Abstract
Background
Recent evidence suggests cross-talk exists between cellular pathways important for pain signaling and ischemia-reperfusion injury. Here we address whether the transient receptor potential ankyrin 1 (TRPA1) channel, important in pain signaling, is present in cardiac myocytes and regulates cardiac ischemia-reperfusion injury.
Methods
For biochemical analysis of TRPA1, techniques including qPCR, western blot, and immunofluorescence were used. To determine how TRPA1 mediates cellular injury, we used an in vivo model of rat cardiac ischemia-reperfusion injury and adult rat isolated cardiac myocytes subjected to hypoxia-reoxygenation.
Results
Our biochemical analysis indicates TRPA1 is within the cardiac myocytes. Further, using a rat in vivo model of cardiac injury, the TRPA1 activators ASP7663 and optovin reduce myocardial injury (45±5%*, 44±8%*, respectively, versus control 66±6% infarct size/area at risk, n=6/group, mean±SD, *P<0.001). TRPA1 inhibition also blocked the infarct size sparing effects of morphine. In isolated cardiac myocytes, the TRPA1 activators ASP7663 and optovin reduce cardiac myocyte cell death when given during reoxygenation (20±3%*, 22±4%*, versus 36±3%, % of dead cells per field, n=6/group, mean±SD, *P<0.05). For a rat in vivo model of cardiac injury, the infarct size sparing effect of TRPA1 activators also occurs during reperfusion.
Conclusions
Our data suggests that TRPA1 is present within the cardiac myocytes and is important in regulating myocardial reperfusion injury. The presence of TRPA1 within the cardiac myocytes may potentially explain why certain pain relievers that can block TRPA1 activation, such as cyclooxygenase-2 (COX-2) inhibitors or some non-steroidal anti-inflammatory drugs (NSAIDs), could be associated with cardiovascular risk.
Introduction
Over 200 million opioid prescriptions are written annually in the United States to treat pain.1 These drugs have serious unwanted side effects including abuse, dependence, and addiction. As a result, an opioid epidemic within the United States is shifting medical practice towards prescribing non-opioid analgesics or opioid adjuvants to reduce opioid use intraoperatively, postoperatively, and in the clinic.2, 3 Further, developing novel analgesics to replace opioids within medical practice is a continued focus for scientists.
For these reasons, understanding how the pathways of pain signaling may contribute to protection of organs from ischemia-reperfusion injury is important since cellular cross-talk exists between these two pathways.3 Non-narcotic analgesics may also have a deleterious effect by blocking the endogenous mechanisms which reduce ischemia-reperfusion injury of organs. For example, inhibition of cyclooxygenase 2 (COX-2) experimentally blocks the natural ability of the heart to protect against ischemia-reperfusion injury.4, 5 Clinically, in addition to cyclooxygenase-2 inhibitors, the cardiac safety of some non-steroidal anti-inflammatory drugs (NSAIDs) has also been recently questioned.2, 3
Since the heart unlike other organs also possesses neuroendocrine qualities, pain receptors from the transient receptor potential family (TRP) could potentially exist within the cardiac myocytes. In particular, transient receptor potential ankyrin 1 (TRPA1) inhibitors are being explored and developed as an alternative to opioids for pain control.6 Further, the TRPA1 receptor is also modulated by different pain relievers including NSAIDs, cyclooxygenase-2 inhibitors, and acetaminophen.7–9
The TRPA1 receptor, besides regulating pain, also serves multiple functions within the cell.10, 11 In particular, TRPA1 functions as a sensor that is activated by reactive aldehydes and is modulated when intracellular changes in oxygen levels occur.12, 13 Both factors are important in regards to organ ischemia-reperfusion injury. In particular, the production of reactive aldehydes as a result of the break-down of lipid membranes is considered a critical mediator of cellular injury.13
However, little is known whether the TRPA1 receptor exists in the cardiac myocyte and if TRPA1 contributes to regulating injury during cardiac ischemia-reperfusion. If TRPA1 is present in the cardiac myocyte, this could explain why some non-opioid analgesics block natural pathway(s) of protection from ischemia-reperfusion injury. This would also be important to understand when developing drugs targeting TRPA1 to provide analgesia. Here we address the question whether TRPA1 is present in the cardiac myocyte and further if this receptor is important in mediating ischemia-reperfusion injury.
Materials and methods
Procedures and protocols were approved by the Animal Care and Use Committee at Stanford University, Stanford, California. All animal studies conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals (8th edition, 2011) Eight to 10 week old male Sprague-Dawley rats (Charles River) were used for the studies outlined.
Pharmacological Agents
The TRPA1 receptor activators, ASP 7663 (ASP, 3 mg/kg in vivo, 3 µM in vitro) and optovin (1 mg/kg in vivo, 1 µM in vitro), in addition to the TRPA1 inhibitors, TCS 5861528 (TCS, 1 mg/kg in vivo, 1mM in vitro) and AP 18 (AP, 1 mg/kg in vivo, 1mM in vitro), were dissolved in DMSO. Doses were chosen for TRPA1 activators and inhibitors based upon data for these compounds provided within the manufacturer insert (Tocris, Bristol, United Kingdom). Morphine (0.3 mg/kg IV bolus) was dissolved in saline and the dose determined from our prior studies.14, 15
Biochemical Studies
Biochemical studies consisted of quantitative PCR, western blot, and immunofluorescence.
For quantitative PCR, adult rat cardiac myocytes, left ventricle heart tissue, and H9C2 cell samples were stored in RNAlater (Sigma, Saint Louis, Missouri) in −80°C and homogenized in 1ml of TRI Reagent (Molecular Research Center, Cincinnati, Ohio). Total RNA was isolated from tissue and cell homogenates using RNeasy Mini Kit 50 (Qiagen, Hilden, Germany). cDNA synthesis was performed with the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, California). Quantitative PCR was performed using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, California). The 20µL reactions contained 10µL TaqMan Fast Universal PCR Master Mix (2X), 1µL of the specific TaqMan assay, 1µL cDNA, and water to 20µL. Cycling parameters were 30 s initial setup at 95 °C, followed by 40 cycles of 95 °C for 3 s and 30 s at 61 °C (ABI 7500 Fast, Applied Biosystems, Foster City, California). Primers used are as follows: TRPA1 forward: GCTTCTGCAAGACATCAGCG, TRPA1 reverse: CCTCTCCATCTGGCAGCAAA, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward: CTCAGTTGCTGAGGAGTCCC, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reverse: ATTCGAGAGAAGGGAGGGCT.
For western blot, adult cardiac myocytes, left ventricle tissue, and H9C2 cells were used. The left ventricles of male Sprague-Dawley rats were excised, finely minced with scissors, and homogenized with mannitol-sucrose lysis buffer (210mM mannitol, 70mM sucrose, 5mM 3-N-morpholino propanesulfonic acid (MOPS), 1mM EDTA with pH 7.4, protease/phosphatase inhibitors and 1% Triton X (Sigma, Saint Louis, Missouri). Homogenates were centrifuged at 800g for 5 minutes to remove cellular debris and the supernatant kept as the total fraction. Adult cardiac myocytes and H9C2 cells were also lysed in mannitol-sucrose buffer. For the 3 types of samples used (adult cardiac myocytes, left ventricle heart tissue and H9C2 cells), total protein content was determined by Bradford assay with 35µg of each homogenate run on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels. Membrane proteins were transferred to polyvinylidene difluoride membrane and probed overnight at 4 °C for specific antibodies to TRPA1 (1:500 dilution in 5% milk-TBST, Novus, Littleton, Colorado) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:1000 dilution in 5% milk-TBST, Sigma, Saint Louis, Missouri). The next day, membranes were washed and incubated in secondary anti-rabbit antibody for 2 hours (1:1000 for TRPA1 and 1:3000 for glyceraldehyde 3-phosphate dehydrogenase in 5% milk, ThermoScientific, Waltham, Massachusetts). Membranes were developed in enhanced chemiluminescent reagent and images were acquired by using an Azure Biosystems c300.
For immunofluorescence, isolated cardiomyocytes were plated on pre-coated laminin (Invitrogen, Carlsbad, California) glass coverslips (2 µg/cm2) for 24 hours and fixed with 2% buffered paraformaldehyde for 10 minutes at room temperature. The fixed cells were washed 3 times at 5 minute intervals with PBS. The cells were blocked and permeabilized with blocking buffer (40mM HEPES, 3% Dry Milk, phosphate buffered saline and 0.1% Triton-X 100) for 30 minutes at room temperature. Cells were incubated with TRPA1 primary antibody (1:250 dilution) in blocking buffer for one hour at room temperature. Excess antibody was removed by washing samples with phosphate buffered saline 3 times at 5 minutes intervals. The cells were then incubated with donkey anti-rabbit Alexa Fluor 488 (Invitrogen, Carlsbad, California, 1:500 dilution) in blocking buffer for 2 hours at room temperature. To remove excess secondary antibody, the cells were washed 6 times at 5 minutes intervals. The nucleus was stained with dye 4’,6-diamidino-2-phenylindole (DAPI, 1µg/ml) diluted in phosphate buffered saline for 20 minutes. Cells were then washed for 10 minutes with phosphate buffered saline and mounted in ProLong gold reagent anti-fade (Invitrogen, Carlsbad, California) for microscopic imaging.
In Vivo Myocardial Infarction Rodent Model
The model is described in a number of publications.1, 14, 15 After obtaining body weight, rats were anesthetized with Inactin (thiobutabarbital, 100mg/kg intraperitoneal, Sigma, Saint Louis, Missouri). A tracheotomy was performed in addition to cannulation of the carotid artery and internal jugular vein to measure blood pressure and to administer drugs, respectively. Rats were placed on a ventilator (30–40 breaths per minute, tidal volume 8mL/kg) and adjusted to maintain a normal pH (7.35–7.45) and end tidal CO2 (35–45mmHg) by a blood gas machine (Radiometer ABL-80, Cleveland, Ohio). Body temperature was monitored with a rectal thermometer (Thermalert TH-5) and maintained at 36–38°C by heating pads and heat lamps. The heart was exposed by an incision in the fourth intercostal space, the pericardium excised and a suture was placed around the left anterior descending coronary artery (6-0 prolene, Ethicon, Somerville, New Jersey). After surgical manipulation and adjustment of the ventilator settings based upon blood gas analysis, rodents were allowed to stabilize for 30 minutes prior to initiation of the experimental protocol.
The experimental protocol had fourteen treatment groups, which are described in detail throughout the manuscript. Based upon our prior studies where a power analysis with α = 0.05 and 80% power to detect at least a 15% difference, a minimum of 6 experiments are required.14 Rodents were randomized to experimental treatment groups and all rats were subjected to 30 minutes of left anterior descending (LAD) coronary artery occlusion followed by 2 hours of reperfusion. Following reperfusion, the left anterior descending coronary artery was again occluded and the heart negatively stained for the area at risk by injection of patent blue dye (Sigma, St. Louis, Missouri) given through the internal jugular vein. The heart was then excised, both atria and the right ventricle were removed, and the left ventricle was cut into 5 equal slices to create cross-sections from apex to base. The slices were separated into normal zone and area at risk, both followed by incubation in 1% triphenyl tetrazolium chloride (TTC) to measure viability of myocardial tissue. Viable tissue stained red, while non-viable tissue remained unstained or white. Infarct size as a percentage of area at risk was determined gravimetrically. Heart rate, blood pressure, and rate pressure product were monitored and calculated throughout the experimental protocol using PowerLab monitoring system (AD Instruments, MLS060/8 PowerLab 4/35). We defined rate pressure product (RPP) as the product of heart rate and systolic blood pressure.
In Vitro cardiac myocytes hypoxia and reoxygenation injury model
Adult rat primary cardiac myocytes were isolated from male Sprague-Dawley rat hearts by enzymatic dissociation as previously described.16 The isolated cardiac myocytes were plated in 4% FBS Medium 199 on laminin coated plates (2µg/cm2) for 2 hours. The plating medium was changed to serum free Medium (1% BSA Medium 199) to remove non-myocytes. Cardiomyocytes were incubated at 37 °C in 5% CO2 for 24 hours prior to experiments in 24 well plates with a seeding density of 5×104/mL.
After 24 hours, serum free media 199 was changed the morning of the study 1 hour prior to hypoxia and remained on the cells until completion of the study. Experiments were divided into two sub-sets: a normoxic control group and a hypoxia-reoxygenation group. In the hypoxia-reoxygenation groups, cardiac myocytes were subjected to either DMSO, a TRPA1 activator (optovin, 1µM or ASP 3µM) or a TRPA1 inhibitor (TCS, 1µM, AP, 1µM) immediately before reoxygenation. For the normoxic control group, cardiac myocytes were also treated with dimethyl sulfoxide (DMSO), a TRPA1 activator or a TRPA1 inhibitor. Hypoxia was induced by placing the plates into an anaerobic gas pouch (BD Biosciences, GasPak EZ Gas Generating Pouch Systems, Sparks, Maryland) for 2 hours. The pouch creates an anaerobic environment where the oxygen level within the pouch is less than 0.1%.17 The cells were then removed from the anaerobic gas pouches and reoxygenated within the cell culture incubator for an additional 4 hours.
To determine cell death in this model, trypan blue exclusion and LDH release were used in separate biological replicates. For the trypan blue experiments after 4 hours of reoxygenation, trypan blue was added to adult cardiac myocytes at a final concentration of 0.04%. To distinguish viable cells from dead cells, two digital images for each experiment were acquired using a camera (Nikon Coolpix 8800) attached to an adapter (MM99 adapter S/N: 1925, Martin Microscope Company, Easley, South Carolina) connected to the microscope (Motic AE21, Xiameng, China). All images were taken within three minutes of trypan blue application to minimize variability associated with changes in the ratio of stained/unstained cells over time. Cell death was determined by a person blinded to the experimental groups that was provided the digital images. The number of trypan blue positive cells were counted and further expressed as a percentage of the total cells in the image. Approximately 300 cells were counted per well from 2 images. A total of 6 experiments per group were performed from 2 biological replicates. The LDH release was measured as previously described and quantified as the ratio of LDH release after 4 hours of reoxygenation to total LDH.18
Statistical Analysis
All data were shown as mean ± SD. For analysis of in vivo and cardiac myocyte models of ischemia-reperfusion or ischemia-reoxygenation, a one-way analysis of variance followed by Bonferroni correction for multiplicity was used in order to compare each group to the control group. A two-way analysis of variance was used to determine significance for hemodynamic parameters. For differences between two groups, a two tailed t-test was performed. Statistical analysis was performed using GraphPad Prism 6. P < 0.05 was considered statistically significant.
Results
A total of 107 rats were used for the study. Twenty-one rats were used to isolate adult cardiac myocytes to determine the TRPA1 expression, conduct ischemia-reoxygenation experiments, and to obtain heart left ventricle. One prep was excluded due to complications with cannulating the aorta for cardiac myocyte isolation. Further, 86 rats were used for the in vivo experiments for completion of 84 successful experiments. Two rats were excluded for this portion of the study with one rat due to intractable ventricular fibrillation during reperfusion (ASP at reperfusion group) and another rat secondary to a small area at risk per left ventricle (AP+MOR group). No differences in the percentage of the area at risk per left ventricle were noted for any of the groups (Table 1). Further no differences in hemodynamics including heart rate, blood pressure, and rate pressure product occurred between any of the treatment groups (Table 1). Within the group where DMSO was given during reperfusion, significant differences were noted in the heart rate, mean arterial pressure and rate pressure product at 2 hours of reperfusion when compared to baseline values (Table 1).
Table 1. Number of animals used, area at risk per left ventricle %, and hemodynamic values measured.
Described are the groups, number of animals per group (n), area at risk per left ventricle percent (AAR/LV), and hemodynamics acquired for the in vivo studies.
| Groups | n | AAR/LV% | Baseline | 15 min ischemia | 2 hr reperfusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | MAP | RPP | HR | MAP | RPP | HR | MAP | RPP | |||
| DMSO | 6 | 41±9 | 398±20 | 98±12 | 47±5 | 407±37 | 89±21 | 42±7 | 397±27 | 80±11 | 43±8 |
| Optovin | 6 | 40±6 | 419±32 | 104±21 | 54±9 | 437±22 | 102±22 | 52±10 | 425±13 | 79±14 | 43±7 |
| ASP | 6 | 41±9 | 422±41 | 114±11 | 60±5 | 428±33 | 105±28 | 52±13 | 415±25 | 89±12 | 49±6 |
| MOR | 6 | 37±5 | 410±35 | 109±14 | 53±9 | 407±37 | 104±14 | 49±9 | 374±19 | 73±10 | 37±6 |
| TCS + MOR | 6 | 42±3 | 420±38 | 104±12 | 53±9 | 407±35 | 92±26 | 44±11 | 398±44 | 73±8 | 42±8 |
| AP+MOR | 6 | 40±9 | 423±14 | 107±14 | 54±5 | 378±28 | 107±31 | 48±15 | 388±29 | 77±11 | 40±8 |
| TCS | 6 | 36±4 | 427±30 | 111±16 | 57±8 | 424±25 | 109±27 | 54±10 | 404±23 | 83±15 | 47±7 |
| AP | 6 | 40±8 | 425±32 | 103±22 | 53±13 | 421±34 | 100±43 | 50±22 | 397±25 | 75±14 | 41±10 |
| DMSO at rep | 6 | 39±3 | 421±33 | 105±11 | 50±8 | 447±36 | 96±15 | 48±9 | 370±28† | 73±18† | 34±9† |
| Optovin at rep | 6 | 40±8 | 413±43 | 108±16 | 54±8 | 434±36 | 100±24 | 54±13 | 388±31 | 84±20 | 42±9 |
| ASP at rep | 6 | 45±7 | 421±12 | 104±8 | 52±4 | 423±22 | 94±25 | 49±14 | 395±33 | 81±15 | 42±8 |
Heart rate (HR), mean blood pressure (MAP), and rate pressure product (RPP) defined as the product of heart rate and systolic blood pressure, were assessed at baseline, during ischemia, and at 2 hours of reperfusion. Rate pressure product (RPP) was calculated as the product of heart rate and systolic blood pressure.
Data presented as mean ± SD (n=6). No significant differences were found between groups.
P<0.05 vs. Baseline.
AAR/LV = area at risk per left ventricle percent, AP= AP18, ASP = ASP 7663, HR = heart rate, MAP = mean arterial pressure, MOR = morphine, n= number of animals per group, RPP = rate pressure product, rep = reperfusion, TCS = TCS 5861528
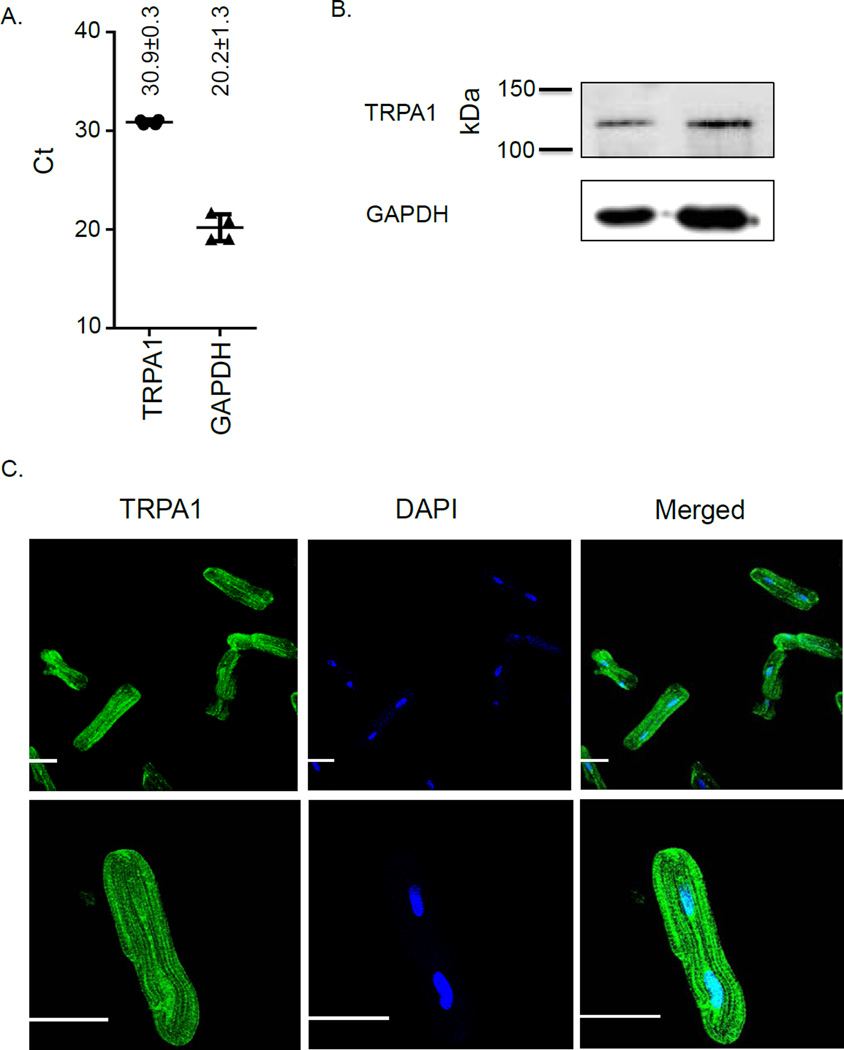
To initially address whether TRPA1 is present in the heart, we performed quantitative PCR and western blot on the cardiac myocytes, the left ventricle derived stable cell line, H9C2, and heart homogenate from the left ventricle. Both PCR and protein expression detected TRPA1 in the cardiac myocytes (Figure 1A and Figure 1B). Further, by immunofluorescence, isolated adult cardiac myocytes also displayed TRPA1 expression (Figure 1C). These findings were further supported by detecting TRPA1 in the H9C2 cell line and homogenate of left ventricle (Supplemental Digital Content 1, qPCR showing presence of TRPA1 in H9C2 cell line and homogenate of left ventricle and Supplemental Digital Content 2 showing western blot).
Figure 1. Biochemical evidence TRPA1 is present in cardiac myocytes.
A. qPCR of cardiac myocytes with 4 biological replicates. B. Western blot of cardiac myocytes representative of 4 biological replicates. C. Immunofluorescence for TRPA1 in cardiac myocytes and nuclear staining with 4’,6-diamidino-2-phenylindole (DAPI). Top panel shows multiple cardiomyocytes per field (20×). Lower panel shows a single cardiomyocyte under higher magnification (63×). GAPDH = glyceraldehyde 3-phosphate dehydrogenase, Ct = cycle threshold, kDa = kilodalton, White bar in images equals 30µm.
TRPA1 activators, including ASP 7663 and optovin, are selective for TRPA1 (Figure 2A). We gave these agents prior to ischemia in an in vivo rodent model of heart attack injury (Figure 2B). ASP 7663 and optovin reduced myocardial damage when compared to the vehicle DMSO (ASP7663 45±5%*, optovin 44±8%*, versus DMSO 66±6%, %infarct size/area of risk, n=6/group, mean±SD, *P<0.001 versus DMSO, Figure 2C, and 2D). Together, these data suggest that TRPA1 is present in the cardiac myocyte and TRPA1 activators can modulate injury from a heart attack.
Figure 2. TRPA1 activators reduce myocardial infarct size.
A. Chemical structure of TRPA1 activators. B. Experimental protocol for myocardial ischemia-reperfusion studies. Rats were given a TRPA1 activator (ASP or optovin) or vehicle (DMSO) 5 minutes prior to 30 minutes of left anterior descending coronary artery ligation to cause ischemia followed by 2 hours of reperfusion. C. Infarct size per area at risk percentage for each experimental group. Data points represent individual biological results for each experiment in addition to values presented as mean ± SD (n=6), *P<0.001 versus DMSO group. D. Representative images of left ventricle area at risk. Infarcted areas are unstained and remain white while viable tissue is stained red by triphenyltetrazolium chloride (TTC). DMSO= dimethyl sulfoxide, ASP= ASP7663
TRPA1 is also selectively modulated by the reactive aldehyde, cinnamaldehyde (Supplemental Digital Content 3A, showing chemical structure of cinnamaldehyde). Unlike the other TRPA1 activators tested that are not reactive aldehydes (ASP 7663 and optovin), cinnamaldehyde given at 0.01mg/kg or 0.1mg/kg increased blood pressure dose-dependently immediately upon administration (cinnamaldehyde 0.01mg/kg 124±10*mmHg, cinnamaldehyde 0.1mg/kg 134±12*mmHg compared to DMSO 92±2mmHg 5 minutes after administration, n=6/group, *P<0.01 versus DMSO, Supplemental Digital Content 3B, showing mean arterial blood pressures after cinnamaldehyde administration). Further, cinnamaldehyde did not affect myocardial infarct size (cinnamaldehyde 0.01mg/kg: 61±3%, cinnamaldehyde 0.1mg/kg: 61±5% versus control 62±3%, n=6/group, Supplemental Digital Content 3C and 3D, showing infarct size data and representative images of infarct size). For these studies no differences were noted between groups for the area at risk per left ventricle percentage. In addition, no changes were noted between groups for the recorded hemodynamics at baseline, 15 minutes of ischemia and at 2 hours of reperfusion (Supplemental Digital Content 4, showing a table of hemodynamics for the cinnamaldehyde portion of the study).
Transient receptor potential channel family members are also known to co-localize with opioid receptors in the nervous system, and opioids activate TRPA1.19 Thus, we further questioned whether morphine decreases myocardial injury by TRPA1 activation. Selective inhibitors of TRPA1 include AP 18 and TCS 5861528 (Figure 3A). We tested whether these TRPA1 inhibitors affect the ability for morphine to reduce myocardial injury in an in vivo rodent heart attack injury model (Figure 3B). When either TRPA1 inhibitor was given prior to morphine, the ability for morphine to decrease heart damage was blocked (morphine 44±5%*, TCS + morphine 62±5%#, AP + morphine 65±6%#, versus DMSO 66±6%, *P<0.001 versus all groups, #P<0.001 versus morphine, Figure 3C and 3D). No effect on myocardial infarct size was noted when either TRPA1 inhibitor was given alone (Figure 3C and Figure 3D).
Figure 3. Opioid-induced reduction of myocardial infarct size is mediated by TRPA1.
A. Chemical structure of two TRPA1 inhibitors(TCS and AP). B. Experimental protocol for myocardial ischemia-reperfusion studies. DMSO or morphine (MOR) wasgiven 5 minutes prior to ischemia. The two TRPA1 inhibitors (TCS and AP) were given 10 minutes before morphine treatment. C. Infarct size per area at risk percentage. Data presented as mean ± SD (n=6), *P<0.001 vs. DMSO, #P<0.001 vs. MOR. For comparison purposes, the DMSO group data presented in Figure 2 is also presented here in this figure. D. Representative images of left ventricle area at risk for each group. AP = AP18, DMSO = dimethyl sulfoxide, MOR = morphine, TCS = TCS 5861528
Since the data we obtained was in an in vivo rodent model, the effect seen could potentially be due to modulation of the nervous system, rather than a direct effect on the cardiac myocyte itself. Therefore, we further determined how TRPA1 modulates cellular death of cardiac myocytes when independent of the nervous system. We isolated primary cardiac myocytes from adult rat hearts and the following day subjected the cardiac myocytes to either sham or hypoxia-reoxygenation. Primary adult cardiac myocytes are viable in cell culture for 48 hours, and as also shown by others,20 a percentage of cell death will occur for a sham-treated group. We administered the TRPA1 activators and inhibitors without the presence of hypoxia-reoxygenation (Supplemental Digital Content 5A, describing experimental protocol). Without hypoxia-reoxygenation, these agents showed no differences in cell death by % of LDH release (Sham 14±6, DMSO 14±3, ASP 13±4, Optovin 13±2, AP 15±7, TCS 14±7, %LDH release of total amount in cells, n=6/group, Supplemental Digital Content 5B which describes LDH release data). Further no differences were noted in trypan blue exclusion when compared to untreated or DMSO treat cardiac myocytes (Sham 11±2, DMSO 11±2, ASP 11±4, Optovin 10±2, AP 10±2, TCS 11±2, % trypan blue positive cells, n=6/group, Supplemental Digital Content 5C–D, which provides images and quantification of trypan blue exclusion assay). We also tested the TRPA1 inhibitors when given prior to reoxygenation (Supplemental Digital Content 6A describing experimental protocol). Similar to the in vivo model findings, the TRPA1 inhibitors did not affect LDH release (Sham 13±2, DMSO 49±11*, AP 49±6*, TCS 49±10*, n=6/group, *P<0.05 versus sham, Supplemental Digital Content 6B, which describes LDH release data). The TRPA1 inhibitors also did not change the number of trypan blue positive cells when compared to DMSO treated adult cardiac myocytes (Sham 9±3, DMSO 38±3*, AP 33±3*, TCS 33±4*, n=6/group, *P<0.05 versus sham, Supplemental Digital Content 6C–D, which provides images and quantification of trypan blue exclusion assay).
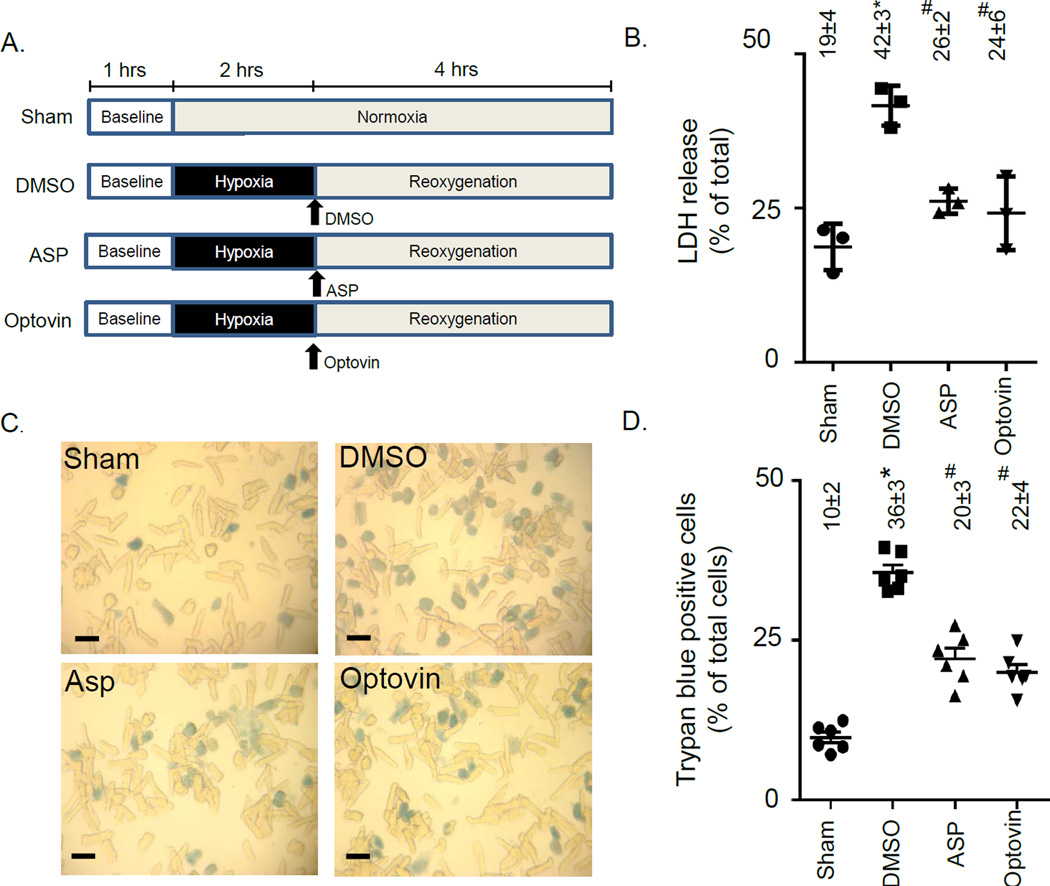
Further, we tested whether the TRPA1 activators affected cardiac myocyte viability when the TRPA1 activators were given prior to reoxygenation (Figure 4A). We determined when either TRPA1 activator was given, the amount of LDH released was less when compared to vehicle treated adult rat cardiac myocytes (ASP 26±2#%, optovin 24±6#%, versus DMSO 42±3*% and sham 19±4%, % of LDH release, n=3/group, mean ± SE, *P<0.05 versus all other groups, #P<0.05 versus sham or DMSO, Figure 4B). Further, the percentage of cardiac myocytes that died was significantly reduced by almost 40% when assessed by a trypan blue exclusion assay (ASP 20±3#%, optovin 22±4#%, versus DMSO 36±3*% and sham 10±2%, % of trypan blue positive cells, n=6/group, mean ± SE, *P<0.05 versus all other groups, #P<0.05 versus sham or DMSO, Figure 4C and 4D). Together, these data suggest that TRPA1 activators are effective agents to reduce injury from hypoxia-reoxygenation when TRPA1 activators are directly applied to the cardiac myocyte prior to reoxygenation.
Figure 4. Activation of TRPA1 at reoxygenationin isolated adult cardiac myocytes reduces cell death.
A. Experimental protocol for cardiac myocyte hypoxia-reoxygenation studies. The two TRPA1 activators (ASP and optovin) were given immediately after hypoxia. B. Percentage of LDH release for each experimental group (n=3/group). C. Representative images of trypan blue positive and negative cardiac myocytes for each group (black bar represents 50µm). D. Percentage of dead cells for each experimental group (n=6/group). Data points represent individual biological results for each experiment in addition to values presented as mean ± SD, *P<0.05 vs. SHAM, #P<0.05 vs. DMSO. DMSO = dimethyl sulfoxide, ASP = ASP7663
About 50% of the myocardial damage can be attributed to reperfusion and accordingly, drugs to limit reperfusion injury are importantly needed.21, 22 Therefore, we further determined whether administration of a TRPA1 activator just prior to reperfusion also reduces cellular death in an in vivo rodent heart attack injury model (Experimental protocol Figure 5A). Interestingly, TRPA1 activators when given prior to reperfusion reduced myocardial injury (ASP 42±8*, optovin 43±7*, DMSO 60±5, n=6/group, *P<0.001 versus DMSO, Figure 5B, 5C). The reduction in heart damage seen was also equal to that when the agents were given prior to ischemia (Figure 2C); implying that the beneficial effect of TRPA1 activation occurs mainly during reperfusion to limit reperfusion injury.
Figure 5. TRPA1 activation reduces infarct size at reperfusion.
A. Experimental protocol for myocardial ischemia-reperfusion studies.A TRPA1 activator (ASP and optovin) was given 5 minutes prior to reperfusion. B. Infarct size per area at risk percentage. Data points represent individual biological results for each experiment in addition to values presented as mean ± SD (n=6), *P<0.05 versus DMSO. C. Representative images of left ventricle area at risk for each group. DMSO = dimethyl sulfoxide, ASP=ASP7663
Discussion
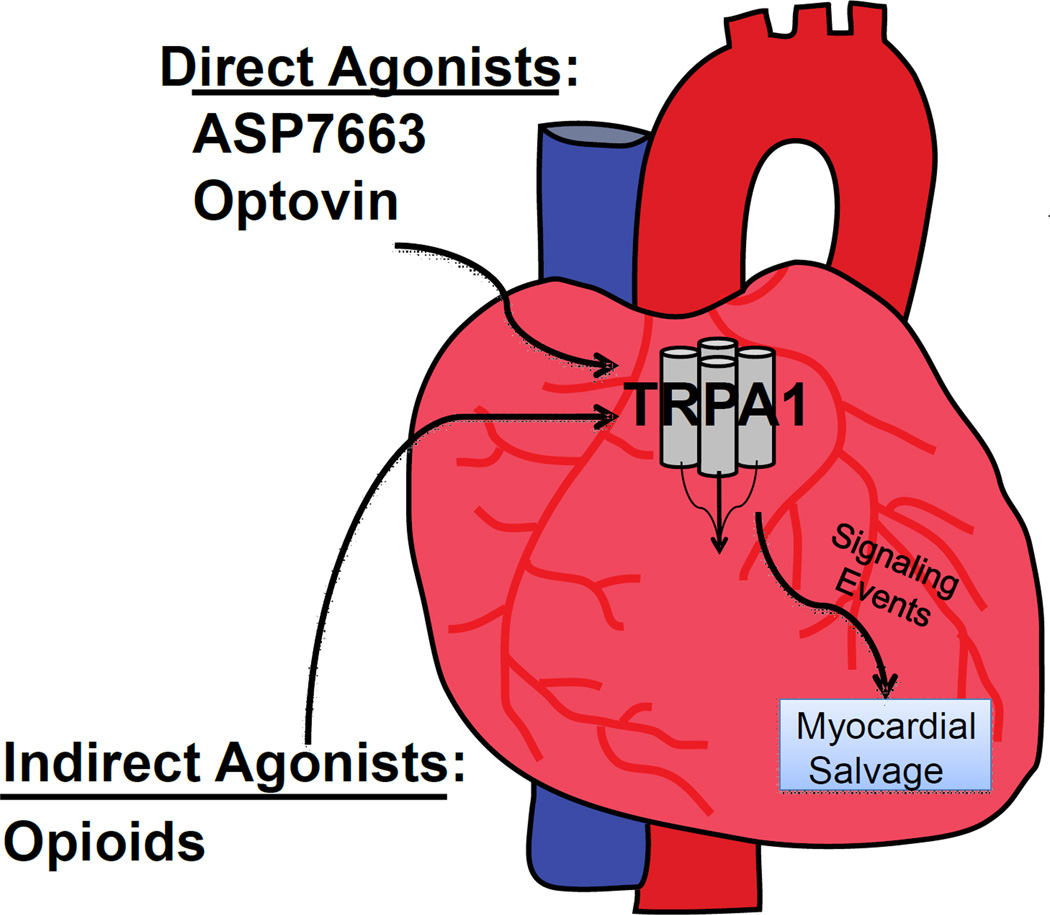
Here we report that TRPA1 is present within the cardiac myocytes and regulates cardiac reperfusion injury. This finding is important since it suggests that the TRPA1 receptor, largely considered only in the cells of the nervous system,23 also contributes an important physiological role in the heart which regulates cellular damage from ischemia-reperfusion injury (Figure 6).
Figure 6. Hypothetical pathway summary.
Activation of TRPA1 prior to or during ischemia within the cardiac myocyte reduces ischemia-reperfusion injury.
TRPA1 can also be modulated by different pain relievers including NSAIDs, acetaminophen, and cyclooxygenase-2 (COX-2).7–9 The presence and function of TRPA1 within the cardiac myocyte must be considered since drugs specifically targeting TRPA1 for pain relief may be detrimental to the heart when at risk for ischemia-reperfusion. If TRPA1 is inhibited, this should be considered when selecting pain relievers for patients during the perioperative period and when treating acute or chronic pain conditions. This will be particularly important in the future when more specific TRPA1 small molecules are designed as analgesics.
Prostaglandins including A1, A2, and J2 can activate neuronal TRPA1.9 One can surmise that some NSAIDs, COX-2 inhibitors, and acetaminophen modulate TRPA1 by altering the production of arachidonic acid metabolites which occur during reperfusion injury. This is supported since either prostaglandin A1 and J2, when given exogenously, reduce myocardial infarct size in experimental models.24, 25 Although further studies are needed, NSAIDs or acetaminophen, by limiting the production of prostaglandins interacting with TRPA1, may potentially block this mechanism which reduces cellular injury.
Opioid-dependent activation of TRPA1 is reported in neuronal cells.19 Receptors associated with pain relief, such as the opioid receptor family, are also present in the cardiac myocytes.26, 27 When activated by opioids, opioid receptors contribute an initiation of a signaling cascade that reduces damage from ischemia-reperfusion injury.28, 29 Our study suggests that opioids, frequently given by anesthesiologists for analgesia, reduce myocardial injury via a TRPA1-dependent mechanism. This finding suggests adjuvants to opioids, which may block TRPA1, may limit the beneficial effects of opioids in regards to reducing reperfusion injury. We previously showed that aspirin, unlike ibuprofen, can block the ability for opioids to reduce myocardial reperfusion injury.30 Although more studies are needed, potentially other myocardial salvaging techniques (such as using volatile anesthetics31 or implementing remote conditioning31) may also have a reduced efficacy for limiting myocardial ischemia-reperfusion injury when TRPA1 is inhibited.
Reactive aldehydes, produced at the highest levels during the initial minutes of reperfusion, are established to modify critical cysteines present on TRPA1 which in turn regulate function.12 These cysteine modifications, which occur through Michael adduct addition, can change the cellular gating of calcium by TRPA1.32 For our study, the binding site for optovin is identified to be within a region of critical cysteines present on TRPA1 that is also essential for reactive aldehyde-induced TRPA1 activation.12, 31 Although further studies will be needed, the TRPA1 activators we used may reduce injury during a heart attack by occupying critical cysteines of TRPA1; limiting the amount of irreversible reactions which occur by reactive aldehydes during the initial minutes of reperfusion.
In contrast to the reversible TRPA1 activators used, cinnamaldehyde, the fragrant component of cinnamon oil, is a reactive aldehyde. Administering this reactive aldehyde prior to ischemia-reperfusion injury resulted in an increase in blood pressure, which was previously shown by a prior study from others in rodents. This particular study described the cardiovascular hemodynamic responses when cinnamaldehyde was given were lost in TRPA1 knockout mice; suggesting the specificity for cinnamaldehyde within the cardiovascular system to interact with TRPA1.33 We further extend the findings for this study by describing how cinnamaldehyde does not reduce myocardial injury unlike the reversible TRPA1 agonists given. It is also interesting to note the blood pressure elevation seen for cinnamaldehyde did not occur for the reversible TRPA1 activators optovin and ASP7663. Although more work is needed, we believe this difference in effect seen by cinnamaldehyde (with an end result of increased blood pressure and lack of infarct size reduction compared to the other TRPA1 agonists optovin and ASP7663) is secondary to the aldehyde that is part of the chemical structure of cinnamaldehyde. The aldehyde may form an irreversible adduct by Michael addition as opposed to the other activators, optovin and ASP7663, which may instead cause a reversible modification of TRPA1.
Our study should be interpreted within the context of several potential limitations. With the discovery of TRPA1 within the cardiac myocyte, more in-depth molecular analysis is needed. This includes determining the critical cysteines within TRPA1 that are important in regulating cellular injury. Further, we administered optovin intravenously without optogenetic activation of the drug prior to delivery as described in a previous study.31 Since ultraviolet light in this prior study was used to activate optovin, we can only assume the effect we see for our studies, which is similar to another TRPA1 agonist, ASP 7763, is possibly secondary to the reactive oxygen species produced during reperfusion converting optovin into an active form. This is an interesting idea which will require further study, and could suggest a means to develop therapeutic modulators of cellular injury that are selectively cleaved into an active form only during ischemia-reperfusion injury.
Our findings can also lead to developing more cardiac safe analgesics that will not block endogenous pathways involving TRPA1, which are important for cardiac protection. Further, once the pathway is understood, design of agents may be possible to provide a beneficial effect of pain relief, with a secondary benefit of reducing reperfusion injury. This could improve upon using opioids to reduce myocardial ischemia-reperfusion injury, which have deleterious side-effects which can lead to addiction, overdose, and death.34
In summary, this report describes the presence of TRPA1 in the heart and how TRPA1 agonists can reduce damage from cardiac reperfusion injury. This may need to be considered when developing drugs to target TRPA1 for analgesia. Further, these findings identify that adjuvants given with opioids that target TRPA1 may block the ability for opioids to reduce myocardial injury. This is important when considering treatment strategies for patients with acute or chronic pain who may have a potential risk of suffering a heart attack.
Supplementary Material
Brief Summary Statement.
We describe in rodents the presence of TRPA1 within the cardiac myocyte and an essential role for TRPA1 activation in reducing myocardial infarct size during ischemia-reperfusion injury.
Acknowledgments
Funding Sources: This work is supported by Stanford University Department of Anesthesiology, Perioperative and Pain Management, Stanford, California, USA (ERG), National Institutes of Health, National Institute of General Medicine T32 training award GM089626 (SLM) and National Heart Lung and Blood Institute HL109212 (ERG), USA
Footnotes
Conflict of Interest: None
References
- 1.Gross ER, Hsu AK, Urban TJ, Mochly-Rosen D, Gross GJ. Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase c. Basic research in cardiology. 2013;108:381. doi: 10.1007/s00395-013-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective cox-2 inhibitors. Jama. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 3.Coxib, traditional NTC. Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossoni G, Muscara MN, Cirino G, Wallace JL. Inhibition of cyclo-oxygenase-2 exacerbates ischaemia-induced acute myocardial dysfunction in the rabbit. British journal of pharmacology. 2002;135:1540–1546. doi: 10.1038/sj.bjp.0704585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei H, Karimaa M, Korjamo T, Koivisto A, Pertovaara A. Transient receptor potential ankyrin 1 ion channel contributes to guarding pain and mechanical hypersensitivity in a rat model of postoperative pain. Anesthesiology. 2012;117:137–148. doi: 10.1097/ALN.0b013e31825adb0e. [DOI] [PubMed] [Google Scholar]
- 7.Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, Bucher B, Galzi JL, Sterner O, Bevan S, Hogestatt ED, Zygmunt PM. Trpa1 mediates spinal antinociception induced by acetaminophen and the cannabinoid delta(9)-tetrahydrocannabiorcol. Nature communications. 2011;2:551. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- 8.Hu H, Tian J, Zhu Y, Wang C, Xiao R, Herz JM, Wood JD, Zhu MX. Activation of trpa1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Archiv : European journal of physiology. 2010;459:579–592. doi: 10.1007/s00424-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor trpa1. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential a1 is a sensory receptor for multiple products of oxidative stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor trpa1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y. Trpa1 underlies a sensing mechanism for o2. Nature chemical biology. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- 14.Gross ER, Hsu AK, Gross GJ. Acute methadone treatment reduces myocardial infarct size via the delta-opioid receptor in rats during reperfusion. Anesthesia and analgesia. 2009;109:1395–1402. doi: 10.1213/ANE.0b013e3181b92201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Small BA, Lu Y, Hsu AK, Gross GJ, Gross ER. Morphine reduces myocardial infarct size via heat shock protein 90 in rodents. BioMed research international. 2015;2015:129612. doi: 10.1155/2015/129612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM. Protection of adult rat cardiac myocytes from ischemic cell death: Role of caveolar microdomains and delta-opioid receptors. American journal of physiology. Heart and circulatory physiology. 2006;291:H344–H350. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- 17.Keysar SB, Trncic N, Larue SM, Fox MH. Hypoxia/reoxygenation-induced mutations in mammalian cells detected by the flow cytometry mutation assay and characterized by mutant spectrum. Radiation research. 2010;173:21–26. doi: 10.1667/RR1838.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YS, Song YS, Giffard RG, Chan PH. Biphasic role of nuclear factor-kappa b on cell survival and cox-2 expression in sod1 tg astrocytes after oxygen glucose deprivation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1076–1088. doi: 10.1038/sj.jcbfm.9600261. [DOI] [PubMed] [Google Scholar]
- 19.Forster AB, Reeh PW, Messlinger K, Fischer MJ. High concentrations of morphine sensitize and activate mouse dorsal root ganglia via trpv1 and trpa1 receptors. Molecular pain. 2009;5:17. doi: 10.1186/1744-8069-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez L, Thiebaut PA, Paillard M, Ducreux S, Abrial M, Crola Da Silva C, Durand A, Alam MR, Van Coppenolle F, Sheu SS, Ovize M. The sr/er-mitochondria calcium crosstalk is regulated by gsk3beta during reperfusion injury. Cell death and differentiation. 2015;22:1890. doi: 10.1038/cdd.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hausenloy DJ, Yellon DM. Targeting myocardial reperfusion injury--the search continues. The New England journal of medicine. 2015;373:1073–1075. doi: 10.1056/NEJMe1509718. [DOI] [PubMed] [Google Scholar]
- 22.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. The New England journal of medicine. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 23.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. Anktm1, a trp-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 24.Wayman NS, Hattori Y, McDonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, Chatterjee PK, Thiemermann C. Ligands of the peroxisome proliferator-activated receptors (ppar-gamma and ppar-alpha) reduce myocardial infarct size. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- 25.Zingarelli B, Hake PW, Mangeshkar P, O'Connor M, Burroughs TJ, Piraino G, Denenberg A, Wong HR. Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-gamma ligands, 15-deoxy-delta12,14-prostaglandin j2 and ciglitazone, in reperfusion injury: Role of nuclear factor-kappab, heat shock factor 1, and akt. Shock. 2007;28:554–563. doi: 10.1097/shk.0b013e31804f56b9. [DOI] [PubMed] [Google Scholar]
- 26.Sobanski P, Krajnik M, Shaqura M, Bloch-Boguslawska E, Schafer M, Mousa SA. The presence of mu-, delta-, and kappa-opioid receptors in human heart tissue. Heart and vessels. 2014;29:855–863. doi: 10.1007/s00380-013-0456-5. [DOI] [PubMed] [Google Scholar]
- 27.Bell SP, Sack MN, Patel A, Opie LH, Yellon DM. Delta opioid receptor stimulation mimics ischemic preconditioning in human heart muscle. Journal of the American College of Cardiology. 2000;36:2296–2302. doi: 10.1016/s0735-1097(00)01011-1. [DOI] [PubMed] [Google Scholar]
- 28.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovascular research. 2006;70:212–221. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka K, Kersten JR, Riess ML. Opioid-induced cardioprotection. Current pharmaceutical design. 2014;20:5696–5705. doi: 10.2174/1381612820666140204120311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross ER, Hsu AK, Gross GJ. Acute aspirin treatment abolishes, whereas acute ibuprofen treatment enhances morphine-induced cardioprotection: Role of 12-lipoxygenase. The Journal of pharmacology and experimental therapeutics. 2004;310:185–191. doi: 10.1124/jpet.103.064667. [DOI] [PubMed] [Google Scholar]
- 31.Kokel D, Cheung CY, Mills R, Coutinho-Budd J, Huang L, Setola V, Sprague J, Jin S, Jin YN, Huang XP, Bruni G, Woolf CJ, Roth BL, Hamblin MR, Zylka MJ, Milan DJ, Peterson RT. Photochemical activation of trpa1 channels in neurons and animals. Nature chemical biology. 2013;9:257–263. doi: 10.1038/nchembio.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate trpa1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 33.Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain SD. Evidence for the pathophysiological relevance of trpa1 receptors in the cardiovascular system in vivo. Cardiovascular research. 2010;87:760–768. doi: 10.1093/cvr/cvq118. [DOI] [PubMed] [Google Scholar]
- 34.Okie S. A flood of opioids, a rising tide of deaths. The New England journal of medicine. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.