Abstract
IMPORTANCE
Magnetic resonance imaging (MRI) has the advantage of imaging swallow function at any anatomical level without changing the position of patient, which can provide detailed information than modified barium swallow, by far the gold standard of swallow evaluation.
OBJECTIVE
To investigate the use of real-time MRI in the evaluation of swallow function of patients with tongue cancer.
DESIGN, SETTING, AND PARTICIPANTS
Real-time MRI experiments were performed on a Signa Excite HD 1.5-T scanner (GE Healthcare), with gradients capable of 40-mT/m (milli-Tesla per meter) amplitudes and 150-mT/m/ms (mT/m per millisecond) slew rates. The sequence used was spiral fast gradient echo sequence. Four men with base of tongue or oral tongue squamous cell carcinoma and 3 age-matched healthy men with normal swallowing participated in the experiment.
INTERVENTIONS
Real-time MRI of the midsagittal plane was collected during swallowing. Coronal planes between the oral tongue and base of tongue and through the middle of the larynx were collected from 1 of the patients.
MAIN OUTCOMES AND MEASURES
Oral transit time, pharyngeal transit time, submental muscle length change, and the distance change between the hyoid bone and anterior boundary of the thyroid cartilage were measured frame by frame during swallowing.
RESULTS
All the measurable oral transit and pharyngeal transit times of the patients with cancer were significantly longer than the ones of the healthy participants. The changes in submental muscle length and the distance between the hyoid bone and thyroid cartilage happened in concert for all 60 normal swallows; however, the pattern differed for each patient with cancer. To our knowledge, the coronal view of the tongue and larynx revealed information that has not been previously reported.
CONCLUSIONS AND RELEVANCE
This study has demonstrated the potential of real-time MRI to reveal critical information beyond the capacity of traditional videofluoroscopy. Further investigation is needed to fully consider the technique, procedure, and standard scope of applying MRI to evaluate swallow function of patients with cancer in research and clinic practice.
Oral and oropharyngeal cancers are the sixth most common cancer in the world. Estimated annual incidence is 405 300 cases worldwide, excluding nasopharyngeal tumors, and 40 250 adults in the United States.1 Primary treatments include surgery, radiation, chemotherapy, and combinations.2 Each may result in short- and long-term (often permanent) morbidity because of disfigurement, highly viscous saliva, trismus, dysphagia, and voice or resonance problems.3
Functional rehabilitation of swallowing is critical for quality of life following cancer treatment. Videofluoroscopy (VF) using both lateral and anteroposterior views is widely accepted as the gold standard for direct assessment of swallowing function4; however, the radiation exposure and the composite image of all anatomical structures limit its application. Real-time magnetic resonance imaging offers a noninvasive visualization of swallowing function without exposure to radiation or any known biohazards. In addition, swallowing function can be imaged at any anatomical level in the sagittal, coronal, and transaxial views without changing the patient’s position. Real-time MRI is an important new tool in evaluation and has the potential to improve rehabilitation for posttreatment patients with head and neck cancer. Therefore, the purpose of this pilot study was to investigate the use of real-time MRI to evaluate swallow function of posttreatment patients with tongue cancer.
Methods
Participants
After obtaining institutional review board approval from the University of Southern California and informed written patient consent, four men with base of tongue (BOT) or oral tongue (OT) squamous cell carcinoma and 3 age-matched healthy men with normal swallowing participated in this study (Table). All 4 patients with cancer had undergone partial glossectomy, primary reconstruction of tongue and floor of mouth with free flap, modified radical neck dissection, and radiation therapy. Patient 2 was diagnosed as having recurrent BOT cancer several days before data collection.
Table.
Participant Characteristics
| Participant (All Male) | Age, ya | Cancer Stage (Type) | Time Elapsed Since Cancer Treatment | Speech and Swallowing Therapy | Diet |
|---|---|---|---|---|---|
| Normal 1 | 54 | None | NA | None | Oral |
| Normal 2 | 60 | None | NA | None | Oral |
| Normal 3 | 67 | None | NA | None | Oral |
| Patient 1 | 66 | T2N1M0 (BOT) | 5 y | Concurrent with radiation therapy | Oral |
| Patient 2 | 64 | T3N1M0 (BOT) | 7 y | 6-mo after radiation therapy | Oral |
| Patient 3 | 70 | T4N1M0 (OT) | 1.5 mo | None | Oral |
| Patient 4 | 55 | T3N1M0 (BOT) | 1.5 y | Concurrent with radiation therapy | Oral |
Abbreviations: BOT, base of tongue; NA, not applicable; OT, oral tongue.
In healthy participants, mean (SD) age was 60.3 (6.5) years; and in the patients with tongue cancer, 63.8 (6.3) years.
Real-Time MRI
Experiments with MRI were performed on a Signa Excite HD 1.5-T scanner (GE Healthcare), with gradients capable of 40-mT/m (milli-Tesla per meter) amplitudes and 150-mT/m/ms (mT/m per millisecond) slew rates. The sequence used was spiral fast gradient echo sequence.5 A body coil was used for radiofrequency transmission. A custom 4-channel upper airway receiver coil array was used for radiofrequency signal reception. In the 4-channel receiver coil array, 2 coil elements are anterior and the other 2 coil elements are posterior to the head and neck. Participants lie supine on the magnet table wearing foam ear plugs to reduce scanner noise. Head movements were minimized with foam padding and an elastic strap. Real-time midsagittal scans of the oral and pharyngeal cavities during swallowing were collected from all participants using a custom real-time imaging software.6 Coronal planes between the OT and BOT and through the middle of the larynx were collected from patient 4 (Figure 1A and Figure 2A). In-plane spatial resolution was 3 × 3 mm, and slice thickness was 5 mm. Video 1, Video 2, Video 3, and Video 4 were obtained with a frame rate of 23 to 24 frames per second after sliding window reconstruction.
Figure 1. Lingual Groove Formation.
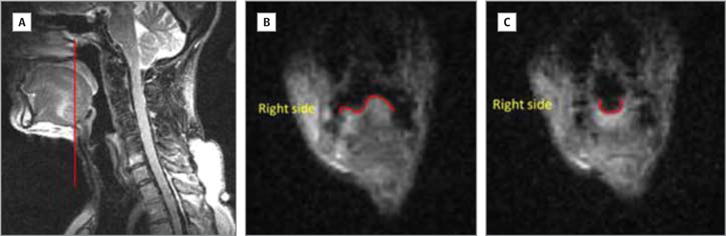
A, Sagittal schematic showing the location of the coronal slice in panels B and C. B, Lingual asymmetry during quiet breathing. C, Symmetrical lingual surface during swallowing; lingual groove is visible.
Figure 2. Adduction of the Vocal Folds.
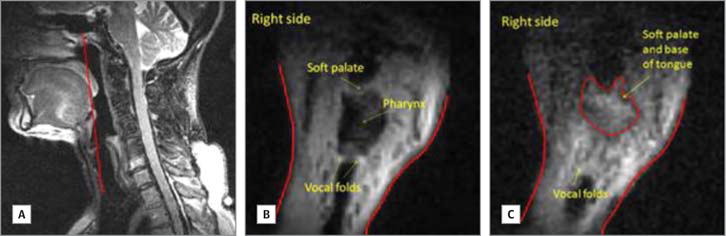
A, Sagittal schematic showing the location of the coronal slice viewed in panels B and C. B, Vocal folds opening during quiet breathing. C, Vocal folds closing during swallowing; weakness on the left side.
We could find no previous literature regarding the type of bolus consistency and volume a patient with oral/oropharyngeal cancer can consume safely in supine position. Therefore, we asked the patients to consume the amount of yogurt closest to their daily swallowing experience. We estimated that the yogurt given was a pudding/paste consistency (5 to 10 mL).
Data Analysis and Statistical Design
Magnetic resonance images of the swallows were viewed and measured frame by frame. Observations included tongue shape change in both sagittal and coronal planes, oral transit time (OTT) and pharyngeal transit time (PTT). Oral transit time was defined as the duration of time for the bolus to move through the oral cavity, measured from the first backward movement of the bolus until the head of the bolus passed the tongue base at the level of the tip of soft palate, and PTT was defined as the time required for the bolus to move through the pharynx, measured from the time the head of the bolus passed the tongue base at the level of the tip of the soft palate until the tail of the bolus left the cricopharyngeal region. These definitions differ slightly from the classic definitions of OTT and PTT developed for use with VF,7 because the ramus of the mandible is not visible on a mid-sagittal slice; the chosen landmark provides an available alternative in a similar location.
Submental muscle length (SM) and the distance between the hyoid bone and thyroid cartilage (TH) were measured frame by frame during swallowing (Figure 3). The SM was measured from the most inferior point of the mandible to the most anterior-inferior point of the hyoid bone. The TH was measured from the most anterior-inferior point of the hyoid bone to the anterior point of the anterior commissure of the vocal folds, since this is the most visible point of the larynx on MRI. Data were analyzed by a senior dysphagia researcher. Twenty percent of the data was remeasured 2 weeks later to establish intrarater reliability, and separately, a speech-language pathologist with more than 20 years of experience conducting approximately 10 VFs per week for interrater reliability testing.
Figure 3. The SM and TH Measurement on a Schematic Mid-Sagittal Image.
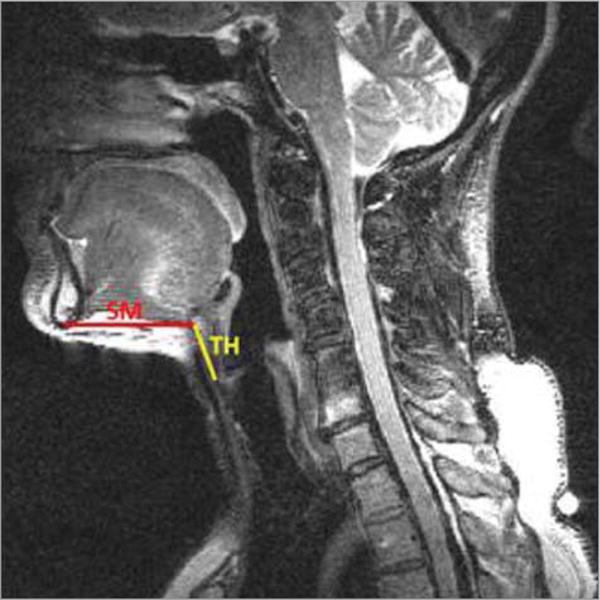
SM indicates submental muscle length; TH, the distance between the hyoid bone and thyroid cartilage.
Intraclass correlation coefficients were used to test reliability. Paired t tests were used to compare timing and muscle length of the healthy participants with those of each patient with tongue cancer.
Results
There were no statistically significant differences between measurements made 2 weeks apart by the primary investigator (intraclass correlation coefficient, 0.94), nor between the 2 investigators (intraclass correlation coefficient, 0.89).
A total of 60 swallows were collected from the 3 healthy participants (17, 22, and 21). Because there was no significant intersubject difference for OTT, PTT, SM, or TH, the 60 swallows were pooled for further analysis. The pooled mean (SD) OTT was 0.26 (0.06) seconds, and the pooled mean (SD) PTT was 0.51 (0.05) seconds. The length change of SM and TH happened in concert for all 60 normal swallows (Figure 4A). The tongue shape change of a normal swallow on the mid-sagittal plane is shown in Figure 5.
Figure 4. The SM and TH Length Change Over a Representative Swallow.
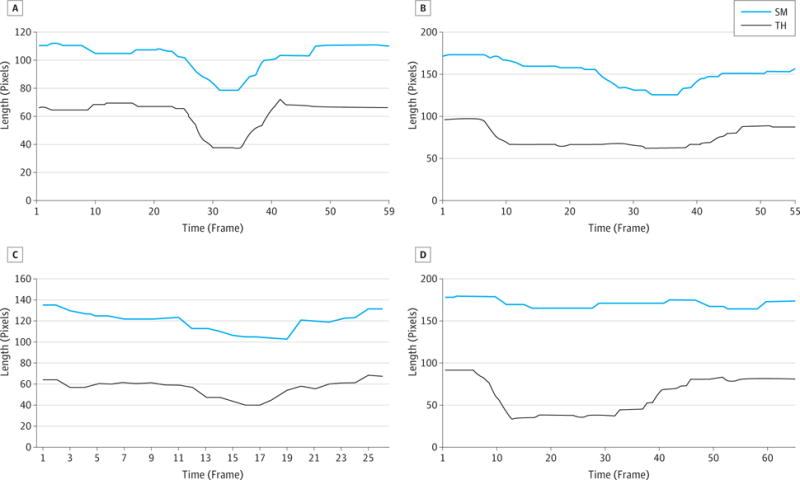
A, Normal swallow. The length change in SM and TH happened in concert. B, Swallow by patient 1. The TH contracted much earlier than the SM, and the contraction lasted much longer. C, Swallow by patient 2. The length change of SM and TH happened almost simultaneously. D, Swallow by patient 4.
No obvious SM length change during swallowing, while the TH shortened for approximately 0.45 s. SM indicates submental muscle length; and TH, the distance between the hyoid bone and thyroid cartilage.
Figure 5. Tongue Shape Change During the Oral Stage of a Representative Normal Swallow.
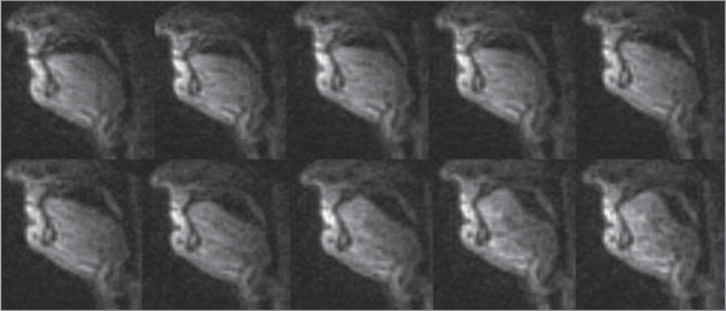
The bolus was visualized to be transferred from the tip of the tongue to the base of the tongue.
Fourteen swallows were collected from patient 1, who could comfortably swallow less than 5 mL (Video 1). Four or more serial swallows occurred for each bolus. The tongue shape change was similar with normal on the mid-sagittal plane during the oral stage of swallowing. The mean (SD) OTT across swallows was 0.42 (0.07) seconds, and the mean (SD) PTT was 0.65 (0.03) seconds, which was longer than the times for the healthy participants (P < .001 for both). The TH contracted much earlier than the SM, and the contraction lasted much longer (Figure 4B).
Fifteen swallows were collected from patient 2, who could comfortably swallow less than 5 mL (Video 2). Although the patient was able to transfer the bolus from the oral cavity to the pharynx without premature spillage, the amplitude of tongue shape change was visually estimated to be one-quarter to one-third of the normal one presented in Figure 5 on the mid-sagittal plane during the oral stage of swallowing; therefore, OTT was not measurable. Contact between the BOT and posterior pharyngeal wall was observed during pharyngeal stage. The mean (SD) PTT was 0.67 (0.06) seconds, which was longer than the times for the healthy participants (P < .001). As with the healthy participants, the length change of SM and TH happened almost simultaneously (Figure 4C).
Fourteen swallows were collected from patient 3, who demonstrated fibrosis of the OT (Figure 6 and Video 3), with absent lingual range of motion. Owing to a fear of choking, this patient could only perform dry swallows in the MRI scanner; the oral transit stage was missing. Serial swallows occurred. The OTT could not be measured because of the lack of bolus and limited range of motion of the tongue. The PTT was measured based on the transfer of visible saliva on MRI. The mean (SD) PTT was 0.63 (0.05) seconds, which was significantly longer than the times for the healthy participants (P < .001). There was no obvious SM length change during swallowing, whereas the TH shortened for approximately 0.45 seconds (11 frames; Figure 4D).
Figure 6. Oral Tongue Fibrosis in Patient 3.
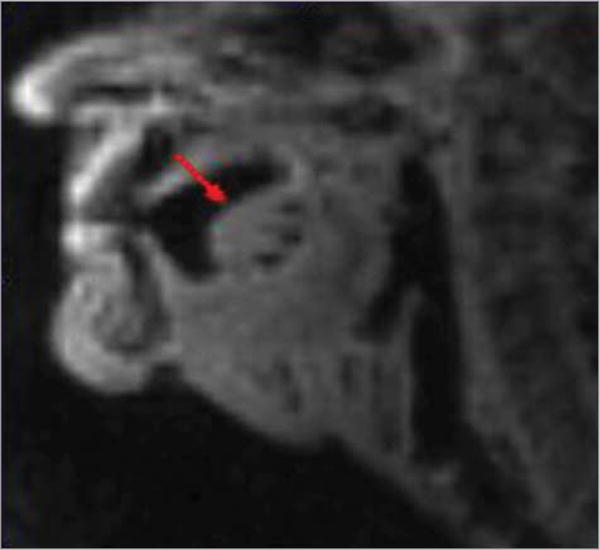
Free flap fibrosis due to radiation exposure.
Eleven swallows were collected from patient 4, who could comfortably swallow 5- to 10-mL boluses. One serial swallow occurred for 8 of 11 boluses. Oral stage tongue shape change on the mid-sagittal plane was within normal limits. The mean (SD) OTT was 0.44 (0.04) seconds, and the mean (SD) PTT was 1.07 (0.08) seconds, which was longer than the times for the healthy participants (P < .001 for both). The SM and TH length changes were not measureable because poor image quality.
Images were also acquired in the coronal plane from patient 4. On a slice between the OT and BOT (Figure 1A), the tongue surface showed asymmetry during quiet breathing, with the reconstructed left side higher than the normal right side (Figure 1B). During the oral transit stage, the tongue surface appeared symmetrical while the bolus was passing through (Figure 1C and Video 4). On a plane through the middle of the larynx (Figure 2A), there was obvious asymmetry of head and neck soft tissue during quiet breathing (Figure 2B). During swallowing, although the vocal folds on both sides showed superior displacement, the displacement on the right side was greater than the left side (Figure 2C).
Discussion
Magnetic resonance imaging is one of the most commonly used medical imaging technologies because it can provide better contrast resolution than other methods.8 In addition, unlike computed tomographic scans and traditional x-ray films, MRI uses strong magnetic fields and nonionizing radiation, and there is no solid evidence of health risk.9 With the advent of fast-imaging techniques, MRI can provide high-quality images of structures undergoing physiological motion. Real-time MRI, a rapidly maturing technology, has steadily improved visualization of the anatomical and physiological characteristics of normal swallows.10–13 With improving spatial and temporal resolution, more recent work has shifted from qualitative description to quantification of physiological events. Measurement of bolus transit times, for instance, was limited by temporal resolution until recently.14 The study most similar to ours in temporal and spatial resolution13 found OTT and PTT values comparable to those from the VF-based literature, but it included only healthy young adult participants.
Real-time MRI is also increasingly being investigated for the evaluation of dysphagia, such as that associated with head and neck cancer and its treatment. Previous studies using this technique to analyze swallowing function of patients with head and neck cancer15–20 have described posttreatment tissue changes and pathological and compensatory differences in swallow physiologic events between patients and controls consistent with VF-based studies, but their data have been primarily qualitative. Our results extend previous findings, extend to older adults and those with cancer, and quantify the temporal pattern of swallowing events and degree of muscle length changes.
Consistent with previous reports from VF-based studies,21,22 the OTTs and PTTs of patients with cancer were significantly longer than those of healthy participants, most likely because of dysfunction of the reconstructed tongue and floor of the mouth and complications of radiation and chemoradiation therapy. Changes in the lengths of muscles ordinarily responsible for displacing the hyoid and larynx differed between patients with tongue cancer and healthy participants, for whom hyoid and laryngeal displacement happened in concert. Posttreatment patients with tongue cancer were more variable: 1 patient displayed simultaneity, 1 patient showed no hyoid movement, laryngeal displacement preceded hyoid movement in a third patient, and hyoid displacement was visible but not measurable in the fourth. Although reduced hyoid motion in patients with head and neck cancer has been reported and reviewed23 previously, VF does not permit measurement of muscle length changes; use of real-time MRI enhances investigation of the causes of this altered motion.
All 4 patients with tongue cancer had undergone partial glossectomy and primary free flap reconstruction of the floor of mouth and tongue, meaning that the submental muscles and part of the intrinsic tongue musculature were excised and replaced by noncontracting soft tissue. It has long been believed that hyoid displacement during swallowing is due to the contraction of submental muscles.24–27 However, 3 of our 4 patients with tongue cancer showed some hyoid displacement during swallowing, despite loss of all submental muscle tissue bilaterally. This displacement might be explained by contraction of the remaining intrinsic tongue muscles working on the hyoid bone via the soft tissue connecting them. This pilot study cannot explain the variety of patterns of hyoid and laryngeal displacement owing to the heterogeneity of site and size of the disease among our few patients.
Analysis of the coronal plane between the OT and BOT for 1 patient (Figure 5) revealed that the lingual surface appeared higher on the reconstructed side during quiet breathing but was symmetrical while the bolus was passing through the image plane. The primary purpose of tongue reconstruction after partial glossectomy is to avoid tissue loss to support optimal oral function. In this study, fasciocutaneous free flaps from sternocleidomastoid and radial forearm were used to reconstruct the tongue and floor of the mouth. Such flaps have been reported to be associated with recovery of adequate speech and swallowing function.28 Our results suggest that with the flap filling the space of excised floor of mouth and tongue tissue, the remaining intrinsic tongue muscle may be able to compensate to achieve a safe oral stage of swallowing.
True vocal fold closure and asymmetrical movement were visible on a coronal plane through the larynx of 1 patient (Figure 6). Vocal fold closure during swallowing is critical for airway protection29–31 but is difficult to observe with VF. Real-time MRI provides a novel method for evaluation of deglutitive airway protection.
How comparable are our physiological data to those from the gold standard (VF) and other real-time MRI studies with similar spatiotemporal resolution? Any comparison of OTTs and PTTs across studies must be made with caution: definitions, bolus volumes, and consistencies have all varied. Comparisons with VF data entail the additional differences of supine patient position and the need for a definition dependent on mid-sagittal landmarks. With these limitations, our healthy participants’ OTTs and PTTs appeared comparable to those seen using VF, with healthy individuals of similar ages swallowing similarly sized liquid barium boluses,32,33 and lower than those shown by similarly aged adults swallowing cookies.34 Our healthy participants’ OTTs and PTTs were slightly lower than those of a previous study,13 which used real-time MRI with supine healthy young adult participants but without defining their measurement criteria.
The importance of the positional difference between supine real-time MRI and upright VF for physiology assessment, safety, and subjective difficulty swallowing are unclear. In the supine position, bolus motion is congruent with gravity in the oral phase and perpendicular to it in the pharyngeal phase, reversed from the habitual upright position. The timing of muscle activation for airway protection is not affected.35 Owing to the variety of definitions, bolus volumes, directness of measurement, and quality of bolus consistency description across studies, the effects on bolus movement are less clear. Oral transit time may be altered,36,37 but for PTT there are conflicting results and possibly an interaction with bolus consistency.38–40 Because a postural effect on swallowing safety is generally accepted,41 the effect of supine posture on risk of aspiration is not often investigated. Supine swallowing without aspiration during real-time MRI may be possible even in those with significant dysphagia,15 though the certainty of this result is limited by temporal resolution. Position-based subjective differences in swallowing difficulty have not been assessed but may be exacerbated in people with dysphagia. Therefore, the pros and cons of using MRI to evaluate swallowing function should be carefully considered for each patient. As upright (open) MRI becomes more widely available, its use for swallowing evaluation42 may negate this potential disadvantage.
Experimental design limitations included inability to detect residual material after swallow (due to the use of noncontrast boluses) and lack of data outside the chosen slices. Regarding the first, our patient sample is very likely to have had residue in the oral and pharyngeal cavities.22 Each patient used multiple swallows for each bolus, which may have indicated patient awareness of residue but cannot guarantee its successful clearance. Regarding the second, this study collected images (slices) of the midsagittal plane. Information from other planes can be clinically critical, eg, parasagittal planes for evaluation of pyriform sinus residuals. Future studies should include a range of clinically relevant slices and add contrast to boluses when residue is suspected.
As a preliminary study, our major goal is to inspect the possibility of using MRI to evaluate the swallowing of patients with head and neck cancer. Owing to its novelty, no previous literature was available for guidance, and our approach is highly experimental in various aspects. We selected 4 patients with oral/oropharyngeal cancer with dysphagia who were not willing to receive an extra dose of radiation, but formal swallow evaluation via medical imaging technique was still required for speech language pathologists to acquire sufficient information to perform adequate therapy. Currently, there are no established protocols for MRI. Except assuming that swallowing in the supine position would be more difficult than in the upright position for our patients, we do not have enough data to predict what kind of bolus consistency and how much volume a patient can consume safely. Therefore, we did not enforce the control of bolus volume. Instead, we asked the patients to consume the amount of yogurt closest to their daily swallowing experience. In addition, using MRI to evaluate a swallow does not allow us to test some of the existing therapeutic maneuvers (which may help patients to perform a safe swallow) because of head movement limitation during MRI scanning. However, with more understanding about the physiologic characteristics of swallows in the supine position, specially designed therapeutic maneuvers might be created. So far, MRI has not been used to evaluate swallowing clinically. There are fewer MRI facilities than x-ray imaging suites across the United States, and even fewer capable of using MRI to evaluate swallowing because analyzing MRI on the planes other than mid-sagittal plane requires additional training of a speech language pathologist. The cost of MRI is expected to be higher than of VF. The average cost of MRI is approximately $2500 and approximately $500 for x-ray imaging in our facility. A careful evaluation of cost-effectiveness is warranted, taking into consideration diagnostic accuracy of swallowing analysis using MRI or VF, treatment planning needs, and other factors. In this study, we revealed some interesting findings through MRI data analysis that VF usually is not able to provide, such as lingual groove and vocal folds closure during swallowing. Such capabilities may help us better understand the pathophysiologic characteristics of head and neck cancer and design appropriate therapeutic maneuvers. More work is still needed to further explore the potential of real-time MRI to improve evaluation of dysphagia.
Advantages of MRI over VF include nonionizing radiation, improved soft-tissue visualization, and availability of images from any angle without repositioning the patient, with spatial and temporal resolution now comparable to VF. Examinations with VF and real-time MRI of the same patients close in time could clarify comparability of the 2 evaluation modalities and establish norms using the definitions necessary in real-time MRI.
In conclusion, this study used real-time MRI with high spatial and temporal resolution to improve the quality of anatomical and physiological data on swallowing in healthy participants and posttreatment patients with cancer. Novel findings include hyoid movement in the absence of submental musculature and new views of asymmetry and partial compensation in these patients. For patients with dysphagia, VF may provide insufficient information for a full understanding of the pathophysiologic characteristics or may be contraindicated in repeated examinations because of cumulative radiation exposure. This study has demonstrated the potential of real-time MRI to reveal critical information beyond the capacity of traditional VF. Its usage may enhance the effectiveness of functional rehabilitation.
Supplementary Material
Acknowledgments
Funding/Support: This study was partially sponsored by the National Institute on Deafness and Other Communication Disorders (NIDCD, grant DC007124, “Dynamics of Vocal Tract Shaping”).
Role of the Sponsor: The sponsor played a role in the process of collection, management, analysis, and interpretation of the data.
Footnotes
Video at jamaotolaryngology.com
Author Contributions: Dr Zu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zu, Narayanan, Kim, Nayak, Sinha.
Acquisition of data: Zu, Narayanan, Kim, Nayak, Villegas, Ouyoung, Sinha.
Analysis and interpretation of data: Zu, Narayanan, Bronson-Lowe, Ouyoung, Sinha.
Drafting of the manuscript: Zu, Kim, Nayak, Villegas, Ouyoung, Sinha,
Critical revision of the manuscript for important intellectual content: Zu, Narayanan, Nayak, Bronson-Lowe, Sinha.
Statistical analysis: Zu, Sinha.
Obtained funding: Zu, Nayak, Sinha.
Administrative, technical, or material support: Zu, Narayanan, Kim, Nayak, Villegas, Ouyoung, Sinha.
Study supervision: Zu, Narayanan, Nayak, Sinha.
Conflict of Interest Disclosures: None reported.
References
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.List MA, D’Antonio LL, Cella DF, et al. The performance status scale for head and neck cancer patients and the functional assessment of cancer therapy-head and neck scale. a study of utility and validity. Cancer. 1996;77(11):2294–2301. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2294::AID-CNCR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Clark JR, Frei E., III Chemotherapy for head and neck cancer: progress and controversy in the management of patients with M0 disease. Semin Oncol. 1989;16(4 suppl 6):44–57. [PubMed] [Google Scholar]
- 4.Stoeckli SJ, Huisman TA, Seifert B, Martin-Harris BJ. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia. 2003;18(1):53–57. doi: 10.1007/s00455-002-0085-0. [DOI] [PubMed] [Google Scholar]
- 5.Narayanan S, Nayak K, Lee S, Sethy A, Byrd D. An approach to real-time magnetic resonance imaging for speech production. J Acoust Soc Am. 2004;115(4):1771–1776. doi: 10.1121/1.1652588. [DOI] [PubMed] [Google Scholar]
- 6.Santos JM, Wright GA, Pauly JM. Flexible real-time magnetic resonance imaging framework. Conf Proc IEEE Eng Med Biol Soc. 2004;2:1048–1051. doi: 10.1109/IEMBS.2004.1403343. [DOI] [PubMed] [Google Scholar]
- 7.Logemann JA. Evaluation and Treatment of Swallowing Disorders. 2nd. Austin, TX: Pro-Ed, Inc; 1998. [Google Scholar]
- 8.Prasad VP. Magnetic Resonance Imaging: Methods and Biologic Applications. Totowa, NJ: Human Press; 2006. p. 3. [Google Scholar]
- 9.Liney G. MRI in Clinical Practice. London, England: Springer-Verlag London Limited; 2006. p. 17. [Google Scholar]
- 10.Hartl DM, Albiter M, Kolb F, Luboinski B, Sigal R. Morphologic parameters of normal swallowing events using single-shot fast spin echo dynamic MRI. Dysphagia. 2003;18(4):255–262. doi: 10.1007/s00455-003-0007-9. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostara A, Stoeckli S, Weber OM, Kollias SS. Evaluation of the anatomical and functional properties of deglutition with various kinetic high-speed MRI sequences. J Magn Reson Imaging. 2001;14(2):194–199. doi: 10.1002/jmri.1172. [DOI] [PubMed] [Google Scholar]
- 12.Breyer T, Echternach M, Arndt S, et al. Dynamic magnetic resonance imaging of swallowing and laryngeal motion using parallel imaging at 3 T. Magn Reson Imaging. 2009;27(1):48–54. doi: 10.1016/j.mri.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Olthoff A, Frahm J. Real-time magnetic resonance imaging of normal swallowing. J Magn Reson Imaging. 2012;35(6):1372–1379. doi: 10.1002/jmri.23591. [DOI] [PubMed] [Google Scholar]
- 14.Panebianco V, Ruoppolo G, Pelle G, et al. Morpho-functional patterns of physiologic oropharyngeal swallowing evaluated with dynamic fast MRI. Eur Arch Otorhinolaryngol. 2010;267(9):1461–1466. doi: 10.1007/s00405-010-1232-0. [DOI] [PubMed] [Google Scholar]
- 15.Albiter M, Petrow P, Kolb F, Bretagne E, Luboinski B, Sigal R. Swallowing study with kinetic MRI using a single shot fast spin echo sequence in healthy volunteers and patients treated for head and neck cancer [in French] J Radiol. 2003;84(3):311–316. [PubMed] [Google Scholar]
- 16.Fauvet F, Charpiot A, Schultz P, et al. Cine-MRI contribution to assess swallowing mechanism and oro-pharyngeal dysphagia [in French] Rev Laryngol Otol Rhinol (Bord) 2008;129(2):85–90. [PubMed] [Google Scholar]
- 17.Hartl DM, Kolb F, Bretagne E, Marandas P, Sigal R. Cine magnetic resonance imaging with single-shot fast spin echo for evaluation of dysphagia and aspiration. Dysphagia. 2006;21(3):156–162. doi: 10.1007/s00455-006-9026-7. [DOI] [PubMed] [Google Scholar]
- 18.Hartl DM, Kolb F, Bretagne E, Bidault F, Sigal R. Cine-MRI swallowing evaluation after tongue reconstruction. Eur J Radiol. 2010;73(1):108–113. doi: 10.1016/j.ejrad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Kitano H, Asada Y, Hayashi K, Inoue H, Kitajima K. The evaluation of dysphagia following radical surgery for oral and pharyngeal carcinomas by cine-magnetic resonance imaging (Cine-MRI) Dysphagia. 2002;17(3):187–191. doi: 10.1007/s00455-002-0057-4. [DOI] [PubMed] [Google Scholar]
- 20.Kreeft AM, Rasch CR, Muller SH, Pameijer FA, Hallo E, Balm AJM. Cine MRI of swallowing in patients with advanced oral or oropharyngeal carcinoma: a feasibility study. Eur Arch Otorhinolaryngol. 2012;269(6):1703–1711. doi: 10.1007/s00405-011-1861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 22.Pauloski BR, Logemann JA, Rademaker AW, et al. Speech and swallowing function after anterior tongue and floor of mouth resection with distal flap reconstruction. J Speech Hear Res. 1993;36(2):267–276. doi: 10.1044/jshr.3602.267. [DOI] [PubMed] [Google Scholar]
- 23.Lazarus CL. Effects of chemoradiotherapy on tongue function in patients with head and neck cancer. Perspectives Swallowing Swallowing Disord (Dysphagia) 2009;18(23):55–60. [Google Scholar]
- 24.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19(1):44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 25.Crary MA, Carnaby Mann GD, Groher ME. Biomechanical correlates of surface electromyography signals obtained during swallowing by healthy adults. J Speech Lang Hear Res. 2006;49(1):186–193. doi: 10.1044/1092-4388(2006/015). [DOI] [PubMed] [Google Scholar]
- 26.Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR Am J Roentgenol. 1990;154(5):953–963. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- 27.Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol (1985) 1999;86(5):1663–1669. doi: 10.1152/jappl.1999.86.5.1663. [DOI] [PubMed] [Google Scholar]
- 28.Haughey BH, Taylor SM, Spector JG, Fuller D. Outcomes and techniques of fasciocutaneous flap reconstruction of the tongue and floor of mouth. Arch Otolaryngol. 2002;128:1388–1395. doi: 10.1001/archotol.128.12.1388. [DOI] [PubMed] [Google Scholar]
- 29.Calcaterra TC. Laryngeal suspension after supraglottic laryngectomy. Arch Otolaryngol. 1971;94(4):306–309. doi: 10.1001/archotol.1971.00770070498003. [DOI] [PubMed] [Google Scholar]
- 30.Heitmiller RF, Tseng E, Jones B. Prevalence of aspiration and laryngeal penetration in patients with unilateral vocal fold motion impairment. Dysphagia. 2000;15(4):184–187. doi: 10.1007/s004550000026. [DOI] [PubMed] [Google Scholar]
- 31.Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia. 1999;14(4):228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- 32.Hamlet SL, Muz J, Patterson R, Jones L. Pharyngeal transit time: assessment with videofluoroscopic and scintigraphic techniques. Dysphagia. 1989;4(1):4–7. doi: 10.1007/BF02407396. [DOI] [PubMed] [Google Scholar]
- 33.Tracy JF, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugler C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1989;4(2):90–94. doi: 10.1007/BF02407151. [DOI] [PubMed] [Google Scholar]
- 34.Blonsky ER, Logemann JA, Boshes B, Fisher HB. Comparison of speech and swallowing function in patients with tremor disorders and in normal geriatric patients: a cinefluorographic study. J Gerontol. 1975;30(3):299–303. doi: 10.1093/geronj/30.3.299. [DOI] [PubMed] [Google Scholar]
- 35.Barkmeier JM, Bielamowicz S, Takeda N, Ludlow CL. Laryngeal activity during upright vs. supine swallowing. J Appl Physiol (1985) 2002;93(2):740–745. doi: 10.1152/japplphysiol.00380.2001. [DOI] [PubMed] [Google Scholar]
- 36.Gramiak R, Kelley MLJ, Jr, Gravina RF. Nasal pressure changes during swallowing; an analysis of 1,219 swallows in 88 healthy subjects. Am J Roentgenol Radium Ther Nucl Med. 1967;99(3):562–576. [PubMed] [Google Scholar]
- 37.Inagaki D, Miyaoka Y, Ashida I, Ueda K, Yamada Y. Influences of body posture on duration of oral swallowing in normal young adults. J Oral Rehabil. 2007;34(6):414–421. doi: 10.1111/j.1365-2842.2007.01737.x. [DOI] [PubMed] [Google Scholar]
- 38.Ingervall B, Lantz B. Significance of gravity on the passage of bolus through the human pharynx. Arch Oral Biol. 1973;18(3):351–356. doi: 10.1016/0003-9969(73)90158-1. [DOI] [PubMed] [Google Scholar]
- 39.Dejaeger E, Pelemans W, Ponette E, Vantrappen G. Effect of body position on deglutition. Dig Dis Sci. 1994;39(4):762–765. doi: 10.1007/BF02087420. [DOI] [PubMed] [Google Scholar]
- 40.Perry JL, Bae Y, Kuehn DP. Effect of posture on deglutitive biomechanics in healthy individuals. Dysphagia. 2012;27(1):70–80. doi: 10.1007/s00455-011-9340-6. [DOI] [PubMed] [Google Scholar]
- 41.Rasley A, Logemann JA, Kahrilas PJ, Rademaker AW, Pauloski BR, Dodds WJ. Prevention of barium aspiration during videofluoroscopic swallowing studies: value of change in posture. AJR Am J Roentgenol. 1993;160(5):1005–1009. doi: 10.2214/ajr.160.5.8470567. [DOI] [PubMed] [Google Scholar]
- 42.Honda Y, Hata N. Dynamic imaging of swallowing in a seated position using open-configuration MRI. J Magn Reson Imaging. 2007;26(1):172–176. doi: 10.1002/jmri.20992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


