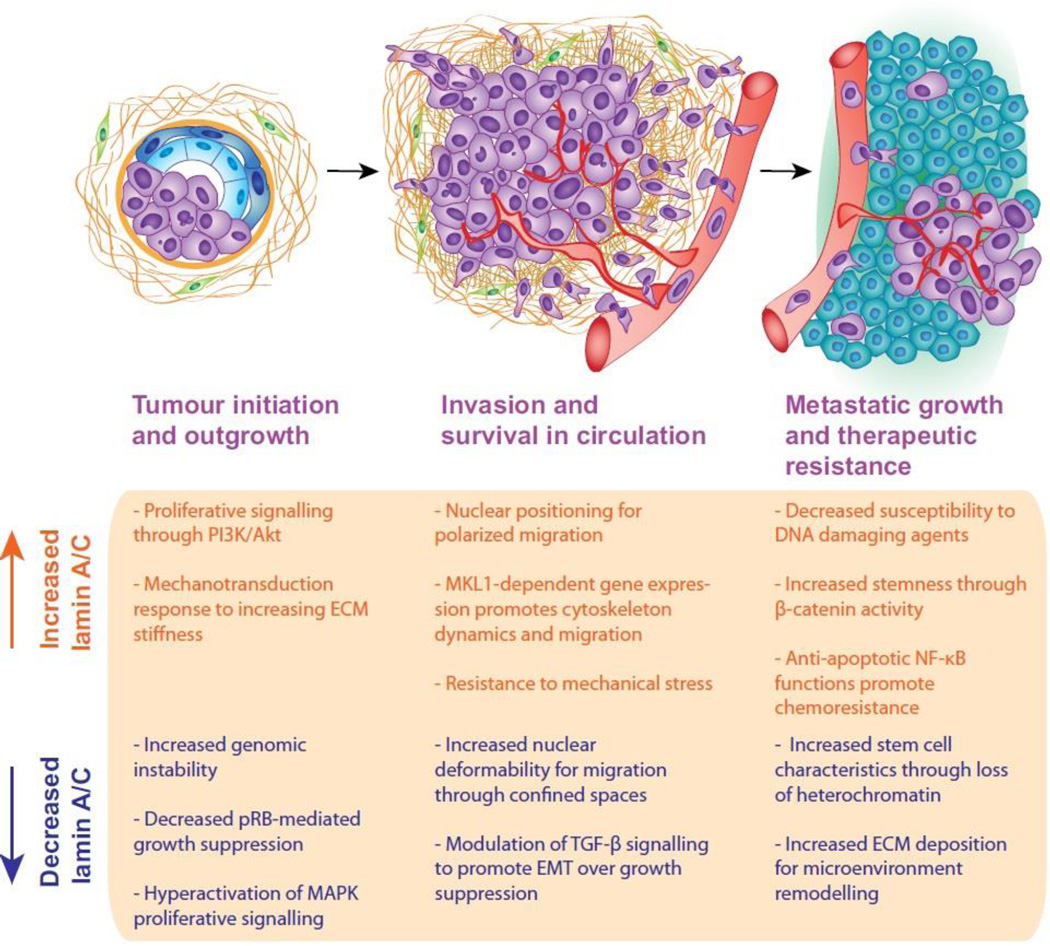
Enhanced stiffness in the tumour microenvironment would be expected to affect lamin A/C levels, which could further promote tumour progression. Importantly, increased tissue rigidity is not a uniform characteristic of tumours and has been shown to vary between subtypes and within single tumours (Acerbi et al., 2015). In breast cancers, increased rigidity is commonly observed at the tumour edge, with high stiffness and greater variability in the invasive front (Acerbi et al., 2015; Plodinec et al., 2012; Provenzano et al., 2006). These findings, along with the ability of increased stiffness to promote EMT-like phenotypes, support the hypothesis that tissue rigidity promotes invasion and metastasis (Pickup et al., 2014; Wei and Yang, 2016). However, invasive breast tumours have more heterogeneity in tissue stiffness than benign tumours, which have a more uniform increase in stiffness over normal tissue (Plodinec et al., 2012). Interestingly, studies of stiffness in mouse mammary tumour models found that increased metastasis was associated with more compliant tumours, and lung metastases display lower stiffness than matched primary tumours (Fenner et al., 2014; Plodinec et al., 2012). The idea that A-type lamins may be regulated by rigidity in these different environments is particularly exciting when viewed from the perspective of lamin A/C controlling differentiation and plasticity. During tumourigenesis, increased tissue-stiffness could modulate lamin A/C levels and intranuclear distribution, thereby disturbing chromatin organization and gene expression. During metastasis, outgrowth at the secondary site is thought to be the rate-limiting step in the metastatic cascade, and could require a switch from an EMT to a MET phenotype (Luzzi et al., 1998; Tsai and Yang, 2013). Thus, phenotypic flexibility could be the true driver of tumour progression, and may be directly linked to changes in lamin levels and intranuclear organization. In this scenario, it is attractive to imagine that increased rigidity may promote invasion and escape from the primary tumour, but perhaps a softer microenvironment at the secondary site leads to decreased lamin A/C levels that support the enhanced stem cell-associated plasticity required for outgrowth and metastasis (Fig. 4).
Figure 4. Potential roles for lamin A/C in tumour initiation and progression.
Both increased and decreased levels of lamin A/C have been observed in cancer and related to patient prognosis. The complexity of lamin A/C functions and signalling networks suggests that the role of lamin A/C in tumour progression may vary with stage of disease, tissue of origin, and mutational landscape of a given tumour. Possible functions for increased and decreased lamin A/C levels during tumour progression are depicted here, and warrant further investigation towards understanding the role of lamin A/C in cancer. Importantly, many of these lamin-related functions could contribute to multiple stages of cancer, but are each shown here only once for simplicity.
Conclusions and future perspectives
Both increased and decreased lamin A/C levels have been associated with poor prognosis in human cancers. This paradox could arise from tissue-specific functions, variation in expression within a single tumour, or dynamic changes in response to biochemical or mechanical signals throughout tumour progression (Fig. 4). Regardless, this discrepancy warrants further investigation to understand in what contexts increased or decreased lamin A/C promotes tumour progression. The infrequency of cancer-associated deletions or mutations in the LMNA gene suggests that selective pressures exist for retention of a minimum level of expression or the ability to upregulate lamin A/C for cellular responses to the changing tumour environment. It is still unclear whether lamin A/C levels in cancer are a dynamic representation of conditions in the tumour at a particular time, and thus a reflection of response to transformed signalling pathways and microenvironmental conditions, or whether altered lamin A/C levels in cancer are uncoupled from normal regulatory mechanisms and can independently initiate oncogenic changes.
The NE participates in complex regulatory loops, and determining the function of lamin A/C in cancer will require studies that can distinguish between the causes and consequences of altered lamin A/C in particular tumour types and disease stages. Lamin A/C influences EMT and stem cell phenotypes, which are interconnected cellular programs and important mediators of metastasis and therapeutic resistance (Mani et al., 2008; Shuang et al., 2014). It will be pertinent to determine whether lamin A/C expression in CSCs and metastatic subpopulations is different compared to the bulk primary tumour, and whether this is associated with specific therapeutic vulnerabilities. The use of inducible systems to modify lamin levels at specific stages of in vivo tumour xenograft experiments could be instrumental in answering some of these unresolved questions. Tissue-specific alteration of lamin A/C in transgenic mouse models will also be critical to definitively determine the function of lamin A/C during the full scope of disease progression from normal tissue to tumour initiation and through to metastasis.
One exciting future direction is the potential exploitation of lamin A/C in targeted therapies. It is possible that with a better understanding of lamin A/C regulation and functions, altered lamin levels and organization could serve as a biomarkers for response to specific therapeutics. Lamin A/C influences pathways known to have roles in both tumour suppression and tumour progression, such as TGF-β, Notch, HSP90, and NF-κB. It is intriguing to imagine that changes in lamin A/C levels and/or organization in cancer act to promote a shift towards the tumourigenic functions of these pathways, and therefore modulating lamin A/C expression could be a therapeutic approach for restoration of tumour suppressor function. Additionally, understanding the role of lamin A/C expression in metastasis could lead to improvements in therapy and patient outcome. For example, cells with lower lamin A/C levels may self-select during invasion in tissue environments due to enhanced nuclear deformability, but are also more susceptible to NE rupture, and may have diminished DNA damage response capabilities. This suggests that inhibiting DNA repair pathways could be an effective strategy to eliminate cells in the process of metastasizing, and tumours with low lamin A/C levels could be particularly susceptible to DNA damaging agents. Thus, while many open questions remain regarding the role of lamins in tumour initiation and progression, rapidly emerging findings suggest that we are entering an exciting area of growth and discovery as lamin A/C is increasingly appreciated as a central node regulating multiple aspects of the tumourigenic process, with important diagnostic, prognostic, and therapeutic applications.
Acknowledgments
The authors apologize to all researchers whose work could not be cited due to space constraints. This work was supported by awards from the National Institutes of Health [R01 HL082792], the National Science Foundation [CBET-1254846], and the Department of Defense [Breast Cancer Breakthrough BC150580]. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation or the National Institutes of Health.
Abbreviations
- 12-LOX
12-lipoxygenase
- 2D
two-dimensional
- 3D
three-dimensional
- CDK
cyclin-dependent kinase
- CSC
cancer stem cell
- ECM
extracellular matrix
- EDMD
Emery-Dreifuss muscular dystrophy
- EMT
epithelial-mesenchymal transition
- ERK1/2
extracellular signal-regulated kinase 1/2
- ESCRT
endosomal sorting complex required for transport
- GI
gastrointestinal
- HGPS
Hutchinson-Gilford progeria syndrome
- HSP90
heat shock protein 90
- INM
inner nuclear membrane
- iPSC
induced pluripotent stem cell
- JNK
c-Jun N-terminal kinase
- KASH
Klarsicht, ANC-1, and Syne homology
- LAP2α
lamina-associated polypeptide 2α
- LINC
linker of nucleoskeleton and cytoskeleton
- MAPK
Mitogen activated protein kinase
- MEF
mouse embryonic fibroblast
- MKL1
Megakaryoblastic leukemia protein-1
- MMP
matrix metalloproteases
- NAT
N-acetyltransferase
- NE
nuclear envelope
- ONM
outer nuclear membrane
- PI3K
phosphoinositide 3-kinase
- PP2A
protein phosphatase 2A
- pRB
retinoblastoma protein
- SREBP-1
sterol regulatory element-binding protein 1
- SRF
serum response factor
- SUN
Sad1 and UNC-84
- TGF-β
transforming growth factor-β
- TRF2
telomere repeat-binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015;7:1120–1134. doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Achari C, Praveen D, Roy KR, Reddy GV, Reddanna P. Inhibition of 12-LOX and COX-2 reduces the proliferation of human epidermoid carcinoma cells (A431) by modulating the ERK and PI3K–Akt signalling pathways. Exp Dermatol. 2009;18:939–946. doi: 10.1111/j.1600-0625.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- Agrelo R, Setien F, Espada J, Artiga MJ, Rodriguez M, Perez-Rosado A, Sanchez-Aguilera A, Fraga MF, Piris MA, Esteller M. Inactivation of the lamin A/C gene by CpG island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-cell lymphoma. J Clin Oncol. 2005;23:3940–3947. doi: 10.1200/JCO.2005.11.650. [DOI] [PubMed] [Google Scholar]
- Alberio R, Johnson AD, Stick R, Campbell KH. Differential nuclear remodeling of mammalian somatic cells by Xenopus laevis oocyte and egg cytoplasm. Exp Cell Res. 2005;307:131–141. doi: 10.1016/j.yexcr.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Aljada A, Doria J, Saleh AM, Al-Matar SH, AlGabbani S, Shamsa HB, Al-Bawab A, Ahmed AA. Altered Lamin A/C splice variant expression as a possible diagnostic marker in breast cancer. Cell Oncol (Dordr) 2016;39:161–174. doi: 10.1007/s13402-015-0265-1. [DOI] [PubMed] [Google Scholar]
- Andres V, Gonzalez JM. Role of A-type lamins in signaling, transcription, and chromatin organization. J Cell Biol. 2009;187:945–957. doi: 10.1083/jcb.200904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, Lacene E, Fromes Y, Toussaint M, Mura AM, Keller DI, Amthor H, Isnard R, Malissen M, Schwartz K, Bonne G. Mouse model carrying H222P–Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science. 2013;340:864–867. doi: 10.1126/science.1235038. [DOI] [PubMed] [Google Scholar]
- Barati MT, Rane MJ, Klein JB, McLeish KR. A proteomic screen identified stress-induced chaperone proteins as targets of Akt phosphorylation in mesangial cells. Journal of Proteome Research. 2006;5:1636–1646. doi: 10.1021/pr0502469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt EJ, Fijneman RJ, van den Berg EG, Bril H, Delis-van Diemen PM, Tijssen M, van Essen HF, de Lange-de Klerk ES, Belien JA, Stockmann HB, Meijer S, Meijer GA. Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence. Eur J Cancer. 2011;47:1837–1845. doi: 10.1016/j.ejca.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Bermeo S, Vidal C, Zhou H, Duque G. Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/beta-catenin pathway. J Cell Biochem. 2015;116:2344–2353. doi: 10.1002/jcb.25185. [DOI] [PubMed] [Google Scholar]
- Bertacchini J, Beretti F, Cenni V, Guida M, Gibellini F, Mediani L, Marin O, Maraldi NM, de Pol A, Lattanzi G, Cocco L, Marmiroli S. The protein kinase Akt/PKB regulates both prelamin A degradation and Lmna gene expression. FASEB J. 2013;27:2145–2155. doi: 10.1096/fj.12-218214. [DOI] [PubMed] [Google Scholar]
- Bertrand AT, Ziaei S, Ehret C, Duchemin H, Mamchaoui K, Bigot A, Mayer M, Quijano-Roy S, Desguerre I, Laine J, Ben Yaou R, Bonne G, Coirault C. Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J Cell Sci. 2014;127:2873–2884. doi: 10.1242/jcs.144907. [DOI] [PubMed] [Google Scholar]
- Blanco R, Munoz P, Flores JM, Klatt P, Blasco MA. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev. 2007;21:206–220. doi: 10.1101/gad.406207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslavsky RL, Stewart CL, Worman HJ. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2006;15:653–663. doi: 10.1093/hmg/ddi480. [DOI] [PubMed] [Google Scholar]
- Boyd J, Pienta KJ, Getzenberg RH, Coffey DS, Barrett JC. Preneoplastic alterations in nuclear morphology that accompany loss of tumor suppressor phenotype. J Natl Cancer Inst. 1991;83:862–866. doi: 10.1093/jnci/83.12.862. [DOI] [PubMed] [Google Scholar]
- Broers JL, Machiels BM, Kuijpers HJ, Smedts F, van den Kieboom R, Raymond Y, Ramaekers FC. A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol. 1997;107:505–517. doi: 10.1007/s004180050138. [DOI] [PubMed] [Google Scholar]
- Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, Baaijens FP, Ramaekers FC. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- Bronshtein I, Kepten E, Kanter I, Berezin S, Lindner M, Redwood AB, Mai S, Gonzalo S, Foisner R, Shav-Tal Y, Garini Y. Loss of lamin A function increases chromatin dynamics in the nuclear interior. Nat Commun. 2015;6:8044. doi: 10.1038/ncomms9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru T, Clarke C, McGrew MJ, Sang HM, Wilmut I, Blow JJ. Rapid induction of pluripotency genes after exposure of human somatic cells to mouse ES cell extracts. Exp Cell Res. 2008;314:2634–2642. doi: 10.1016/j.yexcr.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati G, Maletta F, Asioli S, Annaratone L, Sapino A, Marchio C. “To be or not to be in a good shape”: diagnostic and clinical value of nuclear shape irregularities in thyroid and breast cancer. Adv Exp Med Biol. 2014;773:101–121. doi: 10.1007/978-1-4899-8032-8_5. [DOI] [PubMed] [Google Scholar]
- Bussolati G, Marchio C, Gaetano L, Lupo R, Sapino A. Pleomorphism of the nuclear envelope in breast cancer: a new approach to an old problem. J Cell Mol Med. 2008;12:209–218. doi: 10.1111/j.1582-4934.2007.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M, Misteli T. Nongenetic functions of the genome. Science. 2016;352:aad6933. doi: 10.1126/science.aad6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A, Kao YR, Cho S, Harada T, Shin JW, Discher DE. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol. 2014;24:1909–1917. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario J, Sudhakar S, Navarro S, Reddy S, Comai L. Perturbation of wild-type lamin A metabolism results in a progeroid phenotype. Aging Cell. 2008;7:355–367. doi: 10.1111/j.1474-9726.2008.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekaite L, Rantala JK, Bruun J, Guriby M, Agesen TH, Danielsen SA, Lind GE, Nesbakken A, Kallioniemi O, Lothe RA, Skotheim RI. MiR-9, -31, and -182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia. 2012;14:868–879. doi: 10.1593/neo.121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenni V, Bertacchini J, Beretti F, Lattanzi G, Bavelloni A, Riccio M, Ruzzene M, Marin O, Arrigoni G, Parnaik V, Wehnert M, Maraldi NM, de Pol A, Cocco L, Marmiroli S. Lamin A Ser404 is a nuclear target of Akt phosphorylation in C2C12 cells. J Proteome Res. 2008;7:4727–4735. doi: 10.1021/pr800262g. [DOI] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Koshy ST, Branco da Cunha C, Shin JW, Verbeke CS, Allison KH, Mooney DJ. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Zhang Y, Frock RL, Lewis SM, Molinie B, Ho YJ, Myers DR, Choi VW, Compagno M, Malkin DJ, Neuberg D, Monti S, Giallourakis CC, Gostissa M, Alt FW. Genome-wide translocation sequencing reveals mechanisms of chromosome breaks and rearrangements in B cells. Cell. 2011;147:107–119. doi: 10.1016/j.cell.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KH, Factor RE, Ullman KS. The nuclear envelope environment and its cancer connections. Nat Rev Cancer. 2012;12:196–209. doi: 10.1038/nrc3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Collas P. Sequential PKC- and Cdc2-mediated phosphorylation events elicit zebrafish nuclear envelope disassembly. J Cell Sci. 1999;112(Pt 6):977–987. doi: 10.1242/jcs.112.6.977. [DOI] [PubMed] [Google Scholar]
- Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Davidson PM, Denais C, Bakshi MC, Lammerding J. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell Mol Bioeng. 2014;7:293–306. doi: 10.1007/s12195-014-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Las Heras JI, Schirmer EC. The nuclear envelope and cancer: a diagnostic perspective and historical overview. Adv Exp Med Biol. 2014;773:5–26. doi: 10.1007/978-1-4899-8032-8_1. [DOI] [PubMed] [Google Scholar]
- De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J, Manders EM, Verstraeten VL, van Steensel MA, Marcelis CL, van den Wijngaard A, Vaux DJ, Ramaekers FC, Broers JL. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum Mol Genet. 2011;20:4175–4186. doi: 10.1093/hmg/ddr344. [DOI] [PubMed] [Google Scholar]
- Denais C, Lammerding J. Nuclear mechanics in cancer. Adv Exp Med Biol. 2014;773:435–470. doi: 10.1007/978-1-4899-8032-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denais CM, Gilbert RM, Isermann P, McGregor AL, Te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Dilly AK, Ekambaram P, Guo Y, Cai Y, Tucker SC, Fridman R, Kandouz M, Honn KV. Platelet-type 12-lipoxygenase induces MMP9 expression and cellular invasion via activation of PI3K/Akt/NF-kappaB. Int J Cancer. 2013;133:1784–1791. doi: 10.1002/ijc.28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingal PC, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, Discher DE. Fractal heterogeneity in minimal matrix models of scars modulatesstiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat.Mater. 2015;14:951–960. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerschuk CM, Beyers N, Coxson HO, Wiggs B, Hogg JC. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol (1985) 1993;74:3040–3045. doi: 10.1152/jappl.1993.74.6.3040. [DOI] [PubMed] [Google Scholar]
- Dorner D, Vlcek S, Foeger N, Gajewski A, Makolm C, Gotzmann J, Hutchison CJ, Foisner R. Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J Cell Biol. 2006;173:83–93. doi: 10.1083/jcb.200511149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson LJ, Holt MR, Wheeler MA, Wehnert M, Parsons M, Ellis JA. Defects in cell spreading and ERK1/2 activation in fibroblasts with lamin A/C mutations. Biochim Biophys Acta. 2009;1792:810–821. doi: 10.1016/j.bbadis.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Espada J, Varela I, Flores I, Ugalde AP, Cadinanos J, Pendas AM, Stewart CL, Tryggvason K, Blasco MA, Freije JM, Lopez-Otin C. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J Cell Biol. 2008;181:27–35. doi: 10.1083/jcb.200801096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- Evangelisti C, Bernasconi P, Cavalcante P, Cappelletti C, D’Apice MR, Sbraccia P, Novelli G, Prencipe S, Lemma S, Baldini N, Avnet S, Squarzoni S, Martelli AM, Lattanzi G. Modulation of TGFbeta 2 levels by lamin A in U2-OS osteoblast-like cells: understanding the osteolytic process triggered by altered lamins. Oncotarget. 2015;6:7424–7437. doi: 10.18632/oncotarget.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner J, Stacer AC, Winterroth F, Johnson TD, Luker KE, Luker GD. Macroscopic stiffness of breast tumors predicts metastasis. Sci Rep. 2014;4:5512. doi: 10.1038/srep05512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro A, Eufemi M, Cervoni L, Marinetti R, Turano C. Glycosylated forms of nuclear lamins. FEBS Lett. 1989;257:241–246. doi: 10.1016/0014-5793(89)81543-1. [DOI] [PubMed] [Google Scholar]
- Ferrera D, Canale C, Marotta R, Mazzaro N, Gritti M, Mazzanti M, Capellari S, Cortelli P, Gasparini L. Lamin B1 overexpression increases nuclear rigidity in autosomal dominant leukodystrophy fibroblasts. FASEB J. 2014;28:3906–3918. doi: 10.1096/fj.13-247635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4:e1000039. doi: 10.1371/journal.pgen.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AH. The diagnostic pathology of the nuclear envelope in human cancers. Adv Exp Med Biol. 2014;773:49–75. doi: 10.1007/978-1-4899-8032-8_3. [DOI] [PubMed] [Google Scholar]
- Fischer AH, Bond JA, Taysavang P, Battles OE, Wynford-Thomas D. Papillary thyroid carcinoma oncogene (RET/PTC) alters the nuclear envelope and chromatin structure. Am J Pathol. 1998;153:1443–1450. doi: 10.1016/S0002-9440(10)65731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500. doi: 10.1101/gad.1364906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiova G, Bartova E, Raska I, Krejci J, Kozubek S. Chromatin changes induced by lamin A/C deficiency and the histone deacetylase inhibitor trichostatin A. Eur J Cell Biol. 2008;87:291–303. doi: 10.1016/j.ejcb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- Gibbs-Seymour I, Markiewicz E, Bekker-Jensen S, Mailand N, Hutchison CJ. Lamin A/C-dependent interaction with 53BP1 promotes cellular responses to DNA damage. Aging Cell. 2015;14:162–169. doi: 10.1111/acel.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massague J. A FoxO-Smad synexpression group in human keratinocytes. Proc Natl Acad Sci U S A. 2006;103:12747–12752. doi: 10.1073/pnas.0605333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Chen P, Li L, Tan H, Zhou J, Zhou Y, Yang X, Wu X. Loss of lamin A but not lamin C expression in epithelial ovarian cancer cells is associated with metastasis and poor prognosis. Pathol Res Pract. 2015;211:175–182. doi: 10.1016/j.prp.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Navarro-Puche A, Casar B, Crespo P, Andres V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J Cell Biol. 2008;183:653–666. doi: 10.1083/jcb.200805049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, Morgado-Palacin L, Gapud EJ, Sleckman BP, Sullivan T, Sage J, Stewart CL, Mai S, Gonzalo S. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 2009;28:2414–2427. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S. DNA damage and lamins. Adv Exp Med Biol. 2014;773:377–399. doi: 10.1007/978-1-4899-8032-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y, Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem. 2015;84:131–164. doi: 10.1146/annurev-biochem-060614-034115. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y, Medalia O. Lamins: the structure and protein complexes. Curr Opin Cell Biol. 2015;32:7–12. doi: 10.1016/j.ceb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Guarda A, Bolognese F, Bonapace IM, Badaracco G. Interaction between the inner nuclear membrane lamin B receptor and the heterochromatic methyl binding protein, MeCP2. Exp Cell Res. 2009;315:1895–1903. doi: 10.1016/j.yexcr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, van Steensel B. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Bell EH, Mischel P, Chakravarti A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr Pharm Des. 2014;20:2619–2626. doi: 10.2174/13816128113199990486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harada T, Swift J, Irianto J, Shin JW, Spinler KR, Athirasala A, Diegmiller R, Dingal PC, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol. 2014;204:669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughian JM, Reno EM, Thorne AM, Bradford AP. Protein kinase C alpha-dependent signaling mediates endometrial cancer cell growth and tumorigenesis. Int J Cancer. 2009;125:2556–2564. doi: 10.1002/ijc.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Roux KJ, Wong ES, Mounkes LC, Mutalif R, Navasankari R, Rai B, Cool S, Jeong JW, Wang H, Lee HS, Kozlov S, Grunert M, Keeble T, Jones CM, Meta MD, Young SG, Daar IO, Burke B, Perantoni AO, Stewart CL. Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev Cell. 2010;19:413–425. doi: 10.1016/j.devcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer MW. The nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000539. doi: 10.1101/cshperspect.a000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Lammerding J. Lamins at a glance. J Cell Sci. 2012;125:2087–2093. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska JM, Kowalski AK, Wilson KL. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2004;2:E231. doi: 10.1371/journal.pbio.0020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn KV, Tang DG, Gao X, Butovich IA, Liu B, Timar J, Hagmann W. 12-lipoxygenases and 12(S)-HETE: role in cancer metastasis. Cancer Metastasis Rev. 1994;13:365–396. doi: 10.1007/BF00666105. [DOI] [PubMed] [Google Scholar]
- Houben F, Willems CH, Declercq IL, Hochstenbach K, Kamps MA, Snoeckx LH, Ramaekers FC, Broers JL. Disturbed nuclear orientation and cellular migration in A-type lamin deficient cells. Biochim Biophys Acta. 2009;1793:312–324. doi: 10.1016/j.bbamcr.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Hutchison CJ. The role of DNA damage in laminopathy progeroid syndromes. Biochem Soc Trans. 2011;39:1715–1718. doi: 10.1042/BST20110700. [DOI] [PubMed] [Google Scholar]
- Ihalainen TO, Aires L, Herzog FA, Schwartlander R, Moeller J, Vogel V. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat Mater. 2015;14:1252–1261. doi: 10.1038/nmat4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irianto J, Pfeifer CR, Ivanovska IL, Swift J, Discher D. Nuclear lamins in cancer. Cellular and Molecular Bioengineering. 2016;9:258–267. doi: 10.1007/s12195-016-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol. 2013;23:R1113–R1121. doi: 10.1016/j.cub.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O’Connor JE, Burke B, Andres V. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20:307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, Harlow E, Kennedy BK. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci U S A. 2004;101:9677–9682. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Thijssen B, McDermott U, Garnett M, Wessels LF, Bernards R. Targeting the RB-E2F pathway in breast cancer. Oncogene. 2016 doi: 10.1038/onc.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Coffinier C, Choe Y, Beigneux AP, Davies BS, Yang SH, Barnes RH, 2nd, Hong J, Sun T, Pleasure SJ, Young SG, Fong LG. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc Natl Acad Sci U S A. 2012;109:E423–E431. doi: 10.1073/pnas.1111780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, Bartel G, Krieger S, Kalt R, Hantusch B, Keller T, Nagy-Bojarszky K, Huttary N, Raab I, Lackner K, Krautgasser K, Schachner H, Kaserer K, Rezar S, Madlener S, Vonach C, Davidovits A, Nosaka H, Hammerle M, Viola K, Dolznig H, Schreiber M, Nader A, Mikulits W, Gnant M, Hirakawa S, Detmar M, Alitalo K, Nijman S, Offner F, Maier TJ, Steinhilber D, Krupitza G. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher P, Hermanns C, Nossek M, Drexler MK, Grosse R, Fischer M, Sarikas A, Penkava J, Lewis T, Prywes R, Gudermann T, Muehlich S. Filamin A interacts with the coactivator MKL1 to promote the activity of the transcription factor SRF and cell migration. Sci Signal. 2015;8:ra112. doi: 10.1126/scisignal.aad2959. [DOI] [PubMed] [Google Scholar]
- Klampfl T, Bogner E, Bednar W, Mager L, Massudom D, Kalny I, Heinzle C, Berger W, Stattner S, Karner J, Klimpfinger M, Furstenberger G, Krieg P, Marian B. Up-regulation of 12(S)-lipoxygenase induces a migratory phenotype in colorectal cancer cells. Exp Cell Res. 2012;318:768–778. doi: 10.1016/j.yexcr.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochin V, Shimi T, Torvaldson E, Adam SA, Goldman A, Pack CG, Melo-Cardenas J, Imanishi SY, Goldman RD, Eriksson JE. Interphase phosphorylation of lamin A. J Cell Sci. 2014;127:2683–2696. doi: 10.1242/jcs.141820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Schafer G, Bu H, Zhang Y, Zhang Y, Klocker H. Lamin A/C protein is overexpressed in tissue-invading prostate cancer and promotes prostate cancer cell growth, migration and invasion through the PI3K/AKT/PTEN pathway. Carcinogenesis. 2012;33:751–759. doi: 10.1093/carcin/bgs022. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Chow MZ, Wang Z, Zhang L, Liu B, Liu X, Zhou Z. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. Proc Natl Acad Sci U S A. 2011;108:12325–12330. doi: 10.1073/pnas.1102789108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu D, Britton S, Demir M, Rodriguez R, Jackson SP. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science. 2014;344:527–532. doi: 10.1126/science.1252651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Welton KL, Smith ED, Kennedy BK. A-type nuclear lamins act as transcriptional repressors when targeted to promoters. Exp Cell Res. 2009;315:996–1007. doi: 10.1016/j.yexcr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Shi H, Poon Z, Nyan LM, Kaushik T, Shivashankar GV, Chan JK, Lim CT, Han J, Van Vliet KJ. Multivariate biophysical markers predictive of mesenchymal stromal cell multipotency. Proc Natl Acad Sci U S A. 2014;111:E4409–E4418. doi: 10.1073/pnas.1402306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Jung JJ, Jeung HC, Noh SW, Oh BK, Kim KY, Kim TS, Chung HC, Roh JK, Rha SY. Methylation status of lamin A/C in gastric cancer cell lines. Hepatogastroenterology. 2012;59:1313–1318. doi: 10.5754/hge11610. [DOI] [PubMed] [Google Scholar]
- Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-beta1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23:781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum Mol Genet. 2005;14:437–445. doi: 10.1093/hmg/ddi040. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, Pei D, Pendas AM, Cadinanos J, Lopez-Otin C, Tse HF, Hutchison C, Chen J, Cao Y, Cheah KS, Tryggvason K, Zhou Z. Genomic instability in laminopathy-based premature aging. Nat Med. 2005;11:780–785. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Le Berre M, Lautenschlaeger F, Maiuri P, Callan-Jones A, Heuze M, Takaki T, Voituriez R, Piel M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell. 2015;160:659–672. doi: 10.1016/j.cell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11:769–777. doi: 10.1093/hmg/11.7.769. [DOI] [PubMed] [Google Scholar]
- Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011;208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem. 2011;286:26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Xu X, Liu X, Peng Y, Zhang B, Wang L, Luo H, Peng X, Li G, Tian W, He M, Li X. Predictive value of miR-9 as a potential biomarker for nasopharyngeal carcinoma metastasis. Br J Cancer. 2014;110:392–398. doi: 10.1038/bjc.2013.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E, Oldenburg AR, Delbarre E, Freberg CT, Duband-Goulet I, Eskeland R, Buendia B, Collas P. Lamin A/C-promoter interactions specify chromatin state-dependent transcription outcomes. Genome Res. 2013;23:1580–1589. doi: 10.1101/gr.159400.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhija E, Jokhun DS, Shivashankar GV. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc Natl Acad Sci U S A. 2016;113:E32–E40. doi: 10.1073/pnas.1513189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ. Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell. 2002;13:4401–4413. doi: 10.1091/mbc.E02-07-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, Hausmanowa-Petrusewicz I, Ramaekers FC, Broers JL, Blankesteijn WM, Salpingidou G, Wilson RG, Ellis JA, Hutchison CJ. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 2006;25:3275–3285. doi: 10.1038/sj.emboj.7601230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli AM, Bortul R, Tabellini G, Faenza I, Cappellini A, Bareggi R, Manzoli L, Cocco L. Molecular characterization of protein kinase C-alpha binding to lamin A. J Cell Biochem. 2002;86:320–330. doi: 10.1002/jcb.10227. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Hieda M, Yokoyama Y, Nishioka Y, Yoshidome K, Tsujimoto M, Matsuura N. Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med. 2015;4:1547–1557. doi: 10.1002/cam4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A, Ciccarelli C, De Cesaris P, Scoglio A, Bouche M, Molinaro M, Aquino A, Zani BM. PKCalpha-mediated ERK, JNK and p38 activation regulates the myogenic program in human rhabdomyosarcoma cells. J Cell Sci. 2002;115:3587–3599. doi: 10.1242/jcs.00037. [DOI] [PubMed] [Google Scholar]
- McGregor AL, Hsia CR, Lammerding J. Squish and squeeze-the nucleus as a physical barrier during migration in confined environments. Curr Opin Cell Biol. 2016;40:32–40. doi: 10.1016/j.ceb.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, Treisman R. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat Cell Biol. 2009;11:257–268. doi: 10.1038/ncb1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcon G, Kozlov S, Cutler DA, Sullivan T, Hernandez L, Zhao P, Mitchell S, Nader G, Bakay M, Rottman JN, Hoffman EP, Stewart CL. Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum Mol Genet. 2006;15:637–651. doi: 10.1093/hmg/ddi479. [DOI] [PubMed] [Google Scholar]
- Mitchell MJ, Denais C, Chan MF, Wang Z, Lammerding J, King MR. Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress. Am J Physiol Cell Physiol. 2015;309:C736–C746. doi: 10.1152/ajpcell.00050.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Mayanagi T, Sobue K. Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J Cell Biol. 2007;179:1027–1042. doi: 10.1083/jcb.200708174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H, Barcellos-Hoff MH. TGF-beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a003277. doi: 10.1101/cshperspect.a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchir A, Pavlidis P, Decostre V, Herron AJ, Arimura T, Bonne G, Worman HJ. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007;117:1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchir A, Reilly SA, Wu W, Iwata S, Homma S, Bonne G, Worman HJ. Treatment with selumetinib preserves cardiac function and improves survival in cardiomyopathy caused by mutation in the lamin A/C gene. Cardiovasc Res. 2012a;93:311–319. doi: 10.1093/cvr/cvr301. [DOI] [PubMed] [Google Scholar]
- Muchir A, Wu W, Choi JC, Iwata S, Morrow J, Homma S, Worman HJ. Abnormal p38alpha mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum Mol Genet. 2012b;21:4325–4333. doi: 10.1093/hmg/dds265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchir A, Wu W, Worman HJ. Reduced expression of A-type lamins and emerin activates extracellular signal-regulated kinase in cultured cells. Biochim Biophys Acta. 2009;1792:75–81. doi: 10.1016/j.bbadis.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myant K, Qiao X, Halonen T, Come C, Laine A, Janghorban M, Partanen JI, Cassidy J, Ogg EL, Cammareri P, Laitera T, Okkeri J, Klefstrom J, Sears RC, Sansom OJ, Westermarck J. Serine 62-Phosphorylated MYC Associates with Nuclear Lamins and Its Regulation by CIP2A Is Essential for Regenerative Proliferation. Cell Rep. 2015;12:1019–1031. doi: 10.1016/j.celrep.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem AS, Zhu Y, Di WL, Marmiroli S, O’Shaughnessy RF. AKT1-mediated Lamin A/C degradation is required for nuclear degradation and normal epidermal terminal differentiation. Cell Death Differ. 2015;22:2123–2132. doi: 10.1038/cdd.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardella M, Guglielmi L, Musa C, Iannetti I, Maresca G, Amendola D, Porru M, Carico E, Sessa G, Camerlingo R, Dominici C, Megiorni F, Milan M, Bearzi C, Rizzi R, Pirozzi G, Leonetti C, Bucci B, Mercanti D, Felsani A, D’Agnano I. Down-regulation of the Lamin A/C in neuroblastoma triggers the expansion of tumor initiating cells. Oncotarget. 2015;6:32821–32840. doi: 10.18632/oncotarget.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Neumann S, Schneider M, Daugherty RL, Gottardi CJ, Eming SA, Beijer A, Noegel AA, Karakesisoglou I. Nesprin-2 interacts with {alpha}-catenin and regulates Wnt signaling at the nuclear envelope. J Biol Chem. 2010;285:34932–34938. doi: 10.1074/jbc.M110.119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta RT, Jameson SA, Kudlow BA, Conlan LA, Kennedy BK. Stabilization of the retinoblastoma protein by A-type nuclear lamins is required for INK4A–mediated cell cycle arrest. Mol Cell Biol. 2006;26:5360–5372. doi: 10.1128/MCB.02464-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K, Hosoe Y, Nakajima N. c-Jun and Sp1 family are critical for retinoic acid induction of the lamin A/C retinoic acid-responsive element. Biochem Biophys Res Commun. 2004;320:487–492. doi: 10.1016/j.bbrc.2004.05.191. [DOI] [PubMed] [Google Scholar]
- Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgis P. TGFbeta Signaling in Tumor Initiation, Epithelial-to-Mesenchymal Transition, and Metastasis. J Oncol. 2015;2015:587193. doi: 10.1155/2015/587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada LA, McQueen PG, Misteli T. Tissue-specific spatial organization of genomes. Genome Biol. 2004;5:R44. doi: 10.1186/gb-2004-5-7-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pegoraro G, Kubben N, Wickert U, Gohler H, Hoffmann K, Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol. 2009;11:1261–1267. doi: 10.1038/ncb1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekovic V, Gibbs-Seymour I, Markiewicz E, Alzoghaibi F, Benham AM, Edwards R, Wenhert M, von Zglinicki T, Hutchison CJ. Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell. 2011;10:1067–1079. doi: 10.1111/j.1474-9726.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- Pekovic V, Harborth J, Broers JL, Ramaekers FC, van Engelen B, Lammens M, von Zglinicki T, Foisner R, Hutchison C, Markiewicz E. Nucleoplasmic LAP2alpha-lamin A complexes are required to maintain a proliferative state in human fibroblasts. J Cell Biol. 2007;176:163–172. doi: 10.1083/jcb.200606139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Graf S, Flicek P, Kerkhoven RM, van Lohuizen M, Reinders M, Wessels L, van Steensel B. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 2004;14:64–69. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61:591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, Oertle P, Hyotyla JT, Aebi U, Bentires-Alj M, Lim RY, Schoenenberger CA. The nanomechanical signature of breast cancer. Nat Nanotechnol. 2012;7:757–765. doi: 10.1038/nnano.2012.167. [DOI] [PubMed] [Google Scholar]
- Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–748. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- Pombo A, Dillon N. Three-dimensional genome architecture: players and mechanisms. Nat Rev Mol Cell Biol. 2015;16:245–257. doi: 10.1038/nrm3965. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S, Jiang ZL. PDGF-BB and TGF-{beta}1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci U S A. 2011;108:1908–1913. doi: 10.1073/pnas.1019219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, Lautenschlaeger F, Voituriez R, Lennon-Dumenil AM, Manel N, Piel M. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–362. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashmi RN, Eckes B, Glockner G, Groth M, Neumann S, Gloy J, Sellin L, Walz G, Schneider M, Karakesisoglou I, Eichinger L, Noegel AA. The nuclear envelope protein Nesprin-2 has roles in cell proliferation and differentiation during wound healing. Nucleus. 2012;3:172–186. doi: 10.4161/nucl.19090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky D, Wirtz D, Hodzic D. Nuclear envelope in nuclear positioning and cell migration. Adv Exp Med Biol. 2014;773:471–490. doi: 10.1007/978-1-4899-8032-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KL, Feinberg AP. Higher order chromatin organization in cancer. Semin Cancer Biol. 2013;23:109–115. doi: 10.1016/j.semcancer.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- Redwood AB, Perkins SM, Vanderwaal RP, Feng Z, Biehl KJ, Gonzalez-Suarez I, Morgado-Palacin L, Shi W, Sage J, Roti-Roti JL, Stewart CL, Zhang J, Gonzalo S. A dual role for A-type lamins in DNA double-strand break repair. Cell Cycle. 2011;10:2549–2560. doi: 10.4161/cc.10.15.16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rober RA, Sauter H, Weber K, Osborn M. Cells of the cellular immune and hemopoietic system of the mouse lack lamins A/C: distinction versus other somatic cells. J Cell Sci. 1990;95(Pt 4):587–598. doi: 10.1242/jcs.95.4.587. [DOI] [PubMed] [Google Scholar]
- Rober RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Calvo F, Gonzalez JM, Casar B, Andres V, Crespo P. ERK1/2 MAP kinases promote cell cycle entry by rapid, kinase-independent disruption of retinoblastoma-lamin A complexes. J Cell Biol. 2010;191:967–979. doi: 10.1083/jcb.201004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, Olins AL, Herrmann H, Weitz DA, Lammerding J. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J Biol Chem. 2013;288:8610–8618. doi: 10.1074/jbc.M112.441535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarinen I, Mirtti T, Seikkula H, Bostrom PJ, Taimen P. Differential Predictive Roles of A- and B-Type Nuclear Lamins in Prostate Cancer Progression. PLoS One. 2015;10:e0140671. doi: 10.1371/journal.pone.0140671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore-Bianchi A, Ardini E, Bosotti R, Amatu A, Valtorta E, Somaschini A, Raddrizzani L, Palmeri L, Banfi P, Bonazzina E, Misale S, Marrapese G, Leone A, Alzani R, Luo D, Hornby Z, Lim J, Veronese S, Vanzulli A, Bardelli A, Martignoni M, Davite C, Galvani A, Isacchi A, Siena S. Sensitivity to Entrectinib Associated With a Novel LMNA-NTRK1 Gene Fusion in Metastatic Colorectal Cancer. J Natl Cancer Inst. 2016;108:djv306. doi: 10.1093/jnci/djv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med. 2005;11:440–445. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schape J, Prausse S, Radmacher M, Stick R. Influence of lamin A on the mechanical properties of amphibian oocyte nuclei measured by atomic force microscopy. Biophys J. 2009;96:4319–4325. doi: 10.1016/j.bpj.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg MA, Chiquet-Ehrismann R, Asparuhova MB. Megakaryoblastic leukemia protein-1 (MKL1): Increasing evidence for an involvement in cancer progression and metastasis. Int J Biochem Cell Biol. 2010;42:1911–1914. doi: 10.1016/j.biocel.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell. 2013;152:1365–1375. doi: 10.1016/j.cell.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc Natl Acad Sci U S A. 2013;110:18892–18897. doi: 10.1073/pnas.1304996110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuang ZY, Wu WC, Xu J, Lin G, Liu YC, Lao XM, Zheng L, Li S. Transforming growth factor-beta1-induced epithelial-mesenchymal transition generates ALDH-positive cells with stem cell properties in cholangiocarcinoma. Cancer Lett. 2014;354:320–328. doi: 10.1016/j.canlet.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001;61:6307–6312. [PubMed] [Google Scholar]
- Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A. 2003;100:8430–8435. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DN, Domaradzki T, Hofmann WA, Wilson KL. Lamin A tail modification by SUMO1 is disrupted by familial partial lipodystrophy-causing mutations. Mol Biol Cell. 2013;24:342–350. doi: 10.1091/mbc.E12-07-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DN, Wilson KL. Partners and post-translational modifications of nuclear lamins. Chromosoma. 2013;122:13–31. doi: 10.1007/s00412-013-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Hunt CR, Pandita RK, Kumar R, Yang CR, Horikoshi N, Bachoo R, Serag S, Story MD, Shay JW, Powell SN, Gupta A, Jeffery J, Pandita S, Chen BP, Deckbar D, Lobrich M, Yang Q, Khanna KK, Worman HJ, Pandita TK. Lamin A/C depletion enhances DNA damage-induced stalled replication fork arrest. Mol Cell Biol. 2013;33:1210–1222. doi: 10.1128/MCB.01676-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvortsov S, Schafer G, Stasyk T, Fuchsberger C, Bonn GK, Bartsch G, Klocker H, Huber LA. Proteomics profiling of microdissected low- and high-grade prostate tumors identifies Lamin A as a discriminatory biomarker. J Proteome Res. 2011;10:259–268. doi: 10.1021/pr100921j. [DOI] [PubMed] [Google Scholar]
- Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, Herrmann H, Blum H, Engelkamp D, Stewart CL, Leonhardt H, Joffe B. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–598. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Somech R, Shaklai S, Geller O, Amariglio N, Simon AJ, Rechavi G, Gal-Yam EN. The nuclear-envelope protein and transcriptional repressor LAP2beta interacts with HDAC3 at the nuclear periphery, and induces histone H4 deacetylation. J Cell Sci. 2005;118:4017–4025. doi: 10.1242/jcs.02521. [DOI] [PubMed] [Google Scholar]
- Stewart C, Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987;51:383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Pfaller K, Stossel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol. 2002;118:117–125. doi: 10.1046/j.0022-202x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- Stubenvoll A, Rice M, Wietelmann A, Wheeler M, Braun T. Attenuation of Wnt/beta-catenin activity reverses enhanced generation of cardiomyocytes and cardiac defects caused by the loss of emerin. Hum Mol Genet. 2015;24:802–813. doi: 10.1093/hmg/ddu498. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori Y, Tamura Y, Kataoka Y, Cui Y, Seo S, Kanazawa T, Kurokawa K, Yamada H. Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur J Neurosci. 2007;25:1653–1662. doi: 10.1111/j.1460-9568.2007.05450.x. [DOI] [PubMed] [Google Scholar]
- Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, Wakefield LM. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K, Finley RL, Jr, Nie D, Honn KV. Identification of 12-lipoxygenase interaction with cellular proteins by yeast two-hybrid screening. Biochemistry. 2000;39:3185–3191. doi: 10.1021/bi992664v. [DOI] [PubMed] [Google Scholar]
- Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- Tilgner K, Wojciechowicz K, Jahoda C, Hutchison C, Markiewicz E. Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of beta-catenin. J Cell Sci. 2009;122:401–413. doi: 10.1242/jcs.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berlo JH, Voncken JW, Kubben N, Broers JL, Duisters R, van Leeuwen RE, Crijns HJ, Ramaekers FC, Hutchison CJ, Pinto YM. A-type lamins are essential for TGF-beta1 induced PP2A to dephosphorylate transcription factors. Hum Mol Genet. 2005;14:2839–2849. doi: 10.1093/hmg/ddi316. [DOI] [PubMed] [Google Scholar]
- van Tintelen JP, Tio RA, Kerstjens-Frederikse WS, van Berlo JH, Boven LG, Suurmeijer AJ, White SJ, den Dunnen JT, te Meerman GJ, Vos YJ, van der Hout AH, Osinga J, van den Berg MP, van Veldhuisen DJ, Buys CH, Hofstra RM, Pinto YM. Severe myocardial fibrosis caused by a deletion of the 5’ end of the lamin A/C gene. J Am Coll Cardiol. 2007;49:2430–2439. doi: 10.1016/j.jacc.2007.02.063. [DOI] [PubMed] [Google Scholar]
- Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, Tryggvason K, Freije JM, Lopez-Otin C. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437:564–568. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- Vargas JD, Hatch EM, Anderson DJ, Hetzer MW. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus. 2012;3:88–100. doi: 10.4161/nucl.18954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A, Alvarez-Reyes M, Bridger JM, Broers JL, Ramaekers FC, Wehnert M, Morris GE, Whitfield WGF, Hutchison CJ. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J Cell Sci. 2001;114:2577–2590. doi: 10.1242/jcs.114.14.2577. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13:e83–e89. doi: 10.1016/S1470-2045(11)70257-1. [DOI] [PubMed] [Google Scholar]
- Verstraeten VL, Renes J, Ramaekers FC, Kamps M, Kuijpers HJ, Verheyen F, Wabitsch M, Steijlen PM, van Steensel MA, Broers JL. Reorganization of the nuclear lamina and cytoskeleton in adipogenesis. Histochem Cell Biol. 2011;135:251–261. doi: 10.1007/s00418-011-0792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigouroux C, Auclair M, Dubosclard E, Pouchelet M, Capeau J, Courvalin JC, Buendia B. Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene. J Cell Sci. 2001;114:4459–4468. doi: 10.1242/jcs.114.24.4459. [DOI] [PubMed] [Google Scholar]
- Wang AS, Kozlov SV, Stewart CL, Horn HF. Tissue specific loss of A-type lamins in the gastrointestinal epithelium can enhance polyp size. Differentiation. 2015;89:11–21. doi: 10.1016/j.diff.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Ward IM, Minn K, van Deursen J, Chen J. p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol Cell Biol. 2003;23:2556–2563. doi: 10.1128/MCB.23.7.2556-2563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SC, Yang J. Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial-Mesenchymal Transition. Trends Cell Biol. 2016;26:111–120. doi: 10.1016/j.tcb.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelin B, Bakker GJ, Friedl P. Intravital third harmonic generation microscopy of collective melanoma cell invasion. IntraVital. 2012;1:32–43. doi: 10.4161/intv.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis ND, Cox TR, Rahman-Casans SF, Smits K, Przyborski SA, van den Brandt P, van Engeland M, Weijenberg M, Wilson RG, de Bruine A, Hutchison CJ. Lamin A/C is a risk biomarker in colorectal cancer. PLoS One. 2008;3:e2988. doi: 10.1371/journal.pone.0002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V, Pavlick D, Brennan T, Yelensky R, Crawford J, Ross JS, Miller VA, Malicki D, Stephens PJ, Ali SM, Ahn H. Evaluation of a Congenital Infantile Fibrosarcoma by Comprehensive Genomic Profiling Reveals an LMNA-NTRK1 Gene Fusion Responsive to Crizotinib. J Natl Cancer Inst. 2016;108:djv307. doi: 10.1093/jnci/djv307. [DOI] [PubMed] [Google Scholar]
- Wood AM, Rendtlew Danielsen JM, Lucas CA, Rice EL, Scalzo D, Shimi T, Goldman RD, Smith ED, Le Beau MM, Kosak ST. TRF2 and lamin A/C interact to facilitate the functional organization of chromosome ends. Nat Commun. 2014;5:5467. doi: 10.1038/ncomms6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wu L, Weng D, Xu D, Geng J, Zhao F. Reduced expression of lamin A/C correlates with poor histological differentiation and prognosis in primary gastric carcinoma. J Exp Clin Cancer Res. 2009;28:8. doi: 10.1186/1756-9966-28-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Mouw JK, Weaver VM. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 2011;21:47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Sarge KD. Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J Cell Biol. 2008;182:35–39. doi: 10.1083/jcb.200712124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Radu G, Ju W, Brown WT. Novel progerin-interactive partner proteins hnRNP E1, EGF, Mel 18, and UBC9 interact with lamin A/C. Biochem Biophys Res Commun. 2005;338:855–861. doi: 10.1016/j.bbrc.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Zuela N, Zwerger M, Levin T, Medalia O, Gruenbaum Y. Impaired mechanical response of an EDMD mutation leads to motility phenotypes that are repaired by loss of prenylation. J Cell Sci. 2016;129:1781–1791. doi: 10.1242/jcs.184309. [DOI] [PubMed] [Google Scholar]
- Zuo B, Yang J, Wang F, Wang L, Yin Y, Dan J, Liu N, Liu L. Influences of lamin A levels on induction of pluripotent stem cells. Biol Open. 2012;1:1118–1127. doi: 10.1242/bio.20121586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerger M, Jaalouk DE, Lombardi ML, Isermann P, Mauermann M, Dialynas G, Herrmann H, Wallrath LL, Lammerding J. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum Mol Genet. 2013;22:2335–2349. doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]