Abstract
Study Design Systematic review.
Clinical Questions (1) When used as an assistive device, do wearable exoskeletons improve lower extremity function or gait compared with knee-ankle-foot orthoses (KAFOs) in patients with complete or incomplete spinal cord injury? (2) When used as a rehabilitation device, do wearable exoskeletons improve lower extremity function or gait compared with other rehabilitation strategies in patients with complete or incomplete spinal cord injury? (3) When used as an assistive or rehabilitation device, are wearable exoskeletons safe compared with KAFO for assistance or other rehabilitation strategies for rehabilitation in patients with complete or incomplete spinal cord injury?
Methods PubMed, Cochrane, and Embase databases and reference lists of key articles were searched from database inception to May 2, 2016, to identify studies evaluating the effectiveness of wearable exoskeletons used as assistive or rehabilitative devices in patients with incomplete or complete spinal cord injury.
Results No comparison studies were found evaluating exoskeletons as an assistive device. Nine comparison studies (11 publications) evaluated the use of exoskeletons as a rehabilitative device. The 10-meter walk test velocity and Spinal Cord Independence Measure scores showed no difference in change from baseline among patients undergoing exoskeleton training compared with various comparator therapies. The remaining primary outcome measures of 6-minute walk test distance and Walking Index for Spinal Cord Injury I and II and Functional Independence Measure–Locomotor scores showed mixed results, with some studies indicating no difference in change from baseline between exoskeleton training and comparator therapies, some indicating benefit of exoskeleton over comparator therapies, and some indicating benefit of comparator therapies over exoskeleton.
Conclusion There is no data to compare locomotion assistance with exoskeleton versus conventional KAFOs. There is no consistent benefit from rehabilitation using an exoskeleton versus a variety of conventional methods in patients with chronic spinal cord injury. Trials comparing later-generation exoskeletons are needed.
Keywords: exoskeleton, robotics, rehabilitation, spinal cord injury
Introduction
According to the National Spinal Cord Injury Statistical Center, ∼282,000 persons live with spinal cord injury (SCI) in the United States, and ∼17,000 new SCI cases occur each year.1 SCIs have intense consequences, such as the loss of motor or sensory functions in the lower or upper limbs, depending on the level of injury. Therefore, the rehabilitation of locomotion has always been the key priority for patients suffering from SCIs.2
The greatest functional and neurologic recovery is to be expected during the first year after initial SCI; even with continuous and extensive rehabilitation, further improvements are usually not seen.3
Devices have been developed to assist patients suffering from SCI with mobility and to facilitate locomotion rehabilitation.4 One such device is a powered exoskeleton. Powered exoskeletons are motorized orthoses placed over a person's limb with joint parts corresponding to those of the human body. Their purpose is to facilitate standing and walking, as well as assist in rehabilitation.5
The first powered exoskeleton—the Lokomat (Hocoma, Switzerland)–was a residential (fixed) exoskeleton, also known as a driven gait orthosis.6 However, in recent years, innovative mobility devices have been developed, such as powered lower limb exoskeletons, that help individuals with low-level SCI walk as naturally as possible. Despite the similar frame structure, assistive and rehabilitative exoskeletons differ in their application and clinical objective. Assistive exoskeletons (e.g., Rex-Bionics [Auckland, New Zealand], Wearable Power-Assist Locomotor exoskeleton [WPAL; Fujita Health University, Japan], Re-Walk [Argo Medical Technologies Ltd, Yokneam Ilit, Israel]) allow patients to walk, and rehabilitative exoskeletons (e.g., Lokomat [Hocoma, Switzerland], Hybrid Assistive Limb [HAL; Cyberdyne, Inc., Tsukuba, Japan], Kinesis [Technaid, Madrid, Spain], and to some extent, Ekso-Bionics [Eksobionics Ltd, Richmond, California, USA]) focus on long-term gait improvement. Another difference is the control mechanism of the exoskeletons, such as joystick control (Rex-Bionics, Lokomat, WPAL, Kinesis), posture control (Re-Walk, Ekso-Bionics, Indego [Parker Hannifin Corp., Macedonia, Ohio, USA]), and electromyographic (EMG) control (HAL).
The aim of this systematic review is to determine if powered exoskeletons are effective as assistive and rehabilitation devices in improving locomotion in patients with SCI. We sought to answer the following clinical questions:
When used as an assistive device, do wearable exoskeletons improve lower extremity function or gait compared with knee-ankle-foot orthoses (KAFOs) in patients with complete or incomplete SCI?
When used as a rehabilitation device, do wearable exoskeletons improve lower extremity function or gait compared with other rehabilitation strategies in patients with complete or incomplete SCI?
When used as an assistive or rehabilitation device, are wearable exoskeletons safe compared with KAFOs for assistance or other rehabilitation strategies for rehabilitation in patients with complete or incomplete SCI?
Materials and Methods
Reporting of the methods and results follow the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for reporting systematic reviews.
Study design: Systematic review.
Information sources and search: PubMed, Cochrane, and Embase were searched for publications from database inception to May 2, 2016; bibliographies of included articles were also searched. The search strategy can be found in the online supplementary material.
Eligibility criteria: The inclusion criteria were as follows: (1) SCI resulting in a gait disorder; (2) age ≥18 and <75 years; and (3) randomized controlled trials. The exclusion criteria were as follows: (1) neurologic conditions other than SCI; (2) no neurologic gait disorder; (3) studies where the intervention is a robotic end-effector device (e.g., Gait Trainer GT1 [Reha-Stim Medtec GmbH & Co. KG, Berlin, Germany]); (4) studies measuring only upper extremity outcomes; and (5) studies measuring only physiologic or metabolic outcomes. A more detailed patient, intervention, comparator, and outcome table can be viewed in the online supplementary material.
Outcomes: Primary outcomes include gait outcomes (e.g., walking speed, 6-minute walk test [6MWT], 10-meter walking test [10MWT]), functional improvement (e.g., Functional Independence Measure [FIM], Spinal Cord Independence Measure [SCIM]), and safety (e.g., fracture, pain, cardiopulmonary episodes); secondary outcomes include neurologic improvement, motor strength, bladder and bowel function, spasticity, and requirement of walking aid. A summary table of the primary outcome measures can be found in the online supplementary material.
Data collection process and items: Data was extracted by a single individual and verified independently by a second using a pre-established data abstraction form. We attempted to contact authors of publications in cases where data needed confirmation or clarification. The following data items were sought: study design, time from SCI, injury level, study purpose, and rehabilitation treatment details.
Risk of bias evaluation: Randomized controlled trials were evaluated for risk of bias using criteria to judge articles on therapy. Crossover studies were evaluated for risk of bias using criteria outlined by Ding et al.7 These ratings are described in the online supplementary material.
Analysis and synthesis of results: When possible, the differences between groups' mean change in scores from baseline to follow-up were reported or calculated for continuous outcomes; otherwise, the mean or median differences between groups' scores at last follow-up were calculated.
Overall strength of evidence: We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) System to evaluate the quality of the evidence base for each key question. Details about this system can be found in the online supplementary material.
Results
Study Selection and Characteristics
From among 175 citations identified from our search, we excluded 155 after two individuals reviewed the titles and abstracts. We reviewed the full text of 20 articles and identified 9 randomized trials in 11 publications meeting the inclusion criteria (Fig. 1). Nine studies were excluded. A list of excluded articles and reason for exclusion can be found in the online supplementary material. A majority of the patients in the included studies were male with subacute or chronic incomplete SCI of cervical or thoracic etiology. Among the 11 studies identified, 10 utilized the robotic exoskeleton Lokomat,8 9 10 11 12 13 14 15 16 17 and the remaining study utilized the robotic exoskeleton MBZ-CPM1 (ManBuZhe [TianJin] Rehabilitation Equipment Co. Ltd., PR China).18 Nine of the included randomized trials were of parallel design, and two were of crossover design. Most studies were of moderately high risk of bias (Table 1).
Fig. 1.
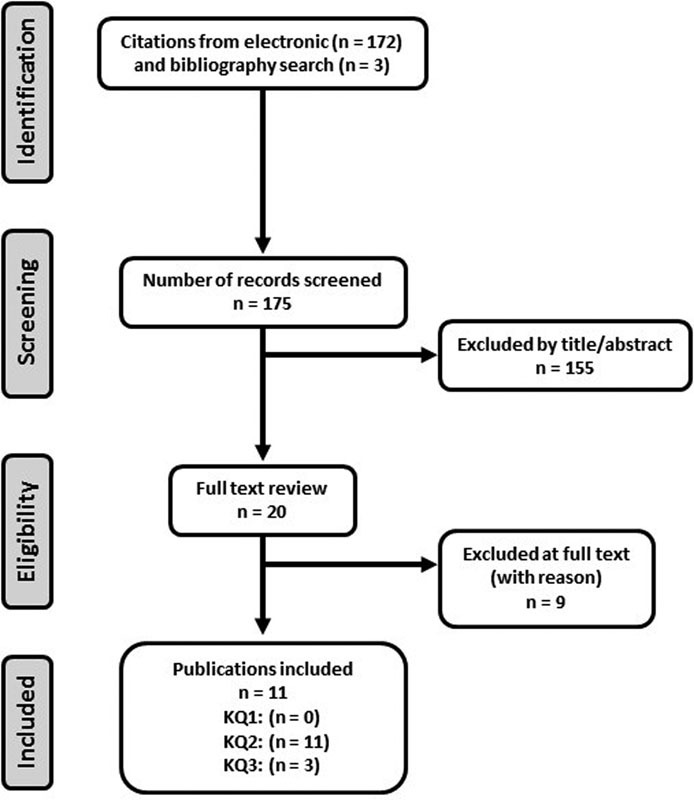
Flow diagram showing results of literature search. Abbreviation: KQ, key question.
Table 1. Spinal cord injury studies characteristics table.
| First author (year), study design | Patient characteristics | Study purpose | Inclusion and exclusion criteria | Intervention (A)a | Comparator (B) | Length of F/U; % F/U (n/N) | Risk of bias | Funding |
|---|---|---|---|---|---|---|---|---|
| Alcobendas-Maestro (2012),8 RCT | No. randomized A: 40 B: 40 Age (y) A: 45.2 ± 15.5 B: 49.5 ± 12.8 Males A: 77.5% B: 78.8% Months from SCI: 4.2 (median) Injury level Levels C1–C8 A: 59% B: 61% Levels T1–T6 A: 19% B: 13% Levels T7–T12 A: 22% B: 26% |
To compare a walking re-education program using exoskeleton to conventional OGT among individuals with iSCI of both traumatic and nontraumatic etiology | Inclusion: C2–T12 SCI; ASIA C or D; traumatic or nontraumatic, nonprogressive lesions; onset <6 mo; age 16–70 y; achieved assisted standing for minimum of 1 wk previously Exclusion: Unstable orthopedic injury; osteoporosis; lesions or ulcers where exoskeleton harness or straps are fitted; joint rigidity; leg length >2 cm; pulmonary heart disease; body weight >150 kg; previous SCI |
Standard physical treatment + robotic-assisted locomotor training via exoskeletonb (30 min) Training duration: 40 sessions/8 wk |
Standard physical treatment + OGT (60 min) Standard PT: joint mobilization, strengthening of supralesional musculature and remaining motor functions, muscle stretching and postural relaxation techniques, trunk stabilization, and practice of self-care skills |
8 wks of F/U; 93.8% (75/80) | Low | Grant from Fondo para la Investigación Sanitaria en Castilla la Mancha- AN/2006/27 |
| Benito-Penalva (2012),9 RCT | No. randomized A: 46 B: 84 Age (y)d A: 45 B: 45 Malesb A: 66.7% B: 68.1% Months from SCId <6 mo: 77.1% 6–12 mo: 7.6% >12 mo: 15.2% Injury level: NR |
To report the clinical improvements in patients with spinal cord injury associated with intensive gait training using electromechanical systems according to patient characteristics | Inclusion: motor incomplete (ASIA C or D), and select motor complete SCI (ASIA A or B) when voluntary movement was present at segments L2 and L3; 18+ y of age; able to tolerate the standing position without orthostasis Exclusion: cardiorespiratory instability, pressure ulcers that interfered with mechanical components of gait devices, spasticity (Ashworth Scale ≥3), severe contractures; weight > 115 kg |
Conventional therapy + robotic-assisted locomotor training via exoskeletonb (20–45 min) Training duration: 5 d a week, 8 wk |
Gait trainer GT 1 + conventional therapies Conventional therapy: details NR |
8 wks of F/U; 80.8% (105/130) | Moderately high | NR |
| Duffell (2015),10 RCT | No. randomized A: 27 B: 29 C: 27 Age (y) A: 46.6 ± 12.6 B: 47.8 ± 13.1 C: 47.4 ± 11.6 Males A: 70.3% B: 65.5% C: 70.3% Months from SCI: 113 ± 111 (mean ± SD) Level of injury: above T10: 100% |
To investigate the effects of locomotor treadmill training and tizanidine on gait impairment in people with incomplete motor SCI, and to understand whether functional levels affect recovery with different interventions | Inclusion: age 18–50 y, motor incomplete SCI (ASIA C or D); level of injury above T10; >12 mo postinjury; able to ambulate; medical clearance; spasticity in the ankle (Modified Ashworth Score ≥1); lower-limb PROM within limits for ambulation Exclusion: sitting tolerance <2 h, existing infection, severe cardiopulmonary disease, concomitant neurologic injury, fractures post-SCI, orthopedic or peripheral nerve injury in the LL |
Robotic-assisted locomotor training via exoskeletonb (30–45 min) Treatment duration: 3 times a week for 4 wk |
B: no training or C: tizanidine (0.03 mg/kg per dose) Treatment duration: 4 times a day for 4 wk |
4 wk; A versus B F/U: 95.4% (54/56), A versus C F/U: 98.1% (53/54) | Moderately high | Supported by the National Institutes of Health and the Craig H. Neilsen Foundation awards to M.M.M. |
| Esclarín-Ruz (2014),11 RCT | No. randomized UMN A: 22 B: 22 LMN A: 22 B: 22 Age (y) UMN A: 43.6 ± 12 B: 44.9 ± 7 LMN A: 36.4 ± 12 B: 42.7 ± 18 Males UMN A: 71.4% B: 61.9% LMN A: 70% B: 80.9% Months from SCI: (mean ± SD) UMN: 4.4 ± 1.8 LMN: 3.6 ± 1.3 Level of injury UMN > Levels C1–C8 A: 57.1% B: 57.1% > Levels T1–6 A: 19% B: 19% > Levels T7–11 A: 24% B: 24% > LMN > Levels T12–L1 A: 67% B: 76% > Levels L2–L3 A: 33% B: 24% |
To compare a walking re-education program with robotic locomotor training plus OGT to conventional OGT in individuals with incomplete UMN or LMN injuries having either traumatic or nontraumatic nonprogressive etiology | Inclusion: C2–T11 SCI ASIA C or D; with only UMN findings; T12–L3 SCI ASIA C or D with only LMN; traumatic and nontraumatic, nonprogressive lesions; onset < 6 mo; age 16–70 y; achieved assisted standing a minimum of 1 wk previously Exclusion: orthopedic injuries that are unstable; osteoporosis with high risk of pathologic fracture; cutaneous lesions and/or pressure ulcers in areas in which the exoskeleton harness or thigh straps are fitted; joint rigidity; asymmetry of lower extremity length > 2 cm; pulmonary or heart disease requiring monitoring during exercise; body weight > 150 kg; history of spinal injury |
Standard physical treatment (30 min) + OGT + robotic-assisted locomotor training via exoskeletonb (30 min) Training duration: daily sessions for a total of 40 sessions over 8 wk |
Conventional OGT (30 min) + standard physical treatment (30 min) Standard physical treatment |
8 wk of F/U (no training): UMN: 95.4% (42/44), LMN: 93.2% (41/44) |
Low | Supported by the Founding for Research of Castilla La Mancha (grant no. PI 2006-45) |
| Field-Fote (2011),12 RCT | No. randomized A: 15 B: 19 C: 22 D: 18 Age (y)d A: 45 ± 8 B: 39.3 ± 14.6 C: 38.5 ± 12.7 D: 42.2 ± 15.7 Malesd A: 85.7% B: 82.3% C: 77.8% D: 73.3% Months from SCId: ≥12: 100% Injury level: above T11: 100% |
The objective of this study was to compare changes in walking speed and distance associated with 4 locomotor training approaches | Inclusion: chronic (≥1 y) SCI; ASIA C or D at or above T10; ability to take at least 1 step with 1 leg; ability to rise to a standing position with moderate assistance (50% effort) from 1 other person Exclusion: current orthopedic problems, history of cardiac condition, or radiographic evidence of hip pathology that could be aggravated by training |
A: robotic-assisted locomotor training via exoskeletonb (60 min) Treatment duration: 5 d/wk for 12 wk; mean number of training sessions completed was 49 ± 7 (range, 27–58) |
B: treadmill-based training with manual assistance (60 min) C: treadmill-based training with stimulation to common peroneal n (60 min) D: OGT with stimulation to common peroneal n (60 min) Manual assistance through step phase |
4 wk of training, 6 mo F/U; 86% (64/74) | Moderately high | Funding provided by National Institutes of Health grant R01HD41487 (to Dr. Field-Fote) and The Miami Project to Cure Paralysis |
| Gorman (2016),13 crossover RCT | No. randomized A: 27 B: 27 In final analysis A: 12 B: 6 Age (y) A: 51.5 ± 12.7 B: 52 ± 15.4 Males A: NR B: NR Months from SCI: ≥12: 100% Level of injury: C4–L2 A: 100% B: 100% |
To assess the effectiveness of robotically assisted BWSTT for improving cardiovascular fitness in chronic motor iSCI | Inclusion: traumatic SCI ≥ 1 y prior to enrollment, age 18–80 y, injury between C4–L2, AISA C or D, able stand at least 30 min Exclusion: uncontrolled hypertension, unstable angina, CHF, COPD, symptomatic PAD, or recent hospitalization (within 3 mo) for medical problem; severe contractures or frequent uncontrolled bouts of autonomic dysreflexia; BMD T-score ≤ − 3.5 |
Robotic-assisted locomotor training via exoskeletonb (20–45 min) Treatment duration: 3 times a week for 3 mo |
Home stretching program (20–25 min) | 3 or 6 moc; 67% (18/27) | Moderately high | Funded by Department of Veterans Affairs Rehabilitation R&D Service Merit Review Award B4027I; study registered at clinicaltrials.gov with the identifier number NCT00385918 |
| Hornby (2005),14 RCT | No. randomized A: NR B: NR C: NR Total: 35 Age (y): NR Males: NR Months from SCI: 0.5–0.9 (range) Level of injury: above T10: 100% |
To study the effects of robotic-assisted BWSTT on individuals with subacute SCI | Inclusion: traumatic or ischemic SCI above T10; injury within 14–180 d; ASIA B, C, or D; required physical assistance from at least one PT to ambulate over ground Exclusion: secondary neurologic injuries, including traumatic brain injury or peripheral nerve damage |
A: robotic-assisted locomotor training via exoskeletonb (30 min) Treatment duration: 3 sessions/wk |
B: Therapist-assisted BWSTT (30 min) C: OGT with mobile suspension system (30 min) |
8 wk training and F/U; 85.7% (30/35) | Moderately high | NR |
| Huang (2015),18 RCT | No. randomized A: 12 B: 12 Age (y) A: 41.7 ± 3.3 B: 38.4 ± 2.3 Males A: 75% B: 58.3% Months from SCI: NR Level of injury: levels T8–L2 A: 100% B: 100% |
To compare the effects of BWSTT and robot-assisted rehabilitation on bowel function in patients with spinal cord injury with respect to defecation time and defecation drug dose (enema) | Inclusion: NR Exclusion: NR |
Robot-assisted locomotor training via exoskeletone + manual therapy (20 min) Treatment duration: 4 times a week for 4 wk |
BWSTT + manual therapy + standard rehabilitation training (20 min) | 4 wk; % F/U NR | Moderately high | Research on Design Theory and Compliant Control for Underactuated Lower-Extremity Rehabilitation Robotic Systems, Code 51175368 |
| Labruyère (2014),15 crossover RCT | No. randomized A: 5 B: 4 Age (y) A: 59 ± 11 B: 59 ± 11 Males A: 55.6% B: 55.6% Months from SCI: 50 ± 56 (mean ± SD) Level of injury Cervical A: 56% B: 56% T1–T6 A: 11% B: 11% T7–T12 A: 33% B: 33% |
To compare changes in a broad spectrum of walking-related outcome measures and pain between robot-assisted gait training and strength training in patients with chronic iSCI, who needed walking assistance | Inclusion: age 18–70 y, chronic iSCI > 1 y; sensorimotor incomplete ASIA C or D; motor level of the lesion C4–T11; walk with at most moderate assistance; cognitive capacity to follow verbal instructions Exclusion: contraindications for training in the exoskeleton system, had injuries limiting training, as well as orthopedic, psychiatric or neurologic diseases, except for the iSCI |
Robot-assisted locomotor training via exoskeletonb (45 min) Treatment duration: 32 total sessions over 8 wk, 16 sessions of each treatment |
Strength training (45 min) | 4 wks of each intervention for a total of 8 wk of training, F/U to 6 mo; 100% (20/20) | Moderately low | International Spinal Research Trust (Clinical Initiative Stage 2, London, UK; grant number CLI06), the Henry Smith Charity (London, UK), and the EMDO Foundation (Zurich, Switzerland) |
| Niu (2014),16 RCT | No. randomized A: 20 B: 20 Age (y) A: 42.2 ± 12.6 B: 49.7 ± 7 Males A: 65% B: 70% Months from SCI: 8.2 ± 7.7 (mean ± SD) Level of injury: above T10: 100% |
To characterize the distinct recovery patterns of gait impairment for SCI subjects receiving exoskeleton training, and to identify significant predictors for these patterns | Inclusion: NR Exclusion: NR |
Robotic-assisted locomotor training via exoskeletonb (45 min) Treatment duration: 3 times a week for 4 wk |
No interventions | 4 wk; F/U NR | Moderately high | National Institutes of Health (R01HD059895]) and the Craig H. Neilsen Foundation awarded to M.M.M. |
| Shin (2014),17 RCT | No. randomized A: 30 B: 30 Age (y) A: 43.2 ± 14.4 B: 48.2 ± 11.5 Males A: 74.1% B: 53.8% Months from SCI: 3.0 ± 2.0 (mean ± SD) Level of injury Cervical A: 51.9% B: 61.5% Thoracic/lumbar A: 48.1% B: 38.5% |
To determine the effect of robotic-assisted gait training compared with OGT | Inclusion: nonprogressive traumatic or nontraumatic SCI, onset <6 mo, ASIA D, age 20–65 y Exclusion: pressure ulcers, severe limitation of ROM of hips and knees, severe cognitive impairment, cardiopulmonary disease requiring monitoring during exercise, LMN lesion, previously undergone robot assisted gait training |
Regular PT (60 min) + robotic-assisted locomotor training via exoskeletonb (40 min) Treatment duration: 1 exoskeleton + 1 conventional treatment session/d, 3 times a week for 4 wk; 2 conventional treatment sessions/d, 2 times a week for 4 wk |
Conventional OGT + regular PT (60 min) Treatment duration: twice a day, 5 times a week for 4 wk |
4 wks; 88.3% (53/60) | Moderately high | NR |
Abbreviations: ASIA, American Spinal Injury Association; BMD, bone mineral density; BWSTT, body weight-supported treadmill training; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; F/U, follow-up; iSCI, incomplete spinal cord injury; LL, lower limb; LMN, lower motor neuron; NR, not reported; OGT, overground training; PAD, peripheral artery disease; PT, physical therapy; RCT, randomized controlled trial; ROM, range of motion; SCI, spinal cord injury; SD, standard deviation; UMN, upper motor neuron.
Unless otherwise noted, training duration is the same for intervention and comparator.
Lokomat (Hocoma, Switzerland).
Three months of follow-up for the robotic group, 6 months of follow-up for control group; the control group crossed over to exoskeleton training after 3 months of home stretching.
Data only for those with data at final analysis.
MBZ-CPM1 (ManBuZhe [TianJin] Rehabilitation Equipment Co. Ltd., PR China).
Efficacy of Wearable Exoskeletons as an Assistive Device
No studies were identified comparing the efficacy of wearable exoskeletons as an assistive device to standard KAFOs in patients with complete or incomplete SCI.
Efficacy of Wearable Exoskeletons as a Rehabilitation Device
Ten-Meter Walk Test
Six randomized controlled trials in eight publications evaluated walking velocity using the 10MWT comparing exoskeleton training to a control group with follow-up ranging from 4 to 12 weeks (Table 2); two measured fast walking velocity,10 12 one self-selected walking velocity,9 one measured both fast and self-selected walking,15 and two did not report the velocity pace for the test.8 14
Table 2. Differences between groups for primary outcomes at various follow-up periodsa .
| First author (year), F/U, and groups | 10MWT (m/s) | 6MWT (m) | WISCI II (0–20 [best]) | FIM-L (2–14 [best]) | SCIM (0–100 [best]) |
|---|---|---|---|---|---|
| Alcobendas-Maestro (2012),8 8 wk A: exoskeleton + PT B: OGT + PT |
Median (IQR) at 8 wk Velocity unclear A: 0.40 (0.20, 0.60) B: 0.30 (0.20, 0.50) p = NS |
Median (IQR) at 8 wk A: 169 (69, 228) B: 91 (51, 179) p < 0.05 |
Median (IQR) at 8 wk A: 16 (9, 19) B: 9 (8, 16) p < 0.05 |
Median (IQR) at 8 wkb
A: 10 (6, 12) B: 7 (5, 10) p < 0.05 |
NR |
| Benito-Penalva (2012),9 8 wk A: exoskeleton + CT B: Gait Trainer GT1 + CT |
Mean Δ from baseline Self-selected velocity A: 0.19 ± 0.03 B: 0.18 ± 0.18 Mean diff (95% CI): A versus B: 0.0 (−0.04, 0.05) |
NR | Mean Δ from baseline A: 5.3 ± 0.7 B: 5.1 ± 0.5 Mean diff (95% CI): A versus B: 0.2 (−0.1, 0.4) |
NR | NR |
| Duffell (2015),10 4 wk A: exoskeleton B: no training C: tizanidine |
% attained MIDc
Fast velocity A: 15% (4/26) B: 23% (6/26) C: 8% (2/25) |
% attained MIDc
A: 8% (2/24) B: 13% (3/23) C: 12% (3/26) |
NR | NR | NR |
| Esclarín-Ruz (2014),11 8 wkd
A: exoskeleton + PT B: OGT + PT |
Mean Δ from baseline Self-selected velocity UMN A: 0.06 ± 0.19 B: 0.03 ± 0.19 LMN A: 0.22 ± 0.17 B: 0.17 ± 0.25 Mean diff (95% CI) UMN, A versus B: 0.03 (−0.10, 0.16) LMN, A versus B: 0.05 (−0.09, 0.19) |
Mean Δ from baseline UMN A: 65.2 ± 70.8 B: 26.1 ± 56.6 LMN A: 74.8 ± 59.7 B: 51.3 ± 79.1 Mean diff (95% CI) UMN, A versus B: 39.1 (−7.4, 85.6) LMN, A versus B: 23.5 (−23.1, 70.2) |
Mean Δ from baseline UMN A: 7.6 ± 3.4 B: 6.1 ± 3.1 LMN A: 6.5 ± 2.5 B: 5.8 ± 2.7 Mean diff (95% CI) UMN, A versus B: 1.5 (−0.5, 3.4) LMN, A versus B: 0.7 (−0.95, 2.25) |
Mean Δ from baseline UMN A: 3.9 ± 1.8 B: 2.1 ± 1.8 LMN A: 2.9 ± 1.8 B: 3.7 ± 1.7 Mean diff (95% CI) UMN, A versus B: 1.8 (0.8, 2.9) LMN, A versus B: −0.8 (−1.8, 0.3) |
NR |
| Field-Fote (2011),12 12 wk A: exoskeleton B: TT with manual assist C: TT with stimulation D: OGT with stimulation |
Mean Δ from baseline Fast velocity A: 0.01 ± 0.06 B: 0.05 ± 0.10 C: 0.05 ± 0.11 D: 0.09 ± 0.17 Mean diff (95% CI) A versus B: −0.04 (−0.10, 0.02) A versus C: −0.04 (−0.10, 0.02) A versus D: −0.08 (−0.17, 0.01) |
NR | NR | NR | NR |
| Hornby (2005),14 8 wk A: exoskeleton B: therapist-assist BWSTT C: OGT |
No significant differences | No significant differences | Mean Δ from baselinee
f
A: 5.8 ± 2.0 B: 5.2 ± 2.0 C: 6.5 ± 2.2 Mean diff at last F/U (95% CI)e A versus B: 0.6 (−1.2, 2.4) A versus C: −0.72 (−2.6, 1.1) |
Mean Δ from baselinef
A: 1.8 ± 0.9 B: 1.9 ± 0.6 C: 2.0 ± 0.5 Mean diff at last F/U (95% CI) A versus B: −0.1 (−0.9, 0.6) A versus C: −0.2 (−0.9, 0.4) |
NR |
| Labruyère (2014),15 8 wk A: exoskeleton B: strength training |
Mean Δ from baseline Self-selected velocity A: 0.04 (SD NR) B: 0.06 (SD NR) Mean diff (95% CI)g: A versus B: 0.02 (–0.04, 0.08), p = NS Mean Δ from baseline Fast velocity A: 0.01 (SD NR) B: 0.14 (SD NR) Mean diff (95% CI)f: A versus B: 0.13 (0.01, 0.25) p = 0.04 |
NR | Mean Δ from baseline A: 0.8 (SD NR) B: 0.4 (SD NR) Mean diff (95% CI)g: A versus B: −0.4 (−1.5, 0.6) p = NS |
NR | Mean Δ from baseline A: 0.8 (SD NR) B: 1.3 (SD NR) Mean diff (95% CI): A versus B: 0.5 (−2.1, 3.2) p = NS |
| Niu (2014),16 4 wkh
i
A: exoskeleton B: no interventions |
Mean Δ from baseline, class Ij
Velocity unclear A: 0.03 ± 0.01 B: 0.01 ± 0.15 Mean diff (95% CI): class I: 0.02 (–0.08, 0.12) Mean Δ from baseline, class IIj Velocity unclear A: 0.12 ± 0.32 B: 0.02 ± 0.13 Mean diff (95% CI): class II: 0.10 (−0.10, 0.29) |
Diff between groups' rate of change over 4 wk (m/wk) Class I: 0.8, p = NS Class II: 2.3, p = NS |
NR | NR | NR |
| Shin (2014),17 4 wk A: exoskeleton + PT B: OGT + PT |
NR | NR | Median (IQR) at 4 wk A: 11 (0, 19) B: 9 (0, 20) p = 0.01 |
NR | Median (IQR) at 4 wk A: 10 (0, 26) B: 9 (0, 33) p = NS |
Abbreviations: 6MWT, 6-minute walk test; 10MWT, 10-meter walk test; BWSTT, body weight-supported treadmill training; CI, confidence interval; CT, conventional training; diff, difference; FIM-L, Functional Independence Measure–Leg; F/U, follow-up; IQR, interquartile range; LMN, lower motor neuron; MID, minimally important difference; NR, not reported; NS, not significant; OGT, overground training; PT, physical therapy; SCIM, Spinal Cord Independence Measure; SD, standard deviation; TT, treadmill training; UMN, upper motor neuron; WISCI II, Walking Index for Spinal Cord Injury II.
Unless otherwise indicated, all values are reported as mean ± SD.
FIM-L evaluated for only walking and stair tasks.
The MID was defined using control group data; this was calculated using the following formula: (1.96 × √2 × standard error of the mean). Participants who achieved a change from baseline equal to or greater than the MID for that test, after 4 weeks of training, were classified as “MID achieved.”
Authors reported WISCI scores (1–20 [best]).
These values were estimated from Fig. 4.
The 95% CIs were estimated from Fig. 4, point estimates were calculated using values in Table 2.
Class I (“low walking capacity class”) included subjects with longer 10MWT and Timed-Up-and-Go (TUG) test times, and shorter 6MWT distances at baseline; class II (“high walking capacity class”) included subjects with shorter 10MWT and TUG test times and longer 6MWT distances.
Values on 10MWT were estimated from Fig. 1.
In general, patients improved slightly with training. However, there were no differences in change from baseline among patients undergoing exoskeleton training compared with treadmill-based training with manual assistance,12 treadmill-based training with electrical stimulation,12 overground ambulation training with or without electrical stimulation,8 12 14 therapist-assisted body weight-supported treadmill training (BWSTT),14 no training,10 treatment with tizanidine (dose = 0.03 mg/kg),10 or the Gait Trainer GT1 with conventional therapy.9 One crossover trial found that fast walking velocity improved significantly more after strength training versus exoskeleton training.15
A subgroup analysis of Duffell et al comparing exoskeleton training to no intervention found that patients classified as high walking capacity at baseline (high 10MWT velocity and Timed-Up-and-Go [TUG] test times; high 6MWT distance) had more improvement in 10MWT times with exoskeleton rehabilitation compared with no intervention at 4 weeks of follow-up; patients with baseline low walking capacity (low 10MWT velocity and TUG test times; low 6MWT distance) showed no difference for either treatment (p for interaction = 0.02).10 16 A subgroup analysis of Alcobendas-Maestro et al comparing exoskeleton training with standard physical treatment to conventional overground training with standard physical treatment found that patient baseline upper or lower motor neuron injury did not modify treatment effect for 10MWT velocity at 8 weeks of follow-up.8 11
6-Minute Walk Test
Three trials in five publications assessed endurance using the 6MWT at 4 or 8 weeks of follow-up. Two found no difference in 6MWT distance among patients undergoing exoskeleton training compared with therapist-assisted BWSTT,14 overground ambulation,14 no training,10 or tizanidine.10 One trial reported a statistically significant median distance following 8 weeks of training in favor of the exoskeleton group compared with overground training plus standard physical therapy.8 With the data provided, it is unclear whether this difference is functionally meaningful.
A subgroup analysis of Duffell et al comparing exoskeleton training with no intervention found that baseline low or high walking capacity did not modify the treatment effect for 6MWT distance at 4 weeks of follow-up.10 16 A subgroup analysis of Alcobendas-Maestro et al comparing exoskeleton training with standard physical treatment to conventional overground training with standard physical treatment found that baseline upper versus lower motor neuron injury did not modify treatment effect for 6MWT distance at 8 weeks of follow-up.8 11
Walking Index for Spinal Cord Injury I and II
Five studies in six publications evaluated the level of walking impairment via Walking Index for Spinal Cord Injury I (WISCI) or II (WISCI II) scores at 4 or 8 weeks of follow-up. One trial found no difference in WISCI scores among patients undergoing exoskeleton training versus therapist-assisted BWSTT or overground ambulation.14 Another found no difference in WISCI II scores among patients undergoing exoskeleton training with conventional therapy versus patients undergoing Gait Trainer GT1 training in conjunction with conventional therapy.9 A third study—a crossover trial—found no differences in WISCI scores comparing exoskeleton training to strength training.15 Two studies found that exoskeleton training in conjunction with regular physiotherapy or standard physical treatment significantly improved WISCI II scores more than conventional overground training in conjunction with regular physiotherapy or standard physical treatment; median scores were 11 and 16 in the exoskeleton group plus physical therapy groups compared with 9 and 9 in the overground training plus physical therapy groups.8 17
A subgroup analysis of Alcobendas-Maestro et al comparing exoskeleton training with standard physical treatment to conventional overground training with standard physical treatment found that baseline upper versus lower motor neuron injury did not modify treatment effect for WISCI II at 8 weeks of follow-up.8 11
Functional Independence Measure–Locomotor
Two trials in three publications assessed locomotive function via Functional Independence Measure–Locomotor (FIM-L) scores at 8 weeks of follow-up. One trial found no difference in FIM-L scores among patients undergoing exoskeleton training, therapist-assisted BWSTT, or overground ambulation training,14 but another trial found that patients undergoing exoskeleton training in conjunction with standard physical treatment significantly improved their FIM-L scores compared with patients undergoing conventional overground training in conjunction with standard physical treatment; median scores at last follow-up were 10 (interquartile range [IQR]: 6 to 12) compared with 7 (IQR: 5 to 10), respectively (p < 0.05).8
A subgroup analysis of Alcobendas-Maestro et al found that patients with upper motor neuron injuries had improved FIM-L scores from treatment with exoskeleton therapy with standard physical treatment compared with conventional overground training with standard physical treatment at 8 weeks of follow-up; patients with lower motor neuron injuries at baseline had no improvement with either treatment (p for interaction = 0.013).8 11
Spinal Cord Independence Measure
Two trials evaluated independence via the SCIM at 4 or 8 weeks of follow-up. There was no difference in SCIM scores between exoskeleton training with regular physiotherapy and conventional overground training with regular physiotherapy at 4 weeks of follow-up in one trial.17 In the second trial, there was no difference between exoskeleton training versus strength training in a crossover design after 4 and 8 weeks.15
Lower Extremity Motor Score
Five trials in six publications evaluated lower extremity motor function via the lower extremity motor score (LEMS) at 4 or 8 weeks of follow-up (Table 3). The results were mixed. One trial found no difference in LEMS between exoskeleton training with regular physiotherapy or conventional overground training with regular physiotherapy.17 A second trial reported that use of the end-effector exoskeleton Gait Trainer GT1 in conjunction with conventional therapy resulted in a greater improvement in the LEMS compared with exoskeleton training in conjunction with conventional therapy; the difference between the mean change scores was –2.17 (95% confidence interval [CI], –2.6 to –1.74).9 However, these results are in contrast to a third trial, which reported exoskeleton training with regular physiotherapy significantly improved LEMS compared with conventional overground training with standard physical treatment; the median score was 40 (IQR: 30 to 45.5) compared with 35 (IQR: 29.7 to 40; p < 0.05).8 Two crossover trials reported no difference between exoskeleton training and strengthening exercises or home stretching exercises.13 15
Table 3. Differences between groups for secondary outcomes at various follow-up periodsa .
| First author (year), follow-up, and groups | Score at follow-up | Difference between treatments |
|---|---|---|
| LEMS 0–50 (best) | ||
| Alcobendas-Maestro (2012),8 8 wk A: exoskeleton + PT B: OGT + PT |
Median (IQR) at 8 wk A: 40 (35, 45.5) B: 35 (29.7, 40) p < 0.05 |
|
| Benito-Penalva (2012),9 8 wk A: exoskeleton + CT B: Gait Trainer GT1 + CT |
Mean Δ from baseline A: 7.1 ± 1.2 B: 9.3 ± 0.9 |
Mean diff (95% CI), A versus B: −2.2 (−2.6, −1.7) |
| Esclarín-Ruz (2014),11 8 wkb
A: exoskeleton + PT B: OGT + PT |
UMN, mean Δ from baseline A: 8.33 ± 6.64 B: 5.28 ± 6.94 LMN, mean Δ from baseline A: 6.15 ± 6.81 B: 2.57 ± 6.6 |
UMN, mean diff (95% CI), A versus B: 3.05(−1.06, 7.16) LMN, mean diff (95% CI), A versus B: 3.58 (−0.53, 7.69) |
| Field-Fote (2011),12 12 wk A: exoskeleton B: TT with manual assist C: TT with stimulation D: OGT with stimulation |
Mean Δ from baseline, left legc
A: 1.2 ± 4.3 B: 1.7 ± 3.5 C: 1.2 ± 3.8 D: 1.1 ± 4.4 |
Mean diff (95% CI), left legc
A versus B: −0.5 (−3.28, 2.28) A versus C: 0.0 (−2.84, 2.84) A versus D: 0.1 (−3.06, 3.26) |
| Gorman (2016),13 3 or 6 mo A: exoskeleton B: home stretching program |
Mean Δ from baselined
A: 0.3 ± 7.06 B: −0.4 ± 5.18 |
Mean diff (95% CI), A versus B: 0.7 (−5.05, 6.45) |
| Hornby (2005),14 8 wk A: exoskeleton B: therapist-assist BWSTT C: OGT |
Mean Δ from baselinee
A: 12.8 ± 2.4 B: 12.6 ± 4 C: 10.8 ± 2.4 |
Mean diff at last F/U (95% CI) A versus B: 0.2 (−2.7, 3.09) A versus C: 2.0 (−0.1, 4.1) |
| Labruyère (2014),15 8 wk A: exoskeleton B: strength training |
Mean Δ from baseline A: 0.7 (SD NR) B: 1 (SD NR) |
Mean diff (95% CI),f A versus B: 0.3 (−0.9, 1.55) p = NS |
| Shin (2014),17 4 wk A: exoskeleton + PT B: OGT + PT |
Median (IQR) at 4 wk A: 37 (20, 49) B: 37 (20, 48) p = NS |
|
| Bowel function | ||
| Huang (2015),18 4 wk A: exoskeleton + manual therapy B: BWSTT + manual therapy + standard rehabilitation |
Defecation time (min), mean Δ from baseline A: −28.5 ± 8.8 B: −14.5 ± 9.8 Enema volume (mL), mean Δ from baseline A: −29.3 ± 7.5 B: −10.8 ± 8.7 |
Mean diff (95% CI): −14 (−21.4, −6.6) Mean diff (95% CI): −18.5 (−24.9, −12.02) |
Abbreviations: BWSTT, body weight-supported treadmill training; CI, confidence interval; CT, conventional training; diff, difference; F/U, follow-up; IQR, interquartile range; LEMS, lower extremity motor score; LMN, lower motor neuron; NR, not reported; NS, not significant; OGT, overground training; PT, physical therapy; TT, treadmill training; UMN, upper motor neuron; SD, standard deviation.
Unless otherwise indicated, all values are reported as mean ± SD.
Right leg lower extremity motor score data also reported, not statistically different from left leg lower extremity motor score data.
It is unclear if authors evaluated the control group after the initial control treatment (12 weeks) or if they were evaluated after crossing over to robotically assisted body weight-supported treadmill training (24 weeks).
These values were estimated from Fig. 4.
The 95% CIs were estimated from Fig. 4, point estimates were calculated using values in Table 2.
A subgroup analysis of Alcobendas-Maestro et al comparing exoskeleton training with standard physical treatment to conventional overground training with standard physical treatment found that baseline upper versus lower motor neuron injury did not modify treatment effect for LEMS at 8 weeks of follow-up.8 11
Defecation Measures
A single trial reported defecation measures via defecation time and enema volume at 4 weeks of follow-up (Table 3). Exoskeleton training in conjunction with manual therapy resulted in better defecation times and enema volumes compared with BWSTT in conjunction with manual therapy and standard rehabilitation; the defecation time difference between mean change scores was –14.0 minutes (95% CI, –21.4 minutes to –6.6 minutes), and the enema volume difference between mean change scores was –18.5 mL (95% CI, –24.9 mL to –12.0 mL).18
Safety of Wearable Exoskeletons
Adverse events were rare. Two trials reported no adverse events at 8 weeks' follow-up among patients receiving exoskeleton training with conventional therapy,9 exoskeleton training alone,15 Gait Trainer GT1 training with conventional therapy at 8 weeks' follow-up,9 or strength training.15 One randomized, crossover trial with follow-up at 3 and 6 months found that skin irritation or abrasion occurred in 11% (2/18) of subjects completing the trial.13 The sites affected included the hip, groin, penis, back, wrist, glutei, and scapula. The authors indicated that this issue was resolved by adding additional padding to the parachute harness setup. The remaining included trials made no explicit mention of adverse events.
Evidence Summary
Exoskeletons as Assistive Devices
There were no studies comparing exoskeletons as assistive devices to currently used orthotics.
Exoskeletons as Rehabilitation Devices
There were no differences in the 10MWT and 6MWT comparing exoskeleton versus non-exoskeleton rehabilitation in patients with SCI. There were mixed results for the WISCI/WISCI II and FIM-L; the majority of studies found no difference between rehabilitation strategies with a few studies reporting significantly improved scores in the exoskeleton group. Patients with a high walking capacity at baseline (high 10MWT velocity and TUG test times; high 6MWT distance) had more improvement in 10MWT but not the 6MWT with exoskeletal rehabilitation compared with non-exoskeleton rehabilitation, and patients classified as low walking capacity showed no difference between treatments. The overall strength of evidence for these findings is low or very low (Table 4).
Table 4. Quality of evidence evaluating exoskeletons as assistive or rehabilitative devices in incomplete or complete SCI.
| Outcome | Follow-up | Studies (N) | Serious risk of bias | Serious inconsistency | Serious indirectness | Serious imprecision | Conclusions | Quality |
|---|---|---|---|---|---|---|---|---|
| Key question 1: exoskeletons as assistive devices | ||||||||
| No comparisons available | No data | |||||||
| Key question 2: exoskeletons as rehabilitative devices | ||||||||
| Exoskeleton versus nonexoskeleton rehabilitation strategiesa | ||||||||
| 10MWT | 8 to 12 wk | 5 RCTs8 9 12 14 15 (N = 319) | Yes (−1) | No | No | Yes (−1) | No significant difference between groups except for 1 high risk of bias crossover study that reported strength training improved speed more than exoskeleton Subgroup analyses: patients with higher versus lower baseline walking capacityb improved more with exoskeleton versus non-exoskeleton rehabilitation; LMN versus UMN did not modify the effect of exoskeleton rehabilitation |
Low |
| 6MWT | 8 wk | 2 RCTs8 14 (N = 115) | No | Yes (−1) | No | Yes (−1) | No significant difference between groups Subgroup analyses: neither higher versus lower baseline walking capacityb nor LMN versus UMN modified the effect of exoskeleton rehabilitation |
Low |
| WISCI/WISCI II | 4 to 8 wk | 5 RCTs8 9 14 15 17 (N = 314) | Yes (−1) | Yes (−1) | No | Yes (−1) | Mixed results; a majority of included studies (3/5) found no statistically significant mean difference in scores between exoskeleton and non-exoskeleton rehabilitation; two studies found that median scores were significantly improved in exoskeleton groups Subgroup analyses: LMN versus UMN did not modify the effect of exoskeleton rehabilitation |
Very low |
| FIM-L | 8 wk | 2 RCTs8 14 (N = 115) | No | Yes (−1) | No | Yes (−1) | Mixed results; one study found no significant difference in mean FIM-L scores between exoskeleton and non-exoskeleton rehabilitation groups, and the other study found that median FIM-L scores were significantly improved in the exoskeleton group Subgroup analyses: patients with UMN versus LMN improved more with exoskeleton versus non-exoskeleton rehabilitation |
Low |
| SCIM | 4 wk | 2 RCTs15 17 (N = 69) | Yes (−1) | No | No | Yes (−1) | No significant difference between groups | Low |
| Exoskeleton versus tizanidine | ||||||||
| 10MWT, Fast velocity | 4 wk | 1 RCT10 (N = 83) | Yes (−1) | Unknown | No | Yes (−1) | No significant difference between groups | Low |
| 6MWT | 4 wk | 1 RCT10 (N = 83) | Yes (−1) | Unknown | No | Yes (−1) | No significant difference between groups | Low |
| Exoskeleton versus no training | ||||||||
| 10MWT, Fast velocity | 4 wk | 1 RCT10 (N = 83) | Yes (−1) | Unknown | No | Yes (−1) | No significant difference between groups | Low |
| 6MWT | 4 wk | 1 RCT10 (N = 83) | Yes (−1) | Unknown | No | Yes (−1) | No significant difference between groups | Low |
| Key question 3: safety of exoskeletons as assistive or rehabilitative devices | ||||||||
| Exoskeletons as rehabilitative devices | ||||||||
| Skin irritation or abrasion | 3 and 6 mo | 1 RCT13 (N = 66) | Yes (−1) | Unknown | No | Yes (−1) | Skin irritation or abrasion associated with exoskeleton use was rare | Low |
| No adverse events | 8 wk | 2 RCTs9 15 (N = 139) | Yes (−1) | No | No | Yes (−1) | No additional adverse events occurred in the two studies that mentioned adverse events explicitly | Low |
| Exoskeletons as assistive devices | ||||||||
| No comparisons available | No data | |||||||
Abbreviations: 6MWT, 6-minute walk test; 10MWT, 10-meter walk test; FIM-L, Functional independence measure- leg; LMN, Lower motor neuron; RCT, randomized controlled trial; SCIM, Spinal Cord Independence Measure; UMN, upper motor neuron; WISCI, Walking Index for Spinal Cord Injury.
Note: Baseline quality rated as follows: high = majority of articles are RCTs; low = majority of articles are observational; upgrade: large magnitude of effect (one or two levels), dose–response gradient (one level), plausible confounding decrease magnitude of effect (one level); downgrade: risk of bias (one or two levels), inconsistency of results (one or two levels), indirectness of evidence (one or two levels), imprecision of effect estimates (one or two levels).
Rehabilitation strategies included overground training with physical therapy, gait training with the GT1 end-effect device with conventional training, treadmill training with manual assistance, treadmill training with stimulation, overground training with stimulation, overground training alone, therapist-assisted bodyweight supported treadmill training, and strength training.
High walking capacity defined by high 10MWT velocity and Timed-Up-and-Go (TUG) test times, and high 6MWT distance at baseline. Low walking capacity defined by low 10MWT velocity and TUG test times, and low 6MWT distance at baseline.
Safety of Exoskeletons
Adverse events were rare; however, they were reported inconsistently.
Illustrative Case
A 29-year-old female acrobat suffered from traumatic SCI after a fall from a 3-m height during an artistic performance. She was instantly unable to move upper and lower limbs.
Diagnosis in the first hospital providing care revealed fractures and fracture dislocation of cervical vertebrae C3 and C4. At the day of admission, she received a decompression operation via laminectomy of the C3 and was immobilized with a cervical collar. On the third postoperative day, the patient was transferred to a level 1 trauma center with an affiliated rehabilitation facility for SCIs.
The neurologic status at the time of admission was American Spinal Injury Association (ASIA) B. Further radiologic diagnostics showed a dislocated fracture and led to dorsal spinal fusion of C2 through C4; additional figures can be seen in the online supplementary material (Figs. S1 and S2).
Due to expected prolonged dependency on respiratory support, the patient received a percutaneous tracheostomy. Subsequent to fracture consolidation and weaning from the respirator, the patient was started on BWSTT using an EMG-controlled exoskeleton (HAL) using the protocol of Aach et al.19
The neurologic status at the time of enrollment was ASIA C; an additional figure can be seen in the online supplementary material (Fig. S3).19 20
Throughout the 12-week intervention, treadmill performance improved significantly in terms of speed, distance, and time on the treadmill. Video demonstration can be seen in the online supplementary material (Video 1). Assessment of functional walking abilities without the exoskeletal device measured by the 10MWT improved significantly as measured by time needed to ambulate a 10-m distance and the assistance needed (WISCI II; Video 2, online supplementary material). The patient regained important motor functions as assessed via LEMS, leading to an improved walking ability that enabled her to ambulate short distances and even over everyday obstacles (e.g., stairs) without personal assistance; a video showing the performance on stairs can be found in the online supplementary material (Video 3). The neurologic status after 12 weeks of HAL BWSTT improved to ASIA D; an additional figure can be seen in the online supplementary material (Fig. S4).19 20
Video 1
Supplemental video of patient in Case 1 which demonstrates pulsatile exophthalmos.
Video 2
Supplemental video of patient in Case 1 which demonstrates pulsatile exophthalmos.
Video 3
Supplemental video of patient in Case 1 which demonstrates pulsatile exophthalmos.
Discussion
In the 1960s and '70s, initial research was conducted in the field of motorized exoskeletons. Due to the size and weight of the technology, it was deemed unfeasible compared with conventional KAFOs. Therefore, the research was discontinued.21 22
However, SCI remained a devastating and disabling condition often resulting in significant gait limitations. To restore mobility, a weight-bearing locomotion training system was introduced into SCI rehabilitation programs. Over the past 15 years, fixed exoskeletons, primarily the Lokomat, emerged as a relatively frequent technology in SCI rehabilitation.
We looked for but were unable to find any studies evaluating exoskeletons as an assistive device compared with conventional KAFOs. With respect to evaluating exoskeletons as a rehabilitation device, we found nine RCTs. Though there was heterogeneity with respect to comparison groups, cointerventions, outcomes, and study design, these nine studies revealed no significant differences in velocity on the 10MWT and SCIM scores comparing exoskeleton to conventional locomotion rehabilitation. There were mixed results among some of the studies with respect to the 6MWT, WISCI, WISCIII, and FIM-L scores.
As new technologies enabled the development of motorized wearable exoskeletons, several types have been introduced to SCI rehabilitation. The available exoskeletons can be subdivided according to their control mode into joystick, posture, and EMG controlled (Table 5).
Table 5. Control and purpose of exoskeletons used with patients with spinal cord injuries.
| Exoskeleton | No. of case series | No. of patients | Purpose |
|---|---|---|---|
| Joystick control | |||
| Rex-Bionics (Rex-Bionics, Auckland, New Zealand) | None | 0 | Assistive mobility device |
| Lokomat (Hocoma, Switzerland) | 830 31 32 33 34 35 36 37 | 159 | Rehabilitation |
| WPAL (Fujita Health University, Japan) | 238 39 | 11 | Assistive mobility device |
| Kinesis (Technaid, Madrid, Spain) | 140 | 3 | Rehabilitation |
| Posture control | |||
| Re-Walk (Argo Medical Technologies Ltd, Yokneam IIit, Israel) | 741 42 43 44 45 46 47 | 66 | Assistive mobility device |
| Ekso-Bionics (Eksobionics Ltd, Richmond, California, USA) | 226 27 | 10 | Rehabilitation |
| Indego (Parker Hannifin Corp., Macedonia, Ohio, USA) | 248 49 | 21 | Assistive mobility device |
| EMG control | |||
| HAL (Cyberdyne, Tsukuba, Japan) | 53 23 24 25 28 | 30 | Rehabilitation |
Abbreviations: EMG, electromyographic; HAL, Hybrid Assistive Limb; WPAL, Wearable Power-Assist Locomotor.
Different applications are possible depending on the control mode of operation. Joystick- and posture-controlled exoskeletons enable the patient to regain mobility while wearing the device to compensate for the functional loss of the lower limbs. In contrast, EMG-controlled devices integrate the patient's voluntary drive in the walking pattern, because it requires an active contribution of the patient's lower limbs. This neuronal feedback may lead to or implicate neuronal plasticity and normalizes cortical excitability. Furthermore, some believe this mechanism leads to increased patient mobility when not wearing the exoskeleton, even in patients with chronic SCI.3 23
There is a certain optimism that exoskeletons, primarily mobile exoskeletons used as rehabilitation devices (e.g., HAL, and to some extent, Ekso-Bionics), have potential to improve walking patterns after the device is removed.3 23 24 25 26 27 28
In our study, adverse events were infrequently reported in literature, with only one trial indicating skin irritation or abrasion from exoskeleton training. There were no reports of falling or pressure sores.
Although case series provide some optimism with respect to the use of exoskeletons in locomotion rehabilitation for SCI (Table 5), this optimism has yet to be validated in randomized trials. Studies of newer exoskeletons comparing them to current rehabilitation strategies are needed.
Conclusion
There is no data to compare locomotion assistance with exoskeleton versus conventional KAFOs. There is no consistent benefit from rehabilitation using an exoskeleton versus a variety of conventional methods in chronic SCI patients. Trials comparing later-generation exoskeletons are needed.
Acknowledgments
Analytic support for this work was provided by Spectrum Research, Inc. with funding from AOSpine International
Footnotes
Disclosures Christian Fisahn: none Mirko Aach: none Oliver Jansen: none Marc Moisi: none Angeli Mayadev: none Krystle T. Pagarigan: none Joseph R. Dettori: none Thomas A. Schildhauer: Consultant (Cyberdyne, Inc., Japan)
Editorial Perspective
Over the last 30 years, the treatment for acute SCI has largely focused on three areas:
Optimizing patient retrieval and resuscitation during the postinjury phase
Timely decompression and surgical stabilization where appropriate
Rehabilitation with efforts concentrating on returning patients to functional independence, relying largely on wheelchairs or bracing with mobilization aids in some patients
Additional areas of SCI care have largely focused on pharmacologic interventions, such as use of high-dose steroids as a powerful anti-inflammatory medication aimed at decreasing expansion of the secondary injury zone. Continued controversy remains about the role of this medication. Further pharmaceuticals and hypothermia, all largely with similar intent to limit secondary injury zone damage, are currently undergoing prospective trials. Taken together all of these efforts have led to improvements of patient survival but have shown to be limited in their potential for further SCI recovery.
Emerging experimental modalities for potential spinal cord repair attempt to stop neuronal apoptosis or induce cell regeneration through stem cell implantation or molecular interventions. Although these therapeutic concepts have raised substantial public interest through sensationalistic media coverage, there is little actual scientifically confirmed evidence that these treatments work in the lab using large mammals or in patients. For the foreseeable future, this important arm of research will need to grapple with a number of overwhelming foundational obstacles prior to providing SCI patients with veritable hope for meaningful SCI recovery.
Robotic devices that augment paralyzed or paraparetic lower extremity function have been a long-held dream of futurists and part of some attempts of patient care since the 1970s. With mainstream treatments as well as cellular treatments having arrived at somewhat of an impasse, there has been a renewed interest in utilizing robotically driven technologies for mobilization and perhaps even as a neurorehabilitation tool. To our knowledge, this is the first systematic review of the current evidence base for use of these devices and categorizes them into more conventional passive mobilization assistive devices versus active neurorehabilitation technologies.
The enclosed assessment provides an important insight into the difficulty of proving recovery in patients with incomplete SCIs. Designing a prospectively randomized trial is challenging due to the limited number of patients and the high degree of variability in their circumstances. Doubters will always be able to challenge any recovery claims by asking about the natural recovery potential of patients if they had not been treated. That said, the investigators and patients appear to be very excited about the potential for improved mobility or even functioning in an upright position.
On a positive scientific note, there seems to be increasing consistency in measuring the function of patients with incomplete SCIs. In addition to the conventional lower extremity motor score, the 10-meter walk test, and the 6-minute walk test, as a general functional measure, WISCI II appears to be more commonly used as an objective performance assessment. Unfortunately, important additional factors such as spasticity, urinary control, sensory recovery, and defecation measures apparently are not yet part of the routine assessments to measure rehabilitation progress. Similarly, patient satisfaction and other patient-reported outcomes were not assessed in these presented studies. To date, the reported results are inconsistent compared with conventional therapy. Longer-term outcomes of affected patients such osteoporosis, pathologic fractures, or skin breakdown are yet to be assessed. Similarly, the persistence of any motor function gains has yet to be established. Nonetheless, these more recent advances in robotics and their human interface in terms of “neurorobotics” stand to offer far more near-term real-life hope for recovery of function and upright mobilization than current established treatment or neuronal cell regeneration therapies. The other main option is based on using robotic devices such as used in form of motorized braces (“exoskeletons”).
Supplementary Material
References
- 1.National Spinal Cord Injury Statistical Center Spinal Cord Injury (SCI) Facts and Figures at a Glance. 2016 Available at: https://www.nscisc.uab.edu/Public/Facts%202016.pdf. Accessed June 13, 2016
- 2.Ditunno P L, Patrick M, Stineman M, Ditunno J F. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46(7):500–506. doi: 10.1038/sj.sc.3102172. [DOI] [PubMed] [Google Scholar]
- 3.Aach M, Cruciger O, Sczesny-Kaiser M. et al. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: a pilot study. Spine J. 2014;14(12):2847–2853. doi: 10.1016/j.spinee.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Aach M, Meindl R C, Geßmann J, Schildhauer T A, Citak M, Cruciger O. [Exoskeletons for rehabilitation of patients with spinal cord injuries. Options and limitations] Unfallchirurg. 2015;118(2):130–137. doi: 10.1007/s00113-014-2616-1. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto H, Sankai Y. Power assist method based on Phase Sequence and muscle force condition for HAL. Adv Robot. 2005;19(7):714–734. [Google Scholar]
- 6.Lünenburger L, Colombo G, Riener R, Dietz V. Biofeedback in gait training with the robotic orthosis Lokomat. Conf Proc IEEE Eng Med Biol Soc. 2004;7:4888–4891. doi: 10.1109/IEMBS.2004.1404352. [DOI] [PubMed] [Google Scholar]
- 7.Ding H, Hu G L, Zheng X Y, Chen Q, Threapleton D E, Zhou Z H. The method quality of cross-over studies involved in Cochrane Systematic Reviews. PLoS One. 2015;10(4):e0120519. doi: 10.1371/journal.pone.0120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcobendas-Maestro M, Esclarín-Ruz A, Casado-López R M. et al. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: randomized controlled trial. Neurorehabil Neural Repair. 2012;26(9):1058–1063. doi: 10.1177/1545968312448232. [DOI] [PubMed] [Google Scholar]
- 9.Benito-Penalva J, Edwards D J, Opisso E. et al. Gait training in human spinal cord injury using electromechanical systems: effect of device type and patient characteristics. Arch Phys Med Rehabil. 2012;93(3):404–412. doi: 10.1016/j.apmr.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Duffell L D, Brown G L, Mirbagheri M M. Interventions to reduce spasticity and improve function in people with chronic incomplete spinal cord injury: distinctions revealed by different analytical methods. Neurorehabil Neural Repair. 2015;29(6):566–576. doi: 10.1177/1545968314558601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esclarín-Ruz A, Alcobendas-Maestro M, Casado-Lopez R. et al. A comparison of robotic walking therapy and conventional walking therapy in individuals with upper versus lower motor neuron lesions: a randomized controlled trial. Arch Phys Med Rehabil. 2014;95(6):1023–1031. doi: 10.1016/j.apmr.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Field-Fote E C, Roach K E. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther. 2011;91(1):48–60. doi: 10.2522/ptj.20090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorman P H, Scott W, York H. et al. Robotically assisted treadmill exercise training for improving peak fitness in chronic motor incomplete spinal cord injury: a randomized controlled trial. J Spinal Cord Med. 2016;39(1):32–44. doi: 10.1179/2045772314Y.0000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornby G T, Campbell D D, Zemon D H, Kahn J H. Clinical and quantitative evaluation of robotic-assisted treadmill walking to retrain ambulation after spinal cord injury. Top Spinal Cord Inj Rehabil. 2005;11(2):1–17. [Google Scholar]
- 15.Labruyère R, van Hedel H J. Strength training versus robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study in patients depending on walking assistance. J Neuroeng Rehabil. 2014;11:4. doi: 10.1186/1743-0003-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu X, Varoqui D, Kindig M, Mirbagheri M M. Prediction of gait recovery in spinal cord injured individuals trained with robotic gait orthosis. J Neuroeng Rehabil. 2014;11:42. doi: 10.1186/1743-0003-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin J C, Kim J Y, Park H K, Kim N Y. Effect of robotic-assisted gait training in patients with incomplete spinal cord injury. Ann Rehabil Med. 2014;38(6):719–725. doi: 10.5535/arm.2014.38.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Q, Yu L, Gu R, Zhou Y, Hu C. Effects of robot training on bowel function in patients with spinal cord injury. J Phys Ther Sci. 2015;27(5):1377–1378. doi: 10.1589/jpts.27.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Multicenter Study on Human Spinal Cord Injury (EMSCI) Welcome to EMSCI's ISNCSCI calculator Available at: https://ais.emsci.org. Accessed June 23, 2016
- 20.Schuld C, Wiese J, Hug A. et al. Computer implementation of the international standards for neurological classification of spinal cord injury for consistent and efficient derivation of its subscores including handling of data from not testable segments. J Neurotrauma. 2012;29(3):453–461. doi: 10.1089/neu.2011.2085. [DOI] [PubMed] [Google Scholar]
- 21.Vodovnik L, Long C II, Reswick J B, Lippay A, Starbuck D. Myo-electric control of paralyzed muscles. IEEE Trans Biomed Eng. 1965;12(3):169–172. doi: 10.1109/tbme.1965.4502374. [DOI] [PubMed] [Google Scholar]
- 22.Vodovnik L, Rebersek S. Information content of myo-control signals for orthotic and prosthetic systems. Arch Phys Med Rehabil. 1974;55(2):52–56. [PubMed] [Google Scholar]
- 23.Sczesny-Kaiser M, Höffken O, Aach M. et al. HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J Neuroeng Rehabil. 2015;12:68. doi: 10.1186/s12984-015-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruciger O, Schildhauer T A, Meindl R C. et al. Impact of locomotion training with a neurologic controlled hybrid assistive limb (HAL) exoskeleton on neuropathic pain and health related quality of life (HRQoL) in chronic SCI: a case study (.) Disabil Rehabil Assist Technol. 2016;11(6):529–534. doi: 10.3109/17483107.2014.981875. [DOI] [PubMed] [Google Scholar]
- 25.Cruciger O, Tegenthoff M, Schwenkreis P, Schildhauer T A, Aach M. Locomotion training using voluntary driven exoskeleton (HAL) in acute incomplete SCI. Neurology. 2014;83(5):474. doi: 10.1212/WNL.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski A J, Bryce T N, Dijkers M P. Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Top Spinal Cord Inj Rehabil. 2015;21(2):110–121. doi: 10.1310/sci2102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kressler J, Thomas C K, Field-Fote E C. et al. Understanding therapeutic benefits of overground bionic ambulation: exploratory case series in persons with chronic, complete spinal cord injury. Arch Phys Med Rehabil. 2014;95(10):1878–1887. doi: 10.1016/j.apmr.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Kubota S, Nakata Y, Eguchi K. et al. Feasibility of rehabilitation training with a newly developed wearable robot for patients with limited mobility. Arch Phys Med Rehabil. 2013;94(6):1080–1087. doi: 10.1016/j.apmr.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Duffell L D, Niu X, Brown G, Mirbagheri M M. Variability in responsiveness to interventions in people with spinal cord injury: do some respond better than others? Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5872–5875. doi: 10.1109/EMBC.2014.6944964. [DOI] [PubMed] [Google Scholar]
- 30.Dietz V, Grillner S, Trepp A, Hubli M, Bolliger M. Changes in spinal reflex and locomotor activity after a complete spinal cord injury: a common mechanism? Brain. 2009;132(Pt 8):2196–2205. doi: 10.1093/brain/awp124. [DOI] [PubMed] [Google Scholar]
- 31.Domingo A, Lam T. Reliability and validity of using the Lokomat to assess lower limb joint position sense in people with incomplete spinal cord injury. J Neuroeng Rehabil. 2014;11:167. doi: 10.1186/1743-0003-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galen S S, Clarke C J, McLean A N, Allan D B, Conway B A. Isometric hip and knee torque measurements as an outcome measure in robot assisted gait training. NeuroRehabilitation. 2014;34(2):287–295. doi: 10.3233/NRE-131042. [DOI] [PubMed] [Google Scholar]
- 33.Mirbagheri M M, Ness L L, Patel C, Quiney K, Rymer W Z. The effects of robotic-assisted locomotor training on spasticity and volitional control. IEEE Int Conf Rehabil Robot. 2011;2011:5.975443E6. doi: 10.1109/ICORR.2011.5975443. [DOI] [PubMed] [Google Scholar]
- 34.Niu X, Varoqui D, Kindig M, Mirbagheri M M. The effect of robot-assisted locomotor training on walking speed. Conf Proc IEEE Eng Med Biol Soc. 2012;2012(12):3858–3861. doi: 10.1109/EMBC.2012.6346809. [DOI] [PubMed] [Google Scholar]
- 35.Schück A, Labruyère R, Vallery H, Riener R, Duschau-Wicke A. Feasibility and effects of patient-cooperative robot-aided gait training applied in a 4-week pilot trial. J Neuroeng Rehabil. 2012;9:31. doi: 10.1186/1743-0003-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz I, Sajina A, Neeb M, Fisher I, Katz-Luerer M, Meiner Z. Locomotor training using a robotic device in patients with subacute spinal cord injury. Spinal Cord. 2011;49(10):1062–1067. doi: 10.1038/sc.2011.59. [DOI] [PubMed] [Google Scholar]
- 37.Varoqui D, Niu X, Mirbagheri M M. Ankle voluntary movement enhancement following robotic-assisted locomotor training in spinal cord injury. J Neuroeng Rehabil. 2014;11:46. doi: 10.1186/1743-0003-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanabe S, Hirano S, Saitoh E. Wearable Power-Assist Locomotor (WPAL) for supporting upright walking in persons with paraplegia. NeuroRehabilitation. 2013;33(1):99–106. doi: 10.3233/NRE-130932. [DOI] [PubMed] [Google Scholar]
- 39.Tanabe S, Saitoh E, Hirano S. et al. Design of the Wearable Power-Assist Locomotor (WPAL) for paraplegic gait reconstruction. Disabil Rehabil Assist Technol. 2013;8(1):84–91. doi: 10.3109/17483107.2012.688238. [DOI] [PubMed] [Google Scholar]
- 40.Del-Ama A J, Gil-Agudo A, Pons J L, Moreno J C. Hybrid gait training with an overground robot for people with incomplete spinal cord injury: a pilot study. Front Hum Neurosci. 2014;8:298. doi: 10.3389/fnhum.2014.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asselin P, Knezevic S, Kornfeld S. et al. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J Rehabil Res Dev. 2015;52(2):147–158. doi: 10.1682/JRRD.2014.02.0060. [DOI] [PubMed] [Google Scholar]
- 42.Benson I, Hart K, Tussler D, van Middendorp J J. Lower-limb exoskeletons for individuals with chronic spinal cord injury: findings from a feasibility study. Clin Rehabil. 2016;30(1):73–84. doi: 10.1177/0269215515575166. [DOI] [PubMed] [Google Scholar]
- 43.Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012;91(11):911–921. doi: 10.1097/PHM.0b013e318269d9a3. [DOI] [PubMed] [Google Scholar]
- 44.Fineberg D B, Asselin P, Harel N Y. et al. Vertical ground reaction force-based analysis of powered exoskeleton-assisted walking in persons with motor-complete paraplegia. J Spinal Cord Med. 2013;36(4):313–321. doi: 10.1179/2045772313Y.0000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talaty M, Esquenazi A, Briceno J E. Differentiating ability in users of the ReWalk(TM) powered exoskeleton: an analysis of walking kinematics. IEEE Int Conf Rehabil Robot. 2013;2013:6.650469E6. doi: 10.1109/ICORR.2013.6650469. [DOI] [PubMed] [Google Scholar]
- 46.Yang A, Asselin P, Knezevic S, Kornfeld S, Spungen A M. Assessment of in-hospital walking velocity and level of assistance in a powered exoskeleton in persons with spinal cord injury. Top Spinal Cord Inj Rehabil. 2015;21(2):100–109. doi: 10.1310/sci2102-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeilig G, Weingarden H, Zwecker M, Dudkiewicz I, Bloch A, Esquenazi A. Safety and tolerance of the ReWalk™ exoskeleton suit for ambulation by people with complete spinal cord injury: a pilot study. J Spinal Cord Med. 2012;35(2):96–101. doi: 10.1179/2045772312Y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans N, Hartigan C, Kandilakis C, Pharo E, Clesson I. Acute cardiorespiratory and metabolic responses during exoskeleton-assisted walking overground among persons with chronic spinal cord injury. Top Spinal Cord Inj Rehabil. 2015;21(2):122–132. doi: 10.1310/sci2102-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartigan C, Kandilakis C, Dalley S. et al. Mobility outcomes following five training sessions with a powered exoskeleton. Top Spinal Cord Inj Rehabil. 2015;21(2):93–99. doi: 10.1310/sci2102-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


