Abstract
Invadopodia are F-actin-rich membrane protrusions that breach basement membrane barriers during cell invasion. Since their discovery more than 30 years ago, invadopodia have been extensively studied in cancer cells in vitro, where great advances in understanding their composition, formation, cytoskeletal regulation, and control of the matrix metalloproteinase MT1-MMP trafficking have been made. In contrast, few studies examining invadopodia have been conducted in vivo, leaving their physiological regulation unclear. Recent live-cell imaging and gene perturbation studies in C. elegans have revealed that invadopodia are formed with a unique invadopodial membrane, defined by its specialized lipid and associated protein composition, which is rapidly recycled through the endolysosome. Here, we provide evidence that the invadopodial membrane is conserved and discuss its possible functions in traversing basement membrane barriers. Discovery and examination of the invadopodial membrane has important implications in understanding the regulation, assembly, and function of invadopodia in both normal and disease settings.
Keywords: invadopodia, endocytic membrane trafficking, invadopodial membrane, C. elegans, cell invasion, basement membrane
Introduction
Directed vesicle trafficking to and from the plasma membrane facilitates the generation of membrane extensions, specialized secretion sites, and rapid delivery and removal of specific proteins from cell membranes. Examples of directed membrane trafficking include include neurite outgrowth (Hausott and Klimaschewski, 2016), neuronal and immunological synapse function (Gonnord et al., 2012), wound healing (Abreu-Blanco et al., 2011), cell division (Shuster and Burgess, 2002), and cell migration (Maritzen et al., 2015). Targeted vesicle delivery requires a source of internal vesicles/membrane, exocytic trafficking machinery, and when vesicles are dynamically recycled, endocytic recycling components (Grant and Donaldson, 2009). This review will highlight recent studies in C. elegans that indicate a role for vesicular trafficking in the regulation of invadopodia, specialized F-actin-rich surface structures that mediate cell invasion through extracellular matrix barriers. Invadopodia in C. elegans undergo dynamic addition of a specialized invadopodial membrane. The invadopodial membrane is specifically associated with invadopodia and contains unique lipid and protein components distinct from the surrounding plasma membrane. During invadopodia breakdown, the invadopodial membrane lipid and protein components are rapidly recycled through endolysosomal vesicles then delivered back to the plasma membrane to form new invadopodia. In this review we will provide a brief history of invadopodia, discuss evidence for the conservation of the invadopodial membrane and focus on the regulation of trafficking and possible functions of the invadopodial membrane. The identification and investigation of this unique membrane is providing a deeper mechanistic understanding of invadopodia formation and cell invasion during normal development and diseases such as cancer.
Background: Invadopodia are specialized subcellular structures that mediate basement membrane and interstitial matrix invasion
Basement membranes are dense, sheet-like forms of extracellular matrix that underlie all epithelia and endothelia and surround muscle, fat, and Schwann cells (Halfter et al., 2015; Yurchenco, 2011). Independent polymeric laminin and type IV collagen networks as well as a number of associated proteins including perlecan and nidogen contribute to basement membrane composition (Yurchenco, 2011). Functionally, basement membranes create tissue barriers, provide structural support, and facilitate filtration, as well as harbor cues for cell differentiation, polarization, and growth (Breitkreutz et al., 2013; Hay, 1981; Poschl et al., 2004; Rasmussen et al., 2012; Suh and Miner, 2013; Yurchenco, 2011). During development and immune cell surveillance, specialized cells acquire the ability to invade basement membrane barriers to allow cell movement into and out of tissues (Kelley et al., 2014; Madsen and Sahai, 2010; Rowe and Weiss, 2008). Misregulation of invasion through basement membranes underlies the pathology of developmental diseases, immune disorders, and cancer (Barsky et al., 1983; Hagedorn and Sherwood, 2011). Given the importance of basement membrane invasion in development, immune function, and human health, there has been great interest in understanding how cells transmigrate basement membrane barriers.
In 1989 Wen-Tien Chen used the term invadopodia to name highly protrusive, matrix-degrading membrane structures, composed of actin regulators and proteases found in transformed embryonic chicken fibroblasts plated on glass slides with a thin coating of matrix—a surface that mimics the 2D topography of basement membranes (Chen, 1989; Even-Ram and Yamada, 2005; Genot and Gligorijevic, 2014; Murphy and Courtneidge, 2011). Since Chen’s initial description, invadopodia have been observed in many metastatic cancer cell lines (Hoshino et al., 2013) and emerged as one of the key subcellular structures that invasive cells use to breach basement membrane barriers (Lohmer et al., 2014; Schoumacher et al., 2013; Schoumacher et al., 2010). Invadopodia also appear to mediate invasion through the more porous type I collagen rich interstitial matrices that reside between cells and tissues. Imaging of invasive cells in in vitro 3D type I collagen matrices has revealed that invadopodia (also referred to as “invadopodia equivalents”) in these environments take on the morphology of long, thin filopodial structures (Li et al., 2010; Tolde et al., 2010; Wolf et al., 2009). Podosomes are another F-actin based membrane-associated structure similar to invadopodia, but are generally not protrusive and are most often associated with non-transformed cells that mediate matrix remodeling events, such as dendritic cells, osteoclasts, macrophages, and vascular smooth muscle cells (Davies and Stossel, 1977; Gawden-Bone et al., 2010; Hoshino et al., 2013; Linder et al., 2011; Murphy and Courtneidge, 2011; Seano et al., 2014; Zambonin-Zallone et al., 1988). In some culture conditions, however, podosomes extend long protrusions that degrade extracellular matrix, suggesting a possible close relationship between podosomes and invadopodia (Gawden-Bone et al., 2010). To help account for such findings, the term invadosomes has recently been adopted to incorporate both structures (Destaing et al., 2011; Linder, 2009; Linder et al., 2011; Saltel et al., 2011), proposing that invadopodia, podosomes, and possibly other actin-based cellular protrusions that bind and degrade extracellular matrix represent a spectrum of molecularly related structures that may adapt and even interchange in response to the microenvironment (Di Martino et al., 2016; McNiven, 2013). In this review, we will be consistent with the bulk of previously published work that defines invadopodia as highly protrusive invasive structures (Linder et al., 2011; Lohmer et al., 2014). Importantly, we include within this definition invadopodia observed during developmental and normal physiological invasion events, recognizing that invadopodia are likely a component of a normal invasion program co-opted by tumor cells (Lohmer et al., 2014; Murphy and Courtneidge, 2011).
Through candidate gene approaches, proteomic analysis, and more recent in vivo genetic screens, approximately 100 genes have been associated with invadopodia formation, function, and breakdown (see Table 1 and references therein). This includes well-studied actin regulators, matrix metalloproteinases (MMPs), signaling pathways, and integrins, as well as genes involved with glycolysis, metabolism, protein degradation, chaperone activity, and protein synthesis for which an exact role in invadopodia formation has not been determined (Attanasio et al., 2011; Hoshino et al., 2013; Lohmer et al., 2016). The breadth of gene families associated with invadopodia likely reflects the complexity and intricate regulation of invadopodia and suggests that many aspects of their function and control remain unknown.
Table 1.
Proteins involved in invadopodial formation and activity
| Gene | Role in invadopodial life cycle | References |
|---|---|---|
| AFAP1 | Actin dynamics | (Dorfleutner et al., 2008) |
| ENAH/VASP** | Actin dynamics | (Hagedorn et al., 2014) |
| ARP2/3** | Actin dynamics | (Yamaguchi et al., 2005) |
| CARMIL2 | Actin dynamics | (Lanier et al., 2015) |
| CALD1 | Actin dynamics | (Mukhopadhyay et al., 2009) |
| CDC42** | Actin dynamics | (Lohmer et al., 2016; Nakahara et al., 2003) |
| ADF/CFL1** | Actin dynamics | (Hagedorn et al., 2014; Yamaguchi et al., 2005) |
| CRP2 | Actin dynamics | (Hoffmann et al., 2016) |
| DRF1-3 | Actin dynamics | (Lizarraga et al., 2009) |
| ERK | Actin dynamics | (Ayala et al., 2008) |
| ETAR | Actin dynamics | (Semprucci et al., 2015) |
| FSCN1 | Actin dynamics | (Vignjevic et al., 2006) |
| FLNA | Actin dynamics | (Takkunen et al., 2010) |
| GRB2 | Actin dynamics | (Oikawa et al., 2008) |
| HIF1A | Actin dynamics | (Md Hashim et al., 2013) |
| ITSN1/2 | Actin dynamics | (Gryaznova et al., 2015) |
| ARHGAP35 | Actin dynamics | (Bravo-Cordero et al., 2011; Nakahara et al., 1998) |
| ARHGEF28 | Actin dynamics | (Bravo-Cordero et al., 2011) |
| PODXL | Actin dynamics | (Lin et al., 2014) |
| RHOC | Actin dynamics | (Bravo-Cordero et al., 2011) |
| TRMP7 | Actin dynamics | (Visser et al., 2013) |
| VCL | Actin dynamics | (Branch et al., 2012) |
| WICH/WIRE | Actin dynamics | (Garcia et al., 2016) |
| ARHGEF7 | Actin dynamics | (Md Hashim et al., 2013) |
| ATX | Actin dynamics | (Harper et al., 2010) |
| EPAC | Actin dynamics | (Harper et al., 2010) |
| PIP5KIα | Actin dynamics | (Yamaguchi et al., 2010) |
| SVIL | Actin dynamics | (Crowley et al., 2009) |
| LAMTOR1 | Actin dynamics | (Hoshino et al., 2009) |
| FGD1 | Actin dynamics | (Ayala et al., 2009) |
| NCK1 | Actin dynamics, Matrix degradation | (Stylli et al., 2009; Yamaguchi et al., 2005) |
| ROCK1/2 | Actin dynamics, Matrix degradation | (Jerrell and Parekh, 2016; Vishnubhotla et al., 2007) |
| TLN1 | Actin dynamics, Matrix degradation | (Beaty et al., 2014; Mueller et al., 1992) |
| KISS1R | Actin dynamics, MMP activation | (Goertzen et al., 2016) |
| TIS21 | Actin dynamics, regulation of ROS | (Choi and Lim, 2013) |
| ARF6 | Cargo transport | (Hashimoto et al., 2004) |
| VAV1 | Cdc42 activation | (Razidlo et al., 2014) |
| β1 and β3 Integrin** | ECM attachment, actin dynamics | (Beaty et al., 2013; Hagedorn et al., 2009; Mueller and Chen, 1991) |
| HDAC6 | EGF signaling, hypoxic conditions | (Rey et al., 2011) |
| PXN | Erk activation | (Badowski et al., 2008; Bowden et al., 1999) |
| EXO70 | Exocyst complex, actin dynamics | (Liu et al., 2009) |
| SEC8 | Exocyst complex, actin dynamics | (Liu et al., 2009) |
| ACC1 | Fatty acid synthesis and de novo lipidogenesis | (Scott et al., 2012) |
| EGF | Induction | (Yamaguchi et al., 2005) |
| MET | Induction | (Rajadurai et al., 2012) |
| NTN1 | Induction | (Hagedorn et al., 2013) |
| PDGF | Induction | (Eckert et al., 2011) |
| ROS | Induction | (Diaz et al., 2009; Gianni et al., 2009) |
| SRC | Induction | (Chen, 1989) |
| TGF-β | Induction | (Eckert et al., 2011; Pignatelli et al., 2012) |
| RPS6KA1 | Induction, hypoxic conditions | (Lucien et al., 2011) |
| ORAI1 | Invadopodia assembly and Matrix degradation | (Sun et al., 2014) |
| STIM1 | Invadopodia assembly and Matrix degradation | (Sun et al., 2014) |
| PLEC | Invadopodia stabilization | (Sutoh Yoneyama et al., 2014) |
| PAK1 | Invadopodial disassembly | (Moshfegh et al., 2014) |
| VIM | Invadopodial elongation | (Schoumacher et al., 2010) |
| Microtubules | Invadopodial elongation | (Schoumacher et al., 2010) |
| IMP3 | Invadopodial formation, multiple mechanisms | (Hwang et al., 2012) |
| EZR | Invadopodial turnover | (Hoskin et al., 2015) |
| RAC1** | Invadopodial turnover | (Moshfegh et al., 2014; Nakahara et al., 2003; Ziel et al., 2009) |
| DPP4 | Matrix degradation | (Chen and Kelly, 2003) |
| HIC-5 | Matrix degradation | (Pignatelli et al., 2012) |
| MMP2 | Matrix degradation | (Deryugina et al., 2002) |
| MMP9 | Matrix degradation | (Nascimento et al., 2010) |
| MT1-MMP (MMP14) | Matrix degradation | (Nakahara et al., 1997; van Hinsbergh et al., 2006) |
| NEDD9 | Matrix degradation | (McLaughlin et al., 2014) |
| NHE-1 | Matrix degradation | (Busco et al., 2010) |
| PRKD1 | Matrix degradation | (Bowden et al., 1999) |
| TWIST1 | Matrix degradation | (Eckert et al., 2011) |
| uPAR | Matrix degradation | (Furmaniak-Kazmierczak et al., 2007) |
| NOX1 | Matrix degradation | (Gianni et al., 2010b) |
| PI3K3CA | Matrix degradation | (Nakahara et al., 2003; Yamaguchi et al., 2011) |
| FAP | Matrix degradation, Protease docking, localization | (Chen, 1996; Monsky et al., 1994) |
| PFN1 | Membrane composition | (Valenzuela-Iglesias et al., 2015) |
| GDI1 | Membrane trafficking | (Lohmer et al., 2016) |
| GAB1 | Met signaling | (Rajadurai et al., 2012) |
| RAB40B | MMP trafficking | (Jacob et al., 2013) |
| WIP | MMP trafficking, actin dynamics | (Garcia et al., 2012) |
| CD44 | MT1-MMP recruitment | (Vikesaa et al., 2006; Zhao et al., 2016) |
| JIP3/4 | MT1-MMP trafficking | (Marchesin et al., 2015) |
| CD147 | MT1-MMP trafficking | (Grass et al., 2012) |
| CDCP1 | MT1-MMP trafficking | (Miyazawa et al., 2013) |
| IQGAP1 | MT1-MMP trafficking | (Sakurai-Yageta et al., 2008) |
| RAB8A | MT1-MMP trafficking | (Bravo-Cordero et al., 2007) |
| RHOA | MT1-MMP trafficking | (Bravo-Cordero et al., 2011) |
| STX4 | MT1-MMP trafficking | (Williams et al., 2014) |
| VAMP7 | MT1-MMP trafficking | (Steffen et al., 2008; Williams et al., 2014) |
| ZF21 | MT1-MMP trafficking | (Hoshino et al., 2013b) |
| YB-1 | MT1-MMP trafficking | (Lovett et al., 2010) |
| KIF3A/B | MT1-MMP trafficking | (Wiesner et al., 2010) |
| KIF5A | MT1-MMP trafficking | (Wiesner et al., 2010) |
| TOM1L1 | MT1-MMP trafficking | (Chevalier et al., 2016) |
| N-WASP** | MT1-MMP trafficking, actin dynamics | (Lorenz et al., 2004; Yamaguchi et al., 2005) |
| CTTN | MT1-MMP trafficking, actin dynamics | (Artym et al., 2006; Bowden et al., 1999; Clark et al., 2007) |
| CLIC3 | MT1-MMP trafficking, cargo selection | (Macpherson et al., 2014) |
| ABL1/2 | MT1-MMP trafficking, EGF signaling | (Beaty et al., 2013; Smith-Pearson et al., 2010; Sun et al., 2009) |
| SNAP23 | MT1-MMP trafficking, Src and EGFR trafficking | (Williams and Coppolino, 2014; Williams et al., 2014) |
| TKS4/5 | MT1-MMP trafficking, Src signaling | (Abram et al., 2003) |
| SYNJ2 | RAC1 activity | (Chuang et al., 2004) |
| SHIP2 | Regulates invadopodia PI(3,4)P2 levels | (Sharma et al., 2013) |
| PDPN | Regulator of Invadopodia maturation | (Martin-Villar et al., 2015) |
| RALB/RALBP1 | Required, unknown mechanism | (Neel et al., 2012) |
| PLS1 | Required, unknown mechanism | (Schoumacher et al., 2010) |
| FAK | Src signaling | (Hauck et al., 2002; Vitale et al., 2008) |
| P130CAS | Src signaling | (Alexander et al., 2008) |
| TP53 | Src signaling | (Mukhopadhyay et al., 2009) |
| ASAP1 | Src signaling | (Bharti et al., 2007) |
| ADRB2 | Src signaling | (Creed et al., 2015) |
| CAPN2 | Src signaling | (Cortesio et al., 2008) |
| ERK1/2 | Src signaling | (Furmaniak-Kazmierczak et al., 2007) |
| PTP1B | Src signaling | (Cortesio et al., 2008) |
| NOXA1 | Src signaling | (Gianni et al., 2010a) |
| Laminin-332 | Src signaling | (Liu et al., 2010) |
| Gαi2 | Src trafficking to invadopodia | (Ward et al., 2015) |
| STX12 | Src, EGF trafficking | (Williams and Coppolino, 2014) |
| ADAM12 | Src/EGF signaling, hypoxic conditions | (Albrechtsen et al., 2011) |
| TGM1/2 | TGF-β signaling | (Lauzier et al., 2012) |
| CAV-1 | Vesicle / membrane trafficking | (Caldieri et al., 2009; Yamaguchi et al., 2009) |
| DNM2 | Vesicle trafficking | (Baldassarre et al., 2003) |
| FBP17 | Vesicle trafficking | (Yamamoto et al., 2011) |
| WASH | Vesicle trafficking | (Monteiro et al., 2013) |
| RAB4 | Vesicle trafficking | (Frittoli et al., 2014) |
| RAB5A | Vesicle trafficking | (Frittoli et al., 2014) |
The proteins listed were identified in numerous cancer cell types and model systems. Rows shaded grey represent proteins with direct roles in invadopodial membrane formation and function.
Identified in numerous species, indicating a conserved mechanism.
Although most studies have examined invadopodia in cancer cells in vitro, recent imaging advances in ex vivo and in vivo settings are establishing their existence and physiological importance in basement membrane invasion in both normal and disease settings (Di Martino et al., 2016; Genot and Gligorijevic, 2014; Lohmer et al., 2014). These studies include examination of cancer cell invasion on isolated rat peritoneum basement membranes (Schoumacher et al., 2010), imaging of vascular invasion by cancer cells in mouse and chicken embryos (Gligorijevic et al., 2012; Leong et al., 2014; Roh-Johnson et al., 2014), examination of intestinal epithelial cell invasion in a reactive oxygen species (ROS) disease model in zebrafish (Seiler et al., 2012), and visualizing anchor cell invasion during organogenesis in C. elegans (Hagedorn et al., 2013). Importantly, studying invadopodia in native contexts is not only confirming the relevance of these structures for cell invasion through basement membrane, but also is revealing new aspects of invadopodia biology. One fascinating example comes from the discovery of the invadopodial membrane in the anchor cell of C. elegans.
Invadopodia in C. elegans are formed from a recycling invadopodial membrane
The C. elegans anchor cell is a specialized uterine cell that initiates uterine-vulval attachment following invasion through underlying basement membrane (Sherwood and Sternberg, 2003). Anchor cell invasion is facilitated by dynamic and highly protrusive F-actin-rich invadopodia that localize to the anchor cell-basement membrane interface (the invasive cell membrane). The basement membrane in C. elegans is highly conserved and all major basement membrane components and receptors found in vertebrates are also present in C. elegans (Kramer, 2005). A suite of unique attributes of C. elegans as a model organism—including fluorescently tagged basement membrane components, anchor cell specific expression of fluorescently tagged proteins, the highly stereotyped nature of invasion, and genetic analysis—have allowed detailed experimental dissection of invadopodia in vivo (Hagedorn et al., 2014; Hagedorn et al., 2013; Lohmer et al., 2016; Lohmer et al., 2014).
Similar to tumor progression, where cancer cell invasion is promoted by signals from neighboring cells such as tumor associated macrophages (Noy and Pollard, 2014; Roh-Johnson et al., 2014), anchor cell invasion is stimulated by the underlying vulval cells (Sherwood and Sternberg, 2003). The vulval cells direct invasion by generating a diffusible cue that activates the Rho GTPase CDC-42 within the anchor cell (Lohmer et al., 2016). Active CDC-42 promotes robust invadopodia formation along the anchor cell-basement membrane interface. These invadopodia breach the basement membrane shortly after secretion of the vulval cue in the early-to-mid L3 larval stage (Hagedorn et al., 2013; Lohmer et al., 2016). Interestingly, several genes encoding G-protein signaling components were identified in the sensitized invadopodial screen that isolated CDC-42 (Lohmer et al., 2016), suggesting that the vulval cue might act through a G-protein coupled receptor pathway.
Anchor cell invadopodia undergo rapid turnover, with an average lifespan of 45 seconds. In contrast, invadopodia seen in cancer cells in culture exist for minutes to hours (Li et al., 2010; Sibony-Benyamini and Gil-Henn, 2012). Invadopodia observed in vitro may lack the appropriate microenvironment required for rapid formation and turnover, or developmental invasion events may be more swift and efficient than cancer cell invasion (Di Martino et al., 2016; Genot and Gligorijevic, 2014). Similar to invadopodia in cancer cell lines, anchor cell invadopodia are dependent on integrin for their formation (Destaing et al., 2010) and are composed of F-actin and a number of actin regulators, including the ADF/cofilin ortholog UNC-60A, the WASP ortholog WSP-1, and the Ena/VASP ortholog UNC-34 (Hagedorn et al., 2014; Hagedorn et al., 2013; Lohmer et al., 2016).
Anchor cell invadopodia have a specialized membrane that is molecularly distinct from the rest of the anchor cell plasma membrane, and is enriched in the phospholipid PI(4,5)P2, the membrane associated Rho GTPases MIG-2 (RhoG), and CED-10 (Rac) (Hagedorn et al., 2014). The invadopodial membrane is highly dynamic as invadopodia depress, penetrate, then cross the basement membrane with a single long protrusion (Hagedorn et al., 2013). This behavior may be common during invasion, as similar single protrusions have been observed when tumor cells transmigrate basement membranes (Hotary et al., 2006; Leong et al., 2014; Schoumacher et al., 2010).
Live-cell imaging and gene perturbation studies have revealed that the anchor cell’s invadopodial membrane is rapidly recycled through the endolysosomal system and targeted back to the invasive cell membrane to form new invadopodia (Fig. 1 and Supplementary video 1). Evidence of this active trafficking is particularly apparent after loss of UNC-60A (cofilin), a key actin regulatory protein that disassembles F-actin filaments (Blanchoin et al., 2014). UNC-60A localizes to invadopodia and is required for anchor cell invasion. Consistent with its role in disassembly of F-actin, loss of UNC-60A leads to a large buildup of F-actin at the invasive cell membrane (Fig. 1). Loss of UNC-60A also dramatically disrupts the invadopodial membrane (Supplementary video 2), as invadopodial membrane components are no longer found at the invasive cell membrane and instead localize with static endolysosome vesicles within the anchor cell. As cofilin is known to break apart cortical F-actin to facilitate the trafficking and exocytosis of vesicles to the plasma membranes of cells (Lee et al., 2009; Mahaffey et al., 2013), these observations suggest that UNC-60A may disassemble cortical F-actin to allow targeting of the invadopodial membrane from the endolysosome to nascent invadopodia at the cell membrane. The endolysosome marker LAMP-1 localizes to invadopodial membrane at the cell surface, and fluorescence loss in photobleaching (FLIP) experiments revealed that LAMP-1 molecules undergo rapid recycling from invadopodial membrane and through the endolysosome system in less than 5 minutes (Hagedorn et al., 2014). The rate of cycling is consistent with the rapid invadopodia turnover rate observed in the anchor cell. Additional evidence of active recycling and targeting of the invadopodial membrane comes from the identification of GDI-1 (Rab GDP dissociation inhibitor) as a regulator of invadopodia and anchor cell invasion (Lohmer et al., 2016). Rab GDIs have a high affinity for GDP-bound Rab proteins and deliver Rabs to specific membrane compartments to help regulate membrane trafficking (Pfeffer, 2013). Loss of GDI-1 in the anchor cell results in dynamic mis-targeting of invadopodial membrane to the apical and lateral membranes of the anchor cell (Fig. 1, Supplementary video 3). Importantly, other trafficking events in the anchor cell—including Integrin receptor localization to the invasive cell membrane, EGF-like ligand (LIN-3) secretion, and deposition of the matrix component hemicentin into the basement membrane—were normal in the anchor cell after loss of UNC-60A (cofilin) or GDI-1 (Hagedorn et al., 2014; Lohmer et al., 2016). These observations suggest that the invadopodial membrane is uniquely trafficked and recycled through the endolysosome and targeted to the cell-basement membrane interface to form invadopodia.
Fig. 1. Models of Cofilin and GDI-1 function in invadopodial membrane trafficking in the C. elegans anchor cell.
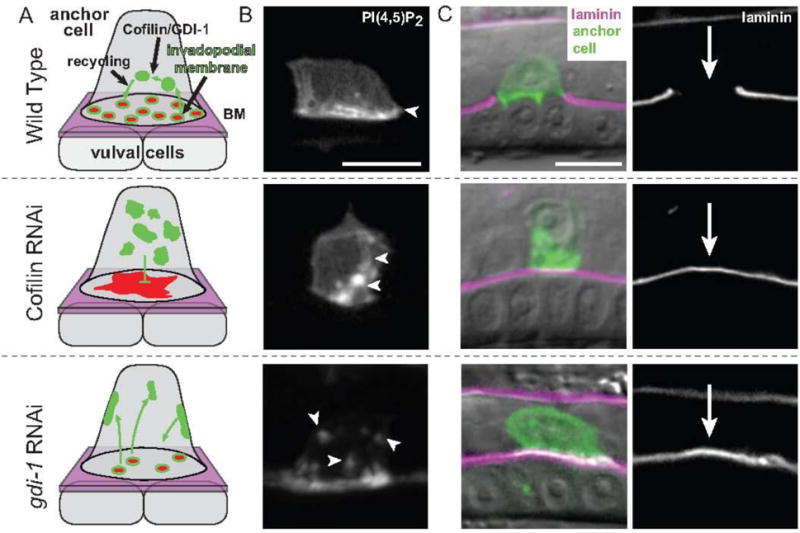
(A) Schematic images of invadopodial membrane trafficking within the anchor cell during anchor cell invasion into the vulval cells. F-actin and actin regulators (red) are coupled with invadopodial membrane trafficking (green) to form invadopodia at the anchor cell-basement membrane (BM, shown in magenta) interface prior to invasion. Both cofilin (encoded by the C. elegans unc-60a gene) and GDI-1 regulate trafficking of the invadopodial membrane through the endolysosome system. RNAi-mediated knockdown of cofilin results in a static, non-trafficked invadopodial membrane that is localized internally, an accumulation of stagnant F-actin along the invasive cell membrane, and an absence of invadopodia formation. Loss of GDI-1 results in mis-targeting of invadopodial membrane to all cell membranes and a reduction in the number of invadopodia. (B) A grayscale image of the invadopodial membrane observed with mCherry::PLCδPH, which binds to the invadopodial membrane component PI(4,5)P2 (highlighted by white arrows). In wild-type animals, the invadopodial membrane is localized along the invasive cell membrane. Loss of cofilin (through cofilin RNAi) results in a static, non-trafficked internal membrane and reduction of GDI-1 protein (through gdi-1 RNAi) causes the invadopodial membrane to be trafficked to all cell membranes. (C) Left, RNAi-mediated loss of the cofilin or GDI-1 results in invasion defects as seen by an inability of the anchor cell (green—visualized with either the invadopodial membrane probe mCherry::PLCδPH or the F-actin probe mCherry::MoeABD) to breach the basement membrane (magenta; visualized with laminin::GFP) overlaid on DIC. Right, a grayscale image of laminin::GFP. Arrow shows position of basement membrane breach in wild-type and a lack of breach after loss of cofilin or GDI-1. Bars, 5 μm.
The specialized invadopodial membrane may be conserved in other invasive cells
There is convincing evidence suggesting that the recycling of the invadopodial membrane observed in C. elegans may be a shared feature of invadopodia. When Chen first characterized invadopodia, he noted the “highly motile membrane” and with transmission electron microscopy (TEM) visualized vesicles associated with invadopodia (Chen, 1989). Subsequent EM studies in melanoma cells also revealed many membrane surface protrusions associated with invadopodia (Baldassarre et al., 2003), and similar internal vesicles (thought to be endosomes) were observed within invadopodia in breast cancer cells (Schoumacher et al., 2010). Live-cell, high-resolution total internal reflection fluorescence (TIRF) microscopy visualized dynamic ruffling and undulation of the invadopodial membrane in breast cancer cells (Artym et al., 2011). Notably, podosomes and integrin based focal adhesions are not associated with similar membrane processes, highlighting the distinct nature of the invadopodial membrane (Artym et al., 2011). In addition to comparable membrane dynamics and vesicle trafficking, PI(4,5)P2, a component of the C. elegans anchor cell invadopodial membrane, also localizes at invadopodia and in vesicular structures surrounding invadopodia in breast cancer cells (Yamaguchi et al., 2010). Furthermore, invadopodia in breast cancer cells are enriched in lipid raft membranes, which are actively trafficked to and from invadopodia (Yamaguchi et al., 2009). Finally, studies on the trafficking of the transmembrane matrix metalloproteinase MT1-MMP, a key protease associated with invadopodia in numerous cancer cell lines, has indicated that MT1-MMP localizes to the invadopodial membrane and is recycled through the endolysosome. MT1-MMP is dynamically internalized by both clathrin- and caveolar-mediated endocytosis and directed via a Rab5 early endosome vesicle trafficking pathway to either a Rab4 fast recycling exocytosis pathway (thought to be a minor component of MT1-MMP recycling) or through Rab7 delivery to late endosome/endolysosome where most MT1-MMP accumulates. Following accumulation in the late endosome/endolysosome, MT1-MMP is trafficked and exocytosed back to invadopodia through an Arf6, endosomal WASH protein, exocyst complex, and V-SNARE protein VAMP-7 mediated mechanism (Frittoli et al., 2014; Linder, 2015; Marchesin et al., 2015; Monteiro et al., 2013; Poincloux et al., 2009; Remacle et al., 2003; Williams and Coppolino, 2011). MT1-MMP is stabilized at invadopodia in the plasma membrane by a direct interaction with invadopodial F-actin, providing a link between the F-actin core of invadopodia and the invadopodial membrane (Yu et al., 2012). MT1-MMP recycling takes approximately one hour in Madin-Darby canine kidney and fibrosarcoma cells, matching the turnover rates of invadopodia in cancer cells (Wang et al., 2004; Williams and Coppolino, 2011). Together these studies suggest that the invadopodial membrane is a unique membrane domain, and that its trafficking through the endolysosome might be a shared feature of invadopodia in normal development and cancer progression.
Function of the invadopodial membrane
Perturbations in the invadopodial membrane impede or block the ability of the anchor cell to invade through the basement membrane, offering strong evidence for the essential function of the invadopodial membrane during cell invasion (Hagedorn et al., 2014; Hagedorn et al., 2013; Lohmer et al., 2016). A key feature of invadopodia that distinguishes them from podosomes is their highly protrusive nature and membrane ruffles (Artym et al., 2011). Active recycling of the invadopodial membrane through the endolysosome may provide a source of new membrane, allowing the invadopodia to rapidly extend into and protrude through basement membrane and interstitial matrices. In other biological events, addition of membrane from the endolysosome is used to mediate plasma membrane repair and neurite outgrowth (Arantes and Andrews, 2006; Reddy et al., 2001). Furthermore, endocytic trafficking is used to add membrane during cytokinesis (Grant and Donaldson, 2009). Adding weight to the notion that the endolysosome delivers membrane to invadopodia, the GTPase Arf6 together with its effectors JIP3 and JIP4 mediate the delivery of endosomes for membrane addition during cytokinesis and regulate exocytosis of MT1-MMP (Marchesin et al., 2015; Montagnac et al., 2009). Thus, the molecular machinery of the vesicular trafficking system is present for rapid membrane addition to invadopodia to allow for its protrusive activity.
The invadopodial membrane may also deliver and concentrate proteases at invadopodia (Frittoli et al., 2011; Scita and Di Fiore, 2010; Trimble and Grinstein, 2015). Many studies in cancer cell lines have focused on the targeted delivery of MT1-MMP to invadopodia, which is thought to be required for basement membrane break down and breach (see Table 1 for genes associated with MT1-MMP trafficking) (Hotary et al., 2006). Interestingly, transmembrane MMPs such as MT1-MMP might be a deuterostome innovation, as protostomes such as C. elegans and Drosophila do not encode these genes (Fanjul-Fernandez et al., 2010). However, C. elegans and Drosophila do encode membrane anchored glycosyl-phosphatidyl inositol (GPI) MMPs (Altincicek et al., 2010; Page-McCaw, 2008), which could be directed to invadopodia via the invadopodial membrane. Vertebrate GPI-anchored MMPs are overexpressed in cancer and strongly implicated with cancer progression, but their trafficking and subcellular localization is poorly understood (Sohail et al., 2008). Whether GPI-anchored MMPs are trafficked to invadopodia is an important unanswered question. Genetic loss of the sole C. elegans GPI-anchored MMP zmp-1, however, only slightly delays anchor cell invasion (Sherwood et al., 2005), strongly suggesting that the invadopodial membrane has additional functions outside of delivering MMP proteases during basement membrane invasion.
Other functions of the invadopodial membrane are also possible. For example, the invadopodial membrane may have a fundamental role in invadopodia assembly. The unique lipid composition of the invadopodial membrane may act as a platform that recruits and activates the various signaling, actin regulatory, and adhesion proteins required for invadopodia formation and turnover (Moshfegh et al., 2014; Yamaguchi and Oikawa, 2010). Endocytic vesicles also are emerging as key signaling centers (Scita and Di Fiore, 2010). Signaling from endosomes that originate from endocytic events at invadopodia might allow rapid feedback and adaption during invasion. Consistent with this possibility, endocytosis of collagen has been observed in macrophages, fibroblasts, and hepatic stellate cells (a liver cell that maintains extracellular matrix homeostasis during liver damage) (Bi et al., 2014). Further, in fibrosarcoma cells fibronectin is internalized into the endolysosome, the same compartment as MT1-MMP (Sung et al., 2011). Intriguingly, collagen endocytosis in hepatic stellate cells leads to the transcriptional upregulation of MMP-9, consistent with a signaling function of endocytosed collagen (Bi et al., 2014). It is thus possible that the invadopodial membrane has numerous essential functions in invadopodia formation, function, and adaptation to the microenvironment.
Summary and outlook
The complexity of F-actin and membrane structures within invadopodia observed by TIRF microscopy lead to invadopodia being referred to as “invasive superstructures” (Artym et al., 2011). Given its impressive and complex structure, it should come as no surprise that we are only beginning to understand the intricacies of invadopodia regulation and function. The discovery of an actively recycled invadopodial membrane reveals another layer of regulation to these fascinating cellular drill bits that breach basement membrane barriers.
To advance our understanding of the invadopodial membrane, it will be important to determine how invadopodial lipids and membrane components beside MT1-MMP are restricted to the invadopodial membrane at the plasma membrane. Targeted exocytosis and endocytosis may play a role in maintaining the invadopodial membrane at the cell surface (Trimble and Grinstein, 2015). In addition, F-actin, which can limit protein and lipid diffusion, is a strong candidate for preventing diffusion of invadopodial membrane components into the broader plasma membrane (Kusumi et al., 2012; Trimble and Grinstein, 2015). Indeed, actin filaments attached to the plasma membrane can even promote ordered lipid raft domains in cells, suggesting that the F-actin core of invadopodia may not only play a role in maintaining, but also in organizing the invadopodial membrane (Dinic et al., 2013). It will also be crucial to examine the trafficking of other invadopodial membrane components in cancer cell lines beyond MT1-MMP and to carefully examine the initial stages of endocytic trafficking of invadopodial constituents in C. elegans (i.e. early endosomes) to determine if the lipids and proteins of the invadopodial membrane are trafficked together as a unit through a complete cycle of endocytic recycling or if they coalesce at the endolysosome (where all components are present in C. elegans) from different routes prior to reaching the cell surface to form invadopodia. Notably, the protease MT3-MMP is co-trafficked with MT1-MMP in Madin-Darby canine kidney cells, supporting the possibility that invadopodial membrane proteins are trafficked jointly (Wang et al., 2004). Rigorous live-cell image analysis of invadopodial membrane component trafficking will also be necessary to further dissect invadopodial membrane regulation. Interestingly, recent live-cell imaging work using TIRF microscopy in human macrophages has revealed that MT1-MMP remains at the membrane in small islets after dissolution of podosomes, and that these islets seed the reemergence of podosomes at these sites (El Azzouzi et al., 2016). Whether a similar memory mechanism is used to direct waves of invadopodia to specific extracellular matrix sites to facilitate invasion will be fascinating to explore. Finally, biochemical isolation of the invadopodial membrane will be key in further elucidating invadopodial membrane composition (Asano et al., 2009). Together, these efforts should ultimately lead to a deeper mechanistic understanding of invadopodial membrane trafficking and function. As evidence in C. elegans suggests that invadopodial membrane recycling is uniquely regulated from other secretion and vesicle trafficking events, this analysis will not only increase our understanding of invadopodia, but also may reveal specialized features of the invadopodial membrane that could be to targeted with novel cancer therapeutics to halt cell invasive behavior.
Supplementary Material
Lateral-view time-lapses show 3D reconstruction of PI(4,5)P2 in wild-type anchor cells. PI(4,5)P2 is visualized with mCherry::PLCδPH (cyan); the basement membrane is visualized with laminin::GFP (magenta). Images were acquired using a spinning-disc confocal microscope (CSU-10 scan head; Yokogawa Corporation of America) mounted on an upright microscope (AxioImager; Carl Zeiss). 40-min time-lapses are shown with time points acquired every 60s. Projections of seven z-sections (step size of 1 μm) are shown. The video plays at 10 frames per second.
Lateral-view time-lapses show 3D reconstruction of PI(4,5)P2 in unc-60a RNAi-treated anchor cells. PI(4,5)P2 is visualized with mCherry::PLCδPH (cyan); the basement membrane is visualized with laminin::GFP (magenta). Images were acquired using a spinning-disc confocal microscope (CSU-10 scan head; Yokogawa Corporation of America) mounted on an upright microscope (AxioImager; Carl Zeiss). 40-min time-lapses are shown with time points acquired every 60 s. Projections of seven z-sections (step size of 1 μm) are shown. The video plays at 10 frames per second.
Lateral-view time-lapses show 3D reconstruction of PI(4,5)P2 in gdi-1 RNAi-treated anchor cells. PI(4,5)P2 is visualized with mCherry::PLCδPH (cyan); the basement membrane is visualized with laminin::GFP (magenta). Images were acquired using a spinning-disc confocal microscope (CSU-10 scan head; Yokogawa Corporation of America) mounted on an upright microscope (AxioImager; Carl Zeiss). 40-min time-lapses are shown with time points acquired every 60 s. Projections of seven z-sections (step size of 1 μm) are shown. The video plays at 10 frames per second.
Acknowledgments
We thank Kacy Gordon for insightful comments on the manuscript and thank Phillipe Chavrier for discussions. ELH is supported by postdoctoral fellowship 129351-PF-16-024-01-CSM from the American Cancer Society. The lab of DRS is supported by National Institutes of Health MIRA award R35GM118049.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Blanco MT, Verboon JM, Parkhurst SM. Single cell wound repair: Dealing with life’s little traumas. Bioarchitecture. 2011;1:114–121. doi: 10.4161/bioa.1.3.17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altincicek B, Fischer M, Luersen K, Boll M, Wenzel U, Vilcinskas A. Role of matrix metalloproteinase ZMP-2 in pathogen resistance and development in Caenorhabditis elegans. Dev Comp Immunol. 2010;34:1160–1169. doi: 10.1016/j.dci.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Arantes RM, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26:4630–4637. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym VV, Matsumoto K, Mueller SC, Yamada KM. Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes. Eur J Cell Biol. 2011;90:172–180. doi: 10.1016/j.ejcb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano A, Selvaraj V, Buttke DE, Nelson JL, Green KM, Evans JE, Travis AJ. Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts. J Cell Physiol. 2009;218:537–548. doi: 10.1002/jcp.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio F, Caldieri G, Giacchetti G, van Horssen R, Wieringa B, Buccione R. Novel invadopodia components revealed by differential proteomic analysis. European journal of cell biology. 2011;90:115–127. doi: 10.1016/j.ejcb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, Luini A, Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Molecular biology of the cell. 2003;14:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky SH, Siegal GP, Jannotta F, Liotta LA. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983;49:140–147. [PubMed] [Google Scholar]
- Bi Y, Mukhopadhyay D, Drinane M, Ji B, Li X, Cao S, Shah VH. Endocytosis of collagen by hepatic stellate cells regulates extracellular matrix dynamics. Am J Physiol Cell Physiol. 2014;307:C622–633. doi: 10.1152/ajpcell.00086.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiological reviews. 2014;94:235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- Breitkreutz D, Koxholt I, Thiemann K, Nischt R. Skin basement membrane: the foundation of epidermal integrity–BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res Int. 2013;2013:179784. doi: 10.1155/2013/179784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. The Journal of experimental zoology. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- Davies WA, Stossel TP. Peripheral hyaline blebs (podosomes) of macrophages. J Cell Biol. 1977;75:941–955. doi: 10.1083/jcb.75.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Curr Opin Cell Biol. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Destaing O, Planus E, Bouvard D, Oddou C, Badowski C, Bossy V, Raducanu A, Fourcade B, Albiges-Rizo C, Block MR. beta1A integrin is a master regulator of invadosome organization and function. Mol Biol Cell. 2010;21:4108–4119. doi: 10.1091/mbc.E10-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino J, Henriet E, Ezzoukhry Z, Goetz JG, Moreau V, Saltel F. The microenvironment controls invadosome plasticity. J Cell Sci. 2016 doi: 10.1242/jcs.182329. [DOI] [PubMed] [Google Scholar]
- Dinic J, Ashrafzadeh P, Parmryd I. Actin filaments attachment at the plasma membrane in live cells cause the formation of ordered lipid domains. Biochim Biophys Acta. 2013;1828:1102–1111. doi: 10.1016/j.bbamem.2012.12.004. [DOI] [PubMed] [Google Scholar]
- El Azzouzi K, Wiesner C, Linder S. Metalloproteinase MT1-MMP islets act as memory devices for podosome reemergence. J Cell Biol. 2016;213:109–125. doi: 10.1083/jcb.201510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Frittoli E, Palamidessi A, Disanza A, Scita G. Secretory and endo/exocytic trafficking in invadopodia formation: the MT1-MMP paradigm. European journal of cell biology. 2011;90:108–114. doi: 10.1016/j.ejcb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Frittoli E, Palamidessi A, Marighetti P, Confalonieri S, Bianchi F, Malinverno C, Mazzarol G, Viale G, Martin-Padura I, Garre M, Parazzoli D, Mattei V, Cortellino S, Bertalot G, Di Fiore PP, Scita G. A RAB5/RAB4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. The Journal of cell biology. 2014;206:307–328. doi: 10.1083/jcb.201403127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawden-Bone C, Zhou Z, King E, Prescott A, Watts C, Lucocq J. Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. Journal of cell science. 2010;123:1427–1437. doi: 10.1242/jcs.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genot E, Gligorijevic B. Invadosomes in their natural habitat. Eur J Cell Biol. 2014;93:367–379. doi: 10.1016/j.ejcb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724–734. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnord P, Blouin CM, Lamaze C. Membrane trafficking and signaling: two sides of the same coin. Seminars in cell & developmental biology. 2012;23:154–164. doi: 10.1016/j.semcdb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nature reviews Molecular cell biology. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn EJ, Kelley LC, Naegeli KM, Wang Z, Chi Q, Sherwood DR. ADF/cofilin promotes invadopodial membrane recycling during cell invasion in vivo. The Journal of cell biology. 2014;204:1209–1218. doi: 10.1083/jcb.201312098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn EJ, Sherwood DR. Cell invasion through basement membrane: the anchor cell breaches the barrier. Current opinion in cell biology. 2011;23:589–596. doi: 10.1016/j.ceb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn EJ, Ziel JW, Morrissey MA, Linden LM, Wang Z, Chi Q, Johnson SA, Sherwood DR. The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivo. The Journal of cell biology. 2013;201:903–913. doi: 10.1083/jcb.201301091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Oertle P, Monnier CA, Camenzind L, Reyes-Lua M, Hu H, Candiello J, Labilloy A, Balasubramani M, Henrich PB, Plodinec M. New concepts in basement membrane biology. Febs J. 2015 doi: 10.1111/febs.13495. [DOI] [PubMed] [Google Scholar]
- Hausott B, Klimaschewski L. Membrane turnover and receptor trafficking in regenerating axons. The European journal of neuroscience. 2016;43:309–317. doi: 10.1111/ejn.13025. [DOI] [PubMed] [Google Scholar]
- Hay ED. Extracellular matrix. J Cell Biol. 1981;91:205s–223s. doi: 10.1083/jcb.91.3.205s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino D, Branch KM, Weaver AM. Signaling inputs to invadopodia and podosomes. Journal of cell science. 2013;126:2979–2989. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes & development. 2006;20:2673–2686. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LC, Lohmer LL, Hagedorn EJ, Sherwood DR. Traversing the basement membrane in vivo: A diversity of strategies. J Cell Biol. 2014;204:291–302. doi: 10.1083/jcb.201311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM. Basement membranes. WormBook. 2005:1–15. doi: 10.1895/wormbook.1.16.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, Kasai RS, Suzuki KG. Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu Rev Cell Dev Biol. 2012;28:215–250. doi: 10.1146/annurev-cellbio-100809-151736. [DOI] [PubMed] [Google Scholar]
- Lee CW, Han J, Bamburg JR, Han L, Lynn R, Zheng JQ. Regulation of acetylcholine receptor clustering by ADF/cofilin-directed vesicular trafficking. Nat Neurosci. 2009;12:848–856. doi: 10.1038/nn.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K, McPherson VA, Postenka CO, Turley EA, Courtneidge SA, Chambers AF, Lewis JD. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8:1558–1570. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]
- Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, Konig I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. Invadosomes at a glance. J Cell Sci. 2009;122:3009–3013. doi: 10.1242/jcs.032631. [DOI] [PubMed] [Google Scholar]
- Linder S. MT1-MMP: Endosomal delivery drives breast cancer metastasis. J Cell Biol. 2015;211:215–217. doi: 10.1083/jcb.201510009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- Lohmer LL, Clay MR, Naegeli KM, Chi Q, Ziel JW, Hagedorn EJ, Park JE, Jayadev R, Sherwood DR. A Sensitized Screen for Genes Promoting Invadopodia Function In Vivo: CDC-42 and Rab GDI-1 Direct Distinct Aspects of Invadopodia Formation. PLoS genetics. 2016;12:e1005786. doi: 10.1371/journal.pgen.1005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmer LL, Kelley LC, Hagedorn EJ, Sherwood DR. Invadopodia and basement membrane invasion in vivo. Cell adhesion & migration. 2014;8:246–255. doi: 10.4161/cam.28406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen CD, Sahai E. Cancer dissemination–lessons from leukocytes. Developmental cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Mahaffey JP, Grego-Bessa J, Liem KF, Jr, Anderson KV. Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development. 2013;140:1262–1271. doi: 10.1242/dev.085316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesin V, Castro-Castro A, Lodillinsky C, Castagnino A, Cyrta J, Bonsang-Kitzis H, Fuhrmann L, Irondelle M, Infante E, Montagnac G, Reyal F, Vincent-Salomon A, Chavrier P. ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. The Journal of cell biology. 2015;211:339–358. doi: 10.1083/jcb.201506002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritzen T, Schachtner H, Legler DF. On the move: endocytic trafficking in cell migration. Cellular and molecular life sciences: CMLS. 2015;72:2119–2134. doi: 10.1007/s00018-015-1855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven MA. Breaking away: matrix remodeling from the leading edge. Trends Cell Biol. 2013;23:16–21. doi: 10.1016/j.tcb.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnac G, Sibarita JB, Loubery S, Daviet L, Romao M, Raposo G, Chavrier P. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol. 2009;19:184–195. doi: 10.1016/j.cub.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Monteiro P, Rosse C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, Desnos C, Formstecher E, Darchen F, Perrais D, Gautreau A, Hertzog M, Chavrier P. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. The Journal of cell biology. 2013;203:1063–1079. doi: 10.1083/jcb.201306162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfegh Y, Bravo-Cordero JJ, Miskolci V, Condeelis J, Hodgson L. A Trio-Rac1-Pak1 signalling axis drives invadopodia disassembly. Nature cell biology. 2014;16:574–586. doi: 10.1038/ncb2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A. Remodeling the model organism: matrix metalloproteinase functions in invertebrates. Seminars in cell & developmental biology. 2008;19:14–23. doi: 10.1016/j.semcdb.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Rasmussen JP, Reddy SS, Priess JR. Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development. 2012;139:2050–2060. doi: 10.1242/dev.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Remacle A, Murphy G, Roghi C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci. 2003;116:3905–3916. doi: 10.1242/jcs.00710. [DOI] [PubMed] [Google Scholar]
- Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L, Condeelis J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33:4203–4212. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends in cell biology. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Saltel F, Daubon T, Juin A, Ganuza IE, Veillat V, Genot E. Invadosomes: intriguing structures with promise. Eur J Cell Biol. 2011;90:100–107. doi: 10.1016/j.ejcb.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Schoumacher M, Glentis A, Gurchenkov VV, Vignjevic DM. Basement membrane invasion assays: native basement membrane and chemoinvasion assay. Methods Mol Biol. 2013;1046:133–144. doi: 10.1007/978-1-62703-538-5_8. [DOI] [PubMed] [Google Scholar]
- Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. The Journal of cell biology. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- Seano G, Chiaverina G, Gagliardi PA, di Blasio L, Puliafito A, Bouvard C, Sessa R, Tarone G, Sorokin L, Helley D, Jain RK, Serini G, Bussolino F, Primo L. Endothelial podosome rosettes regulate vascular branching in tumour angiogenesis. Nature cell biology. 2014;16:931–941. 931–938. doi: 10.1038/ncb3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C, Davuluri G, Abrams J, Byfield FJ, Janmey PA, Pack M. Smooth muscle tension induces invasive remodeling of the zebrafish intestine. PLoS biology. 2012;10:e1001386. doi: 10.1371/journal.pbio.1001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Developmental cell. 2003;5:21–31. doi: 10.1016/s1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Shuster CB, Burgess DR. Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3633–3638. doi: 10.1073/pnas.052342699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibony-Benyamini H, Gil-Henn H. Invadopodia: The leading force. Eur J Cell Biol. 2012;91:896–901. doi: 10.1016/j.ejcb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Sohail A, Sun Q, Zhao H, Bernardo MM, Cho JA, Fridman R. MT4-(MMP17) and MT6-MMP (MMP25), A unique set of membrane-anchored matrix metalloproteinases: properties and expression in cancer. Cancer Metastasis Rev. 2008;27:289–302. doi: 10.1007/s10555-008-9129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol. 2013;9:470–477. doi: 10.1038/nrneph.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, Zhu X, Kaverina I, Weaver AM. Cortactin controls cell motility and lamellipodial dynamics by regulating ECM secretion. Current biology: CB. 2011;21:1460–1469. doi: 10.1016/j.cub.2011.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolde O, Rosel D, Vesely P, Folk P, Brabek J. The structure of invadopodia in a complex 3D environment. Eur J Cell Biol. 2010;89:674–680. doi: 10.1016/j.ejcb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Trimble WS, Grinstein S. Barriers to the free diffusion of proteins and lipids in the plasma membrane. The Journal of cell biology. 2015;208:259–271. doi: 10.1083/jcb.201410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma D, Keski-Oja J, Pei D. Co-recycling of MT1-MMP and MT3-MMP through the trans-Golgi network. Identification of DKV582 as a recycling signal. J Biol Chem. 2004;279:9331–9336. doi: 10.1074/jbc.M312369200. [DOI] [PubMed] [Google Scholar]
- Williams KC, Coppolino MG. Phosphorylation of membrane type 1-matrix metalloproteinase (MT1-MMP) and its vesicle-associated membrane protein 7 (VAMP7)-dependent trafficking facilitate cell invasion and migration. J Biol Chem. 2011;286:43405–43416. doi: 10.1074/jbc.M111.297069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Oikawa T. Membrane lipids in invadopodia and podosomes: key structures for cancer invasion and metastasis. Oncotarget. 2010;1:320–328. doi: 10.18632/oncotarget.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, Fukami K. Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer research. 2009;69:8594–8602. doi: 10.1158/0008-5472.CAN-09-2305. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Yoshida S, Muroi E, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K. Phosphatidylinositol 4,5-bisphosphate and PIP5-kinase Ialpha are required for invadopodia formation in human breast cancer cells. Cancer science. 2010;101:1632–1638. doi: 10.1111/j.1349-7006.2010.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zech T, McDonald L, Gonzalez EG, Li A, Macpherson I, Schwarz JP, Spence H, Futo K, Timpson P, Nixon C, Ma Y, Anton IM, Visegrady B, Insall RH, Oien K, Blyth K, Norman JC, Machesky LM. N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods. J Cell Biol. 2012;199:527–544. doi: 10.1083/jcb.201203025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambonin-Zallone A, Teti A, Carano A, Marchisio PC. The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J Bone Miner Res. 1988;3:517–523. doi: 10.1002/jbmr.5650030507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lateral-view time-lapses show 3D reconstruction of PI(4,5)P2 in wild-type anchor cells. PI(4,5)P2 is visualized with mCherry::PLCδPH (cyan); the basement membrane is visualized with laminin::GFP (magenta). Images were acquired using a spinning-disc confocal microscope (CSU-10 scan head; Yokogawa Corporation of America) mounted on an upright microscope (AxioImager; Carl Zeiss). 40-min time-lapses are shown with time points acquired every 60s. Projections of seven z-sections (step size of 1 μm) are shown. The video plays at 10 frames per second.
Lateral-view time-lapses show 3D reconstruction of PI(4,5)P2 in unc-60a RNAi-treated anchor cells. PI(4,5)P2 is visualized with mCherry::PLCδPH (cyan); the basement membrane is visualized with laminin::GFP (magenta). Images were acquired using a spinning-disc confocal microscope (CSU-10 scan head; Yokogawa Corporation of America) mounted on an upright microscope (AxioImager; Carl Zeiss). 40-min time-lapses are shown with time points acquired every 60 s. Projections of seven z-sections (step size of 1 μm) are shown. The video plays at 10 frames per second.
Lateral-view time-lapses show 3D reconstruction of PI(4,5)P2 in gdi-1 RNAi-treated anchor cells. PI(4,5)P2 is visualized with mCherry::PLCδPH (cyan); the basement membrane is visualized with laminin::GFP (magenta). Images were acquired using a spinning-disc confocal microscope (CSU-10 scan head; Yokogawa Corporation of America) mounted on an upright microscope (AxioImager; Carl Zeiss). 40-min time-lapses are shown with time points acquired every 60 s. Projections of seven z-sections (step size of 1 μm) are shown. The video plays at 10 frames per second.


