Abstract
Investigating sleep in mental disorders has the potential to reveal both disorder-specific and transdiagnostic psychophysiological mechanisms. This meta-analysis aimed at determining the polysomnographic (PSG) characteristics of several mental disorders.
Relevant studies were searched through standard strategies. Controlled PSG studies evaluating sleep in affective, anxiety, eating, pervasive developmental, borderline and antisocial personality disorders, ADHD, and schizophrenia were included. PSG variables of sleep continuity, depth, and architecture, as well as rapid-eye movement (REM) sleep were considered. Calculations were performed with the “Comprehensive Meta-Analysis” and “R” softwares. Using random effects modeling, for each disorder and each variable, a separate meta-analysis was conducted if at least 3 studies were available for calculation of effect sizes as standardized means (Hedges’g). Sources of variability, i.e., sex, age, and mental disorders comorbidity, were evaluated in subgroup analyses.
Sleep alterations were evidenced in all disorders, with the exception of ADHD and seasonal affective disorders. Sleep continuity problems were observed in most mental disorders. Sleep depth and REM pressure alterations were associated with affective, anxiety, autism and schizophrenia disorders. Comorbidity was associated with enhanced REM sleep pressure and more inhibition of sleep depth. No sleep parameter was exclusively altered in one condition; however, no two conditions shared the same PSG profile.
Sleep continuity disturbances imply a transdiagnostic imbalance in the arousal system likely representing a basic dimension of mental health. Sleep depth and REM variables might play a key role in psychiatric comorbidity processes. Constellations of sleep alterations may define distinct disorders better than alterations in one single variable.
Keywords: meta-analysis, sleep continuity, sleep depth, REM sleep, mental disorders
Introduction
Sleep is a fundamental operating state of the central nervous system, occupying up to a third of the human life span. As such, it may be one of the most important psychophysiological processes for brain function and mental health e.g. (Regier, Kuhl, Narrow & Kupfer, 2012; Harvey, Murray, Chandler & Soehner, 2011). Decades of research have shown that sleep disturbances are highly prevalent in mental disorders and have been associated with adverse effects for cognitive, emotional, and interpersonal functioning (e.g. Kahn, Sheppes & Sadeh, 2013; Rasch & Born, 2013; Walker, 2009). While traditional models proposed that distinct sleep alterations would map to specific mental disorders (e.g. Kupfer, Reynolds, Grochocinski, Ulrich & McEachran, 1986; Kupfer, 1976; Kupfer & Foster, 1972), novel models emphasize the transdiagnostical nature of sleep disturbances as a dimension for brain and mental health (e.g. Harvey et al., 2011; Harvey, 2009). Surprisingly, however, sleep characteristics of mental disorders have not yet been sufficiently described, with data being either limited by methodological variance as for major depression or scarce as for most other disorders. The present meta-analysis aims at filling this gap and at discussing the specific versus dimensional role of sleep disturbances in psychopathology both with respect to research and clinical implications.
Sleep and its assessment
For centuries sleep has been conceptualized as a passive state of absolute repose of the brain (e.g. Coriat, 1912). Only in 1953 with the discovery of rapid eye movement (REM) and non-REM (NREM) sleep (Aserinsky & Kleitman, 1953), it became clear that sleep is an active process fundamental for brain function. The question ‘why we sleep’ has started to receive some answers starting from animal research showing the necessity to sleep for survival and key physiological processes, as thermoregulation (e.g. Rechtschaffen, Bergmann, Everson, Kushida, & Gilliand, 1989; Rechtschaffen & Bergmann, 2002). Furthermore, in the last decades, human research on sleep deprivation demonstrated a central role of sleep for mental health, influencing a wide range of cognitive and emotional functions, e.g. memory consolidation and reorganization (e.g. Landmann et al., 2015; Rasch & Born, 2013; Stickgold & Walker, 2013); problem solving and creativity (e.g. Landmann et al., 2014; Wagner, Gais, Haider, Verleger & Born, 2004; Walker, Liston, Hobson & Stickgold, 2002); emotional reactivity and regulation (e.g. Kahn et al., 2013; Baglioni, Spiegelhalder, Lombardo & Riemann, 2010; Walker, 2009); emotional empathy (e.g. Guadagni, Burles, Ferrara & Iaria, 2014); and management of interpersonal conflicts (e.g. Gordon & Chen, 2013).
The gold standard of sleep assessment is polysomnography (PSG) including electrophysiological recordings of brain activity (EEG), muscle activity (EMG), and eye movements (EOG). The recording is scored into different variables defining the continuity and the architecture of sleep. ‘Sleep continuity’ variables relevant for the present meta-analysis are:
Sleep Efficiency Index (SEI): Ratio of Total Sleep Time (TST) to Time in Bed (TIB) x 100 % (or to time from sleep onset until final awakening, i.e. Sleep Period Time - SPT);
Sleep Onset Latency (SOL): Time from lights out until sleep onset (generally defined as first epoch of sleep stage 2)
Total Sleep Time (TST): The total time spent asleep during the recording night;
Number of Awakenings (NA): The total number of awakenings during the night.
Wake After Sleep Onset (WASO): The duration of wake during the night generally defined as the difference between SPT and TST;
‘Sleep architecture’ refers to the distribution of the distinct sleep stages – wake, sleep stage 1, sleep stage 2, slow wave sleep (SWS), rapid eye movement sleep (REM) – that occur in cycles through the night. Sleep architecture variables relevant for the present meta-analysis are:
Total time awake during the night (WAKE): The amount of wake stages as identified through PSG recordings generally presented as percentage of SPT or TST;
Stage 1 (S1): Duration of sleep stage 1 generally presented as percentage of SPT or TST;
Stage 2 (S2): Duration of sleep stage 2 generally presented as percentage of SPT or TST;
Slow Wave Sleep (SWS): Duration of SWS generally presented as percentage of SPT or TST;
Rapid Eye Movement Sleep (REM): Duration of REM generally presented as percentage of SPT or TST.
Finally, different aspects of REM are often further evaluated. REM sleep is a unique state in the sleep-wake cycle characterized by rapid eye movements, a desynchronized EEG (with theta and alpha waves), muscle atonia, and the experience of vivid dreaming. During this sleep state, posture control is lost and autonomic activity is highly unstable, such as sudden intensifications of heart rate and blood pressure occur, breathing becomes irregular and thermoregulation is lowered or suspended (Amici & Zoccoli, 2014). It occurs cyclically throughout sleep in intervals of circa 90 minutes and takes up approximately 20% of the sleep time of healthy adults. Although REM sleep is not divided into stages as NREM sleep (including S1, S2 and SWS), phasic and tonic aspects of this particular sleep stage are often distinguished. Phasic aspects refer to transient and periodic events, such as the rapid eye movements. Phasic events during REM sleep also include peri-orbital integrated potentials, middle ear muscle activity, and skeletal muscle twitches that often appear in correspondence with rapid eye movements. Tonic REM sleep refers to periods in which atonia and desynchronized EEG are present in absence of phasic events (Mallick, Pandi-Perumal, McCarley, & Morrison, 2011). Important REM sleep variables for our work are:
REM Latency (REML): the interval between sleep onset and the onset of the first REM sleep period;
REM Density (REMD): An index that represents the frequency of rapid eye movements during REM sleep.
PSG research in mental disorders psychopathology
PSG research in major depression
The relationship between major depression and sleep has been noted since ancient times. Philosophers and physicians like Plato or Hippocrates already noted that patients afflicted with melancholia complained about sleep disturbances, including problems falling asleep, maintaining sleep, or waking up too early in the morning (described in the book by R. Burton, The Anatomy of Melancholy, first published in 1621). In the last century, the founder of modern psychiatry, Emil Kraepelin (1909), based on clinical observations, proposed that different types of depression may be accompanied by specific forms of sleep disturbances. In his nosology, neurotic (psychological) depression was characterized by problems falling asleep (prolonged sleep latency), whereas endogenous (biological) depression was accompanied by sleep maintenance problems and early morning awakenings. PSG research in psychopathology started in the 1960s with studies showing that major depression was characterized by alterations of sleep continuity, shortened time in SWS and increased REM sleep pressure, i.e. longer REM sleep duration, shortened REM latency, a prolongation of the first REM period, and increased REM density (Riemann, Hohagen, Bahro & Berger, 1994; Berger & Riemann, 1993; Lauer, Riemann, Wiegand & Berger, 1991; Kupfer et al., 1986; Berger, Doerr, Lund, Bronisch & Zerssen, 1982; Kupfer, 1976; Kupfer & Foster, 1972). With respect to REM variables, shortened REM latency was initially proposed to represent the most specific biological marker of depression (Kupfer et al., 1986; Kupfer, 1976; Kupfer & Foster, 1972), while following studies indicated increased REM density as a more specific sleep marker of the disorder (Riemann et al., 1994; Berger & Riemann, 1993; Lauer et al., 1991).
After these pioneer studies, PSG research in major depression continued producing a rich literature, which however is limited by many conflicting findings, probably due to modest sample sizes and methodological variance between studies (Swanson, Hoffmann & Armitage, 2010). Indeed, confounding factors such as sex, age, comorbidity, or medication intake have frequently not been well controlled (Newell, Mairesse, Verbanck & Neu, 2012). A recent meta-analysis of 46 studies reporting PSG recordings in patients with depression compared to control groups aimed at overpassing these limitations by accounting for sampling error across studies and by aggregating data from multiple samples, thus providing a greater statistical power (Pillai, Kalmbach & Ciesla, 2011). The results confirmed REM density as a possible biological marker for the disorder: more specifically, the authors suggested that major depression may be related to a combination of diminished SWS duration and increased REM density. However, in this meta-analytic work the authors did not control whether patients suffered also from other psychopathological conditions commonly associated with depression, as anxiety disorders. Comorbidity being rather the rule than the exception in clinical settings, it is likely that many patients with depression present mixed clinical profiles. Thus, comorbidity may be a relevant factor to consider with respect to sleep physiology associated to distinct disorders.
PSG research in other mental disorders than major depression
While most research focused on depression, less consideration was given to other disorders, with few exceptions, such as a recent meta-analysis evaluating PSG studies in patients with schizophrenia (Chouinard, Poulin, Stip & Godbout, 2004). In this work, 20 studies were evaluated comparing 321 patients with schizophrenia without antipsychotic treatment at the time of sleep recording with 331 healthy controls. Results showed that patients presented with sleep alterations, as increased total time awake and shorter duration of stage 2 sleep during the night, even if never treated. Thus, sleep disruptions seem to be an intrinsic feature of schizophrenia. Disorders comorbidity was however not controlled or further evaluated in subgroup analyses. Moreover, apart from this relevant work on sleep in patients with schizophrenia, sleep characteristics associated with mental disorders different from major depression have been little investigated. Thus, the answer to the question whether sleep variables represent genetic/biological markers of distinct mental disorders is still not clear.
The previous meta-analysis: Benca et al. 1992
The most widely cited analysis which tested the specificity of sleep markers for mental disorders was performed by Benca and co-authors in a meta-analysis published in 1992 (Benca, Obermeyer, Thisted & Gillin, 1992). The authors quantitatively summarized the polysomnographic literature in mental disorders. Data for this meta-analysis included all studies published in English and listed in Index Medicus. The diagnoses of the patient samples were based on available standardized research diagnostic criteria and publications had to report the mean age of the groups. Moreover, the polysomnographic records had to be visually scored by standard criteria (i.e. Rechtschaffen & Kales, 1968). Finally, all patients had to be ill at the time of the study and drug free for at least 14 days before the sleep recordings, although exceptions were made for some studies in which patients had been drug-free for only 7 days. A total of 177 studies were found including data from 7151 patients and controls. The authors considered the following disorders: affective disorders (15 studies, 13 major depression and 2 dysthymic disorder), anxiety disorders (10 studies, 4 generalized anxiety disorder, 4 panic disorder, 1 obsessive compulsive disorder and 1 generalized anxiety disorders or phobias), alcoholism (6 studies), borderline personality disorder (4 studies), dementia (10 studies), eating disorders (8 studies, 6 bulimia and anorexia nervosa and 2 bulimia only), insomnia (7 studies), schizophrenia (12 studies), and narcolepsy (11 studies). The results showed that patients with affective disorders differed significantly from their corresponding healthy comparisons more often than did any other diagnostic category. Moreover, patients with affective disorders differed from healthy controls in all sleep variables considered (including total sleep time, sleep onset latency, sleep efficiency index, SWS duration, REM duration, REM latency, REM density, and other REM variables). Particularly, alterations of REM sleep like shortened REM latency occurred more frequently in patients with affective disorders than in any other psychopathological condition. Nevertheless, it was noted that a shortened REM latency was also associated with schizophrenia. In addition, alterations in any of the sleep variables were not specifically linked to single disorders, thus questioning the specificity of any sleep variable for a particular mental disorder. The single exception was a REM density increase found exclusively in affective disorders, although analyses for this sleep parameter were limited to only some of the disorders due to an insufficient number of studies.
This impressive work still represents the only effort made until now to summarize the biological aspects of sleep in different mental disorders. However, as a first work published around 20 years ago, it includes many limitations which might have affected the results. Specifically, Kupfer and Reynolds (1992) pointed out that the authors did not consider important interfering factors, such as family history of mental disorders in control subjects, different definitions of sleep variables in the studies (considered only for REM latency), subtypes of mental disorders (particularly bipolar depression and different anxiety disorders), studies with overlapping subjects, studies conducted on children, adolescents and elderly. Moreover, the sleep variables considered referred to either only one night or to the average of the nights recorded. As a consequence, no attempt was made to contrast a possible “first night effect”, which is the tendency for individuals to sleep worse during the first night of PSG, or “reverse first night effect”, which may be encountered in some patients with insomnia who sleep better because the maladaptive conditioning between the bed and poor sleep does not generalize to new environments (e.g. Hirscher et al., 2015).
Sleep disturbances as transdiagnostic and dimensional
The results of the meta-analysis from Benca and co-authors did not support the idea that disorder-specific sleep profiles could be observed through polysomnography. Based on the categorical approach to nosology and on the previous finding on REM sleep variable alterations in major depression, this idea was at the time of the publication supported by most sleep researchers. For this reason the findings of this pioneer meta-analysis were initially interpreted with caution and much attention was dedicated to methodological limitations which could have explained the unexpected results.
As in the last decades clinical and research interest in psychopathology focused on a better understanding of comorbidity and psychophysiological mechanisms shared between disorders, the results of the meta-analysis conducted from Benca and co-authors were reinterpreted on the basis of a different theoretical focus. Thus, it inspired modern theories to highlight transdiagnostic and dimensional aspects of sleep disturbances (Harvey et al., 2011). Sleep biology is reciprocally related with emotion regulation and its neurophysiological substrates. Moreover, genetics showed that genes associated with circadian rhythms have been also related to a range of mental disorders. In addition to this, dopaminergic and serotonergic function interplays with circadian and sleep biological mechanisms (Harvey et al., 2011). These new findings have been integrated in a new transdiagnostic and dimensional aetiological and clinical perspective for sleep problems in psychopathology: a) sleep difficulties play a relevant role in facilitating and maintaining mental disorders; b) transdiagnostic treatment of sleep disturbance could be standardly implied in clinical settings (Harvey et al., 2011). A recent study based on 220 patients with post-traumatic stress disorder and problematic alcohol use showed support for the sleep dimensional hypothesis. Presence of insomnia was found to be a transdiagnostic process linked with mental symptoms severity after controlling for emotion dysregulation and depressed mood (Fairholme, Nosen, Nillni, Schumacher, Tull, & Coeffey, 2013).
Sleep, considered as a fundamental operating state of the central nervous system, and occupying up to a third of the human life span, may be one of the most important basic dimensions of brain function and mental health (e.g. Regier et al., 2012; Harvey et al., 2011). Investigating PSG sleep variables in mental disorders has the potential to reveal neurobiological mechanisms of specific disorders (endophenotypic approach) and to evidence neural pathways cutting across diagnostic categories (dimensional approach).
The present meta-analysis
The aim of this meta-analysis was to evaluate nocturnal sleep alterations in mental disorders considering both the endophenotypic and the dimensional approaches. We focused on seven mental disorder categories based on DSM-IV classification (APA, 1994): i.e. affective, anxiety, eating, externalizing (attention-deficit/hyperactivity), pervasive developmental, personality (borderline and antisocial), and schizophrenia disorders. These categories were chosen based on the meta-analysis by Benca et al. (1992). In contrast to the previous work, disorders involving neurologic damage (i.e. dementia and narcolepsy) or substance abuse (i.e. alcoholism) were not considered because we sought to exclude disorders with a known neurobiological or substance-related etiology. Because a meta-analysis on PSG studies in insomnia disorder was recently published (Baglioni et al., 2014), we did not include insomnia disorder here. Instead, we decided to include additional categories such as externalizing and pervasive developmental disorders. Of these, for the first category, we searched only for attention deficit hyperactivity disorder (ADHD) because most sleep research in externalizing disorders focused on this condition (Owens et al., 2013). By adding these two further categories we aimed to address the role of sleep for developmental psychopathology, as this has been recently stressed (e.g. Sadeh, Tikotzky & Kahn, 2014). In line with this choice, within the personality disorders category, we searched also for antisocial personality disorder which is often related to history of ADHD in childhood (McCracken et al., 2000). While sleep problems have been classically linked with depression and anxiety, recent attention has addressed the role of sleep for aggression and impulsivity behaviors especially in adolescence and early adulthood (e.g. Gregory & Sadeh, 2012). Thus, we aimed to evaluate sleep physiology characteristics associated with several mental disorder categories covering various symptomatic profiles.
Methods
This meta-analysis followed MOOSE (Meta-analysis Of Observational Studies in Epidemiology) guidelines (Stroup et al., 2000: see Document S1 in the Supplemental Materials Online).
Study selection
We included nocturnal PSG studies evaluating the disorders noted above but did not consider diurnal studies because of limited data. For inclusion, studies were required to meet the following criteria:
Written in English, German, Italian, Spanish or French;
Diagnosis of mental disorders based on DSM-IV (APA, 1994) or ICD-10 (WHO, 2010);
Discontinuation of psychoactive medication for at least 1 week before and during the PSG examination;
Current episode of mental disorder at the time of the PSG recordings;
Inclusion of a healthy control group;
Report of PSG parameters as means and standard deviations;
Use of standard sleep scoring criteria (Iber et al., 2007; Rechtschaffen & Kales, 1968);
Exclusion of the first sleep laboratory night (i.e. adaptation night) from the analysis;
Report of the average age of participants;
Non-overlap of samples across studies.
We did not include data from unpublished studies in order to focus on those with the most rigorous research methodology subject to peer review.
Search Procedure
We used several strategies to identify our final study sample. First, we conducted computer-based searches using PubMed and PsychInfo according to the following keywords, capturing the title and the abstract: (polysomnogr* OR sleep architecture OR sleep recordings OR sleep stages) AND ((depress* OR affective OR unipolar OR bipolar OR mania) OR (GAD OR anxiety OR posttraumatic stress disorder OR PTSD OR phobia OR panic OR obsessive compulsive disorder OR OCD) OR (attention deficit hyperactivity disorder OR ADHD) OR (autis* OR Asperger OR pervasive developmental disorder) OR (border* OR borderline personality disorder) OR (eating OR anorex* OR bulim*) OR (antisocial personality disorder OR sociopath*) OR (schizophren*)). The search was conducted from January 1992, the date in which the earlier meta-analysis was published (Benca et al., 1992), to July 2015. The first author conducted the literature search in PsycInfo and the third author in PubMed, screening titles and abstracts of potentially eligible studies, collaborating whenever the inclusion or exclusion of one study was doubtful. The first and the second authors examined the full texts and extracted the data for the analyses.
Second, we expanded our search through identifying further studies from the references of the screened full-texts. Third, we contacted authors in the field to obtain further studies and, if needed, to obtain additional information, especially on potential overlaps between samples of different studies (see Acknowledgments).
Data extraction
The literature search lead to the selection of studies evaluating sleep efficiency index (SEI), sleep onset latency (SOL), total sleep time (TST), number of awakenings (NA), wake during the night (WAKE/WASO: we considered these 2 parameters together in one single variable due to the closeness of the 2 definitions and the interchangeable use of the 2 terms in our sample of studies. This decision was made in order to evaluate the largest number of studies possible), REM latency (REML), REM density (REMD), percentages of stage 1 (S1), stage 2 (S2), SWS (SWS), and REM (REM) sleep in the following seven categories of mental disorders:
Affective disorders
The analyses were first conducted for all affective disorders considered together. Afterwards, separate analyses were computed for each specific affective disorder for which a sufficient number of studies was available (at least 3 studies, see paragraphs below for more information on the methodological procedure). Considering our final sample of studies (see Results for details), separate analyses for specific affective disorders were possible to be conducted only for major depression and seasonal affective disorder.
Anxiety disorders
Similarly to affective disorders, first all studies were analyzed together. Afterwards, separate analyses were computed for specific anxiety disorders for which at least 3 studies were available. Analyses could be computed only for panic disorder and Post-Traumatic Stress Disorder (PTSD).
Eating disorders
All studies included in this category focused on anorexia nervosa.
Externalizing disorders
For this category we searched exclusively for Attention-deficit/hyperactivity disorder (ADHD).
Pervasive developmental disorders
All studies included in this category focused either on autistic disorder or Asperger syndrome. We considered these two disorders only separately to evaluate possible differences depending on the degree of cognitive impairment. Moreover, most studies selected for Asperger syndrome included also a group with autistic disorder and compared both patients’ samples with the same control group. This methodological issue was a further reason for analyzing the conditions only separately.
Personality disorders
For this category we focused on borderline personality disorder and antisocial personality disorder. However, for this last condition, only 2 studies were selected in the final database, thus it was not evaluated in meta-analytic computations. Of note, by searching for antisocial personality disorder, we found a study evaluating polysomnography in patients with conduct disorder, which was added in the externalizing disorders category and described in this systematic review. However, this study was not considered in meta-analytic computations.
Schizophrenia
All subtypes were considered together, and no further analysis considering subtypes separately could be conducted due to a lack of a sufficient number of studies.
Table 1 and Table S1 give an overview of descriptive and clinical characteristics of the selected studies, such as demographic information, PSG characteristics, comorbidity in the patient samples, and past personal and family histories of mental disorders in the control samples.
Table 1.
Study characteristics.
| Disorder | N_studies | N_comparisons* | N_patients | N_controls | Age_range | F % | QA (mean ± sd) | QA (median) |
|---|---|---|---|---|---|---|---|---|
| Affective disorders | 43 | 55 | 1627 | 1217 | x< 18 yrs: 6 st. | 48.5 (patients) 44.3 (controls) |
8.56 ± 1.26 | 9 |
| 18 < x < 60 yrs: 29 st. | ||||||||
| x>60 yrs: 2 st. | ||||||||
| >18: 5 st; adolescents + adults: 1 st | ||||||||
| Major depression | 38 | 50 | 1524 | 1128 | x< 18 yrs: 6 st. | 44.9 (patients) 39.8 (controls) |
8.63 ± 1.15 | 8.5 |
| 18 < x < 60 yrs: 25 st. | ||||||||
| x>60 yrs: 2 st. | ||||||||
| >18: 4 st; adolescents + adults: 1 st | ||||||||
| Seasonal affective disorder | 3 | 3 | 55 | 41 | x< 18 yrs: 0 st. | 92.8 (patients) 92.8 (controls) |
7.33 ± 2.52 | 7 |
| 18 < x < 60 yrs: 2 st. | ||||||||
| x>60 yrs: 0 st. | ||||||||
| >18: 1 st | ||||||||
| Mixed unipolar and bipolar affective disorders | 2 | 2 | 48 | 48 | x< 18 yrs: 0 st. | 49.1 (patients) 55.5 (controls) |
9.00 ± 0.00 | 9 |
| 18 < x < 60 yrs: 2 st. | ||||||||
| x>60 yrs: 0 st. | ||||||||
| Anxiety disorders | 21 | 21 | 397 | 409 | x< 18 yrs: 1 st. 18 < x < 60 yrs: 19 st. x>60 yrs: 1 st. |
28.6 (patients) 51.3 (controls) |
8.57 ± 0.81 | 9 |
| Panic disorder | 4 | 4 | 60 | 50 | all 18 < x < 60 yrs | 61.6 (patients) 60.0 (controls) |
8.75 ± 0.50 | 9 |
| Post-traumatic stress disorder | 13 | 13 | 255 | 195 | x< 18 yrs: 0 st. | 21.4 (patients) 33.0 (controls) |
8.46 ± 0.97 | 8 |
| 18 < x < 60 yrs: 12 st. | ||||||||
| x>60 yrs: 1 st. | ||||||||
| Obsessive compulsive disorder | 1 | 1 | 22 | 22 | 18 < x < 60 yrs | 54.5 (patients) 45.5 (controls) |
Score=9 | |
| Specific phobia | 1 | 1 | 19 | 25 | 18 < x < 60 yrs | 47.4 (patients) 36.0 (controls) |
Score=9 | |
| Social phobia | 1 | 1 | 17 | 16 | 18 < x < 60 yrs | 17.6 (patients) 31.3 (controls) |
Score=8 | |
| Mixed anxiety disorders | 1 | 1 | 24 | 101 | < 18 yrs | 58.3 (patients) 46.5 (controls) |
Score=9 | |
| Eating disorders (only studies on anorexia nervosa were found) | 5 | 5 | 58 | 50 | < 18 yrs: 1 st. mixed adolescents and adults: 2 st.; young adults: 2 st |
100 (patients) 100 (controls) |
8.60 ± 1.52 | 8 |
| adults: 2 st | ||||||||
| Externalizing disorders• | 7 | 8.29 ± 0.95 | 8 | |||||
| Attention deficit hyperactivity disorder | 6 | 11 | 128 | 114 | x< 18 yrs: 4 st. 18 < x < 60 yrs: 2 st. x>60 yrs: 0 st. |
17.2 (patients) 28.1 (controls) |
8.50 ± 0.84 | 8 |
| Conduct disorder | 1 | No meta-analysis | 15 | 20 | < 18 yrs: 1 st. | 60.0 (patients) 55.0 (controls) |
Score=7 | |
| Pervasive developmental disorder• | 7 | 8.14±1 .57 | 8 | |||||
| Asperger syndrome | 3 | 3 | 34 | 38 | < 18 yrs: 1 st. | 20.6 (patients) 23.7 (controls) |
8.67 ± 2.08 | 8 |
| 18 < x < 60 yrs: 1 st. | ||||||||
| mixed adolescents and adults: 1 st. | ||||||||
| Autistic disorder | 6 | 7 | 103 | 71 | x< 18 yrs: 4st. | 13.6 (patients) 28.2 (controls) |
7.75 ± 1.26 | 8 |
| mixed adolescents and adults: 2 st. | ||||||||
| Personality disorders• | 6 | 8.5±1. 05 | 8.5 | |||||
| Borderline personality disorder | 5 | 5 | 89 | 85 | 18 < x < 60 yrs | 86.0 (patients) 84.7 (controls) |
8.4 ± 1.14 | 8 |
| Antisocial personality disorder | 1 | No meta-analysis | 19 | 11 | x< 18 yrs: 0 st. | 0.0 (patients) 27.5 (controls) |
Score=7 | |
| 18 < x < 60 yrs: 1 st. | ||||||||
| Schizophrenia disorder | 8 | 10 | 154 | 121 | < 18 yrs: 1 st. 18 < x < 60 yrs: 7 st. |
25.3 (patients) 22.8 (controls)° |
8.5 ± 1.41 | 8 |
Comparisons refer to the number of contrasts available from the studies selected (i.e., some studies were considered in more than one meta-analytic calculation if data were reported separately depending on one or more variables (e.g. mental disorders, gender, age, disorder duration, etc.).
Abbreviations: st=study/studies; yrs= years; QA= quality assessment; sd=standard deviation; F%=percentage of women.
Age range > 18 yrs: including both adults and elderly.
The study of Yang & Winkelman (2006) did not specify how many of patients/controls were females and for that reason it was not included in the calculation of the percentage of female patients with schizophrenia.
Seven main “mental disorders” categories were considered: affective, anxiety, eating, externalizing, pervasive developmental, personality and schizophrenia disorders. Nevertheless, only affective and anxiety disorders were considered also as whole categories, and not only for specific disorders. Indeed, the category “eating disorders” included studies evaluating anorexia nervosa only. No subcategory was found for “schizophrenia” disorder. For “externalizing” disorders only attentional deficit hyperactivity disorder was searched. Although keywords lead to one more study for this category focusing on conduct disorder, this could not be evaluated through meta-analytic computations. For “personality” disorders, borderline and antisocial disorders were searched. However, for this last only one study was selected, thus no analyses were performed. Finally, being 2 of the 3 studies with patients with Asperger syndrome including also a group of patients with autism and comparing both groups with the same control participants, no analyses for the category “pervasive developmental disorders” were performed.
In order to compute meta-analytic parameters for continuous outcome variables, means and standard deviations were used. Table 2 includes the number of studies for each mental disorder and each sleep variable available for use in meta-analytic computations. Most studies reported multiple sleep variables. Of note, for the duration of sleep stages, we considered only studies reporting the value as a percentage of total sleep time or sleep period time (i.e. we did not consider studies reporting the duration in minutes).
Table 2.
Number of comparisons which could be considered for meta-analytic calculations for each disorder and for each sleep variable
| SEI | SOL | TST | NA | WAKE/ WASO(%)1 |
REML | REMD | S1(%) | S2(%) | SWS(%) | REM(%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Sleep Efficiency Index |
Sleep Onset Latency |
Total Sleep Time |
Number of Awakenings |
Wake after sleep onset |
REM Latency |
REM Density |
Duration of stage 1 sleep |
Duration of stage 2 sleep |
Duration of Slow wave sleep |
Duration of REM sleep |
|
| Affective disorders (N=55)2 | 48 | 52 | 36 | 10 | 15 | 52 | 38 | 43 | 42 | 46 | 49 |
| MDD (N=50) | 44 | 48 | 33 | 10 | 15 | 47 | 35 | 40 | 39 | 43 | 45 |
| SAD (N=3) | 3 | 3 | 3 | / | / | 3 | / | / | / | / | 3 |
| Anxiety disorders3 (N=21) | 17 | 19 | 19 | 11 | 3 | 18 | 14 | 14 | 15 | 14 | 17 |
| PD (N=4) | 4 | 4 | 3 | 3 | / | 4 | / | / | 3 | 4 | 3 |
| PTSD (N=13) | 10 | 11 | 13 | 6 | / | 10 | 9 | 9 | 9 | 11 | 11 |
| Eating disorders (N=5)4 | |||||||||||
| Anorexia nervosa (N=5) | 4 | 3 | 3 | / | / | 5 | / | 4 | 4 | 4 | 4 |
| Externalizing disorders (N=6)5 | |||||||||||
| ADHD (N=6) | 6 | 6 | 5 | 4 | 5 | 6 | / | 6 | 6 | 6 | 6 |
| Pervasive developmental disorders (N=10)6 | |||||||||||
| Asperger syndrome (N=3) | 3 | 3 | 3 | / | / | 3 | / | 3 | 3 | 3 | 3 |
| Autistic disorder (N=7) | 7 | 6 | 6 | 6 | 7 | 6 | 3 | 7 | 7 | 7 | 7 |
| Personality disorders (N=5)7 | |||||||||||
| Borderline personality disorder (N=5) | 4 | 5 | 3 | 3 | 4 | 5 | 3 | 5 | 5 | 5 | 5 |
| Schizophrenia8 (N=10) | 10 | 10 | 8 | 6 | 3 | 10 | 9 | 9 | 9 | 10 | 10 |
/ indicates that none or less than 3 studies were available and for this reason no meta-analysis was conducted.
ABBREVIATIONS: MDD= Major Depression Disorder; SAD= Seasonal Affective Disorder; PD= Panic Disorder; PTSD= Post-Traumatic Stress Disorder; AN= Anorexia Nervosa; ADHD= Attention Deficit Hyperactivity Disorder.
WAKE and WASO generally refer to 2 different parameters: while WASO is generally defined as the difference between SPT and TST; WAKE is generally defined as the amount of wake stages as identified through polysomnographic recordings. Nevertheless, in our sample of studies the 2 parameters were often confused, with one study using the first definition for a parameter named WAKE or the other way round. Due to the closeness of the 2 definitions we decide to consider them in one single variable in order to evaluate the largest number of studies possible.
The group “affective disorders” included studies evaluating mixed affective disorders (e.g. mixed unipolar and bipolar affective disorders; this group was not further evaluated); studies focusing on major depression and studies focusing on seasonal affective disorders.
The group “anxiety disorders” included studies evaluating mixed anxiety disorders, social phobia, specific phobia, obsessive compulsive disorder, panic disorder and post-traumatic stress disorder. Because of the number of studies available, only panic disorder and post-traumatic stress disorder could be further evaluated in subgroup analyses.
The group “eating disorders” included studies focusing on anorexia nervosa.
The group “externalizing disorders” included 6 studies focusing on Attention Deficit Hyperactivity Disorder and 1 study evaluating Conduct disorder. Thus, only the 6 studies analyzing PSG in patients with Attention Deficit Hyperactivity Disorder were considered in the meta-analysis.
The group “pervasive developmental disorders” included 7 studies in total, 2 of them included both a group of patients with autism and a group of patients with Asperger syndrome and compared them with the same control group. For this reason, we analyzed the two disorders separately and no analyses for the category “pervasive developmental disorders” were performed.
The group “personality disorders” included 5 studies focusing on Borderline Personality Disorder, and 1 study evaluating Antisocial Personality Disorder. Thus, only the 5 studies analyzing PSG in patients with Borderline Personality Disorder were considered in the meta-analyses.
For schizophrenia, no further subgroups were considered.
Quality assessment
For quality assessment, we referred to Section A of the Critical Appraisal Skills Programme Tool for Case-Control studies (Bradley & Hill, 2001). This section includes 6 questions aimed at assessing the validity of the results. Some questions were adapted for the specific aims of this meta-analysis.
Specifically, the following points were assessed:
Question 1) Did the results address a clear focused issue?
Question 2) Did the authors use an appropriate method to answer this question?
Question 3) Were the cases recruited in an acceptable way? Considering the aims of our work, were the patients assessed via a validated diagnostic interview or not (i.e. through validated questionnaires only)?
Question 4) Were the controls selected in an acceptable way? For our aims, were the controls matched for age and sex?
Question 5) Was the exposure accurately measured to minimize bias? For our work: 5.1.) Was an adaptation night recorded and excluded from the analyses or reported separately? 5.2) Were PSG scorers blind to group assignation? 5.3) Were measurement methods similar in cases and controls? 5.4) Were outcomes measured through standard PSG and scored through standard sleep scoring criteria?
Question 6) What confounding factors were assessed? For our work: 6.1) Were mental disorders comorbidity checked and reported? 6.2) Were interfering variables checked and reported (at least one of body mass index, education level, ethnicity, and socioeconomical status)? 6.3) Was the duration of the psychothropic drug free interval prior to PSG > or = 2 weeks?
In order to calculate a total score, for each of the 11 question, 1 was assigned when the answer was YES and 0 was assigned for NO, higher score (max=11) reflecting better methodological quality.
Statistical analyses: Meta-analytic calculations
In order to evaluate sleep continuity and architecture characteristics, as well as REM variables, related to each disorder, we grouped the 11 specific sleep variables in three main domains, namely:
Sleep continuity: defined by higher sleep efficiency, shorter sleep onset latency, and reduced number of awakenings;
Sleep depth: defined by shorter duration of stage 1 sleep, and longer duration of stage 2 and slow wave sleep;
REM pressure: defined by shorter REM latency, increased REM density, and longer duration of REM sleep.
Meta-analytic calculations for sleep domains were performed using the statistical software package R (http://www.R-project.org/). Effect sizes of single variables (Hedges’g with standard errors) were entered into one meta-analysis for each sleep domain, adjusted for direction (e.g. effect size multiplied by -1 for sleep latency in the sleep continuity domain). Each study could therefore contribute multiple times to the same domain, according to the number of variables the particular study reported in the domain. Robust Variance Estimation (RVE, R package “robumeta”) was used to cater for potentially statistically dependent effect sizes (e.g. Hedges, Tipton, & Johnson, 2010; Tanner-Smith & Tipton, 2014). For this method, results with degrees of freedom < 4 could indicate too few cases for reliable variance estimation. Based on the endophenotypic approach, each sleep variable could be specifically altered in one disorder only. Therefore, a separate meta-analysis for each mental disorder was also conducted considering single sleep variables. Two variables were not considered in sleep domains analyses, but only separately for analyses for each sleep variable: total sleep time, as already included in the definition of sleep efficiency, and WAKE/WASO, as the combination of these two variables together made it complicated to separate these variables for continuity vs architecture. Meta-analytic calculations for each sleep variable were performed using the software “Comprehensive Meta-Analysis” version 3 (Borenstein, Hedges, Higgins & Rothstein, 2005).
For all analyses, significant results were considered with p<0.05, while marginally significant results were considered for p between 0.05 and 0.07.
Effect sizes were calculated as standardized means (Hedges’g). The random-effects model was used because of the considerable heterogeneity between studies (different populations, different settings, etc.). To test for heterogeneity, chi-squared tests and the I2 statistic derived from the chi-squared values were used (Borenstein, Hedges, Higgins & Rothstein, 2009). Meta-analyses were performed when at least 3 studies were available. Possible sources of variability between the studies were controlled through meta-analytic subgroup analyses whenever a sufficient number of studies was available for the subgroup. Sex, age, and mental disorders comorbidity were considered for subgroup analysis. Before performing each meta-analysis, we identified possible outliers by exploring standardized residuals. Studies with standardized residuals > |3|were winsorized, i.e. residuals were reduced to = +/−3. Publication bias was assessed both graphically by using funnel plots and numerically by considering the classical safe-fail number for each significant result evidenced by main analyses.
Results
Figure 1 illustrates the search flow of the studies included in the present meta-analysis. The 91 studies (listed below in the list of references) selected (Table 1) corresponded to 114 different comparisons because some reported data separately for different groups such as women and men, age groups or duration of the disorder (see Table S1 for detailed information about each study). Of those, 55 comparisons referred to affective disorders, 50 evaluating major depression, 3 seasonal affective disorders, and 2 mixed unipolar and bipolar affective disorders. Separate analyses for specific affective disorders could be done only for major depression and seasonal affective disorder. Anxiety disorders were evaluated in 21 comparisons, of which 13 focused on PTSD, 4 on panic disorder, 1 on obsessive-compulsive disorder, 1 on specific phobia, 1 on social phobia, and 1 on mixed anxiety disorders. Consequently, separate analyses for specific anxiety disorders considered only panic disorder and PTSD. Five comparisons investigated polysomnographic characteristics of patients with eating disorders, all presenting anorexia nervosa. The externalizing disorders category included 6 comparisons for ADHD and 1 for conduct disorder. Seven studies were classified in pervasive developmental disorders, resulting in 3 comparisons for Asperger syndrome and 6 for autistic disorder. For personality disorders, we could find 5 comparisons with respect to borderline personality disorder and 1 for antisocial personality disorder. For lack of studies, conduct and antisocial personality disorders were not considered in meta-analytic computations. Finally, 8 studies, including 10 comparisons, were found for schizophrenia.
Figure 1.
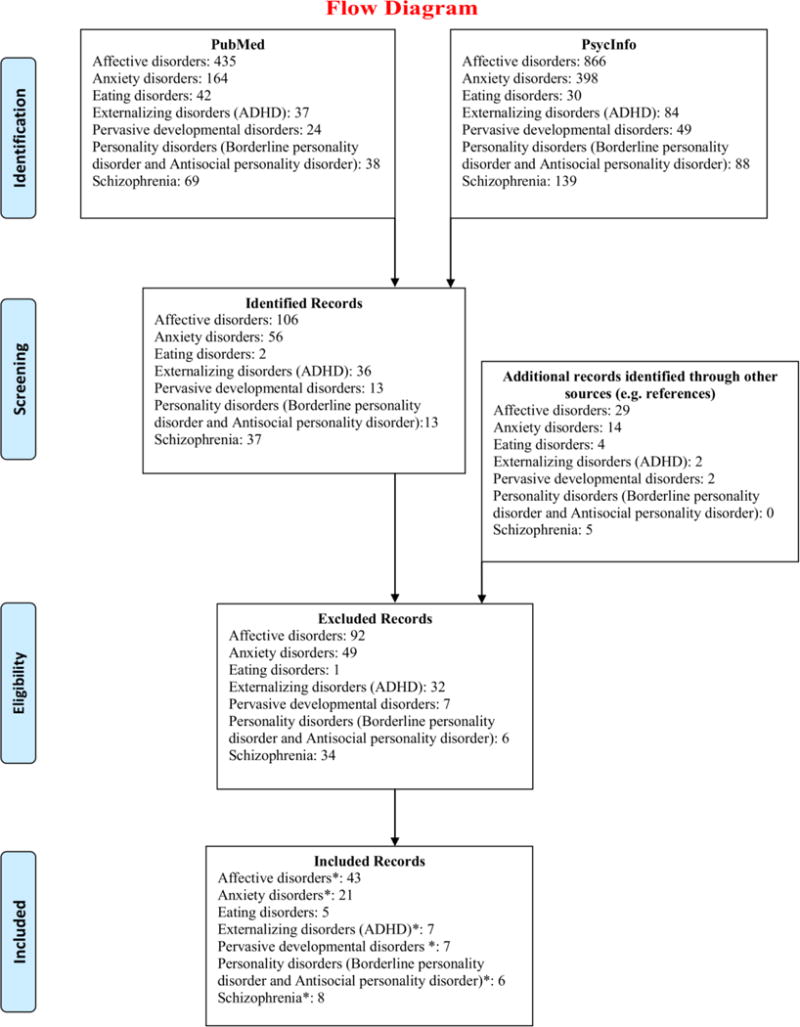
Search flow with respect to each disorder.
*Six studies were considered for more than one disorder: 4 for both affective and anxiety disorders; 1 for affective and borderline personality disorder; and 1 for affective disorders and schizophrenia. Moreover for pervasive developmental disorders, studies focused either on autistic disorder or Asperger syndrome. Two studies included both a group with autistic disorder and a group with Asperger syndrome. In total, we could analyze 6 studies for autistic disorder and 3 for Asperger syndrome. In addition, searching for ‘antisocial personality disorder’, one study was found comparing subjects with conduct disorder with healthy controls, which was added to the list “externalizing disorders”. Finally, some of the included studies compared more than two groups, for example considering sex or age differences, which resulted in a total of 114 comparisons to analyze (from 91 studies). Please refer to Table S1 for detailed information for each included study.
The examination of the full texts led to the exclusion of 205 studies (some studies included more than one sample of patients – e.g. a group with major depression, a group with schizophrenia, and a control group –. For this reason the number of excluded comparisons according to the search flow is 221 and not 205). The excluded studies are listed below in the list of references.
Quality assessment
Appraisal of methodological quality of each study is reported in Table S2 and summarized for disorder categories in Table 1. Of note, Table S2 includes 97 studies as each disorder category was considered independently. All studies were estimated to address a clear focused issue and use an appropriate method to answer to the research’s questions (1 & 2). Eighty-one studies (of 91) based patients’ diagnoses on validated clinical interview, while 10 studies used validated questionnaires. Only 34 of 91 studies matched groups for age and sex. All studies excluded the first night from the analyses as this was an exclusion criterion of our meta-analysis, apart from one study which was included as the authors specifically reported that they tested that the exclusion of the first night from the analyses did not change the results (see Table S1 for more details). About half of the studies (49 of 91) specify that scorers were blind to group assignment, while the remaining 42 either not specify this information or conducted no blind scoring. Six studies of 91 followed slightly different procedural protocols for cases and controls. All included studies measured and scored PSG through standard sleep scoring criteria. Sixty-five studies provided detailed information on mental disorders comorbidity. Only 23 of 91 studies reported information on at least one possible interfering variable, such as body mass index, education level, ethnicity, and socioeconomical status. Finally, 71 of 91 studies required the patient group to be free of psychotropic drug medication for 2 weeks or more prior to PSG.
Global quality scores ranged between 5 and 11. Thirty-four studies scored 8; 24 scored 9; 13 scored 10; other 13 scored 7; 5 scored 11; 2 scored 6 and one study scored 5. As shown in Table 1, median scores for most disorder categories ranged between 8 and 9.
Meta-analyses computations
Results for sleep continuity, sleep depth and REM pressure are graphically summarized in Figure 2. Effect sizes are reported respectively in Table 3 for sleep continuity, Table 4 for sleep depth and Table 5 for REM pressure results. Table S3 reports number of participants, effect sizes and heterogeneity indices for computations conducted for separate sleep variables in each mental disorder.
Figure 2.
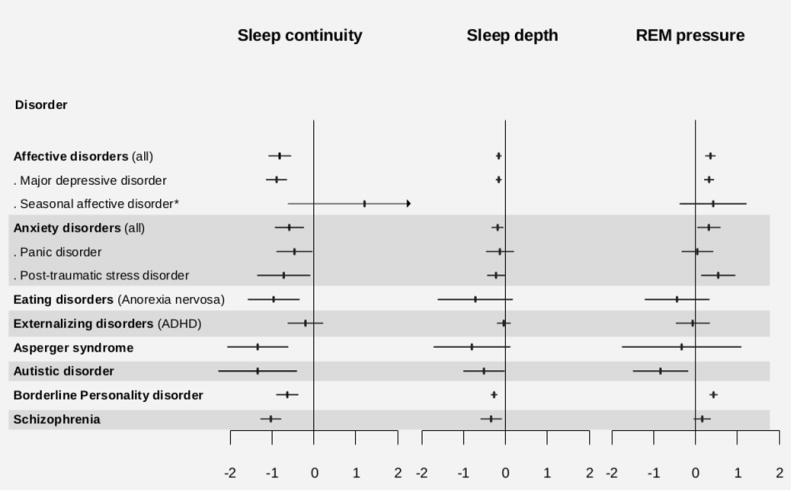
Graphical summary of the main results for sleep domains. Effect sizes and significance values are reported in Tables 3,4 and 5.
*No analyses for sleep depth in seasonal affective disorder (SAD) could be run due to lack of a sufficient number of studies.
Table 3.
Results for sleep continuity domain.
| SLEEP CONTINUITY | MAIN RESULTS | AGE < 18 yrs. | WOMEN | MEN | COMORBIDITY | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | |
| Affective disorders | −0,82 | 0,13 | −6,23 | 48,17 | 0,000 | −1,16 | 0,44 | −2,65 | 7,97 | 0,029 | −0,75 | 0,40 | −1,88 | 9,95 | 0,089 | −0,83 | 0,27 | −3,04 | 15,34 | 0,008 | −0,78 | 0,26 | −3,02 | 15,13 | 0,009 |
| Major depressive disorder | −0,90 | 0,12 | −7,26 | 44,26 | 0,000 | −1,16 | 0,44 | −2,65 | 7,97 | 0,029 | −1,15 | 0,21 | −5,53 | 7,81 | 0,001 | −0,83 | 0,27 | −3,04 | 15,34 | 0,008 | −0,78 | 0,26 | −3,02 | 15,13 | 0,009 |
| Seasonal affective disorder | 1,21 | 0,93 | 1,30 | 1,99 | 0,324 | ||||||||||||||||||||
| Anxiety disorders | −0,59 | 0,17 | −3,43 | 17,80 | 0,003 | −0,92 | 0,41 | −2,22 | 6,07 | 0,068 | −0,58 | 0,24 | −2,45 | 1,96 | 0,136 | ||||||||||
| Panic diosrder | −0,47 | 0,21 | −2,17 | 2,85 | 0,123 | ||||||||||||||||||||
| Post-traumatic stress disorder | −0,72 | 0,32 | −2,27 | 10,27 | 0,046 | −0,92 | 0,41 | −2,22 | 6,07 | 0,068 | |||||||||||||||
| Eating disorder | |||||||||||||||||||||||||
| Anorexia nervosa | −0,96 | 0,31 | −3,10 | 2,74 | 0,060 | −0,96 | 0,31 | −3,10 | 2,74 | 0,060 | |||||||||||||||
| Externalizing disorders | |||||||||||||||||||||||||
| Attentional deficit hyperactivity disorder | −0,20 | 0,21 | −0,97 | 5,89 | 0,372 | −0,15 | 0,17 | −0,88 | 4,09 | 0,429 | −0,24 | 0,25 | −0,96 | 4,05 | 0,389 | ||||||||||
| Pervasive developmental disorders | |||||||||||||||||||||||||
| Asperger syndrome | −1,35 | 0,37 | −3,67 | 1,97 | 0,069 | ||||||||||||||||||||
| Autistic disorder | −1,35 | 0,47 | −2,84 | 5,64 | 0,032 | −0,95 | 0,27 | −3,57 | 3,85 | 0,025 | −1,47 | 0,19 | −7,65 | 1,91 | 0,019 | ||||||||||
| Personality disorders | |||||||||||||||||||||||||
| Borderline Personality disorder | −0,64 | 0,13 | −4,87 | 3,54 | 0,011 | ||||||||||||||||||||
| Schizophrenia | −1,03 | 0,12 | −8,54 | 8,46 | 0,000 | −0,89 | 0,03 | −28,88 | 1,99 | 0,001 | −1,12 | 0,24 | −4,76 | 2,75 | 0,021 | ||||||||||
Abbreviations: ES= effect size (Hedges’g); SE= standard error; t=t-test; dfg=degrees of freedom. Significant results are evidenced in bold if dgf>or=4 (dgf<4 could indicate too few cases for the application of the robust variance estimation method). Marginally significant results (0.05–0.07) are evidenced in bold and italics if dgf> or =4.
Table 4.
Results for sleep depth domain.
| SLEEP DEPTH | MAIN RESULTS | AGE < 18 yrs. | WOMEN | MEN | COMORBIDITY | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | |
| Affective disorders | −0,16 | 0,03 | −4,60 | 38,82 | 0,000 | −0,11 | 0,06 | −1,74 | 8,01 | 0,121 | −0,13 | 0,07 | −1,73 | 7,71 | 0,124 | −0,19 | 0,10 | −2,00 | 9,76 | 0,074 | −0,13 | 0,05 | −2,32 | 12,89 | 0,038 |
| Major depressive disorder | −0,16 | 0,04 | −4,51 | 35,92 | 0,000 | −0,11 | 0,06 | −1,74 | 8,01 | 0,121 | −0,15 | 0,08 | −1,83 | 6,77 | 0,111 | −0,19 | 0,10 | −2,00 | 9,76 | 0,074 | −0,13 | 0,05 | −2,32 | 12,89 | 0,038 |
| Seasonal affective disorder | |||||||||||||||||||||||||
| Anxiety disorders | −0,19 | 0,07 | −2,61 | 14,93 | 0,020 | −0,18 | 0,16 | −1,16 | 4,82 | 0,299 | −0,19 | 0,19 | −1,04 | 1,98 | 0,407 | ||||||||||
| Panic diosrder | −0,13 | 0,17 | −0,78 | 2,64 | 0,498 | ||||||||||||||||||||
| Post-traumatic stress disorder | −0,23 | 0,11 | −2,11 | 8,63 | 0,066 | −0,18 | 0,16 | −1,16 | 4,82 | 0,299 | |||||||||||||||
| Eating disorder | |||||||||||||||||||||||||
| Anorexia nervosa | −0,72 | 0,46 | −1,57 | 2,99 | 0,214 | −0,72 | 0,46 | −1,57 | 2,99 | 0,214 | |||||||||||||||
| Externalizing disorders | |||||||||||||||||||||||||
| Attentional deficit hyperactivity disorder | −0,04 | 0,08 | −0,47 | 4,95 | 0,656 | 0,00 | 0,13 | −0,01 | 2,96 | 0,992 | −0,11 | 0,05 | −2,09 | 2,95 | 0,129 | ||||||||||
| Pervasive developmental disorders | |||||||||||||||||||||||||
| Asperger syndrome | −0,80 | 0,47 | −1,72 | 2,00 | 0,228 | ||||||||||||||||||||
| Autistic disorder | −0,51 | 0,25 | −2,05 | 5,99 | 0,086 | 0,07 | 0,22 | 0,34 | 3,95 | 0,754 | −0,82 | 0,31 | −2,66 | 2,00 | 0,117 | ||||||||||
| Personality disorders | |||||||||||||||||||||||||
| Borderline Personality disorder | −0,27 | 0,04 | −6,53 | 3,93 | 0,003 | ||||||||||||||||||||
| Schizophrenia | −0,34 | 0,13 | −2,65 | 8,42 | 0,028 | −0,13 | 0,31 | −0,42 | 1,89 | 0,720 | −0,57 | 0,22 | −2,64 | 2,94 | 0,079 | ||||||||||
Abbreviations: ES= effect size (Hedges’g); SE= standard error; t=t-test; dfg=degrees of freedom. Significant results are evidenced in bold if dgf>or=4 (dgf<4 could indicate too few cases for the application of the robust variance estimation method). Marginally significant results (0.05–0.07) are evidenced in bold and italics if dgf> or =4.
Table 5.
Results for REM pressure domain.
| REM PRESSURE | MAIN RESULTS | AGE < 18 yrs. | WOMEN | MEN | COMORBIDITY | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | ES | SE | t | dgf | p-Value | |
| Affective disorders | 0,35 | 0,06 | 5,79 | 43,92 | 0,000 | 0,16 | 0,13 | 1,20 | 7,16 | 0,267 | 0,22 | 0,17 | 1,32 | 7,89 | 0,225 | 0,42 | 0,10 | 4,11 | 13,48 | 0,001 | 0,09 | 0,06 | 1,50 | 14,03 | 0,157 |
| Major depressive disorder | 0,32 | 0,06 | 5,34 | 39,74 | 0,000 | 0,16 | 0,13 | 1,20 | 7,16 | 0,267 | 0,12 | 0,18 | 0,66 | 6,35 | 0,531 | 0,42 | 0,10 | 4,11 | 13,48 | 0,001 | 0,07 | 0,06 | 1,15 | 13,22 | 0,272 |
| Seasonal affective disorder | 0,42 | 0,41 | 1,03 | 1,79 | 0,422 | ||||||||||||||||||||
| Anxiety disorders | 0,32 | 0,14 | 2,22 | 17,08 | 0,040 | 0,75 | 0,29 | 2,53 | 5,07 | 0,052 | −0,04 | 0,16 | −0,27 | 1,91 | 0,816 | ||||||||||
| Panic diosrder | 0,04 | 0,19 | 0,22 | 2,57 | 0,841 | ||||||||||||||||||||
| Post-traumatic stress disorder | 0,54 | 0,21 | 2,58 | 9,91 | 0,028 | 0,75 | 0,29 | 2,53 | 5,07 | 0,052 | |||||||||||||||
| Eating disorder | |||||||||||||||||||||||||
| Anorexia nervosa | −0,44 | 0,39 | −1,13 | 3,76 | 0,326 | −0,44 | 0,39 | −1,13 | 3,76 | 0,326 | |||||||||||||||
| Externalizing disorders | |||||||||||||||||||||||||
| Attentional deficit hyperactivity disorder | −0,07 | 0,21 | −0,33 | 4,97 | 0,752 | −0,03 | 0,33 | −0,08 | 2,99 | 0,942 | 0,09 | 0,24 | 0,38 | 2,96 | 0,728 | ||||||||||
| Pervasive developmental disorders | |||||||||||||||||||||||||
| Asperger syndrome | −0,34 | 0,73 | −0,46 | 2,00 | 0,689 | ||||||||||||||||||||
| Autistic disorder | −0,84 | 0,34 | −2,48 | 5,75 | 0,049 | 0,00 | 0,31 | 0,01 | 3,66 | 0,994 | −1,45 | 0,24 | −5,90 | 1,93 | 0,030 | ||||||||||
| Personality disorders | |||||||||||||||||||||||||
| Borderline Personality disorder | 0,43 | 0,05 | 8,84 | 3,72 | 0,001 | ||||||||||||||||||||
| Schizophrenia | 0,16 | 0,10 | 1,51 | 8,16 | 0,169 | −0,25 | 0,06 | −4,02 | 1,94 | 0,060 | 0,06 | 0,10 | 0,66 | 2,94 | 0,559 | ||||||||||
Abbreviations: ES= effect size (Hedges’g); SE= standard error; t=t-test; dfg=degrees of freedom. Significant results are evidenced in bold if dgf>or=4 (dgf<4 could indicate too few cases for the application of the robust variance estimation method). Marginally significant results (0.05–0.07) are evidenced in bold and italics if dgf> or =4.
Sleep continuity disturbance were evidenced in all disorders, with the exception of seasonal affective disorder, panic disorder and ADHD. The result was marginally significant for eating disorder and Asperger syndrome, although this may be dependent on the small number of studies available for these categories (respectively N=5 and N=3). Indeed, degrees of freedoms were < 4 for both these categories. In addition to this, the significant result found for borderline personality disorder showed also degrees of freedom < 4, indicating that more studies are needed with respect to this condition. Analyses for each single variable evidenced some diversions from results for domains. Panic disorder was linked with poorer sleep efficiency, marginally significant longer sleep onset latency and shortened total sleep time compared to controls. Instead, no significant result was evidenced for ADHD in analyses for each sleep variable, similarly to sleep domains analyses. Seasonal affective disorder was associated only with marginally significant shortened total sleep time compared to controls. Finally, sleep onset latency was not statistically different between controls and patients with PTSD, anorexia nervosa, and borderline personality disorder.
Sleep depth was altered in affective, anxiety and schizophrenia disorders. Borderline personality disorder seems also to be associated with reduction of sleep depth, but degrees of freedom were < 4. Within categories, reduction of sleep depth was found in major depression and PTSD (although the result was only marginally significant), but not in panic disorders. Analyses for single sleep variables evidenced no alterations of SWS in patients with major depression, or more generally, in patients with affective disorders. Patients with anxiety disorders, and in particular PTSD, presented shortened SWS, but no alterations in duration of S1 and S2.
REM pressure was increased in affective, anxiety and autistic disorders. Borderline personality disorder seems also to be associated with increased REM pressure, but degrees of freedom were < 4. Within categories, enhanced REM sleep pressure was found in major depression and PTSD, but not in seasonal affective and panic disorders. Analyses for single sleep variables evidenced shortened REML in patients with anxiety disorders, and in particular PTSD, but no alterations in REMD and REM duration. Differently, patients with autism spent shorter time in REM compared to controls, but did not present alterations in REML nor REMD. Finally, patients with borderline personality disorder showed reduced REML compared to controls.
Affective disorders and major depression were associated with alterations in most variables compared to healthy controls (10 of 11), with the exception of SWS duration. Instead, no sleep alteration was observed in ADHD and seasonal affective disorder (apart from marginally significant shortened TST), although analyses for this last condition were limited. Within anxiety disorders, PTSD was associated with severe alterations of sleep continuity, sleep depth and REM variables, while panic disorder was characterized by poor sleep efficiency, latency and quantity only. Anorexia nervosa was associated with sleep discontinuity and lighter sleep, although this last result was only marginally significant. Within pervasive developmental disorders, Asperger syndrome was not associated with alterations of sleep architecture and REM, while autistic patients spent reduced time in REM sleep. Borderline personality disorder was linked with sleep discontinuity and shorter REM latency. Nevertheless, neither patients with anorexia nervosa nor with borderline personality disorder spent more time to fall asleep than controls. Finally, schizophrenia was associated with alteration of sleep continuity, sleep architecture and longer REM latency.
Sample sizes varied relevantly depending on disorder and sleep variable. The largest sample available for calculations related to REM latency for affective disorders included 1597 patients and 1178 controls. Instead, analyses for Asperger syndrome (34 patients vs 24 controls) included the smallest sample size. Moreover, analyses for eating and autistic disorders also referred to small sample sizes (for details see Table S3). Of note, because of lack of sufficient number of studies, we could not run the analyses for number of awakenings in seasonal affective disorder, anorexia nervosa, and Asperger syndrome; for total time awake at night in seasonal affective disorder, panic disorder, PTSD, anorexia nervosa and Asperger syndrome; for REM density in seasonal affective disorder, panic disorder, anorexia nervosa, ADHD, and Asperger syndrome; for duration of stage 1 sleep in panic disorder; and for duration of stage 2 and slow-wave sleep for seasonal affective disorder.
Subgroup analyses
Sex
Analyses were repeated considering only those studies including exclusively women or men or reporting data separately for the two sexes. Results are reported in detail in Tables 3,4,5 and S3. Analyses for women were possible for affective disorders, depression, and anorexia nervosa. Of note, for this last disorder results of the subgroup analyses were identical to the main analyses as all included studies focused exclusively on female samples. Male samples with affective disorders, depression, anxiety disorders, PTSD, and schizophrenia were considered. Of note, within affective disorders, studies reporting data for men focused all on major depression, and, similarly, within anxiety disorders, studies reporting data for men focused all on PTSD.
With respect to depression, sleep continuity and depth, as well as REM pressure were all altered in male patients compared to controls, while in female samples only sleep continuity was found to be disturbed. Similarly, shorter sleep time and increased time awake during the night were found only in male samples with depression. REM sleep variables (REM latency, REM density and REM sleep duration) were all altered only in men with depression, and not in women, for whom only a marginally significant increased REMD was noted. Instead, women, but not men, with depression spent longer time in stage 1 sleep than controls.
Sleep depth was no longer reduced in patients with PTSD and schizophrenia, when focusing only on male populations. Enhanced sleep onset latency was observed in men with PTSD. Longer REM sleep duration was found in men with schizophrenia compared to controls. REM latency was, instead, no longer shorter than controls in male patients with schizophrenia disorders.
Age
To evaluate sleep changes during the life span in the mental disorders considered, the analyses were repeated wherever possible categorizing the studies in 3 groups: < 18/19 years: children and/or adolescents; between 18/19 and 60 years: working age adults; > 60 years: elderly. The working age adults group was evaluated in the majority of the studies, thus, the results related to this age group did not differ substantially from the main results.
Because of lack of sufficient data, we could not run analyses separately for children (< 13 yrs.) vs adolescents (13–18/19 yrs.). Indeed, 17 of 91 studies reported data on pediatric patients, seven of those included young patients with major depression, 1 with anorexia nervosa, 4 with ADHD, 1 with Asperger syndrome, 3 with autistic disorder, and 1 with schizophrenia. Age in the seven studies for major depression ranged between 7 and 18 yrs. One study did not report the age range, but only information on mean (15 yrs.). One study reported data separately for children aged less than 13 yrs. and adolescents (13–17 yrs.). One study included female patients with anorexia nervosa aged between 10 and 17 yrs. All other studies in this category focused on samples of mixed adolescents and young adults. ADHD studies conducted on pediatric samples included age ranges between 5 and 15 yrs. In 1 study including children with autistic disorder, age range was not reported, but only information on mean age (5 yrs.). The other studies focusing on pediatric patients with autistic disorder reported age ranges between 5 and 19 yrs. (including the only study for Asperger syndrome). Finally, one study included patients with schizophrenia aged between 13 and 19 yrs. For detailed information, refer to Table S1.
Separate analyses for the pediatric groups are presented in detail in Tables 3,4,5 and S3. Children and/or adolescents with major depression, presented, in comparison with the main results, only marginally significant poorer sleep continuity compared to controls, but no alterations of sleep depth and REM sleep pressure. Considering specific sleep variables, we found no change in total sleep time, REMD, and duration of S1 and REM sleep compared to controls. Moreover, duration of REML was only marginally shortened compared to healthy controls. Children with autism presented poorer sleep continuity compared to controls, but no alterations in sleep depth and REM pressure. Although these results in sleep domain analyses are limited by degrees of freedom < 4, the same profile was observed considering analyses for each sleep variable. As for main analyses, no significant result was found for ADHD.
Separate analyses for the elderly group were possible to be conducted only for major depression. Compared to main results, findings for elderly individuals with depression, indicated only a marginally significant increased REM density, and no longer altered duration of stages 1 and 2.
Comorbidity excluded
In this subgroup analyses we focused exclusively on studies which carefully excluded all possible mental comorbidities, thus, evaluated specific groups of patients presenting only one diagnosis. We could run these analyses for affective, anxiety, autistic, schizophrenia disorders, depression and ADHD. Of note, within the category of affective disorders, all studies including patients with only one diagnosis referred to major depression. Results are shown in Tables 3,4,5 and S3.
In the absence of comorbidities, major depression was no longer associated with increased REM sleep pressure. Considering each variable separately, results for REM latency, and REM duration showed no significance. S1 duration was also not impaired. Similarly, anxiety disorders without comorbidities were no longer associated with alterations in sleep domains. With respect to each sleep variable, we could observe in patients with anxiety disorders, only poor sleep efficiency and shortened total sleep time. Patients with autism presented enhanced REM latency compared to controls. Of note, REMD could not be calculated for anxiety and autistic disorders. Considering specific sleep variables, REML and SWS duration were no longer shortened in patients with schizophrenia.
Publication bias
Fifty-four funnel plots were visually inspected and related fail-safe classical numbers were calculated for the corresponding significant findings. Summarizing, plots showed few asymmetries, and smaller fail-safe numbers indicating higher publication bias risk were found mostly in association with small study samples. Plots and computations are reported in detail in Document S2.
Discussion
This meta-analysis investigated polysomnographic sleep in several mental disorders and identified both transdiagnostic and disorder-specific sleep alterations. Sleep continuity disturbances cut across current diagnostic entities and were found in all investigated disorders, with the exception of ADHD and seasonal affective disorder. Similarly to Benca et al. (1992), we found that no single sleep variable alteration was specific for one single disorder. Nevertheless no two conditions had the same sleep profile. Sleep depth and REM sleep pressure disturbances were altered in a smaller number of disorders and occurred rarely in a single condition in the absence of comorbidities. For example, even sleep variables that had previously been considered to be related to depression, such as REM latency or duration, were not significantly altered in depression without comorbidity. Sleep architecture and REM sleep variables may be associated with neurobiological pathways underlying different alterations of emotional and cognitive processes, thus, leading to distinct symptoms associations. These results suggest that constellations of sleep alterations may define distinct disorders better than alterations in one single sleep variable.
Sleep and hyperarousal as dimensions for mental health
Findings support the notion of transdiagnostic disruptions of sleep continuity, based on physiological (PSG) data. This implies that the neurobiological balance between arousal and de-arousal is disturbed in most mental disorders and very likely represents a basic dimension for brain function and mental health. Clinically, insomnia, the most prevalent sleep continuity disorder, is known to be highly comorbid with mental and somatic disorders and to increase the risk of some of them, as for example major depression (Baglioni et al., 2011), suicidal behaviors (Biøngaard, Bjerkeset, Romundstad & Gunnel, 2011) and cardiovascular disease (Laugsand, Strand, Platou, Vatten & Janszky, 2013). While classically seen as a predominantly psychological disorder, Riemann et al. (2010; 2012; 2015) pointed out that insomnia is characterized, beyond the better known cognitive/behavioral symptoms, by deviations of neuroendocrine and neuroimmunological variables, as well as electro- and neurophysiological, and functional alterations of the brain, all related to increased levels of psychophysiological arousal. A better understanding of the neurobiological aspects of insomnia may help to identify relevant pathophysiological pathways not only to insomnia but to virtually all mental disorders.
Sleep neural pathways are closely connected and in part overlap with neural pathways regulating affect, cognition and other important brain functions. Sleep can be studied across multiple units of analysis, including genetics, neurophysiology, neurocircuitry, epidemiology, and psychology. The strict categorical approach in psychopathology underestimates the reciprocal influences of neuropsychobiological mechanisms. Comorbidity is indeed the rule and not the exception in mental disorders, which makes it important to spread the focus from specific disorders to psychobiological mechanisms which cut across mental disorders. A dimensional approach to sleep research is in line with the new approach in psychopathology aiming at identifying basic dimensions that cut across diagnostic categories, which should bypass the limitations of the categorical approach embedded in major diagnostic systems (e.g. International Statistical Classification of Diseases and Related Health Problems, ICD-10, World Health Organization, WHO, 2010; and Diagnostic and Statistical Manual of Mental Disorders, DSM-IV, American Psychiatric Association, APA, 1994). These limitations mainly relate to high rates of comorbidity and the neglect of symptoms not included in the primary diagnosis. Major attention to basic dimensions of psychopathology, such as psychomotor activity, mood, anxiety, cognition, suicidal ideation, psychotic symptoms, and sleep-wake functioning, informs the latest revision of DSM (Kupfer, Kuhl & Regier, 2013). DSM-5 (American Psychiatric Association, APA, 2013) follows a new approach, combining categorical and dimensional measurement (Kupfer et al., 2013; Regier et al., 2012). Nevertheless, a great amount of work is necessary to understand which dimensions are crucial for brain and mental health. To this end, the National Institute of Mental Health (NIMH) proposed the Research Domain Criteria (RDoC) Project (Morris, Rumsey & Cuthbert, 2014; Cuthbert & Kozak, 2013; Sanislow et al., 2010) to identify basic dimensions of brain/mind disorders to be studied across multiple units of analysis, from genes to neural circuits to behaviors. Our results suggest that sleep could be investigated within the RDoC concept as a likely basic dimension in mental health.
Further longitudinal studies should evaluate the causal sequences between sleep alterations and changes in relevant aspects of mental health functioning. In particular, it should be better understood the causal interaction between disruption of NREM and REM sleep variables with reduced performance in cognitive daily tasks or alteration of emotional processes, such as heightened emotional reactivity or difficulties in regulating affective responses. Indeed, reduction of sleep depth and increased REM sleep pressure were related to disorders comorbidity. Thus, diverse constellations of sleep architecture and REM sleep alterations may underline specific comorbidities in psychopathology and clarify why some disorders often present together.
Sleep in each mental disorder category
Affective disorders
Most PSG studies focused on major depression. In this meta-analysis we found that major depression was associated with the most severe sleep continuity, sleep depth and REM sleep pressure alterations; in contrast seasonal affective disorder was associated with no alteration in any sleep variable. No analyses could be conducted for bipolar disorder for absence of studies. While Benca et al. (1992) found reduced SWS duration in depression, a result which was confirmed also by a more recent meta-analytic work (Pillai et al., 2011), our study showed no reduction in SWS duration in this group of patients. The different result may depend on procedural differences, i.e. in our study we did not consider data from first PSG night in order to control for the so-called first-night effect (e.g. Hirscher et al., 2015). Slow Wave Sleep disruption could be more evident in patients with major depression in the first night in the laboratory as a result of adaptation to the new environment, more than being a specific feature of the disorder. Future studies should, however, be conducted to assess better this finding, for example through the application of more complex EEG measures such as power spectral analysis, cyclic alternating patterns, or event-related potentials. Subgroup analyses for sex showed more severe sleep impairment in male samples compared to female samples. This may indicate either biological sex differences in the disorder or social differences. For example, men may seek help only when the disorder is severe, while women may seek help earlier. Thusmajor attention should be dedicated to sex differences in future PSG research. When considering only young patients aged less than 18 years old, results showed a slight disruption of sleep continuity in those with depression compared to controls, while no alteration in sleep depth and REM sleep pressure was observed. As sleep architecture and REM variables are related with cognitive and emotional functioning, this result may indicate that sleep disturbances in childhood are less severe and may be associated with better clinical outcome. Because of the limited literature, this can only be said in a speculative way. However, potential clinical implications are so critical, which strongly suggests future PSG research to better explain the role of physiological changes in sleep during development. Future PSG research should be conducted to assess sleep in well-defined samples of children (aged < 13 years) and teens (13–19 years) with depression in controlled studies.
REM variables are strongly altered in major depression, being the only disorder associated with alteration in all three REM variables included (REM latency, REM density and REM sleep duration). Subgroup analyses focusing on only those studies which carefully excluded for mental disorders comorbidity, however, showed that in patients with depression without comorbidity REM latency and REM duration were not altered anymore. In contrast, increased REM density seems to be characteristics of the disorder even when presenting without any comorbidity. Consistent with early theories of depression (see Palagini, Baglioni, Ciapparelli, Gemignani, & Riemann, 2013) and pharmacological studies in healthy subjects (e.g. Nissen et al., 2006), central cholinergic activity and supersensitivity, responsible for the generation of rapid eye movements, may be excessively increased in depression and may represent a relevant neurobiological factor in the regulation of affect. The association between REM sleep variables and affect regulation was evidenced also by neuroimaging studies (van der Helm et al., 2011; Nofzinger et al., 2004). Furthermore, increased REM activity and time are related with suicidal behaviors in individuals with depression (Sabo, Reynolds, Kupfer & Berman, 1991) and in psychotic patients (Keshavan et al., 1994). It could be possible that changes in REM density may precede the onset of a major depression episode by triggering aspects of the emotional functioning. It would be of great interest to investigate whether these possible changes are genetically determined, i.e. they are present from childhood, or precipitate a first depressive episode and persist after the resolution of the episode or are limited to the episode, i.e. are state-dependent. New studies combining physiological (e.g. use of power spectral analysis or neuroimagining techniques) and behavioral (e.g. measures of emotional reactivity or regulation) approaches in patients with mental disorders and healthy controls should clarify the role of REM density for emotional processes.
Anxiety disorders
In our work reduced SWS duration was observed in patients with anxiety disorders, while the previous meta-analysis by Benca et al. (1992) did not report this finding. This is possibly because, in our sample, studies on anxiety disorders specifically focused on PTSD (13 of 21 studies), while the precedent work included mainly studies focusing on generalized anxiety and panic disorders and not on PTSD. We could not perform meta-analyses for generalized anxiety disorder, obsessive compulsive disorder nor phobias. PTSD seems to be linked with all sleep continuity, sleep depth and REM sleep pressure disturbances, while panic disorder mostly with sleep continuity difficulties. These different alterations show that the two disorders present a different sleep physiology indicating that they may be better included in different categorizations. This fits the meta-structure of the current DSM-5 (APA, 2015), that separates PTSD from anxiety disorders, including panic disorder, into different chapters (Regier et al., 2012).
Eating disorders
All studies included in our work focused on anorexia nervosa. Nevertheless, even for this disorder we could evaluate data from only 5 studies. Only marginal significant altered sleep continuity and an increased duration of stage 1 sleep (i.e. light sleep) was evidenced in this condition.
Externalizing disorders
For this category, we focused on ADHD and found no sleep alterations associated to this condition,. As mentioned above, ADHD being a disorder associated with rather hypoarousal instead of hyperarousal, this may explain the results. Four of six studies in this category were conducted on samples of children and/or teens. It could be possible that PSG characteristics of adult patients with ADHD include sleep alterations, but these were not evidenced in our analyses because the study sample for this category mainly focused on pediatric samples. Further PSG studies thus should evaluate and compare different age groups with ADHD.
Pervasive developmental disorders
Asperger syndrome and autistic disorder were found to be associated with diverse sleep alterations. Specifically, Asperger syndrome was linked mainly with disruptions of sleep continuity), while autistic disorder was correlated with both sleep discontinuity and shorter duration of REM sleep. Moreover, autistic disorder in absence of other mental disorders comorbidities was associated with longer REM latency. Diverse alterations associated with REM variables seem to be associated with psychopathology. Consistently, shorter REM duration and increased arousal during REM sleep was also found in insomnia disorder (e.g. Feige et al., 2008; Baglioni et al., 2014). Although REM sleep has been linked with emotional processes (e.g. Van der Helm et al., 2011; Rosales-Lagarde et al., 2012), it is yet not understand how different alterations of REM variables (e.g. increased vs decreased REM sleep pressure) lead to distinct disruptions of emotional processes and psychopathological profiles. The diverse sleep alterations found in the two disorders may indicate that these two conditions do not belong in a common category such as pervasive developmental disorders.
Personality disorders
For this category, we analyzed PSG controlled studies conducted with patients with borderline personality disorder. Results suggest that sleep continuity,sleep depth and REM sleepdisturbances may be associated with this disorder. Moreover, we observed reduced REM latency in patients with borderline personality disorder, while this result was not found previously (Benca et al., 1992). Nevertheless, degress of freedom associated to these analyses also indicate that our sample was too little to draw definite conclusions. Indeed, only 5 studies could be used for our analyses, suggesting that more PSG research in personality disorders is needed.
Schizophrenia
Patients with schizophrenia, compared to controls, showed poor sleep continuity and less deep sleep, but no increased REM sleep pressure. Although REM latency was found to be reduced in this group of patients, no significant result was found when considering schizophrenia in the absence of comorbidities. It is likely that the high comorbidity between schizophrenia and depression could explain this result.
Clinical implications
The results of the present meta-analysis have important conceptual and potential clinical implications. A primary aim of public health is the early identification of risk factors and relevant modulators of the course of illness. The extent of sleep continuity disturbances and their transdiagnostic character foster the concept that the systematic treatment of sleep continuity disturbances in clinical settings may help to improve the course of major mental disorders. Clinical studies showed that the addition of cognitive-behavior therapy for insomnia in standardized interventions protocols of many mental disorders could improve the efficacy of these interventions (e.g. Talbot et al., 2014; Manber et al., 2011; Edinger et al., 2009; Manber et al., 2008). Moreover, considering the longitudinal association between insomnia and depression and mental disorders in general, treating sleep continuity difficulties at an early stage could interrupt the sequential process that gradually reduce the quality of life of people with insomnia and ends in the development of symptoms of psychopathology. Future studies are urgently needed to test this hypothesis. Of note, our results pointed out that classifying mental disorder into more broad categories may not reflect similar neurobiological pathways underlying the single conditions. Indeed different results were noted for major depression and seasonal affective disorder. Similarly, distinct PSG profiles were associated with panic and post-traumatic stress disorders. Finally, Asperger syndrome and autistic disorder seem related to diverse alterations of sleep. These results support DSM 5 changes in disorders classification which for example differentiate trauma from anxiety disorders.
Limitations
Age groups
Although Kupfer and Reynolds (1992) noticed already 20 years ago that sleep at both ends of the life cycle was rarely evaluated in mental disorders this limitation is still valid today. Subgroup analyses focusing on pediatric patients with major depression suggest that the disorder may be less severe in the developmental age, thus it could be possible that clinical interventions may be more effective in early age compared to adult groups. Sleep has been related to development’s outcomes, being linked to brain maturation, learning, memory, temperament, emotional regulation, relational skills, and physical wellbeing. In addition, interventions in early age may have a great impact across the life-span. However PSG research in the developmental age is scarce and future studies should clarify the role of sleep in psychopathology by considering the full life-span perspective.
Sex groups
Similarly, only a few analyses could be conducted for sex subgroups. However, results suggested sex differences in major depression, as well as different results in men sample compared to main analyses in anxiety disorders and schizophrenia. Future PSG research should incorporate separate data presentation/analysesfor sex differences.
Other disorders
PSG research is still largely focusing on major depression, while other mental disorders have been rarely evaluated. We could not run analyses for conditions such as bipolar disorder or generalized anxiety disorder. Our results point out a likely role of sleep in transdiagnostic processes, which strongly recommend future research to assess PSG changes in all categories of mental disorder currently defined. Finally, the future inclusion of somatic disorders as cancer or neurodegenerative disorders will help in better understanding the high comorbidity between mental and somatic diseases and the role of sleep in comorbid processes.
Quality assessment and publication bias
Assessment of methodological quality and of risk of publication bias showed in general that PSG procedures are often conducted through standardized methodologies and scoring guidelines (Iber et al., 2007; Rechtschaffen & Kales, 1968). A possible limitation of our meta-analysis is that we did not search for unpublished studies. As mentioned above, we aimed to focus on most rigorous research which was subject to peer review. Furthermore, PSG studies unlikely do not report negative findings for single variables, as it is common use to include in the manuscript a table with means and standard deviations value for a list of common sleep variables. We evidenced two main limitations in our study sample which future research should overcome:
Some sleep variables have been less frequently considered than others. As an example, we could run only few analyses for REM density, while its biological significance for emotional processes may be relevant and should be better clarified. Whether enhanced REM density may be a specific biological marker for major depression or not, remains still to be defined. Indeed, main results evidenced higher values of REM density in both patients with depression and PTSD compared to controls. However, of the 13 PTSD studies, only one carefully excluded mental disorders comorbidity. Thus, it is likely that comorbidity with depression was frequent in most PTSD studies and could have influenced results. This could however not be tested in our analyses due to insufficient data.
Furthermore, samples characteristics should be more carefully controlled in future research. We could notice that only 34 of 91 studies matched control and patient groups for age and sex. In addition, only 23 of 91 studies collected information on possible confounding variables. In addition, comorbidity with other mental/somatic/sleep disorders should be always reported. Indeed, heterogeneity between the studies was often high (I2>70) and the sources of variability that we could directly investigate did not completely explain this high heterogeneity.
Circadian processes
The available data did not allow consideration of sleep within the context of circadian organization of the sleep-wake cycle. It could be possible that mental disorders differ for circadian timing of major sleep periods and naps, as we know that this explains for example, at least in part, age changes (Cajochen, Münch, Knoblauch, Blatter & Wirz-Justice, 2006).
Quantitative analyses
A further limitation is the general absence of quantitative analyses (e.g. spectral and period/amplitude) of sleep EEG micro-architecture in the data available for this meta-analysis. This may have obscured important windows into mental disorders pathophysiology.
Conclusions
This meta-analysis qualitatively and quantitatively summarized PSG controlled studies conducted in seven mental disorders categories based on DSM-IV classification (APA, 1994): i.e. affective, anxiety, eating, externalizing (attention-deficit/hyperactivity disorder), pervasive developmental, personality (borderline and antisocial personality disorders), and schizophrenia disorders. Sleep continuity disturbances are transdiagnostic in psychopathology, and their treatment in standard care may improve interventions outcomes. Recent clinical studies evidenced effectiveness of cognitive-behavior therapy for insomnia for patients with diverse mental and somatic disorders suffering from sleep disturbance, including depression (e.g. Ashworth et al., 2015), PTSD (e.g. Ho, Chan & Tang, 2015), persistent delusions and hallucinations (e.g. Freeman et al., 2015), and cancer (e.g. Johnson et al., 2015). Sleep architecture and REM variables, defining sleep depth and REM pressure, may play a key role in psychiatric comorbidity through their interaction with cognitive and emotional processes. Different sleep depth and REM alterations may reflect distinct symptomathology, and specific neurobiological and psychological mechanisms should be clarified by future research. A longitudinal approach should be followed to understand how alterations in multiple sleep variables may predict the onset of mental disorders. Future PSG research should be conducted considering all types of mental disorders defined by classificatory diagnostic manuals, including bipolar, generalized anxiety, all personality, and somatization disorders. Furthermore it should address life-span and sex issues and include quantitative EEG analyses as well as consideration of circadian processes.
Supplementary Material
Acknowledgments
The authors wish to thank Prof. R. Armitage, Prof. C. Bastien, Prof. E. Forbes, Prof. J.H. Hudson, Dr. C.J. Meliska, Prof. E. Nofzinger, Prof. B. Parry, and Prof. M. Perlis for their kind replies and for sharing with us additional information about their studies. The authors wish to thank Ms. Zarina Bostanova for the great help with the manuscript’s revision.
Funding information:
The study was funded by the University Medical Center of Freiburg, Germany. Moreover, the study was supported in part by a NIMH grant (P30 MH90333).
Footnotes
Conflict of interest disclosure:
Christoph Nissen received speaker honoraria from Servier. Charles F. Reynolds is part of the Editorial Review Board of the American Association of Geriatric Psychiatry; and he received in the past three years extramural support from: a) National Institute of Health (NIH), b) National Institute of Mental Health (NIMH), c) National Institute on Aging (NIA), d) National Center for Minority Health Disparities (NIMHD), e) National Heart Lung and Blood Institute (NHLBI), f) Center for Medicare and Medicaid Services (CMS), g) Patient Centered Outcomes Research Institute (PCORI), h) John A. Hartford Foundation, i) American Foundation for Suicide Prevention, j) Commonwealth of Pennsylvania, k) Clinical and Translational Science Institute (CTSI), l) National Palliative Care Research Center (NPCRC), m) American Association for Geriatric Psychiatry (for services as associate editor), n) UPMC Endowment in Geriatric Psychiatry (which supports the endowed professorship). Moreover, Charles F. Reynolds received: a) grant support Bristol Meyers Squibb Forrest Labs Lily Pfizer which provide pharmaceutical supplies for NIH sponsored work (the pharmaceutical companies plays no role in the design, analysis and in the reporting of data from Charles F. Reynolds in peer reviewed journals); b) speaker honorarium from Medscape/WEB MD; c) licensed intellectual property as co-inventor for the Psychometric analysis of the Pittsburgh Sleep Quality Index (PSQI): PRO10050447, PI: Dr. Daniel Buysse; d) support for manuscripts by the NIH through grant numbers P60MD000207, P30MH090333, UL1RR024153, UL1TR000005 and by the UPMC Endowment in Geriatric Psychiatry. Dieter Riemann received speaker honoraria from Abbvie. All other authors report no competing interests.
References
- American Psychiatric Association, APA, editor. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Publishing; 1994. [Google Scholar]
- American Psychiatric Association, APA, editor. Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Amici R, Zoccoli G. Adaptation of bodily functions to sleep. Physiological basis of sleep. In: Editors Bassetti C, Dogas Z, Peigneux P, editors. ESRS Sleep Medicine Textbook. European Sleep Research Society; 2014. [Google Scholar]
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility and concomitant phenomena during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Ashworth DK, Sletten TL, Junge M, Simpson K, Clarke D, Cunnington D, Rajaratnam SM. A randomized controlled trial of cognitive behavioral therapy for insomnia: and effective treatment for comorbid insomnia and depression. Journal of Counseling Psychology. 2015;62(2):115–123. doi: 10.1037/cou0000059. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Regen W, Teghen A, Spiegelhalder K, Feige B, Nissen C, Riemann D. Sleep changes in the disorder of insomnia: A meta-analysis of polysomnographic studies. Sleep Medicine Reviews. 2014;18(3):195–213. doi: 10.1016/j.smrv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: A focus on insomnia. Sleep Medicine Reviews. 2010;14(4):227–238. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Archives of General Psychiatry. 1992;49(8):651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Berger M, Riemann D. Symposium: Normal and abnormal REM sleep regulation: REM sleep in depression-an overview. Journal of Sleep Research. 1993;2(4):211–223. doi: 10.1111/j.1365-2869.1993.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Berger M, Doerr P, Lund R, Bronisch T, von Zerssen D. Neuroendocrinological and neurophysiological studies in major depressive disorders: are there biological markers for the endogenous subtype? Biological Psychiatry. 1982;17(11):1217–1242. [PubMed] [Google Scholar]
- Biøngaard JH, Bjerkeset O, Romundstad P, Gunnel D. Sleeping problems and suicide in 75,000 norwegian adults: A 20 year follow-up of the HUNT I Study. Sleep. 2011;34(9):1155–1159. doi: 10.5665/SLEEP.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Comprehensive metaanalysis version 2. Englewood, NJ, USA: Biostat Inc; 2005. [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, editors. Introduction to metaanalysis (statistics in practice) Chichester, West Sussex, UK: Wiley; 2009. [Google Scholar]
- Bradley PMA, Hill A. Critical appraisal skills programme international network: making sense of the evidence. European Journal of Public Health. 2001;11(2):238. [Google Scholar]
- Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA. Sleep and Circadian Rhythm Regulation in Early Parkinson Disease. JAMA Neurology. 2014;71(5):589–595. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R. The anatomy of melancholy. Oxford: H. Cripps; 1621. [Google Scholar]
- Cajochen C, Münch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiology International. 2006;23(1–2):461–474. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: A meta-analysis. Schizophrenia Bulletin. 2004;30(4):957–967. doi: 10.1093/oxfordjournals.schbul.a007145. [DOI] [PubMed] [Google Scholar]
- Coriat IH. The nature of sleep. Journal of Abnormal Psychology. 1912;6:329–367. [Google Scholar]
- Cuthbert BN, Kozak MJ. Constructing Constructs for Psychopathology: The NIMH Research Domain Criteria. Journal of Abnormal Psychology. 2013;122(3):928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Olsen MK, Stechuchak KM, Means MK, Lineberger MD, Kirby A, Carney CE. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: A randomized clinical trial. Sleep. 2009;32(4):499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairholme CP, Nosen EL, Nillni YI, Schumacher JA, Tull MT, Coffey SF. Sleep disturbance and emotion dysregulation as transdiagnostic processes in a comorbid sample. Behavior Research and Therapy. 2013;51(9):540–546. doi: 10.1016/j.brat.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Al-Shajlawi A, Nissen C, Voderholzer U, Hornyak M, Spiegelhalder K, Kloepfer C, Perlis M, Riemann D. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. Journal of Sleep Research. 2008;17(2):180–190. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- Freeman D, Waite F, Startup H, Myers E, Lister R, McInerney J, Harvey AG, Geddes J, Zaiwalla Z, Luengo-Fenandez R, Foster R, Clifton L, Yu LM. Efficacy of cognitive behavioural therapy for sleep improvement in patients with persistent delusions and hallucinations (BEST): a prospective, assessor-blind, randomised controlled pilot trial. Lancet Psychiatry. 2015 doi: 10.1016/S2215-0366(15)00314-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Chen S. The role of sleep in interpersonal conflicts. Do sleepless nights mean worse fights? Psychological and Personality Science. 2013;14(5):1–8. doi: 10.1177/1948550613488952. [DOI] [Google Scholar]
- Gregory AM, Sadeh A. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Medicine Reviews. 2012;16(2):129–136. doi: 10.1016/j.smrv.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Guadagni V, Burles F, Ferrara M, Iaria G. The effects of sleep deprivation on emotional empathy. Journal of Sleep Research. 2014;23(6):657–663. doi: 10.1111/jsr.12192. [DOI] [PubMed] [Google Scholar]
- Harvey AG. A transdiagnostic approach to treating sleep disturbance in psychiatric disorders. Cognitive Behaviour Therapy. 2009;38(Suppl 1):35–42. doi: 10.1080/16506070903033825. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Murray G, Chandler RA, Soehner A. Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clinical Psychology Review. 2011;31(2):225–235. doi: 10.1016/j.cpr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Research Synthesis Methods. 2010;1:39–65. doi: 10.1002/jrsm.5. [DOI] [PubMed] [Google Scholar]; Hirscher V, Unbehaun T, Feige B, Nissen C, Riemann D, Spiegelhalder K. Patients with primary insomnia in the sleep laboratory: Do they present with typical nights of sleep? Journal of Sleep Research. 2015;24(4):383–389. doi: 10.1111/jsr.12280. [DOI] [PubMed] [Google Scholar]
- Ho FY, Chan CS, Tang KN. Cognitive-behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: A meta-analysis of randomized controlled trials. Clinical Psychology Reviews. 2015 doi: 10.1016/j.cpr.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1st. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M, Carlson LE, Garland SN. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Medicine Reviews. 2015 doi: 10.1016/j.smrv.2015.07.001. in press. [DOI] [PubMed] [Google Scholar]
- Ju YS, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM. Sleep quality and preclinical Alzheimer disease. JAMA Neurology. 2013;70(5):587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M, Sheppes G, Sadeh A. Sleep and emotions: Bidirectional links and underlying mechanisms. International Journal of Psychophysiology. 2013;89(2):218–228. doi: 10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Reynolds CF, 3rd, Montrose D, Miewald J, Downs C, Sabo E. Sleep and suicidality in psychotic patients. Acta Psychiatrica Scandinavica. 1994;89(2):122–125. doi: 10.1111/j.1600-0447.1994.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Psychiatrie. Leipzig, Germany: JA Barth; 1909. [Google Scholar]
- Kupfer DJ. REM Latency – Psychobiological marker for primary depressive disease. Biological Psychiatry. 1976;11(2):159–179. [PubMed] [Google Scholar]
- Kupfer DJ, Foster FG. Interval between onset of sleep and rapid-eye-movement sleep as an indicator of depression. Lancet. 1972;2(7779):684–686. doi: 10.1016/s0140-6736(72)92090-9. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Reynolds CF., 3rd Sleep and Psychiatric Disorders. Archives of General Psychiatry. 1992;49(8):669. doi: 10.1001/archpsyc.1992.01820080077011. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Kuhl EA, Regier DA. DSM-5–the future arrived. JAMA. 2013;309(16):1691–1692. doi: 10.1001/jama.2013.2298. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Reynolds CF, 3rd, Grochocinski VJ, Ulrich RF, McEachran A. Aspects of short REM Latency in affective states - A Revisit. Psychiatry Research. 1986;17(1):49–59. doi: 10.1016/0165-1781(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Landmann N, Kuhn M, Maier JG, Spiegelhalder K, Baglioni C, Frase L, Riemann D, Sterr A, Nissen C. REM sleep and memory reorganization: Potential relevance for psychiatry and psychotherapy. Neurobiology of Learning and Memory. 2015;122:28–40. doi: 10.1016/j.nlm.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Landmann N, Kuhn M, Piosczyk H, Feige B, Baglioni C, Spiegelhalder K, Frase L, Riemann D, Sterr A, Nissen C. The reorganisation of memory during sleep. Sleep Medicine Reviews. 2014;18(6):531–541. doi: 10.1016/j.smrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Lauer CJ, Riemann D, Wiegand M, Berger M. From early to late adulthood. Changes in EEG sleep of depressed patients and healthy volunteers. Biological Psychiatry. 1991;29(10):979–993. doi: 10.1016/0006-3223(91)90355-p. [DOI] [PubMed] [Google Scholar]
- Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: A population study. European Heart Journal. 2013;35(21):1382–1393. doi: 10.1093/eurheartj/eht019. [DOI] [PubMed] [Google Scholar]
- Mallick BN, Pandi-Perumal SR, McCarley RW, Morrison AR. Rapid eye movement sleep Regulation and Function. Cambridge University Press; Cambridge: 2011. [Google Scholar]
- Manber R, Bernert RA, Suh S, Novakowski S, Siebern AT, Ong JC. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. Journal of Clinical Sleep Medicine. 2011;7(6):645–652. doi: 10.5664/jcsm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT, Smalley SL, McGough JJ, Crawford L, Del’Homme M, Cantor RM, Liu A, Nelson SF. Evidence for linkage of tandem duplication polymorphism upstream of the dopamine D4 receptor gene (DRD4) with attention deficit hyperactivity disorder (ADHD) Molecular Psychiatry. 2000;5(5):531–536. doi: 10.1038/sj.mp.4000770. [DOI] [PubMed] [Google Scholar]
- Morris SE, Rumsey JM, Cuthbert BN. Rethinking mental disorders: The role of learning and brain plasticity. Restorative Neurology and Neuroscience. 2014;32(1):5–23. doi: 10.3233/RNN-139015. [DOI] [PubMed] [Google Scholar]
- Newell J, Mairesse O, Verbanck P, Neu D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Research. 2012;200(2–3):795–801. doi: 10.1016/j.psychres.2012.07.045. [DOI] [PubMed] [Google Scholar]
- Nissen C, Nofzinger EA, Feige B, Waldheim B, Radosa MP, Riemann D, Berger M. Differential effects of the Muscarinic M1 Receptor Agonist RS-86 and the acetylcholine-esterase inhibitor Donepezil on REM sleep regulation in healthy volunteers. Neuropsychopharmacology. 2006;31:1294–1300. doi: 10.1038/sj.npp.1300906. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Carter C, Luna B, Price JC, Meltzer CC, Miewald JM, Reynolds CF, 3rd, Kupfer DJ. Increased activation of anterior paralimbic and executive cortext from waking to rapid eye movement sleep in depression. Archives of General Psychiatry. 2004;61(7):695–702. doi: 10.1001/archpsyc.61.7.695. [DOI] [PubMed] [Google Scholar]
- Otte JL, Carpenter JS, Manchanda S, Rand KL, Skaar TC, Weaver M, Chernyak Y, Zhong X, Igega C, Landis C. Systematic review of sleep disorders in cancer patients: can the prevalence of sleep disorders be ascertained? Cancer Medicine. 2015;4(2):183–200. doi: 10.1002/cam4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, Gruber R, Brown T, Corkum P, Cortese S, O’Brien L, Stein M, Weiss M. Future research directions in sleep and ADHD: Report of a consensus working group. Journal of Attention Disorders. 2013;17(7):550–564. doi: 10.1177/1087054712457992. [DOI] [PubMed] [Google Scholar]
- Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: State of the art. Sleep Medicine Reviews. 2013;17(5):377–390. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Pillai V, Kalmbach DA, Ciesla JA. A meta-analysis of electroencephalographic sleep in depression: Evidence for genetic biomarkers. Biological Psychiatry. 2011;70(10):912–919. doi: 10.1016/j.biopsych.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Pollmächer T. Insomnia. Comorbidities and special populations. In: Bassetti CL, Dogaŝ Z, Peigneux P, editors. Sleep Medicine Textbook. European Sleep Research Society; 2014. [Google Scholar]
- Rasch B, Born J. About sleep’s role in memory. Physiological Reviews. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep. 1989;12(1):68–87. [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: An update of the 1989 paper. Sleep. 2002;25(1):18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]; Regier DA, Kuhl EA, Narrow WE, Kupfer DJ. Research planning for the future of psychiatric diagnosis. European Psychiatry. 2012;27(7):553–556. doi: 10.1016/j.eurpsy.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Spiker DG, Hanin I, Kupfer DJ. Electroencephalographic sleep, aging, and psychopathology: new data and state of the art. Biological Psychiatry. 1983;18(2):139–155. [PubMed] [Google Scholar]
- Riemann D, Hohagen F, Bahro M, Berger M. Sleep in depression: the influence of age, gender and diagnostic subtype on baseline sleep and the cholinergic REM induction test with RS 86. European Archives of Psychiatry and Clinical Neuroscience. 1994;243(5):279–290. doi: 10.1007/BF02191586. [DOI] [PubMed] [Google Scholar]
- Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegehlhalder K. The neuurobiology of chronic insomnia. Lancet Neurology. 2015;14:547–558. doi: 10.1016/S1474-4422(15)00021-6. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B. REM sleep instability – a new pathway for insomnia? Pharmacopsychiatry. 2012;45(5):167–176. doi: 10.1055/s-0031-1299721. [DOI] [PubMed] [Google Scholar]
- Rosales-Lagarde A, Armony JL, Del Río-Portilla Y, Trejo-Martínez D, Conde R, Corsi-Cabrera Enhanced emotional reactivity after selective REM sleep deprivation in humans: an fMRI study. Frontiers in Behavioral Neuroscience. 2012;18(6):25. doi: 10.3389/fnbeh.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo E, Reynolds CF, 3rd, Kupfer DJ, Berman SR. Sleep, depression, and suicide. Psychiatry Research. 1991;36(3):265–277. doi: 10.1016/0165-1781(91)90025-k. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Tikotzky L, Kahn M. Sleep in infancy and childhood: Implications for emotional and behavioral difficulties in adolescence and beyond. Current Opinion in Psychiatry. 2014;27(6):453–459. doi: 10.1097/YCO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS, Cuthbert BN. Developing Constructs for Psychopathology Research: Research Domain Criteria. Journal of Abnormal Psychology. 2010;119(4):631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Scholtes C, Riemann D. The association between insomnia and cardiovascular diseases. Nature and Science of Sleep. 2010;2:71–78. doi: 10.2147/nss.s7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: Evolving generalization through selective processing. Nature Neuroscience. 2013;16(2):139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Swanson LM, Hoffmann R, Armitage R. Sleep macroarchitecture in depression: Sex differences. Open Sleep Journal. 2010;3:12–18. doi: 10.2174/1874620901003010012. [DOI] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, Perlis ML, Posner DA, Weiss B, Ruoff L, Varbel J, Neylan TC. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: A randomized controlled trial. Sleep. 2014;37(2):327–341. doi: 10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner-Smith EE, Tipton E. Robust variance estimation with dependent effect sizes: Practical considerations including a software tutorial in Stata and SPSS. Research Synthesis Methods. 2014;5:13–30. doi: 10.1002/jrsm.1091. [DOI] [PubMed] [Google Scholar]
- van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experience. Current Biology. 2011;21(23):2029–2032. doi: 10.1016/j.cub.2011.10.052. 2011;21(23):2029–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Duanping L, Bixler EO. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Medicine Reviews. 2013;17(4):241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427(6972):352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annuals of the New York Academy of Sciences. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Cognitive Brain Research. 2002;14(3):317–324. doi: 10.1016/S0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Wetter TC, Collado-Seidel V, Pollmächer T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep. 2000;23(3):361–167. [PubMed] [Google Scholar]
- World Health Organization, editor. International statistical classification of diseases and related health problems. - 10th revision, edition 2010. WHO Library Cataloguing-in-Publication Data; 2010. [Google Scholar]
List of references of the 91 studies included in this meta-analysis
- 1.Armitage R, Emslie GJ, Hoffmann RF, Rintelmann J, Rush A. Delta sleep EEG in depressed adolescent females and healthy controls. Journal of Affective Disorders. 2001;63(1–3):139–148. doi: 10.1016/S0165-0327(00)00194-4. [DOI] [PubMed] [Google Scholar]
- 2.Armitage R, Emslie GJ, Hoffmann RF, Weinberg WA, Kowatch RA, Rintelmann J, Rush A. Ultradian rhythms and temporal coherence in sleep EEG in depressed children and adolescents. Biological Psychiatry. 2000;47(4):338–350. doi: 10.1016/S0006-3223(99)00129-8. [DOI] [PubMed] [Google Scholar]
- 3.Armitage R, Hoffmann RF, Rush AJ. Biological rhythm disturbance in depression: temporal coherence of ultradian sleep EEG rhythms. Psychological Medicine. 1999;29(6):1435–1448. doi: 10.1017/s0033291799001300. [DOI] [PubMed] [Google Scholar]
- 4.Armitage R, Trivedi M, Hoffmann R, Rush AJ. Relationship between objective and subjective sleep measures in depressed patients and healthy controls. Depression and Anxiety. 1997;5(2):97–102. doi: 10.1002/(sici)1520-6394(1997)5:2<97::aid-da6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Arriaga F, Paiva T, Matos-Pires A, Cavaglia F, Lara E, Bastos L. The sleep of non-depressed patients with panic disorder: A comparison with normal controls. Acta Psychiatrica Scandinavica. 1996;93(3):191–194. doi: 10.1111/j.1600-0447.1996.tb10630.x. [DOI] [PubMed] [Google Scholar]
- 6.Bastien CH, Guimond S, St-Jean G, Lemelin S. Signs of insomnia in borderline personality disorder individuals. Journal of Clinical Sleep Medicine. 2008;4(5):462–470. [PMC free article] [PubMed] [Google Scholar]
- 7.Battaglia M, Ferini-Strambi L, Smirne S, Bernadeschi L, Bellodi L. Ambulatory polysomnography of never-depressed borderline subjects: A high-risk approach to rapid eye movement latency. Biological Psychiatry. 1993;33(5):326–334. doi: 10.1016/0006-3223(93)90321-4. [DOI] [PubMed] [Google Scholar]
- 8.Brower KJ, Hoffmann R, Conroy DA, Arnedt JT, Armitage R. Sleep homeostasis in alcohol dependent depressed and healthy control men. European Archives of psychiatry and clinical neuroscience. 2011;261(8):559–566. doi: 10.1007/s00406-011-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown TM, Black B, Uhde TW. The sleep architecture of social phobia. Biological Psychiatry. 1994;35(6):420–421. doi: 10.1016/0006-3223(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 10.Bruni O, Ferri R, Vittori E, Novelli L, Vignati M, Porfirio MC, Curatolo P. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep. 2007;30(11):1577–1585. doi: 10.1093/sleep/30.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunner DP, Kräuchi K, Dijk DJ, Leonhardt G, Haug HJ, Wirz-Justice A. Sleep electroencephalogram in seasonal affective disorder and in control women: Effects of midday light treatment and sleep deprivation. Biological Psychiatry. 1996;40(6):485–496. doi: 10.1016/0006-3223(95)00656-7. [DOI] [PubMed] [Google Scholar]
- 12.Burdick RS, Hoffmann R, Armitage R. Short note: Oral contraceptives and sleep in depressed and healthy women. Sleep. 2002;25(3):347–349. [PubMed] [Google Scholar]
- 13.Clark C, Dupont R, Golshan S, Gillin J, Rapaport MH, Kelsoe JR. Preliminary evidence of an association between increased REM density and poor antidepressant response to partial sleep deprivation. Journal of Affective Disorders. 2000;59(1):77–83. doi: 10.1016/S0165-0327(99)00135-4. [DOI] [PubMed] [Google Scholar]
- 14.Clark CP, Gillin CJ, Golshan S. Do differences in sleep architecture exist between depressives with comorbid simple phobia as compared with pure depressives? Journal of Affective Disorders. 1995;33(4):251–255. doi: 10.1016/0165-0327(94)00096-r. [DOI] [PubMed] [Google Scholar]
- 15.De la Fuente José Manuel, Bobes J, Morlán I, Bascarán MT, Vizuete C, Linkowski P, Mendlewicz J. Is the biological nature of depressive symptoms in borderline patients without concomitant Axis I pathology idiosyncratic? Sleep EEG comparison with recurrent brief, major depression and control subjects. Psychiatry Research. 2004;129(1):65–73. doi: 10.1016/j.psychres.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Della Marca G, Farina B, Mennuni GF, Mazza S, Di Giannantonio M, Spadini V, Mazza M. Microstructure of sleep in eating disorders: Preliminary results. Eating and Weight Disorders. 2004(1):77–80. doi: 10.1007/BF03325049. 9. [DOI] [PubMed] [Google Scholar]
- 17.Delvenne V, Kerkhofs M, Appelboom-Fondu J, Lucas F, Mendlewicz J. Sleep polygraphic variables in anorexia nervosa and depression: A comparative study in adolescents. Journal of Affective Disorders. 1992;25(3):167–172. doi: 10.1016/0165-0327(92)90002-n. [DOI] [PubMed] [Google Scholar]
- 18.Dew MA, Reynolds Charles F, III, Buysse DJ, Houck PR, Hoch CC, Monk TH, Kupfer DJ. Electroencephalographic sleep profiles during depression: Effects of episode duration and other clinical and psychosocial factors in older adults. Archives of General Psychiatry. 1996;53(53):148–156. doi: 10.1001/archpsyc.1996.01830020066008. [DOI] [PubMed] [Google Scholar]
- 19.Diomedi M, Curatolo P, Scalise A, Placidi F, Caretto F, Gigli GL. Sleep abnormalities in mentally retarded autisitc subjects: Down’s syndrome with mental retardation and normal subjects. Brain and Development. 1999;21(8):548–553. doi: 10.1016/s0387-7604(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 20.Dow BM, Kelsoe JR, Gillin JC. Sleep and dreams in Vietnam PTSD and depression. Biological Psychiatry. 1996;39(1):42–50. doi: 10.1016/0006-3223(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 21.Dykierek P, Stadtmüller G, Schramm P, Bahro M, van Calker D, Braus DF, Riemann D. The value of REM sleep parameters in differentiating Alzheimer’s disease from old-age depression and normal aging. Journal of Psychiatric Research. 1998;32(1):1–9. doi: 10.1016/s0022-3956(97)00049-6. [DOI] [PubMed] [Google Scholar]
- 22.Elia M, Ferri R, Musumeci SA, Del Gracco S, Bottita M, Scuderi C, Grubar JC. Sleep in subjects with autistic disorder: A neurophysiological and psychological study. Brain and Development. 2000;22(2):88–92. doi: 10.1016/s0387-7604(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 23.Emslie GJ, Rush AJ, Weinberg WA, Rinterlmann JW, Roffwarg HP. Sleep EEG features of adolescents with major depression. Biological Psychiatry. 1994;36(9):573–581. doi: 10.1016/0006-3223(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 24.Engdahl BE, Eberly RE, Hurwitz TD, Mahowald MW, Blake J. Sleep in a community sample of elderly war veterans with and without posttraumatic stress disorder. Biological Psychiatry. 2000;47(6):520–525. doi: 10.1016/s0006-3223(99)00201-2. [DOI] [PubMed] [Google Scholar]
- 25.Forbes EE, Bertocci MA, Gregory AM, Ryan ND, Axelson DA, Birmaher B, Dahl RE. Objective Sleep in Pediatric Anxiety Disorders and Major Depressive Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(2):148–155. doi: 10.1097/chi.0b013e31815cd9bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germain A, Nielsen TA. Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare suffers. Biological Psychiatry. 2003;54(10):1092–1098. doi: 10.1016/S0006-3223(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 27.Germain A, Nofzinger EA, Kupfer DJ, Buysse DJ. Neurobiology of non-REM sleep in depression - further evidence for hypofrontality and thalamic dysregulation. American Journal of Psychiatry. 2004;161(10):1856–1863. doi: 10.1176/ajp.161.10.1856. [DOI] [PubMed] [Google Scholar]
- 28.Giannotti F, Cortesi F, Cerquiglini A, Vagnoni C, Valente D. Sleep in children with autism with and without autistic regression. Journal of Sleep Research. 2011;20(2):338–347. doi: 10.1111/j.1365-2869.2010.00882.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein MR, Plante DT, Hulse BK, Sarasso S, Landsness EC, Tononi G, Benca RM. Overnight changes in waking auditory evoked potential amplitude reflect altered sleep homeostasis in major depression. Acta Psychiatrica Scandinavica. 2012;125(6):468–477. doi: 10.1111/j.1600-0447.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habukawa M, Uchimura N, Maeda M, Kotorii Nozomu, Maeda H. Sleep findings in young adult patients with posttraumatic stress disorder. Biological Psychiatry. 2007;62(10):1179–1182. doi: 10.1016/j.biopsych.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Herbst E, Metzler TJ, Lenoci M, McCaslin SE, Inslicht S, Marmar CR, Neylan TC. Adaptation effects to sleep studies in participants with and without chronic posttraumatic stress disorder. Psychophysiology. 2010;47(6):1127–1133. doi: 10.1111/j.1469-8986.2010.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann R, Hendrickse W, Rush A, Armitage R. Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Research. 2000;95(3):215–225. doi: 10.1016/S0165-1781(00)00181-5. [DOI] [PubMed] [Google Scholar]
- 33.Hohagen F, Lis S, Krieger S, Winkelmann G, Riemann D, Fritsch-Montero R, Berger M. Sleep EEG of patients with obsessive-compulsive disorder. European Archives of psychiatry and clinical neuroscience. 1994;243(5):273–278. doi: 10.1007/BF02191585. [DOI] [PubMed] [Google Scholar]
- 34.Hubain P, Le Bon O, Vandenhende F, van Wijnendaele R, Linkowski P. Major depression in males: Effects of age, severity and adaptation on sleep variables. Psychiatry Research. 2006;145(2–3):169–177. doi: 10.1016/j.psychres.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Hudson JI, Lipinski JF, Keck PE, JR, Aizley HG, Lukas SE, Rothschild AJ, Kupfer D. Polysomnographic characteristics of young manic patients. Comparison with unipolar depressed patients and normal control subjects. Archives of General Psychiatry. 1992;49(5):378–383. doi: 10.1001/archpsyc.1992.01820050042006. [DOI] [PubMed] [Google Scholar]
- 36.Irwin M, Smith TL, Gillin CJ. Electroencephalographic sleep and natural killer activity in depressed patients and control subjects. Psychosomatic Medicine. 1992;54(1):10–21. doi: 10.1097/00006842-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain, Behavior, and Immunity. 2003;17(5):365–372. doi: 10.1016/S0889-1591(03)00031-X. [DOI] [PubMed] [Google Scholar]
- 38.Keshavan MS, Reynolds CF, Miewald JM, Montrose DM, Sweeney JA, Vasko RC, Kupfer DJ. Delta sleep deficits in schizophrenia: Evidence from automated analyses of sleep. Archives of General Psychiatry. 1998;55(5):443–448. doi: 10.1001/archpsyc.55.5.443. [DOI] [PubMed] [Google Scholar]
- 39.Kirov R, Uebel H, Albrecht B, Banaschewski T, Juliana Yordanova, Rothenberger A. Attention-deficit/hyperactivity disorder (ADHD) and adaptation night as determinants of sleep patterns in children. Eur Child Adolesc Psychiatry. 2012;(21):681–691. doi: 10.1007/s00787-012-0308-3. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi I, Huntley E, Lavela J, Mellman TA. Subjectively and objectively measured sleep with and without posttraumatic stress disorder and trauma exposure. Sleep. 2012;35(7):957–965. doi: 10.5665/sleep.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konofal E, Lecendreux M, Bouvard MP, Mouren-Simeoni MC. High levels of nocturnal activity in children with attention-deficit hyperactivity disorder: A video analysis. Psychiatry and Clinical Neurosciences. 2001;55(2):97–103. doi: 10.1046/j.1440-1819.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 42.Landry P, Marchand L, Mainguy N, Marchand A, Montplaisir J. Electroencephalography during sleep of patients with nocturnal panic disorder. The Journal of Nervous and Mental Disease. 2002;190(8):559–562. doi: 10.1097/00005053-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Lauer CJ, Krieg JC. Weight gain and all-night EEG-sleep in anorexia nervosa. Biological Psychiatry. 1992;31(6):622–625. doi: 10.1016/0006-3223(92)90250-4. [DOI] [PubMed] [Google Scholar]
- 44.Lauer CJ, Krieg JC, Garcia-Borreguero D, Özdaglar A, Holsboer F. Panic disorder and major depression: A comparative electroencephalographic sleep study. Psychiatry Research. 1992;44(1):41–54. doi: 10.1016/0165-1781(92)90068-e. [DOI] [PubMed] [Google Scholar]
- 45.Lauer CJ, Schreiber W, Pollmächer T, Holsboer F, Krieg JC. Sleep in schizophrenia: A polysomnographic study on drug-naive patients. Neuropsychopharmacology. 1997;16(1):51–60. doi: 10.1016/S0893-133X(96)00159-5. [DOI] [PubMed] [Google Scholar]
- 46.Leistedt S, Dumont M, Lanquart JP, Jurysta F, Linkowski P. Characterization of the sleep EEG in acutely depressed men using detrended fluctuation analysis. Clinical Neurophysiology. 2007;118(4):940–950. doi: 10.1016/j.clinph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Limoges É, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain. 2005;128(5):1049–1061. doi: 10.1093/brain/awh425. [DOI] [PubMed] [Google Scholar]
- 48.Lindberg N, Tani P, Appelberg B, Stenberg D, Naukkarinen H, Rimón R, Virkkunen M. Sleep among habitually violent offenders with antisocial personality disorder. Neuropsychobiology. 2003;47(1):198–205. doi: 10.1159/000071215. [DOI] [PubMed] [Google Scholar]
- 49.Lindberg N, Tani P, Sailas E, Virkkala J, Urrila AS, Virkkunen M. Sleep in conduct-disordered adolescents - A polysomnographic and spectral power analysis study: Externalizing disorders. Psychiatry Research. 2008;159(3):339–345. doi: 10.1016/j.psychres.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 50.Lindberg N, Virkkunen M, Tani P, Appelberg B, Rimón R, Porkka-Heiskanen T. Growth hormone-insulin-like growth factor-1 axis, leptin and sleep in anorexia nervosa patients. Neuropsychobiology. 2003;47(2):78–85. doi: 10.1159/000070013. [DOI] [PubMed] [Google Scholar]
- 51.Liscombe MP, Hoffmann RF, Trivedi MH, Parker MK, Rush J, Armitage R. Quantitative EEG amplitude across REM sleep periods in depression: Preliminary report. Journal of psychiatry and neuroscience. 2002;27(1):40–46. [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez J, Hoffmann R, Armitage R. Reduced sleep spindle activity in early-onset and elevated risk for depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(9):934–943. doi: 10.1016/j.jaac.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meliska CJ, Martínez LF, López AM, Sorenson DL, Nowakowski S, Parry BL. Relationship of morningness–eveningness questionnaire score to melatonin and sleep timing, body mass index and atypical depressive symptoms in peri- and post-menopausal women. Psychiatry Research. 2011;188(1):88–95. doi: 10.1016/j.psychres.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mellman TA, David D, Kulick-Bell R, Hebding J, Nolan B. Sleep disturbance and its relationship to psychiatry morbidity after hurricane Andrew. American Journal of Psychiatry. 1995;152(1):1659–1663. doi: 10.1176/ajp.152.11.1659. [DOI] [PubMed] [Google Scholar]
- 55.Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20(1):46–51. doi: 10.1093/sleep/20.1.46. [DOI] [PubMed] [Google Scholar]
- 56.Miano S, Bruni O, Elia M, Trovato A, Smerieri A, Verrillo E, Ferri R. Sleep in children with autistic spectrum disorder: A questionnaire and polysomnographic study. Sleep Medicine. 2007;9(9):64–70. doi: 10.1016/j.sleep.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Miano S, Donfrancesco R, Bruni O, Ferri R, Galiffa S, Pagani J, Villa MP. NREM sleep instability is reduced in children with attention-deficit/hyperactivity disorder: Externalizing disorders. Sleep. 2006;29(6):797–803. [PubMed] [Google Scholar]
- 58.Modell S, Ising M, Holsboer F, Lauer CJ. The Munich vulnerability study on affective disorders: stability of polysomnographic findings over time. Biological Psychiatry. 2002;52(5):430–437. doi: 10.1016/S0006-3223(02)01398-7. [DOI] [PubMed] [Google Scholar]
- 59.Moeller FG, Gillin J, Irwin M, Golshan S, Kripke DF, Schuckit M. A comparison of sleep EEGs in patients with primary major depression and major depression secondary to alcoholism. Journal of Affective Disorders. 1993;27(1):39–42. doi: 10.1016/0165-0327(93)90095-2. [DOI] [PubMed] [Google Scholar]
- 60.Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory Markers and Sleep Disturbance in Major Depression. Psychosomatic Medicine. 2005;67(2):187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 61.Nobili L, Baglietto MG, Beelke M, de Carli F, Di Comite R, Fiocchi I, Ferrillo F. Impairment of the production of delta sleep in anorectic adolescents. Sleep. 2004;27(8):1553–1559. doi: 10.1093/sleep/27.8.1553. [DOI] [PubMed] [Google Scholar]
- 62.Nofzinger EA, Schwartz RM, Reynolds CF, Thase ME, Jennings J, Frank E, Kupfer DJ. Correlation of nocturnal penile tumescence and daytime affect intensity in depressed men. Psychiatry Research. 1993;49(2):139–150. doi: 10.1016/0165-1781(93)90101-L. [DOI] [PubMed] [Google Scholar]
- 63.Palchikov VE, Zolotarev Dmitry Y, Danilenko Konstantin V, Putilov AA. Effects of the Seasons and of Bright Light Administered at Different Times of Day on Sleep EEG and Mood in Patients with Seasonal Affective Disorder. Biological Rhythm Research. 1997;28(2):166–184. doi: 10.1076/brhm.28.2.166.12994. [DOI] [Google Scholar]
- 64.Philipsen A, Feige B, Al-Shajlawi A, Schmahl C, Bohus M, Richter H, Riemann D. Increased delta power and discrepancies in objective and subjective sleep measurements in borderline personality disorder. Journal of Psychiatric Research. 2005;39(5):489–498. doi: 10.1016/j.jpsychires.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Philipsen A, Feige B, Hesslinger B, Ebert D, Carl C, Hornyak M, Riemann D. Sleep in adults with attention-deficit/hyperactivity disorder: A controlled polysomnographic study including spectral analysis of the sleep EEG. Sleep. 2005;28(7):877–884. doi: 10.1093/sleep/28.7.877. [DOI] [PubMed] [Google Scholar]
- 66.Prihodova I, Paclt I, Kemlink D, Nevsimalova S. Sleep microstructure is not altered in children with attention-deficit/hyperactivtiy disorder (ADHD) Physiology Research. 2012;61(1):125–133. doi: 10.33549/physiolres.932225. [DOI] [PubMed] [Google Scholar]
- 67.Raboni M, Alonso F, Tufik S, Suchecki D. Improvement of mood and sleep alterations in posttraumatic stress disorder patients by eye movement desensitization and reprocessing. Frontiers in behavioral neuroscience. 2014;8(209) doi: 10.3389/fnbeh.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rao U, Dahl RE, Ryan ND, Birmaher B, Williamson DE, Rao R, Kaufman J. Heterogeneity in EEG sleep findings in adolescent depression: unipolar versus bipolar clinical course. Journal of Affective Disorders. 2002;70(3):273–280. doi: 10.1016/S0165-0327(01)00396-2. [DOI] [PubMed] [Google Scholar]
- 69.Riemann D, Hohagen F, Bahro M, Berger M. Sleep in depression: the influence of age, gender and diagnostic subtype on baseline sleep and the cholinergic REM induction test with RS 86. European archives of psychiatry and clinical neuroscience. 1994;243(5):279–290. doi: 10.1007/BF02191586. [DOI] [PubMed] [Google Scholar]
- 70.Riemann D, Kammerer J, Löw H, Schmidt MH. Sleep in adolescents with primary major depression and schizophrenia: A pilot study. Journal of child psychology and psychiatry, and allied disciplines. 1995;36(2):313–326. doi: 10.1111/j.1469-7610.1995.tb01827.x. [DOI] [PubMed] [Google Scholar]
- 71.Röschke J, Mann K. The sleep EEG’s microstructure in depression: Alterations of the phase relations between EEG rhythms during REM and NREM sleep. Sleep Medicine. 2002;3(6):501–505. doi: 10.1016/s1389-9457(02)00134-x. [DOI] [PubMed] [Google Scholar]
- 72.Röschke J, Prentice-Cuntz T, Wagner P, Mann K, Frank C. Amplitude frequency characteristics of evoked potentials during sleep: An analysis of the brain’s transfer properties in depression. Biological Psychiatry. 1996;40(8):736–743. doi: 10.1016/0006-3223(95)00495-5. [DOI] [PubMed] [Google Scholar]
- 73.Röschke J, Wagner P, Mann K, Prentice-Cuntz T, Frank C. An analysis of the brain’s transfer properties in schizophrenia: Amplitude frequency characteristics and evoked potentials during sleep. Biological Psychiatry. 1998;43(7):503–510. doi: 10.1016/S0006-3223(97)00223-0. [DOI] [PubMed] [Google Scholar]
- 74.Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biological Psychiatry. 1994;35(3):195–202. doi: 10.1016/0006-3223(94)91152-5. [DOI] [PubMed] [Google Scholar]
- 75.Rotenberg VS, Indursky P, Kayumov L, Sirota P, Melamed Y. The relationship between subjective sleep estimation and objective sleep variables in depressed patients. International Journal of Psychophysiology. 2000;37(3):291–297. doi: 10.1016/S0167-8760(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 76.Schredl M, Paul F, Reinhard I, Ebner-Priemer UW, Schmahl C, Bohus M. Sleep and dreaming in patients with borderline personality disorder: A polysomnographic study. Psychiatry Research. 2012;30(2–3):430–436. doi: 10.1016/j.psychres.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz PJ, Rosenthal NE, Kajimura N, Han L, Turner EH, Bender C, Wehr TA. Ultradian oscillations in cranial thermoregulation and electroencephalographic slow-wave activity during sleep are abnormal in humans with annual winter depression. Brain Research. 2000;866(1–2):152–167. doi: 10.1016/S0006-8993(00)02271-X. [DOI] [PubMed] [Google Scholar]
- 78.Shipley JE, Schteingart DE, Tandon R, Pande AC, Grunhaus L, Haskett RF, Starkman MN. EEG sleep in cushing’s disease and cushing’s syndrome: comparison with patients with major depressive disorder. Biological Psychiatry. 1992;32(2):146–155. doi: 10.1016/0006-3223(92)90017-T. [DOI] [PubMed] [Google Scholar]
- 79.Sobanski E, Schredl M, Kettler N, Alm B. Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: A controlled polysomnographic study. Sleep. 2008;31(3):375–381. doi: 10.1093/sleep/31.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stein MB, Enns MW, Kryger MH. Sleep in nondepressed patients with panic disorder: II. Polysomnographic assessment of sleep architecture and sleep continuity. Journal of Affective Disorders. 1993;28(1):1–6. doi: 10.1016/0165-0327(93)90071-q. [DOI] [PubMed] [Google Scholar]
- 81.Swanson LM, Hoffmann RF, Armitage R. Sleep macroarchitecture in depression: Sex differences. The Open Sleep Journal. 2010;3(3):12–18. doi: 10.2174/1874620901003010012. [DOI] [Google Scholar]
- 82.Tandon R, Shipley JE, Taylor S, Greden JF, Eiser A, DeQuardo J, Goodson J. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Archives of General Psychiatry. 1992;49(3):185–194. doi: 10.1001/archpsyc.1992.01820030017003. [DOI] [PubMed] [Google Scholar]
- 83.Tani P, Lindberg N, Nieminen-von Wendt T, von Wendt L, Virkkala J, Appelberg B, Porkka-Heiskanen T. Sleep in young adults with Asperger syndrome. Neuropsychobiology. 2004;50(2):147–152. doi: 10.1159/000079106. [DOI] [PubMed] [Google Scholar]
- 84.Thase ME, Reynolds Charles F, III, Frank E, Jennings J, Nofzinger EA, Fascizka AL, Kupfer DJ. Polysomnographic studies of unmedicated depressed men before and after cognitive behavioral therapy. American Journal of Psychiatry. 1994;151(11):1615–1622. doi: 10.1176/ajp.151.11.1615. [DOI] [PubMed] [Google Scholar]
- 85.van Liempt S, Arends J, Cluitmans PJ, Westenberg HG, Kahn RS, Vermetten E. Sympathetic activity and hypothalamo-pituitary–adrenal axis activity during sleep in post-traumatic stress disorder: A study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2012;38(1):155–165. doi: 10.1016/j.psyneuen.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 86.Wickniak A, Antczak J, Wierzbicka A, Jernajczyk W. Alterations in pattern of rapid eye movement activity during REM sleep in depression. Acta Neurobiologiae Experimentalis. 2002;62(4):243–250. doi: 10.55782/ane-2002-1441. [DOI] [PubMed] [Google Scholar]
- 87.Wojnar J, Brower KJ, Dopp R, Wojnar M, Emslie G, Rintelmann J, Armitage R. Sleep and body mass index in depressed children and healthy controls. Sleep Medicine. 2010;11(3):295–301. doi: 10.1016/j.sleep.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woodward SH, Murburg MM, Bliwise DL. PTSD-related hyperarousal assessed during sleep. Psychology and Behavior. 2000;70(70):197–203. doi: 10.1016/s0031-9384(00)00271-7. [DOI] [PubMed] [Google Scholar]
- 89.Yang C, Winkelman JW. Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophrenia Research. 2006;82(2–3):251–260. doi: 10.1016/j.schres.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 90.Yetkin S, Aydin H, Özgen F. Polysomnography in patients with posttraumatic stress disorder. Psychiatry and Clinical Neurosciences. 2010;64(64):309–317. doi: 10.1111/j.1440-1819.2010.02084.x. [DOI] [PubMed] [Google Scholar]
- 91.Yetkin S, Aydin H, Özgen F, Sütcigil L, Bozkurt A. Sleep architecture in schizophrenia patients. Turkish Journal of Psychiatry. 2011;22(1):1–8. [PubMed] [Google Scholar]
List of references of the 205 studies excluded in this meta-analysis
- 1.Akinci G, Oztura I, Hiz S, Akdogan O, Karaarslan D, Ozek H, Akay A. Sleep Structure in Children With Attention-Deficit/Hyperactivity Disorder. Journal of child neurology. 2015:1–6. doi: 10.1177/0883073815573318. [DOI] [PubMed] [Google Scholar]
- 2.Alfano CA, Reynolds K, Scott N, Dahl RE, Mellman TA. Polysomnographic sleep patterns of non-depressed, non-medicated children with generalized anxiety disorder. Journal of Affective Disorders. 2013;147(1–3):379–384. doi: 10.1016/j.jad.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JL, Rosen LN, Mendelson WB, Jacobsen FM, Skwerer RG, Joseph-Vanderpool JR, Rosenthal NE. Sleep in fall/winter seasonal affective disorder: Effects of light and changing seasons. Journal of Psychosomatic Research. 1994;38(4):323–337. doi: 10.1016/0022-3999(94)90037-X. [DOI] [PubMed] [Google Scholar]
- 4.Antonijevic IA, Murck H, Frieboes RM, Uhr M, Steiger A. On the role of menopause for sleep-endocrine alterations associated with major depression. Psychoneuroendocrinology. 2003;28(3):401–418. doi: 10.1016/s0306-4530(02)00031-8. [DOI] [PubMed] [Google Scholar]
- 5.Arana-Lechuga Y, Nuñez-Ortiz R, Terán-Pérez G, Castillo-Montoya C, Jiménez-Anguiano A, Gonzalez-Robles RO, Velázquez-Moctezuma J. Sleep-EEG patterns of school children suffering from symptoms of depression compared to healthy controls. World Journal of Biological Psychiatry. 2008;9(2):115–120. doi: 10.1080/15622970701216665. [DOI] [PubMed] [Google Scholar]
- 6.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatrica Scandinavica. 2007;115(Suppl 433):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 7.Armitage R, Calhoun J, Rush A, Roffwarg HP. Comparison of the delta EEG in the first and second non-REM periods in depressed adults and normal control. Psychiatry Research. 1992;41(1):65–72. doi: 10.1016/0165-1781(92)90019-Y. [DOI] [PubMed] [Google Scholar]
- 8.Armitage R, Hoffmann R, Emslie G, Rintelmann J, Robert J. Sleep microarchitecture in childhood and adolescent depression: Temporal coherence. Clinical EEG and Neuroscience. 2006;37(1):1–9. doi: 10.1177/155005940603700103. [DOI] [PubMed] [Google Scholar]
- 9.Armitage R, Hoffmann R, Fitch T, Trivedi M, Rush AJ. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: group and sex effects. Sleep. 2000;23(5):607–617. [PubMed] [Google Scholar]
- 10.Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Research. 2000;95(3):201–213. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 11.Armitage R, Roffwarg HP, Rush AJ, Calhoun JS, Purdy DG, Giles DE. Digital period analysis of sleep EEG in depression. Biological Psychiatry. 1992;31(1):52–68. doi: 10.1016/0006-3223(92)90006-l. [DOI] [PubMed] [Google Scholar]
- 12.Asaad T, Okasha T, Okasha A. Sleep EGG findings in ICD-10 borderline personality disorder in Egypt. Journal of Affective Disorders. 2002;71(1–3):11–18. doi: 10.1016/s0165-0327(01)00357-3. [DOI] [PubMed] [Google Scholar]
- 13.Avery DH, Shah SH, Eder DN, Wildschiodtz G. Nocturnal sweating and temperature in depression. Acta Psychiatrica Scandinavica. 1999;100(4):295–301. doi: 10.1111/j.1600-0447.1999.tb10864.x. [DOI] [PubMed] [Google Scholar]
- 14.Bardwell WA, Moore P, Ancoli-Israel S, Dimsdale JE. Does obstructive sleep apnea confound sleep architecture findings in subjects with depressive symptoms? Biological Psychiatry. 2000;48(10):1001–1009. doi: 10.1016/S0006-3223(00)00887-8. [DOI] [PubMed] [Google Scholar]
- 15.Benson KL, Sullivan EV, Lim KO, Lauriello J, Zarcone Vincent P, Jr, Pfefferbaum A. Slow wave sleep and computed tomographic measures of brain morphology in schizophrenia. Psychiatry Research. 1996;60(2–3):125–134. doi: 10.1016/0165-1781(96)02705-9. [DOI] [PubMed] [Google Scholar]
- 16.Benson KL, Zarcone Vincent P., Jr Rapid eye movement sleep eye movements in schizophrenia and depression. Archives of General Psychiatry. 1993;50(6):474–482. doi: 10.1001/archpsyc.1993.01820180076008. [DOI] [PubMed] [Google Scholar]
- 17.Bertocci MA, Dahl RE, Williamson DE, Iosif AM, Birmaher B, Axelson D, Ryan ND. Subjective Sleep Complaints in Pediatric Depression: A Controlled Study and Comparison With EEG Measures of Sleep and Waking. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(11):1158–1166. doi: 10.1097/01.chi.0000179057.54419.17. [DOI] [PubMed] [Google Scholar]
- 18.Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, da Silva Miozzo Ilsis Cristine, de Barba Maria Emília Ferreira, Menna Barreto Sérgio Saldanha. Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Medicine. 2011;12(1):70–75. doi: 10.1016/j.sleep.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Bioulac S, Chaufton C, Taillard J, Claret A. Excessive Daytime Sleepiness in Adult Patients With ADHD as Measured by the Maintenance of Wakefulness Test, an Electrophysiologic Measure. Journal of Clinical Psychiatry. 2015;76(7):943–948. doi: 10.4088/JCP.14m09087. [DOI] [PubMed] [Google Scholar]
- 20.Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. Sleep in lifetime posttraumatic stress disorder - A community-based polysomnographic study. Archives of General Psychiatry. 2004;61:508–516. doi: 10.1001/archpsyc.61.5.508. [DOI] [PubMed] [Google Scholar]
- 21.Brown TM, Boudewyns PA. Periodic limb movements of sleep in combat veterans with posttraumatic stress disorder. Journal of Traumatic Stress. 1996;9(1):129–136. doi: 10.1007/BF02116838. [DOI] [PubMed] [Google Scholar]
- 22.Buckley AW, Rodriguez AJ, Jennison K, Buckley J, Thurm A, Sato S, Swedo S. REM sleep percentage in children with autism compared with children with developmental delay and typical development. Archives of Pediatric and Adolescent Medicine. 2010;164(11):1032–1037. doi: 10.1001/archpediatrics.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley AW, Sassower K, Rodriguez AJ, Jennison K, Wingert K, Buckley J, Swedo S. An open label trial of donepezil for enhancement of rapid eye movement sleep in young children with autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology. 2011;21(4):353–357. doi: 10.1089/cap.2010.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buljan R, Hrabric K, Jukic V, Bisko A. Disturbed sleep in war veterans according to overnight polysomnography. Likecnicki vjesnik. 2008;130(3–4):101–103. [PubMed] [Google Scholar]
- 25.Cartwright R, Agargun MY, Kirkby J, Friedman JK. Relation of dreams to waking concerns. Psychiatry Research. 2006;141(3):261–270. doi: 10.1016/j.psychres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Cartwright R, Baehr E, Kirkby J, Pandi-Perumal SR, Kabat J. REM sleep reduction, mood regulation and remission in untreated depression. Psychiatry Research. 2003;121(2):159–167. doi: 10.1016/S0165-1781(03)00236-1. [DOI] [PubMed] [Google Scholar]
- 27.Chen X-s, Zhang M-d, Lou F-y, Wang H-x, Wang J-j, Liang J-h, Liu X-w. Lieferschein. Zhonghua-yixue-zazhi. 2006:2467–2470. [Google Scholar]
- 28.Choi J, Yoon IY, Kim HW, Chung S, Yoo HJ. Differences between Objective and Subjective Sleep Measures in Children with Attention Deficit Hyperactivity Disorder. Journal of Clinical Sleep Medicine. 2010;6(6):589–595. [PMC free article] [PubMed] [Google Scholar]
- 29.Clark C. Is there a relationship between delta sleep at night and afternoon cerebral blood flow, assessed by HMPAO-SPECT in depressed patients and normal control subjects? Preliminary data. Psychiatry Research: Neuroimaging. 1998;84(2–3):89–99. doi: 10.1016/S0925-4927(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 30.Cohen DJ, Begley A, Alman JJ, Cashmere DJ, Pietrone RN, Seres RJ, Germain A. Quantitative electroencephalography during rapid eye movement (REM) and non-REM sleep in combat-exposed veterans with and without post-traumatic stress disorder. Journal of Sleep Research. 2013;22(1):76–82. doi: 10.1111/j.1365-2869.2012.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper J, Tyler L, Wallace I, Burgess KR. No evidence of sleep apnea in children with attention deficit hyperactivity disorder. Clinical pediatrics. 2004;43(7):609–614. doi: 10.1177/000992280404300704. [DOI] [PubMed] [Google Scholar]
- 32.Coplan JD, Wolk SI, Goetz RR, Ryan ND, Dahl RE, Mann JJ, Weissman MM. Nocturnal growth hormone secretion studies in adolescents with or without major depression re-examined: Integration of adult clinical follow-up data. Biological Psychiatry. 2000;47:594–604. doi: 10.1016/s0006-3223(00)00226-2. [DOI] [PubMed] [Google Scholar]
- 33.Cowdin N, Kobayashi I, Mellman TA. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Experimental Brain Research. 2014;232:1479–1485. doi: 10.1007/s00221-014-3857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. Journal of the American Geriatrics Society. 2001;49(9):1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 35.Dahl RE, Ryan ND, Matty MK, Birmaher B, Al-Shabbout M, Williamson DE, Kupfer DJ. Sleep onset abnormalities in depressed adolescents. Biological Psychiatry. 1996;39(6):400–410. doi: 10.1016/0006-3223(95)00190-5. [DOI] [PubMed] [Google Scholar]
- 36.Dahl RE, Ryan ND, Perel J, Birmaher B, Al-Shabbout M, Nelson B, Puig-Antich J. Cholinergic REM induction test with arecoline in depressed children. Psychiatry Research. 1994;51(3):269–282. doi: 10.1016/0165-1781(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 37.Daoust AM, Limoges É, Bolduc C, Mottron L, Godbout R. EEG spectral analysis of wakefulness and REM sleep in high functioning autistic spectrum disorders. Clinical Neurophysiology. 2004;115(6):1368–1373. doi: 10.1016/j.clinph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 38.De Bellis Michael D, Dahl RE, Perei JM, Birmaher B, Al-Shabbout M, Williamson Douglas E, Nelson BR, Ryan ND. Nocturnal ACTH, Cortisol, Growth Hormone, and Prolactin Secretion in Prepubertal Depression. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(9):1130–1138. doi: 10.1097/00004583-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 39.De la Fuente José Manuel, Bobes J, Vizuete C, Mendlewicz J. Sleep-EEG in borderline patients without concomitant major depression: a comparison with major depressives and normal control subjects. Psychiatry Research. 2001;105(1–2):87–95. doi: 10.1016/S0165-1781(01)00330-4. [DOI] [PubMed] [Google Scholar]
- 40.De la Fuente José Manuel, Staner L, Kerkhofs M, Linkowski P, Mendlewicz J. Polysomnographic characteristics in recurrent brief depression: A comparative study with major depression and controls. Acta Psychiatrica Belgica. 1992;92(3):179. [PubMed] [Google Scholar]
- 41.Dotan Y, Suraiya S, Pillar G. Sleep spindles in post traumatic stress disorder - significant importance of selective serotonin reuptake inhbitors. Harefuah. 2008;147(10):763–767. [PubMed] [Google Scholar]
- 42.Douglass AB, Benson K, Hill EM, Zarcone VP., JR Markovian analysis of phasic measures of REM sleep in normal, depressed, and schizophrenic subjects. Biological Psychiatry. 1992;31(6):542–559. doi: 10.1016/0006-3223(92)90241-q. [DOI] [PubMed] [Google Scholar]
- 43.Douglass AB, Shipley JE, Haines RF, Scholten RC, Dudley E, Tapp A. Schizophrenia, narcolepsy, and HLA-DR 15, DQ6. Biological Psychiatry. 1993;34:773–780. doi: 10.1016/0006-3223(93)90066-m. [DOI] [PubMed] [Google Scholar]
- 44.Dresler M, Kluge M, Pawlowski M, Schüssler P, Steiger A, Genzel L. A double dissociation of memory impairments in major depression. Journal of Psychiatric Research. 2011;45(12):1593–1599. doi: 10.1016/j.jpsychires.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Eidelman P, Talbot LS, Gruber J, Hairston I, Harvey AG. Sleep architecture as correlate and predictor of symptoms and impairment in inter-episode bipolar disorder: taking on the challenge of medication effects. Journal of Sleep Research. 2010;19(4):516–524. doi: 10.1111/j.1365-2869.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engdahl BE, Dikel TN, Eberly RE, Blank A., Jr Posttraumatic stesss disorder in a community group of former prisoners of war: A normative response to severe trauma. American Journal of Psychiatry. 1997;154(11):1576–1581. doi: 10.1176/ajp.154.11.1576. [DOI] [PubMed] [Google Scholar]
- 47.Farina B, Della Marca G, Grochochinski VJ, Mazza M, Buysse DJ, Di Giannantonio M, Frank E. Microstructure of sleep in depressed patients according to the cyclic alternating pattern. Journal of Affective Disorders. 2003;77(3):227–235. doi: 10.1016/S0165-0327(02)00147-7. [DOI] [PubMed] [Google Scholar]
- 48.Ferini-Strambi L, Bellodi L, Oldani A, Bertella S, Smirne S, Battaglia M. Cyclic alternating pattern of sleep electroencephalogram in patients with panic disorder. Biological Psychiatry. 1996;40(3):225–227. doi: 10.1016/0006-3223(96)84505-7. [DOI] [PubMed] [Google Scholar]
- 49.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Tononi G. Reduced sleep spindle activity in schizophrenia patients. American Journal of Psychiatry. 2007;164(3):483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 50.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Tononi G. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. American Journal of Psychiatry. 2010;167(11):1139–1148. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferri R, Bruni O, Novelli L, Picchietti MA, Picchietti DL. Time structure of leg movement activity during sleep in attention-deficit/hyperactivity disorder and effects of levodopa. Sleep Medicine. 2013:359–366. doi: 10.1016/j.sleep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Fisher A, Woodward SH. Cardiac stability at differing levels of temporal analysis in panic disorder, posttraumatic stress disorder, and healthy controls. Psychophysiology. 2014;51:80–87. doi: 10.1111/psyp.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biological Psychiatry. 2006;59(1):24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forest G, Poulin J, Daoust AM, Lussier I, Stip E, Godbout R. Attention and non-REM sleep in neuroleptic-naive persons with schizophrenia and control participants. Psychiatry Research. 2007;149:33–40. doi: 10.1016/j.psychres.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Franzen PL, Woodward SH, Bootzin RR, Germain A, Colrain IM. K-complexes are not preferentially evoked to combat sounds in combat-exposed Vietnam veterans with and without post-traumatic stress disorder. International Journal of Psychophysiology. 2012;83:393–398. doi: 10.1016/j.ijpsycho.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friess E, Modell S, Brunner H, Tagaya H, Lauer C, Holsboer F, Ising M. The Munich vulnerability study on affective disorders: microstructure of sleep in high-risk subjects. European archives of psychiatry and clinical neuroscience. 2008;258(5):285–291. doi: 10.1007/s00406-007-0795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frölich J, Lehmkuhl G, Wiater A. Schlafstörungen bei hyperkinetischen Kindern - Polysomnografische Untersuchungen zur Schlaf-struktur und -architektur. Zeitschrift für Kinder und Jugendpsychiatrie und Psychotherapie. 2005;33(3):205–216. doi: 10.1024/1422-4917.33.3.205. [DOI] [PubMed] [Google Scholar]
- 58.Fuller KH, Waters WF, Binks PG, Anderson T. Generalized anxiety And sleep architecture - A polysomnographic investigation: Anxiety and sleep. Sleep. 1997;20(5):370–376. doi: 10.1093/sleep/20.5.370. [DOI] [PubMed] [Google Scholar]
- 59.Fuller KH, Waters WF, Scott O. An investigation of slow-wave sleep processes in chronic PTSD patients. Journal of Anxiety Disorders. 1994;8(3):227–236. doi: 10.1016/0887-6185(94)90004-3. [DOI] [Google Scholar]
- 60.Galland BC, Tripp EG, Gray A, Taylor BJ. Apnea-hypopnea indices and snoring in children diagnosed with ADHD: A matched case-control study. Sleep and Breathing. 2010;15(3):455–462. doi: 10.1007/s11325-010-0357-0. [DOI] [PubMed] [Google Scholar]
- 61.Galland BC, Tripp EG, Taylor BJ. The sleep of children with attention deficit hyperactivity disorder on and off methylphenidate: a matched case-control study. Journal of Sleep Research. 2010;19:366–373. doi: 10.1111/j.1365-2869.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 62.Gann H, van Calker D, Feige B, Cloot O, Brück R, Berger M, Riemann D. Polysomnographic comparison between patients with primary alcohol dependency during subacute withdrawal and patients with a major depression. European Archives of Psychiatry and Clinical Neurosciences. 2004;254(4):263–271. doi: 10.1007/s00406-004-0494-1. [DOI] [PubMed] [Google Scholar]
- 63.Germain A, Buysse DJ, Shear MK, Fayyad R, Austin C. Clinical correlates of poor sleep quality in posttraumatic stress disorder. Journal of Traumatic Stress. 2004;17(6):477–484. doi: 10.1007/~10960-004-57%-6. [DOI] [PubMed] [Google Scholar]
- 64.Germain A, Hall M, Shear MK, Nofzinger EA, Buysse DJ. Ecological study of sleep disruption in PTSD: A pilot study. Annals of the New York Academy of Sciences. 2006;1071:438–441. doi: 10.1196/annals.1364.038. [DOI] [PubMed] [Google Scholar]
- 65.Germain A, Hall M, Shear MK, Nofzinger EA, Buysse DJ. Sleep disruption in PTSD: A pilot study with home-based polysomnography. Sleep and Biological Rhythms. 2006;4:286–289. doi: 10.1111/j.1479-8425.2006.00230.x. [DOI] [Google Scholar]
- 66.Gillin CJ, Sohn J-W, Stahl Stephen M, Lardon M, Kelsoe JR, Rapaport MH, Ruiz C, Golshan S. Ipsapirone, a 5-HT1A agonist, suppresses REM sleep equally in unmedicated depressed patients and normal controls. Neuropsychopharmacology. 1996;15:109–115. doi: 10.1016/0893-133X(95)00159-B. [DOI] [PubMed] [Google Scholar]
- 67.Godbout R, Bergeron C, Limonges É, Stip E, Mottron L. A laboratory study of sleep in Asperger’s syndrome. NeuroReport. 2000;11(1):127–130. doi: 10.1097/00001756-200001170-00025. [DOI] [PubMed] [Google Scholar]
- 68.Göder R, Aldenhoff JB, Boigs M, Braun S, Koch J, Fritzer G. Delta power in sleep in relation to neuropsychological performance in healthy subjects and schizophrenia patients. The Journal of Neuropsychiatry and Clinical Neuroscience. 2006;18(4):529–535. doi: 10.1176/jnp.2006.18.4.529. [DOI] [PubMed] [Google Scholar]
- 69.Göder R, Boigs M, Braun S, Friege L, Fritzer G, Aldenhoff JB, Hinze-Selch D. Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. Journal of Psychiatric Research. 2004;38(6):591–599. doi: 10.1016/j.jpsychires.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Goetz RR, Wolk SI, Coplan JD, Ryan ND, Weissman MM. Premorbid polysomnographic signs in depressed adolescents: a reanalysis of EEG sleep after longitudinal followup in adulthood. Biological Psychiatry. 2001;49(11):930–942. doi: 10.1016/S0006-3223(00)01092-1. [DOI] [PubMed] [Google Scholar]
- 71.Golan N, Shahar E, Ravid S, Pilar G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep. 2004;27(2):261–266. doi: 10.1093/sleep/27.2.261. [DOI] [PubMed] [Google Scholar]
- 72.Goraya JS, Cruz M, Valencia I, Kaleyias J, Khurana D, Hardison HH, Kothare SV. Sleep study abnormalities in children with attention deficit hyperactivity disorder. Pediatric Neurology. 2009;40(1) doi: 10.1016/j.pediatrneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 73.Gregory AM, Cousins JC, Forbes EE, Trubnick L, Ryan ND, Axelson DA, Dahl RE. Sleep items in the Child Behavior Checklist: A comparison with sleep diaries, actigraphy, and polysomnography. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(5):499–507. doi: 10.1016/j.jaac.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grissom EM, Brubaker B, Capdvila Oscar Sans, Hawley Wayne R, Gozal D. Eye movement during REM sleep in children with attention deficit hyperactivity disorder. Developmental Neuropsychology. 2009;34(5):552–559. doi: 10.1080/87565640903133475. [DOI] [PubMed] [Google Scholar]
- 75.Gruber R, Fonti L, Bergmame Lana, Wiebe S, Amsel P, Frenette S. Contributions of circadian tendencies and behavioral problems to sleep onset problems of children with ADHD. BMC Psychiatry. 2012;(12) doi: 10.1186/1471-244X-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gruber R, Xi T, Frenette S, Robert M, Vannasinh P, Carrier J. Sleep disturbances in prepubertal children with attention deficit hyperactivity disorder: A home polysomnography study. Sleep. 2009;32(3):343–350. doi: 10.1093/sleep/32.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hornung OP, Regen F, Warnstedt C, Anghelescu I, Danker-Hopfe H, Heuser I, Lammers C-H. Declerative and procedural memory consolidation during sleep in patients with borderline personality disorder. Journal of Psychiatric Research. 2008;42(8):653–658. doi: 10.1016/j.jpsychires.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Hu Y-q, Xie G-r, Yang K. Sleep electroencephalogram physiological characteristics in patients with depression and insomnia. Chinese Journal of Clinical Psychology. 2010;18(1):53–55. [Google Scholar]
- 79.Huang Y-S, Chen N-H, Hsueh-Yu L, Wu Y-Y, Chao C-C, Guilleminault C. Sleep disorders in Taiwanese children with attention deficit/hyperactivity disorder. Journal of Sleep Research. 2004;13:269–277. doi: 10.1111/j.1365-2869.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 80.Huang YS, Guilleminault C, Li H-Y, Yang C-M, Wu Y-Y, Chen N-H. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: A treatment outcome study. Sleep Medicine. 2007;8(1):18–30. doi: 10.1016/j.sleep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 81.Hubain P, Souery D, Jönck L, Staner L, van Veeren C, Kerkhofs M, Linkowski P. Relationship between the Newcastle scale and sleep polysomnographic variables in major depression: a controlled study. European Neuropsychopharmacology. 1995;5(2):129–134. doi: 10.1016/0924-977X(95)00011-D. [DOI] [PubMed] [Google Scholar]
- 82.Hudson JI, Lipinski JF, Keck PE, Aizley HG, Vuckovic A, Zierk KC, Pope HG. Polysomnographic characteristics of schizophrenia in comparison with mania and depression. Biological Psychiatry. 1993;34(3):191–193. doi: 10.1016/0006-3223(93)90391-p. [DOI] [PubMed] [Google Scholar]
- 83.Hurwitz TD, Mahowald MW, Kuskowski M, Engdahl BE. Polysomnographic sleep is not clinically impaired in Vietnam combat veterans with chronic posttraumatic stress disorder. Biological Psychiatry. 1998;44(10):1066–1073. doi: 10.1016/s0006-3223(98)00089-4. [DOI] [PubMed] [Google Scholar]
- 84.Husain AM, Mebust Kimberly A, Carwile ST, Miller PP, Radtke RA. Depression in sleep disorders clinics. Sleep and Breathing. 1997;2:73–75. doi: 10.1007/BF03038869. [DOI] [PubMed] [Google Scholar]
- 85.Ilanković A, Damjanović A, Ilanković V, Filipović B, Janković S, Ilanković N. Polysomnographic sleep patterns in depressive, schizophrenic and healthy subjects. Psychiatra Danubina. 2014;26(1):20–26. [PubMed] [Google Scholar]
- 86.Iorio G, Marciano F, Martino M, Kemali D. Statistical comparison of transition sleep variables in depressed and normal subjects. European Psychiatry. 1994;9:95–1000. [Google Scholar]
- 87.Kaminski M, Blinowska K, Szelenberger W. Investigation of coherence structure and EEG activity propagation during sleep. Acta Neurobiologiae Experimentalis. 1995;55(3):213–219. doi: 10.55782/ane-1995-1078. [DOI] [PubMed] [Google Scholar]
- 88.Kaplana KA, Talbota L, Gruberb J, Harveya A. Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar Disorders. 2012;14(8):870–879. doi: 10.1111/bdi.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keshavan MS, Miewald J, Haas G, Sweeney J, Ganguli R, Reynolds CF. Slow-wave sleep and symptomatology in schizophrenia and related psychotic disorders. Journal of Psychiatric Research. 1995;29(4):303–314. doi: 10.1016/0022-3956(95)00023-x. [DOI] [PubMed] [Google Scholar]
- 90.Keshavan MS, Reynolds CF, Montrose D, Miewald J, Downs C, Sabo EM. Sleep and suicidality in psychotic patients. Acta Psychiatrica Scandinavica. 1994;89(2):122–125. doi: 10.1111/j.1600-0447.1994.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 91.Keshavan MS, Tandon R. Sleep abnormalities in schizophrenia: pathophysiological significance. Psychological Medicine. 1993;23(4):831–835. doi: 10.1017/s0033291700026313. [DOI] [PubMed] [Google Scholar]
- 92.Kirov R, Kinkelbur J, Banaschewski T, Rothenberger A. Sleep patterns in children with attention-deficit/hyperactivity disorder, tic disorder, and comorbidity. Journal of child psychology and psychiatry, and allied disciplines. 2007;48:561–570. doi: 10.1111/j.1469-7610.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 93.Kirov R, Kinkelbur J, Heipke S, Kostanecka-Endress T, Westhoff M, Cohrs S, Rothenberger A. Is there a specific polysomnographic sleep pattern in children with attention deficit/hyperactivity disorder? Journal of Sleep Research. 2004;13(1):87–93. doi: 10.1111/j.1365-2869.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- 94.Kisley MA, Olincy A, Robbins E, Polk SD, Adler LE, Waldo MC, Freedman R. Sensory gating impairment associated with schizophrenia persists into REM sleep. Psychphysiology. 2003;40(1):29–38. doi: 10.1111/1469-8986.00004. [DOI] [PubMed] [Google Scholar]
- 95.Klein E, Koren D, Arnon I, Lavie P. No evidence of sleep disturbance in post-traumatic stress disorder: A polysomnographic study in injured victims of traffic incidents. Israel Journal of Psychiatry and related sciences. 2002;39(1):3–10. [PubMed] [Google Scholar]
- 96.Klein E, Koren D, Arnon I, Lavie P. Sleep complaints are not corrobaorated by objective sleep measures in post-traumatic stress disorder: A 1-year prospective study in survivors of motor vehicle crashes. Journal of Sleep Research. 2003;12:35–41. doi: 10.1046/j.1365-2869.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 97.Kluge M, Schüssler P, Dresler M, Yassouridis A, Steiger A. Sleep onset REM periods in obsessive compulsive disorder. Psychiatry Research. 2007;152(1):29–35. doi: 10.1016/j.psychres.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Koenigsberg HW, Pollak CP, Fine J, Kakuma T. Lactate sensitivity in sleeping panic disorder patients and healthy controls. Biological Psychiatry. 1992;32(6):539–542. doi: 10.1016/0006-3223(92)90222-l. [DOI] [PubMed] [Google Scholar]
- 99.Koenigsberg HW, Pollak CP, Fine J, Kakuma T. Cardiac and respiratory activity in panic disorder: Effects of sleep and sleep lactate infusions. American Journal of Psychiatry. 1994;151(8):1148–1152. doi: 10.1176/ajp.151.8.1148. [DOI] [PubMed] [Google Scholar]
- 100.Kooij JS, Middelkoop HA, van Giles K, Buitelaar, Jan K. The effect of stimulants on nocturnal motor activity and sleep quality in adults with ADHD: An open-label case-control study. Journal of Clinical Psychiatry. 2001;62(12):952–956. doi: 10.4088/jcp.v62n1206. [DOI] [PubMed] [Google Scholar]
- 101.Koorengevel KM, Beersma DG, Den Boer JA, Van den Hoofdakker Rutger H. Sleep in seasonal affective disorder patients in forced desynchrony: An explorative study. Journal of Sleep Research. 2002;11(4):347–356. doi: 10.1046/j.1365-2869.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 102.Krakow B, Melendrez D, Pedersen B, Johnston L, Hollifield M, Germain A, Schrader R. Complex insomnia: Insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biological Psychiatry. 2001;49(11):948–953. doi: 10.1016/s0006-3223(00)01087-8. [DOI] [PubMed] [Google Scholar]
- 103.Kutcher S, Williamson P, Marton P, Szalai J. REM latency in endogenously depressed adolescents. British Journal of Psychiatry. 1992;161:399–402. doi: 10.1192/bjp.161.3.399. [DOI] [PubMed] [Google Scholar]
- 104.Landolt H-P, Gilin C. Similar sleep EEG topography in middle-aged depressed patients and healthy controls. Sleep. 2005;28(2):239–247. doi: 10.1093/sleep/28.2.239. [DOI] [PubMed] [Google Scholar]
- 105.Lauer CJ, Riemann D, Wiegand M, Berger M. From early to late adulthood. Changes in EEG sleep of depressed patients and healthy volunteers. Biological Psychiatry. 1991;29(10):979–993. doi: 10.1016/0006-3223(91)90355-p. [DOI] [PubMed] [Google Scholar]
- 106.Lauer CJ, Schreiber W, Holsboer F, Krieg JC. In quest of identifying vulnerability markers for psychiatric disorders by all-night polysomnography. Archives of General Psychiatry. 1995;52(2):145–153. doi: 10.1001/archpsyc.1995.03950140063009. [DOI] [PubMed] [Google Scholar]
- 107.Lavie P, Katz N, Pillar G, Zinger Y. Elevated awakening thresholds during sleep: Characteristics of chronic war-related posttraumatic stress disorder patients. Biological Psychiatry. 1998;44(10):1060–1065. doi: 10.1016/s0006-3223(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 108.Le Bon O, Hoffmann R, Staner L, Armitage R. Relationships between the number of ultradian cycles and key sleep variables in outpatients with major depressive disorder. Psychiatry Research. 2009;165:1–2. doi: 10.1016/j.psychres.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 109.Lecendreux M, Konofal E, Bouvard MP, Falissard B, Mouren-Simeoni MC. Sleep and alertness in children with ADHD. Journal of child psychology and psychiatry, and allied disciplines. 2000;41(6):803–812. [PubMed] [Google Scholar]
- 110.Lee JH, Woo JI, Meltzer HY. Effects of clozapine on sleep measures and sleep-associated changes in growth hormone and cortisol in patients with schizophrenia. Psychiatry Research. 2001;103(2–3):157–166. doi: 10.1016/s0165-1781(01)00284-0. [DOI] [PubMed] [Google Scholar]
- 111.Leistedt SJ, Coumans N, Dumont M, Lanquart JP, Stam CJ, Linkowski P. Altered sleep brain functional connectivity in acutely depressed patients. Human Brain Mapping. 2009;30(7):2207–2219. doi: 10.1002/hbm.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Léveillé C, Barbeau EB, Bolduc C, Limoges É, Berthiaume C, Chevrier Élyse, Mottron Laurent, Godbout R. Enhanced connectivity between visual cortex and other regions of the brain in autism: A REM sleep EEG coherence study. Autism Research. 2010;3(5):280–285. doi: 10.1002/aur.155. [DOI] [PubMed] [Google Scholar]
- 113.Lewis CF, Tandon R, Shipley JE, DeQuardo JR, Jibson M, Taylor SF, Goldman M. Biological predictors of suicidality in schizophrenia. Acta Psychiatrica Scandinavica. 1996;94(6):416–420. doi: 10.1111/j.1600-0447.1996.tb09883.x. [DOI] [PubMed] [Google Scholar]
- 114.Lindberg N, Tani P, Porkka-Heiskanen T, Appelberg B, Rimón R, Virkkunen M. ADHD and sleep in homicidal men with antisocial personality disorder. Neuropsychobiology. 2004;50(1):41–47. doi: 10.1159/000077940. [DOI] [PubMed] [Google Scholar]
- 115.Lindberg N, Tani P, Sailas E, Putkonen H, Takala P, Urrila AS, Virkkunen M. Sleep architecture in homicidal women with antisocial personality disorder - A preliminary study. Psychiatry Research. 2006;145(1):67–73. doi: 10.1016/j.psychres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 116.Lopes MC, Quera-Salva MA, Guilleminault C. Non-REM sleep instability in patients with major depressive disorder: Subjective improvement and improvement of non-REM sleep instability with treatment (Agomelatine) Sleep Medicine. 2007;9(1):33–41. doi: 10.1016/j.sleep.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 117.Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep. 2006;29(12):1563–1571. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- 118.Mann K, Rossbach W, Müller MJ, Müller-Siecheneder F, Pott T, Linde I, Hiemke C. Nocturnal hormone profiles in patients with schizophrenia treated with olanzapine. Psychoneuroendocrinology. 2006;31(2):256–264. doi: 10.1016/j.psyneuen.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley EJ, Stickgold R. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. Journal of Psychiatric Research. 2010;44(2):112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mehl RC, O’Brien LM, Jones JH, Dreisbach JK, Mervis CB, Gozal D. Correlates of Sleep and Pediatric Bipolar Disorder. Sleep. 2006;29(2):193–197. doi: 10.1093/sleep/29.2.193. [DOI] [PubMed] [Google Scholar]
- 121.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. American Journal of Psychiatry. 2002;159(10):1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 122.Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biological Psychiatry. 2004;55(9):953–956. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 123.Mellman TA, Kulick-Bell R, Ashlock LE, Nolan B. Sleep events among veterans with combat-related posttraumatic stress disorder. American Journal of Psychiatry. 1995;152:110–115. doi: 10.1176/ajp.152.1.110. [DOI] [PubMed] [Google Scholar]
- 124.Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biological Psychiatry. 1995;38(3):174–179. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- 125.Moo Estrella Jesús Antonio, Valencia Flores M, Ulloa Flores Rosa Elena, Ostrosky Solís F, Reyes Lagunes I. Estructura del sueño y funciones ejecutivas en niños con depresión. Salud Mental. 2011;34(5):459–468. [Google Scholar]
- 126.Murck H, Held K, Ziegenbein M, Künzel H, Koch K, Steiger A. The Renin-Angiorensin-Aldosterone system in patients with depression compared to controls - a sleep endocrine study. BMC Psychiatry. 2003;3:15. doi: 10.1186/1471-244X-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neylan TC, van Kammen DP, Kelley ME, Peters JL. Sleep in schizophrenic patients on and off haloperidol therapy. Clinically stable vs relapsed patients. Archives of General Psychiatry. 1992;49(8):643–649. doi: 10.1001/archpsyc.1992.01820080051008. [DOI] [PubMed] [Google Scholar]
- 128.Neylan TC, Lenoci M, Maglione ML, Rosenlicht NZ, Metzler TJ, Otte C, Marmar CR. Delta sleep response to metyrapone in post-traumatic stress disorder. Neuropsychopharmacology. 2003;28(9):1666–1676. doi: 10.1038/sj.npp.1300215. [DOI] [PubMed] [Google Scholar]
- 129.Nishino S, Mignot E, Benson KL, Zarcone Vincent P., Jr Cerebrospinal fluid prostaglandins and corticotropin releasing factor in schizophrenics and controls: Relationship to sleep architecture. Psychiatry Research. 1998;78(3):141–150. doi: 10.1016/s0165-1781(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 130.Nishino S, Ripley B, Mignot E, Benson KL, Zarcone Vincent P., Jr CSF hypocretin-1 levels in schizophrenics and controls: Relationship to sleep architecture. Psychiatry Research. 2002;110(1):1–7. doi: 10.1016/s0165-1781(02)00032-x. [DOI] [PubMed] [Google Scholar]
- 131.Nobili L, Baglietto MG, de Carli F, Savoini M, Schiavi G, Zanotto E, de Negri M. A quantified analysis of sleep electroencephalography in anorectic adolescents. Biological Psychiatry. 1999;45(6):771–775. doi: 10.1016/s0006-3223(98)00107-3. [DOI] [PubMed] [Google Scholar]
- 132.Nofzinger EA, Nichols Thomas E, Meltzer CC, Price J, Steppe DA, Miewald JM, Moore RY. Changes in forebrain function from waking to REM sleep in depression: preliminary analyses of [18F]FDG PET studies. Psychiatry Research. 1999;91(2):59–78. doi: 10.1016/s0925-4927(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 133.Nofzinger EA, Schwartz RM, Reynolds Charles F, III, Thase ME, Jennings J, Frank E, Kupfer DJ. 1994Affect intensity and phasic REM sleep in depessed.pdf. Journal of Consulting and Clinical Psychology. 1994;62(1):83–91. doi: 10.1037//0022-006x.62.1.83. [DOI] [PubMed] [Google Scholar]
- 134.O’Brien LM, Holbrook CR, Mervis CB, Klaus CJ, Bruner JL, Raffield TJ, Gozal D. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111(3):554–563. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- 135.O’Brien LM, Ivanenko A, McLaughlin Carbtree V, Holbrook CR, Bruner JL, Klaus CJ, Gozal D. Sleep disturbances in children with attention deficit hyperactivity disorder. Pediatric Research. 2003;54(2):237–243. doi: 10.1203/01.PDR.0000072333.11711.9A. [DOI] [PubMed] [Google Scholar]
- 136.O’Brien LM, Ivanenko A, McLaughlin Carbtree V, Holbrook CR, Bruner JL, Klaus CJ, Gozal D. The effect of stimulants on sleep characteristics in children with attention deficit/hyperactivity disorder. Sleep Medicine. 2003;4(4):309–316. doi: 10.1016/S1389-9457(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 137.Orff H, Meliska C, Lopez A. Polysomnographic evaluation of sleep quality and quantitative variables in women as a function of mood, reproductive status, and age. Dialogs in clinical neuroscience. 2012;14(4):413–424. doi: 10.31887/DCNS.2012.14.4/hjorff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Otte C, Lenoci M, Metzler TJ, Yehuda R, Marmar CR, Neylan TC. Hypothalamic-pituitary-adrenal axis activity and sleep in posttraumatic stress disorder. Neuropsychopharmacology. 2005;30(6):1173–1180. doi: 10.1038/sj.npp.1300676. [DOI] [PubMed] [Google Scholar]
- 139.Otte C, Lenoci M, Metzler TJ, Yehuda R, Marmar CR, Neylan TC. Effects of metyrapone on hypothalamic-pituitary-adrenal axis and sleep in women with post-traumatic stress disorder. Biological Psychiatry. 2007;61(8):952–956. doi: 10.1016/j.biopsych.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 140.Parry BL, Meliska CJ, Martinez F, Basavaraj N, Zirpoli GG, Sorenson DL, Kripke DF. Menopause: Neuroendocrine changes and hormone replacement therapy. Journal of the American Medical Women’s Association (JAMWA) 2004;59(2):135–145. [PubMed] [Google Scholar]
- 141.Parry BL, Mostofi N, LeVeau B, Nahum HC, Golshan S, Laughlin GA, Gillin J. Sleep EEG studies during early and late partial sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Psychiatry Research. 1999;85(2):127–143. doi: 10.1016/S0165-1781(98)00128-0. [DOI] [PubMed] [Google Scholar]
- 142.Patriquin M, Mellman TA, Glaze D, Alfano C. Polysomnographic Sleep Characteristics of Generally-Anxious and Healthy Children Assessed in the Home Environment. Affective Disorders. 2014;161:79–83. doi: 10.1016/j.jad.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Perlis ML, Smith MT, Andrewas PJ, Off H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 144.Picchietti DL, Underwood DJ, Farris WA, Walters AS, Shah MM, Dahl RE, Hening WA. Further studies on periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. Movement Disorders. 1999;14(6):1000–1007. doi: 10.1002/1531-8257(199911)14:6<1000::aid-mds1014>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 145.Picchietti MA, Picchietti DL, England SJ, Walters AS, Couvadelli BV, Lewin DS, Hening W. Children show individual night-to-night variability of periodic limb movements in sleep. Sleep. 2009;32(4):530–535. doi: 10.1093/sleep/32.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Poulin J, Daoust AM, Forest G, Stip E, Godbout R. Sleep architecture and its clinical correlates in first episode and neuroleptic-naive patients with schizophrenia. Schizophrenia Research. 2003;62(1–2):147–153. doi: 10.1016/S0920-9964(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 147.Prehn-Kristensen A, Göder R, Fischer J, Wilhelm I, Seeck-Hirschner M, Aldenhoff J, Baving L. Reduced sleep-associated consolidation of declarative memory in attention-deficit/hyperactivity disorder. Sleep Medicine. 2011;12(7):672–679. doi: 10.1016/j.sleep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 148.Prehn-Kristensen A, Molzow I, Munz M, Wilhelm I, Müller K, Freytag D, Baving L. Sleep restores daytime deficits in procedural memory in children with attention-deficit/hyperactivity disorder. Research in Developmental Disabilities. 2011;32(6):2480–2488. doi: 10.1016/j.ridd.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 149.Prihodova I, Paclt I, Kemlink D, Skibova J, Ptacek R, Nevsimalova S. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactivity disorder: A two-night polysomnographic study with a multiple sleep latency test. Sleep Medicine. 2010;11(9):922–928. doi: 10.1016/j.sleep.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 150.Rao U, Dahl RE, Ryan ND, Birmaher B, Williamson DE, Giles DE, Nelson B. The relationship between longitudinal clinical course and sleep and cortisol changes in adolescent depression. Biological Psychiatry. 1996;40(6):474–484. doi: 10.1016/0006-3223(95)00481-5. [DOI] [PubMed] [Google Scholar]
- 151.Rao U, Ryan ND, Birmaher B, Dahl RE, Williamson DE, Kaufman J, Nelson B. Unipolar depression in adolescents: Clinical outcome in adulthood. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34(5):566–578. doi: 10.1097/00004583-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 152.Rao U, Ryan ND, Dahl RE, Birmaher B, Rao R, Williamson DE, Perei JM. Factors associated with the development of substance use disorder in depressed adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(9):1109–1117. doi: 10.1097/00004583-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 153.Riemann D, Hohagen F, Krieger S, Gann H, Müller WE, Olbrich R, Berger M. Cholinergic REM induction test: Muscarinic supersensitivity underlies polysomnographic findings in both depression and schizophrenia. Journal of Psychiatric Research. 1994;28(3):195–210. doi: 10.1016/0022-3956(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 154.Riemann D, König A, Hohagen F, Kiemen A, Voderholzer U, Backhaus J, Berger M. How to preserve the antidepressive effect of sleep deprivation: A comparison of sleep phase advance and sleep phase delay. European archives of psychiatry and clinical neuroscience. 1999;249(5):231–237. doi: 10.1007/s004060050092. [DOI] [PubMed] [Google Scholar]
- 155.Robert JJ, Hoffmann RF, Emslie GJ, Hughes C, Rintelmann J, Moore J, Armitage R. Sex and age differences in sleep macroarchitecture in childhood and adolescent depression. Sleep. 2006;29(3):351–358. doi: 10.1093/sleep/29.3.351. [DOI] [PubMed] [Google Scholar]
- 156.Robinson D, Walsleben J, Pollack S, Lerner G. Nocturnal polysomnography in obsessive-compulsive disorder. Psychiatry Research. 1998;80(3):257–263. doi: 10.1016/s0165-1781(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 157.Röschke J, Aldenhoff JB. Estimation of the dimensionality of sleep-EEG data in schizophrenics. European archives of psychiatry and clinical neuroscience. 1993;242(4):191–196. doi: 10.1007/BF02189962. [DOI] [PubMed] [Google Scholar]
- 158.Röschke J, Fell J, Beckmann P. Nonlinear analysis of sleep EEG in depression: Calculation of the largest lyapunov exponent. European Archives of psychiatry and clinical neuroscience. 1995;245(1):27–35. doi: 10.1007/BF02191541. [DOI] [PubMed] [Google Scholar]
- 159.Röschke J, Mann K, Fell J. Nonlinear EEG dynamics during sleep in depression and schizophrenia. International Journal of Neuroscience. 1994;75(3–4):271–284. doi: 10.3109/00207459408986309. [DOI] [PubMed] [Google Scholar]
- 160.Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17(8):723–732. doi: 10.1093/sleep/17.8.723. [DOI] [PubMed] [Google Scholar]
- 161.Ross RJ, Ball WA, Sanford LD, Morrison AR, Dinges DF, Silver SM, McGinnis DE. Rapid eye movement sleep changes during the adaptation night in combat veterans with posttraumatic stress disorder. Biological Psychiatry. 1999;45(7):938–941. doi: 10.1016/s0006-3223(98)00233-9. [DOI] [PubMed] [Google Scholar]
- 162.Rotenberg VS, Kayumov L, Indursky P, Hadjez J, Kimhi R, Sirota P, Elizur A. Rem sleep in depressed patients: Different attempts to achieve adaptation. Journal of Psychosomatic Research. 1997;42(6):565–575. doi: 10.1016/S0022-3999(97)00012-3. [DOI] [PubMed] [Google Scholar]
- 163.Rotenberg VS, Hadjez J, Kimhi R, Indurski P, Sirota P, Mosheva T, Elizur A. First night effect in depression: New data and a new approach. Biological Psychiatry. 1997;42(4):267–274. doi: 10.1016/S0006-3223(96)00343-5. [DOI] [PubMed] [Google Scholar]
- 164.Rotenberg VS, Shamir E, Barak Y, Indursky P, Kayumov L, Mark M. REM sleep latency and wakefulness in the first sleep cycle as markers of major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26(6):1211–1215. doi: 10.1016/S0278-5846(02)00216-6. [DOI] [PubMed] [Google Scholar]
- 165.Saletu B, Anderer P, Brandstätter N, Frey R, Grünberger J, Klösch G, Zeitholfer J. Insomnia in generalized anxiety disorder: Polysomnographic, psychometric and clinical investigations before, during and after therapy with a long-versus a short-half-life benzodiazepine (Quazepam versus Triazolam) Neuropsychobiology. 1994;29(2):69–90. doi: 10.1159/000119067. [DOI] [PubMed] [Google Scholar]
- 166.Saletu B, Klösch G, Gruber G, Anderer P, Udomratn P, Frey R. First-night-effects on generalized anxiety disorder (GAD)-based insomnia: Laboratory versus home sleep recordings: Disrupted nocturnal sleep-Insomnia and hypnotics. Sleep. 1996;19(9):691–697. [PubMed] [Google Scholar]
- 167.Saletu-Zyhlarz GM, Abu-Bakr MH, Anderer P, Gruber G, Mandl M, Strobl R, Saletu B. Insomnia in depression: Differences in objective and subjective sleep and awakening quality to normal controls and acute effects of trazodone. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26(2):249–260. doi: 10.1016/S0278-5846(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 168.Saletu-Zyhlarz GM, Anderer P, Berger P, Gruber G, Oberndorfer S, Saletu B. Nonorganic insomnia in panic disorder: Comparative sleep laboratory studies with normal controls and placebo-controlled trials with alprazolam. Human Psychopharmacology. 2000;15(4):241–254. doi: 10.1002/1099-1077(200006)15:4<241::AID-HUP164>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 169.Saletu-Zyhlarz GM, Saletu B, Anderer P, Brandstätter N, Frey R, Gruber G, Linzmayer L. Nonorganic insomnia in generalized anxiety disorder: 1. Controlled studies on sleep, awakening and daytime vigilance utilizing polysomnography and EEG mapping. Neuropsychobiology. 1997;36(3):117–129. doi: 10.1159/000119373. [DOI] [PubMed] [Google Scholar]
- 170.Salín-Pascual RJ, Drucker-Colín R. A novel effect of nicotine on mood and sleep in major depression. NeuroReport. 1998;9(1):57–60. doi: 10.1097/00001756-199801050-00012. [DOI] [PubMed] [Google Scholar]
- 171.Schaltenbrand N, Lengelle R, Toussaint M, Luthringer R, Carelli G, Jacqmin A, Macher JP. Sleep stage scoring using the neural network model: Comparison between visual and automatic analysis in normal subjects and patients. Sleep. 1996;19(1):26–35. doi: 10.1093/sleep/19.1.26. [DOI] [PubMed] [Google Scholar]
- 172.Sekimoto M. Cortical regional differences of delta waves during all-night sleep in schizophrenia. Schizophrenia Research. 2011;126(1–3):284–290. doi: 10.1016/j.schres.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 173.Sekimoto M, Kato M, Watanabe T, Kajmura N, Takahashi K. Reduced frontal asymmetry of delta waves during all-night sleep in schizophrenia. Schizophrenia Bulletin. 2007;33(6):1307–1311. doi: 10.1093/schbul/sbl069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sharpley AL, Bhagwagar Z, Hafizi S, Whale W Richard, Gijsman HJ, Cowen PJ. Risperidone augmentation decreases rapid eye movement sleep and decreases wake in treatment-resistant depressed patients. Journal of Clinical Psychiatry. 2003;64(2):192–196. doi: 10.4088/jcp.v64n0212. [DOI] [PubMed] [Google Scholar]
- 175.Silvestri R, Gagliano A, Aricò I, Calarese T, Cedro C, Bruni O, Bramandi P. Sleep disorders in children with attention-deficit/hyperactivity disorder (ADHD) recorded overnight by video-polysomnography. Sleep Medicine. 2009;10:1132–1138. doi: 10.1016/j.sleep.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 176.Sloan EP, Natarajan M, Baker B, Dorian P, Mironov D, Barr A, Shapiro CM. Nocturnal and daytime panic attacks - Comparison of sleep architecture, heart rate variability, and response to sodium lactate challenge. Biological Psychiatry. 1999;45(10):1313–1320. doi: 10.1016/s0006-3223(98)00158-9. [DOI] [PubMed] [Google Scholar]
- 177.Sobanski E, Alm B, Hennig O, Riemann D, Feige B, Schultz L. Daytime Sleepiness in Adults With ADHD: A Pilot Trial With a Multiple Sleep Latency Test. Journal of Attention Disorders. 2014;1(7) doi: 10.1177/1087054714529456. [DOI] [PubMed] [Google Scholar]
- 178.Souery D, Hubain P, Joenck L, van Veeren C, Kerkhofs M, Staner L, Linkowski P. Validation of the Newcastle scale through sleep polysomnographic studies in major depression: comparison with age matched controls. Acta Psychiatrica Belgica. 1994;94(2):110. [PubMed] [Google Scholar]
- 179.Steiger A, von Bardeleben U, Guldner J, Lauer C, Rothe B, Holsboer F. The sleep eeg and nocturnal hormonal secretion studies on changes during the course of depression and on effects of cns-active drugs. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1993;17(1):125–137. doi: 10.1016/0278-5846(93)90037-S. [DOI] [PubMed] [Google Scholar]
- 180.Stein MB, Millar TW, Larsen DK, Krieger MH. Irregular breathing during sleep in patients with panic disorder. American Journal of Psychiatry. 1995;152:1168–1173. doi: 10.1176/ajp.152.8.1168. [DOI] [PubMed] [Google Scholar]
- 181.Stephens RJ. REM sleep and aggressive behaviour in children with Tourette’s syndrome (TS), attention deficit hyperactivity disorder (ADHD) and comorbid TS and ADHD. Depamnent of Hurnan Development and Applied Psychology; Toronto, Canada: 2001. [Google Scholar]
- 182.Stephens RJ, Chung S, Jovanovic D, Guerra R, Stephens B, Sandor P, Shapiro CM. Relationship between polysomnographic sleep architecture and behavior in medication-free children with TS, ADHD, TS and DAHD, and controls. Jornal of developmental and behavioral pediatrics. 2013 doi: 10.1097/DBP.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 183.Szklo-Coxe M, Young T, Peppard PE, Finn LA, Benca RM. Prospective Associations of Insomnia Markers and Symptoms With Depression. American Journal of Epidemiology. 2010;171(6):709–720. doi: 10.1093/aje/kwp454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Taillard J, Lemoine P, Boule P, Drogue M, Mouret J. Sleep and heart rate circadian rhythm in depression: The necessity to separate. Chronobiology International. 1993;10(1):63–72. doi: 10.3109/07420529309064483. [DOI] [PubMed] [Google Scholar]
- 185.Tekell JL, Hoffmann R, Hendrickse W, Greene RW, Rush AJ, Armitage R. High frequency EEG activity during sleep: characteristics in schizophrenia and depression. Clinical EEG and Neuroscience. 2005;36(1):25–35. doi: 10.1177/155005940503600107. [DOI] [PubMed] [Google Scholar]
- 186.Thase ME, Kupfer DJ, Fascizka AL, Buysse DJ, Simons AD, Frank E. Identifying an abnormal electroencephalographic sleep profile to characterize major depressive disorder. Biological Psychiatry. 1997;41(9):964–973. doi: 10.1016/S0006-3223(96)00259-4. [DOI] [PubMed] [Google Scholar]
- 187.Thase ME, Reynolds CF, Jennings J, Frank E, Garamoni GL, Nofzinger EA, Kupfer DJ. Diminished nocturnal penile tumescence in depression: A replication study. Biological Psychiatry. 1992;31(11):1136–1142. doi: 10.1016/0006-3223(92)90158-V. [DOI] [PubMed] [Google Scholar]
- 188.Ulmer CS, Sutherland M, Edinger JD, Davidson J, Connor KM, Zhang W, Krystal AD. REM sleep bout duration and frequency in PTSD. Journal of Aggression, Maltreatment and Trauma. 2012;21:67–76. doi: 10.1080/10926771.2012.630339. [DOI] [Google Scholar]
- 189.Valdizán-Usón JR, Abril-Villalba B, Méndez-García M, Sans-Capdevila O. Polisomnograma nocturno en el autismo infantil sin epilepisa. Revista de Neurologia. 2002;34(12):1101–1105. [PubMed] [Google Scholar]
- 190.Voderholzer U, Hohagen F, Klein T, Jungnickel J, Kirschbaum C, Berger M, Riemann D. Impact of sleep deprivation and subsequent recovery sleep on cortisol in unmedicated depressed patients. American Journal of Psychiatry. 2004;161(8):1404–1410. doi: 10.1176/appi.ajp.161.8.1404. [DOI] [PubMed] [Google Scholar]
- 191.Voderholzer U, Riemann D, Huwig-Poppe C, Kuelz AK, Kordon A, Bruestle K, Hohagen F. Sleep in obsessive compulsive disorder: Polysomnographic studies under baseline conditions and after experimentally induced serotin deficiency. European Archives of psychiatry and clinical neuroscience. 2007;257(3):173–182. doi: 10.1007/s00406-006-0708-9. [DOI] [PubMed] [Google Scholar]
- 192.Waldrop AE, Back SE, Sensenig A, Brady KT. Sleep disturbances associated with posttraumatic stress disorder and alcohol dependence. Addictive Behaviors. 2008;33(2):328–335. doi: 10.1016/j.addbeh.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wallace DM, Shafazand S, Ramos AR, Carvalho DZ, Gardener H, Lorenzo D, Wohlgemuth WK. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: An exploratory study. Sleep Medicine. 2011;12(9):850–859. doi: 10.1016/j.sleep.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 194.Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation. Biological Psychiatry. 2012;71(2):154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wickniak A, Riemann D, Kiemen A, Voderholzer U, Jernajczyk W. Comparison between eye movement latency and REM sleep parameters in major depression. European archives of psychiatry and clinical neuroscience. 2000;250(1):48–52. doi: 10.1007/s004060050009. [DOI] [PubMed] [Google Scholar]
- 196.Wiebe S, Carrier J, Frenette S, Gruber R. Sleep and sleepiness in children with attention deficit/hyperactivity disorder and controls. Journal of Sleep Research. 2013;(22):41–49. doi: 10.1111/j.1365-2869.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 197.Williamson DE, Dahl RE, Birmaher B, Goetz RR, Nelson B, Ryan ND. Stressful life events and EEG sleep in depressed and normal control adolescents. Biological Psychiatry. 1995;37(12):859–865. doi: 10.1016/0006-3223(94)00240-4. [DOI] [PubMed] [Google Scholar]
- 198.Woodward SH, Bliwise DL, Friedman MJ, Gusman DF. First night effects in post-traumatic stress disorder inpatients. Sleep. 1996;19(4):312–317. doi: 10.1093/sleep/19.4.312. [DOI] [PubMed] [Google Scholar]
- 199.Woodward SH, Bliwise DL, Friedman MJ, Gusman DF. Subjective versus objective sleep in Vietnam combat veterans hospitalized for PTSD. Journal of Traumatic Stress. 1996;9(1):137–143. doi: 10.1007/BF02116839. [DOI] [PubMed] [Google Scholar]
- 200.Woodward SH, Friedman MJ, Bliwise DL. Sleep and depression in combat-related PTSD inpatients. Biological Psychiatry. 1996;39(3):189–192. doi: 10.1016/0006-3223(95)00104-2. [DOI] [PubMed] [Google Scholar]
- 201.Woodward SH, Leskin GA, Sheikh JI. Movement during sleep: Associations with posttraumatic stress disorder, nightmares, and comorbid panic disorder. Sleep. 2002;25(6):669–676. [PubMed] [Google Scholar]
- 202.Woodward SH, Stegman WK, Pavao JR, Arsenault NJ, Hartl TI, Drescher KD, Weaver C. Self-selection bias in sleep and psychophysiological studies of posttraumatic stress disorder. Journal of Traumatic Stress. 2007;20(4):619–623. doi: 10.1002/jts.20236. [DOI] [PubMed] [Google Scholar]
- 203.Yang AC, Yang CH, Hong CJ, Tsai SJ, Kuo CH, Peng CK, Thomas RJ. Sleep state instabilities in major depressive disorder: Detection and quantification with electrocardiogram-based cardiopulmonary coupling analysis. Psychophysiology. 2011;48(2):285–291. doi: 10.1111/j.1469-8986.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yeragani VK, Cashmere D, Miewald J, Tancer M, Keshavan MS. Decreased coherence in higher frequency ranges (beta and gamma) between central and frontal EEG in patients with schizophrenia: A preliminary report. Psychiatry Research. 2006;141(1):53–60. doi: 10.1016/j.psychres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 205.Zarcone Vincent P, Jr, Benson KL. Middle ear muscle activity (MEMA) in schizophrenia using a noninvasive technique. Sleep. 1995;18(4):266–271. doi: 10.1093/sleep/18.4.266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


