Abstract
Although peroxynitrite is harmful to the host, the beneficial effects of peroxynitrite are less well understood. We explored the role of peroxynitrite in the host immune response to Coxsackievirus infection. Peroxynitrite inhibits viral replication in vitro, in part by inhibiting viral RNA entry into the host cell. Nitrotyrosine, a marker for peroxynitrite production, is colocalized with viral antigens in the hearts of infected mice but not control mice. Nitrotyrosine coprecipitates with the viral polypeptide VP1 as well. Guanidinoethyl disulfide, a scavenger of peroxynitrite, blocks peroxynitrite inhibition of viral replication in vitro and permits an increase in viral replication in vivo. These data suggest that peroxynitrite is an endogenous effector of the immune response to viruses.
Keywords: nitric oxide, picornavirus, superoxide, virus, nitrotyrosine
Nitric oxide (NO) is an effector of the innate immune response to viral infection (1–3). NO inhibits the replication of a wide variety of viruses, but the mechanisms by which NO inhibits viral replication are not completely understood (4–7). NO inhibits HIV replication at a step after reverse transcriptase that may include inactivation of the HIV protease (8, 9). NO decreases DNA and protein synthesis of vaccinia virus by unknown mechanisms (4, 10). NO delays assembly of virions of vaccinia virus (11). A few viral targets of NO have been defined. For example, NO decreases Epstein–Barr virus replication by targeting the Zta transcription factor (12). In addition, NO inhibits Coxsackievirus replication by S-nitrosylation of viral cysteine proteases (13, 14). However, NO may inhibit viral replication by other mechanisms as well.
NO may also inhibit viral replication indirectly by forming reactive nitrogen intermediates (2). Peroxynitrite, formed by the reaction of NO and superoxide, is a powerful oxidant produced during inflammation in animals and humans (15–21). Peroxynitrite oxidation of proteins leads to the formation of nitrotyrosine (22), which is found during viral infection (23–25). Peroxynitrite in high concentrations can be harmful to the host, oxidizing lipids, oxidizing DNA, and oxidizing or nitrating proteins (18, 21, 26). However, peroxynitrite in low concentrations may be beneficial to the host during pathogen infection. Indirect evidence supporting this theory is the existence of peroxiredoxins in bacteria that rapidly detoxify peroxynitrite (27). We hypothesized that peroxynitrite inhibits viral replication.
Methods
Materials. Peroxynitrite was purchased from Upstate Biotechnology (Lake Placid, NY). Guanidinoethyl disulfide (GED), l-nitroarginine methyl ester (l-NAME), and d-NAME were purchased from Sigma–Aldrich. Coxsackievirus B3 (CVB3) Nancy strain was the generous gift of Charles Gauntt (University of Texas Health Science Center, San Antonio). C3H/HeNHsd mice were from Harlan Laboratories (Indianapolis).
Cell Culture and CVB3 Propagation. HeLa cells (American Type Culture Collection) were cultured in 10% FCS containing MEM (Invitrogen). To propagate CVB3, HeLa cell monolayers were infected with CVB3 at a multiplicity of infection (MOI) of 10 for 1 h at 37°C. Infected cells were then incubated in 2% FCS containing MEM at 37°C. To measure the amount of virus, infected cells and culture medium were frozen and thawed three times and centrifuged at 913 × g for 10 min; supernatants were then quantitated by the plaque assay as described in ref. 13. The titer of the CVB3 stock was 2.0 × 108 plaque-forming units (pfu)/ml.
Peroxynitrite Treatment of CVB3. CVB3 particles (107 pfu/ml) were treated with peroxynitrite at various concentrations for a total of three doses because of the short half-life of peroxynitrite. HeLa cell monolayers were then infected with peroxynitrite-treated CVB3 for 1 h at 37°C and then incubated in 2% FCS containing MEM at 37°C for 8 h. Infected cells and culture medium were frozen and thawed three times, centrifuged at 913 × g for 10 min, and then supernatants were quantitated by the plaque assay as described above.
Preparation of Radioactively Labeled CVB3. To make radiolabeled CVB3, we added 19.4 MBq/ml (525 μCi/ml) of trans-35S label (a mixture of [35S]methionine and [35S]cysteine at 43.5 TBq/mmol) (ICN) at 3 h after infection of a confluent monolayer of HeLa cells with CVB3 at a MOI of 10. At 8 h after infection, HeLa cells and medium of infected HeLa cells were frozen and thawed three times and centrifuged at 2,793 × g for 10 min. The supernatant was collected and then centrifuged at 100,000 × g for 90 min, producing a pellet containing 35S-labeled CVB3 with a specific activity of 0.021 cpm/pfu.
Binding Assay of CVB3 to HeLa Cells. HeLa cell monolayers were incubated with 35S-labeled CVB3 at a MOI of 40 (0.021 cpm/pfu) at 4°C for 30 min. The cells were washed with MEM and lysed with 2% SDS. [35S]CVB3 in the samples was measured with a liquid scintillation counter. Competition with a nonlabeled CVB3 was performed by incubating HeLa cells with labeled CVB3 at a MOI of 40 (0.021 cpm/pfu) and nonlabeled CVB3 at a MOI of 2,000.
Isolation of CVB3 RNA and Its Transfection into the HeLa Cells. CVB3 RNA was isolated by using the QIAamp viral RNA mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Viral RNA was transfected into the HeLa cells by using Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. After 48 h of incubation, infected cells and culture medium were frozen and thawed three times and centrifuged at 913 × g for 10 min, and the supernatants were quantitated by the plaque assay as described above.
Isolation of CVB3 RNA and Its Amplification by RT-PCR. HeLa cells were infected with peroxynitrite- or GED/peroxynitrite-treated CVB3 at a MOI of 10 for 1 h at 37°C. Infected cells were scraped from the plates and then centrifuged at 20,357 × g for 2 min. The pellet was suspended in PBS, frozen and thawed three times, and then ultracentrifuged at 100,000 × g for 90 min to separate cytosol from membranes. RNA was harvested from cytosolic fractions with the QIAamp viral RNA mini kit (Qiagen). RT-PCR was performed by using the OneStep RT-PCR kit (Qiagen) according to the manufacturer's instructions. RNA from cytosolic fractions was reverse-transcribed at 50°C for 30 min and then subsequently amplified by using 30 cycles of PCR under the following conditions: 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 20 seconds. The following primers were used: sense primer, 5′-ACTCTGCAGCGGAACCGACTA (positions 526–546 in the CVB3 cDNA sequence); antisense primer, 5′-GCTGTATTCAACTTAACAATG (positions 738–758 in the CVB3 cDNA sequence) (13). The PCR products were analyzed by 2% agarose gel electrophoresis and visualized with ethidium bromide staining.
Immunoblot Analysis. A solution of CVB3 in Hanks' balanced salt solution (107 pfu/ml) was treated with various concentrations of peroxynitrite as described above, fractionated by SDS/PAGE, and then transferred to a poly(vinylidene difluoride) membrane (Bio-Rad). Nontreated CVB3 was loaded as a negative control, and nitrated superoxide dismutase (Upstate Biotechnology) was used as a positive control. Coxsackievirus polypeptide viral protein 1 (VP1) was detected with a monoclonal antibody to enterovirus VP1 (NovoCastra, Newcastle, U.K.) at a dilution of 1/500. Expression of nitrated proteins was detected by antibodies to nitrotyrosine (Upstate Biotechnology) at a concentration of 0.5 μg/ml. Membranes were washed, hybridized with the appropriate secondary antibody conjugated to horseradish peroxidase (Amersham Pharmacia), and developed with a chemiluminescent system (Amersham Pharmacia) according to the manufacturer's instructions.
Immunoprecipitation with Anti-Nitrotyrosine Antibody. For immunoprecipitation of infected cell lysates, CVB3 in Hanks' balanced salt solution (107 pfu/ml) was treated with different concentrations of peroxynitrite and then incubated with 10 μg of antibody to nitrotyrosine (Upstate Biotechnology) at 4°C for 18 h, precipitated at 4°C with 20 μl of protein A agarose (Sigma–Aldrich), washed, and fractionated by SDS/PAGE. For immunoprecipitation of infected murine tissue, mice were infected as above and VP1 and nitrotyrosine coimmunoprecipitation were analyzed by incubating heart lysates with antibody to nitrotyrosine (Upstate Biotechnology), precipitating with Sepharaose A/G beads, washing precipitants with lysis buffer, and then immunoblotting precipitants with antibody to VP1 (NovoCastra).
Immunohistochemistry. Hearts were harvested from mice, fixed in 4% formalin in PBS, embedded in paraffin, and sectioned. Sections were mounted on slides and stained with antibody to VP1 (NovoCastra) and developed with Fast red, or stained with antibody to nitrotyrosine (Upstate Biotechnology), and developed with diaminobenzidine. Double-staining was performed with a kit according to the manufacturer's instructions (DAKO)
Measurement of Peroxynitrite Scavenging by GED. CVB3 in Hanks' balanced salt solution (107 pfu/ml) was treated with GED at various concentrations, then treated or not with peroxynitrite for a total of three doses, and then HeLa cell monolayers were infected for 1 h at 37°C. Infected cells were then incubated in 2% FCS containing MEM at 37°C for 8 h. Infected cells and culture medium were frozen and thawed three times, centrifuged at 913 × g for 10 min, and then supernatants were quantitated by the plaque assay as described above, and immunoprecipitation and immunoblotting for VP1 was performed as described above.
CVB3 Infection of Mice. Male C3H/HeNHsd mice (Harlan Laboratories) aged 3–4 weeks and weighing 15 g were inoculated i.p. with 103 pfu/ml of CBV3 in 0.2 ml of MEM. Infection of this strain of mice with CBV3 (Nancy) results in a nonlethal myocarditis. Some mice were treated with 0.1–1.0 mg/ml l-NAME in the drinking water 1 d before infection. Mice were then infected with 103 pfu of CVB3 i.p. Treatment with control or l-NAME continued for 5 d, and then the hearts were harvested. Some hearts were analyzed for viral titers as above. Other hearts were fixed in formalin, embedded in paraffin, and sectioned; sections of heart were stained with hematoxylin/eosin, antibody to nitrotyrosine, and antibody to VP1.
GED Administration to Mice. To determine the effect of peroxynitrite on viral infection, mice were treated or not with GED. GED (Sigma) was suspended in PBS and stored at 4°C. Mice were injected i.p. twice a day on days 0–3 of infection with GED at 0, 10, or 30 mg·kg–1·d–1 (n = 3). CVB3 was injected on day 0 as above. Mice were killed 5 d after infection, and 10% (wt/vol) heart homogenates were prepared in MEM. The amount of virus was quantitated by the plaque assay as described above.
Results
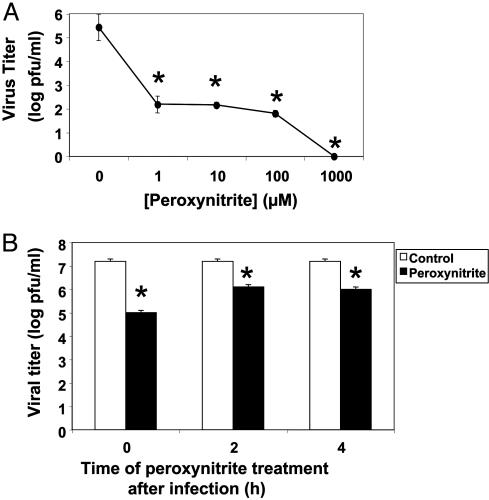
Pretreatment of Virus with Peroxynitrite Decreases Viral Replication. We first explored the effects of peroxynitrite on viral replication. We pretreated CVB3 with increasing amounts of peroxynitrite, added pretreated virus to HeLa cells at a MOI of 10, harvested virus after 8 h, and measured the amount of virus by the plaque assay. Peroxynitrite decreases viral replication in a dose-dependent manner (Fig. 1A). Concentrations of 1 μM peroxynitrite decreases viral replication by >1,000-fold. As little as 0.01 μM peroxynitrite decreases viral replication by 100-fold (Fig. 6, which is published as supporting information on the PNAS web site). These data show that peroxynitrite inhibits viral replication.
Fig. 1.
Peroxynitrite decreases CVB3 replication. (A) Dose–response. CVB3 was pretreated with increasing doses of peroxynitrite and then titered on HeLa cells (n = 3 ± SD) *, P < 0.01 versus 0 μM peroxynitrite. (B) HeLa cells were infected with CVB3 (MOI = 10) and treated with 100 μM peroxynitrite at various times after infection. Eight hours after infection, the amount of virus produced was measured by titering (n = 3 ± SD) *, P < 0.01 versus control.
We next determined whether or not peroxynitrite inhibits viral replication if it is administered during the viral life cycle instead of immediately before infection. We infected HeLa cells with CVB3 at a MOI of 10 and then treated infected cells with 100 μM peroxynitrite at 0, 2, or 4 h after infection. The amount of virus was measured as above. Peroxynitrite inhibits viral infection most when added at the time of infection (Fig. 1B). However, peroxynitrite still has an inhibitory effect on viral replication even when added 2 or 4 h after infection. These data show that peroxynitrite inhibits CVB3 replication.
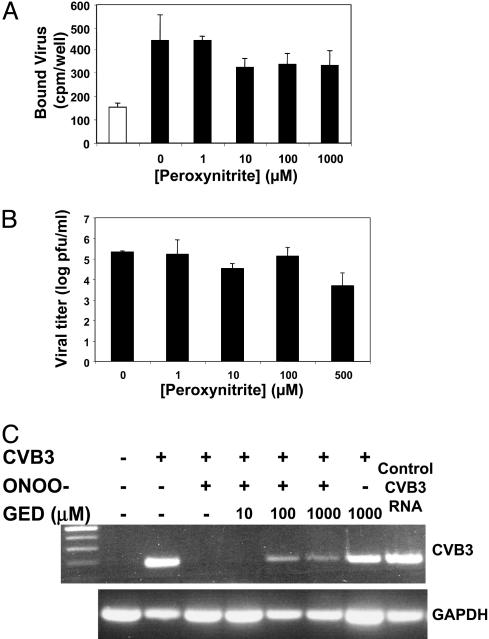
Mechanism of Peroxynitrite Antiviral Effect. To explore the mechanism by which peroxynitrite inhibits viral replication, we first examined the effect of peroxynitrite on virion binding to host cells. We prepared radiolabeled CVB3, pretreated the virus with increasing concentrations of peroxynitrite, and then added radiolabeled virus to HeLa cells at 4°C for 20 min. We washed the cells and then measured the amount of bound virus. (As a control, excess nonlabeled virus was added to cells incubated with radiolabeled virus.) Peroxynitrite has a minimal effect on CVB3 binding to HeLa cells (Fig. 2A). Although 1 μM peroxynitrite decreases binding by 5% (Fig. 2 A), 1 μM peroxynitrite decreases viral replication by >1,000-fold (Fig. 1 A). Thus, the slight decrease in viral binding is unlikely to account for the large decrease in viral replication observed after peroxynitrite treatment.
Fig. 2.
Peroxynitrite inhibits RNA entry into cells but does not affect viral binding or viral RNA. (A) Peroxynitrite has no significant effect on CVB3 binding. HeLa cells were incubated with [35S]CVB3 at 4°C for 20 min and washed. The amount of bound virus was then measured in a scintillation counter. As a control, 50-fold excess nonlabeled virus was added to labeled virus (white bar) (n = 3 ± SD; P > 0.1 for all peroxynitrite samples versus 0 μM). (B) Peroxynitrite has a minor effect on viral RNA. CVB3 RNA was pretreated with increasing doses of peroxynitrite and transfected into HeLa cells. The amounts of virus in cell lysates prepared 8 h after transfection were quantified by the plaque assay (n = 3 ± SD). (C) Peroxynitrite blocks viral RNA entry into host cells. CVB3 was pretreated or not with 1 μM peroxynitrite and incubated with HeLa cells (MOI = 10) for 1 h at 37°C. HeLa cells were then harvested, cytoplasm was collected from cell lysates by ultracentrifugation, and total RNA was isolated and analyzed by RT-PCR for CVB3 RNA. Some virions were pretreated with the peroxynitrite scavenger GED before exposure to peroxynitrite. ONOO–, peroxynitrite. This experiment was repeated twice with similar results.
Because peroxynitrite might cause oxidative damage to nucleic acids, we next examined the effect of peroxynitrite on viral RNA. We purified viral RNA from CVB3, pretreated this viral RNA with increasing concentrations of peroxynitrite, transfected the viral RNA into HeLa cells, and measured the amount of viral replication as above. Peroxynitrite has a minimal effect on viral RNA (Fig. 2B).
We hypothesized that peroxynitrite blocks viral RNA entry into host cells. To test this hypothesis, we pretreated CVB3 with 1 μM peroxynitrite; we then added the pretreated virus to HeLa cells at a MOI of 10 and incubated them together for 1 h at 37°C. Total RNA was prepared from cytosol of HeLa cells and analyzed by RT-PCR by using primers specific for CVB3. CVB3 RNA is absent from cells that are not infected (Fig. 2C). CVB3 RNA is present in cells infected with CVB3. However, CVB3 RNA is absent in cells infected with peroxynitrite-treated CVB3 (Fig. 2C). We then added peroxynitrite to CVB3 that had been pretreated with GED, a peroxynitrite scavenger. GED partially decreases the effect of peroxynitrite on viral RNA entry into HeLa cells (Fig. 2C). These data show that peroxynitrite inhibits viral replication in part by blocking viral RNA entry into host cells.
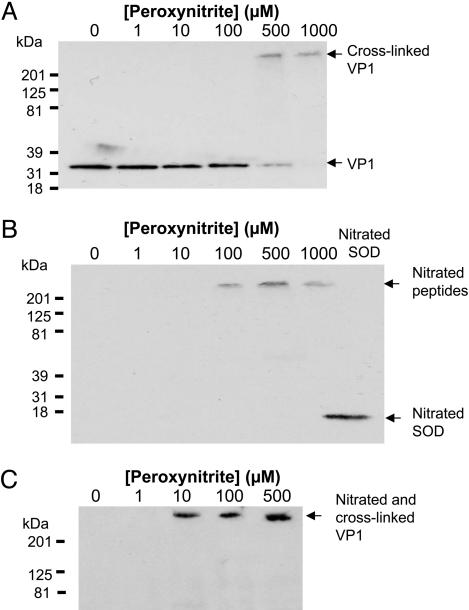
Peroxynitrite Nitrates and Cross-Links Viral Capsid Polypeptides. Because peroxynitrite can nitrate tyrosine and form dityrosine crosslinkages, we hypothesized that peroxynitrite chemically modifies viral capsid polypeptides. To determine whether or not peroxynitrite cross-links CVB3 capsid polypeptides, we analyzed viral polypeptides by immunoblotting. CVB3 was treated with peroxynitrite as above, fractionated by SDS/PAGE, and immunoblotted with antibody to VP1. Untreated CVB3 contains VP1 at the expected molecular weight of ≈33 kDa (Fig. 3A). Increasing concentrations of peroxynitrite diminish the intensity of the 31-kDa band. As the 31-kDa band diminishes, a new band appears at a high molecular weight (Fig. 3A). These data suggest that peroxynitrite cross-links viral capsid polypeptides.
Fig. 3.
Peroxynitrite nitrates and cross-links CVB3 capsid proteins. (A) Immunoblot for VP1. CVB3 was treated with peroxynitrite, fractionated by SDS/PAGE, and immunoblotted with antibody to VP1. (B) Immunoblot for nitrotyrosine. CVB3 was treated with peroxynitrite, fractionated by SDS/PAGE, and immunoblotted with antibody to nitrotyrosine. Nitrated SOD was used as a positive control. (C) Immunoprecipitation for nitrotyrosine followed by immunoblot for VP1. CVB3 was treated with peroxynitrite, immunoprecipitated with antibody to nitrotyrosine, and immunoblotted with antibody to VP1.
Peroxynitrite might cross-link peptides in part by forming nitrotyrosine, which in turn can lead to dityrosine bridges. Accordingly, we searched for nitrotyrosine residues on viral polypeptides. We treated CVB3 with increasing concentrations of peroxynitrite, fractionated the treated CVB3 by SDS/PAGE, and immunoblotted with antibody to nitrotyrosine. Nontreated virus does not contain nitrotyrosine residues. However, peroxynitrite treatment leads to the formation of nitrotyrosine residues (Fig. 3B).
To show that the nitrotyrosine residues are located on viral polypeptides, we immunoprecipitated virus treated with peroxynitrite. We treated CVB3 with peroxynitrite, immunoprecipitated the mixture with antibody to nitrotyrosine, fractionated precipitants on SDS/PAGE, and immunoblotted them with antibody to VP1. Nontreated CVB3 does not contain nitrated VP1 (Fig. 3C). In contrast, peroxynitrite treatment leads to the formation of nitrotyrosine residues on VP1 (Fig. 3C). Taken together, these data show that peroxynitrite nitrates and cross-links viral polypeptides in vitro.
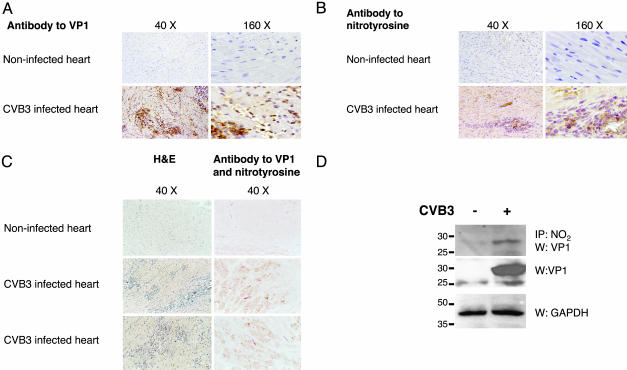
Nitrotyrosine and Viral Polypeptides Are Colocalized in Inflammatory Foci of Infected Mouse Hearts. We next determined whether peroxynitrite is produced in the same region as CVB3 is located in vivo. We infected 4-week-old male C3H/HeNHsd mice with 103 pfu of CVB3, killed the mice 5 d after infection, and performed single and double staining on sections of heart with antibodies to nitrotyrosine and VP1. Foci of inflammation and viral peptide VP1 expression are present in the hearts of infected animals but not in control animals (Fig. 4A). Nitrotyrosine is present in hearts of infected animals but absent from control animals (Fig. 4B). Nitrotyrosine and viral protein VP1 are colocalized in infected mice but absent from control mice (Fig. 4C). Areas of nitrotyrosine and VP1 colocalization occur in foci of inflammation (Fig. 4C).
Fig. 4.
Nitrotyrosine and viral proteins colocalize and coprecipitate in infected mice. Mice were infected with 103 pfu of CVB3; hearts were harvested 5 d after infection; and serial sections of the heart were stained for VP1, nitrotyrosine, or both. (A) VP1 expression. VP1 is localized to individual inflammatory cells and to foci of inflammation. (B) Nitrotyrosine localization. Nitrotyrosine localizes to foci of inflammation. (C) VP1 and nitrotyrosine colocalization. Serial sections of the heart were stained with hematoxylin/eosin or with antibodies to both VP1 (red) and nitrotyrosine (brown). Nitrotyrosine and VP1 colocalize to foci of inflammation. Double-staining was performed on 3–4 mice per group with similar results. (D) VP1 and nitrotyrosine coimmunoprecipitate. Hearts from mice infected or not as above were lysed, immunoprecipitated with antibody to nitrotyrosine, and immunoblotted with antibody to VP1 (Top). Lysates from the same mice were also immunoblotted with antibody to VP1 (Middle) or GAPDH (Bottom). This experiment was repeated twice with similar results.
We next explored whether or not viral peptides are nitrated in vivo. Mice were infected or not with CVB3 as above and killed 5 d after infection, and then heart lysates were prepared. Heart lysates were immunoprecipitated with antibody to nitrotyrosine, and precipitants were immunoblotted with antibody to VP1. VP1 coprecipitates with nitrotyrosine (Fig. 4D). These data imply that peroxynitrite is synthesized in regions in which CVB3 is located and suggest that VP1 is nitrated in vivo.
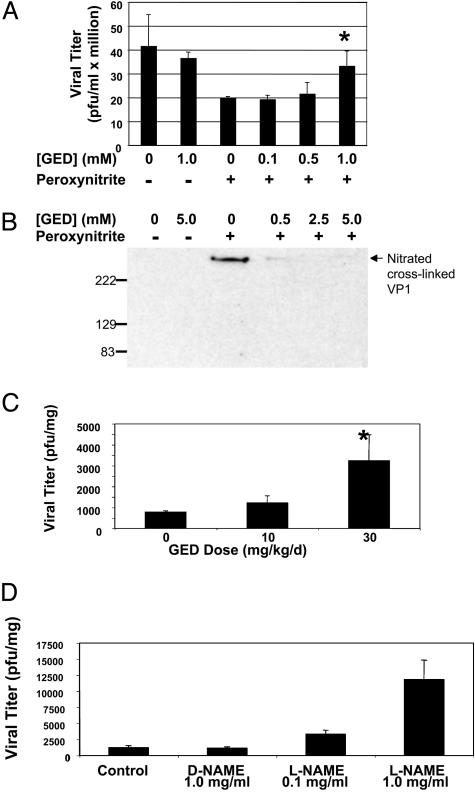
Treatment of Infected Mice with Peroxynitrite Scavenger GED Increases Viral Replication. If peroxynitrite is an innate immune effector, then scavengers of peroxynitrite would be expected to permit an increase in viral replication. We first explored the effect of GED, a peroxynitrite scavenger, on peroxynitrite inhibition of viral replication in vitro (28–31). CVB3 was pretreated with or without 0.0–1.0 mM GED and 100 μM peroxynitrite was added. The treated CVB3 was then added to HeLa cells at an MOI of 1. Virus was harvested after 8 h of infection and quantitated as above. Peroxynitrite inhibits viral replication as shown previously. However, GED blocks the inhibitory effects of peroxynitrite in a dose-responsive manner (Fig. 5A). GED itself has no effect on viral replication.
Fig. 5.
The peroxynitrite scavenger GED permits increased viral replication. (A) GED blocks peroxynitrite inhibition of viral replication in cells. CVB3 was pretreated with or without GED and then treated or not with 100 μM peroxynitrite. HeLa cells were then infected with the pretreated CVB3 for 8 h, and the amount of virus replication was measured by the plaque assay. (B) GED blocks peroxynitrite nitration of viral peptide VP1 in vitro. Shown is an immunoprecipitation for nitrated VP1. CVB3 was pretreated with or without GED and then treated or not with 500 μM peroxynitrite, immunoprecipitated with antibody to nitrotyrosine, and immunoblotted with antibody to VP1. (C) The peroxynitrite scavenger GED permits an increase in viral replication in mice. Mice were treated with the peroxynitrite scavenger GED from days 0–3. On day 0, mice were infected with 103 pfu of CVB3, and the amount of virus was measured in the heart 5 d after infection. (n = 3 ± SD) *, P < 0.05 for 30 versus 0 mg·kg–1·d–1. (D) The NOS inhibitor L-NAME permits an increase in viral replication in mice. Mice were pretreated for 1 d with increasing amounts of L-NAME orally and then infected with 103 pfu of CVB3 i.p. Treatment with control or L-NAME continued for 5 d, and then the hearts were harvested and analyzed for viral titers as above (n = 3 ± SD).
To show that GED blocks the formation of nitrotyrosine residues caused by peroxynitrite, we immunoprecipitated virus treated with or without GED (from 0–5.0 mM) and with or without peroxynitrite (500 μM). We immunoprecipitated the treated CVB3 with antibody to nitrotyrosine and fractionated precipitants on SDS/PAGE. Immunoblot analysis was performed with antibody to VP1. Nontreated CVB3 does not contain nitrated VP1 (Fig. 5B). The peroxynitrite treatment leads to the formation of nitrotyrosine residues on VP1 (Figs. 5B and 3B). However, GED blocks the formation of nitrotyrosine residues by peroxynitrite on VP1 (Fig. 5B).
To show the biological significance of peroxynitrite, we next treated infected mice with and without GED. Mice were injected i.p. twice a day on days 0–3 of infection with GED at 0, 10, or 30 mg·kg–1·d–1 (n = 3). CVB3 was injected on day 0 as above. Mice were killed 5 d after infection, and 10% (wt/vol) heart homogenates were prepared in MEM. The amount of virus was then quantitated by the plaque assay as described above. GED treatment permits an increase in viral replication in hearts in a dose-dependent manner (Fig. 5C).
One mechanism by which peroxynitrite is produced is by the reaction of NO and superoxide (15). If peroxynitrite inhibits viral replication, then virus replication should increase in mice unable to synthesize the precursors of peroxynitrite. Accordingly, we measured CVB3 replication in mice unable to make NO. Mice were pretreated with the NO synthase (NOS) inhibitor l-NAME in the drinking water and then infected with CVB3. NOS inhibition continued for 5 d until the mice were killed, and the amount of virus in hearts was measured by the plaque assay. NAME permits an increase in viral replication (Fig. 5D). These results demonstrate that virus replication increases during the inhibition of synthesis of NO, a peroxynitrite precursor. These data complement our previous studies, which show that CVB3 replication increases in NOS2 knock-out mice (32, 33). Taken together, these data show that GED blocks peroxynitrite inhibition of CVB3 replication in vitro and in vivo.
Discussion
The major finding of this study is that peroxynitrite inhibits viral replication. As little as 1 μM peroxynitrite decreases viral replication by 1,000-fold. Peroxynitrite appears to inhibit the entry of viral RNA into the host cell. Finally, inhibition of peroxynitrite production leads to an increase in viral replication in vivo. These data suggest a mechanism by which a host immune effector disrupts the viral life cycle.
Peroxynitrite appears to inhibit viral replication most when applied to the virus before infection. Peroxynitrite inhibits viral RNA entry into the host cell, but not virion binding to the host nor the viral RNA genome. The mechanism by which peroxynitrite inhibits viral RNA entry into the host cell may involve chemical modification of the viral capsid. Peroxynitrite nitration and cross-linking of capsid polypeptides may inhibit viral uncoating, which is necessary for viral entry into the host cell.
Peroxynitrite not only decreases viral replication when added to virus before infection (Fig. 1 A), but peroxynitrite also inhibits viral replication when added to cells during infection (Fig. 1B). The other host and viral targets of peroxynitrite have not been identified. Potential viral targets of peroxynitrite include proteases and RNA-dependent RNA polymerase; host enzymes necessary for viral replication may also be inhibited by peroxynitrite (9, 14). However, the greatest effect of peroxynitrite is during the early stages of viral replication.
Peroxynitrite is produced by the reaction of NO with superoxide (21). CVB3 infection triggers the infiltration into the heart of macrophages that express NOS2 and synthesize NO (34). Other potential sources of NO include NOS3 expressed in endothelial cells and cardiac myocytes (35). Monocytes infiltrating into the infected myocardium can also produce superoxide by the NADPH oxidase (36). Additional sources of superoxide include vascular smooth muscle cells that express the vascular oxidase Mox1 (37). Thus, a variety of cells in the heart can be stimulated to produce the precursors of peroxynitrite. Our data show that pharmacologic inhibition of NOS permits an increase in viral replication (Fig. 5D). These data complement our previous studies that show that genetic ablation of the NOS2 allele also allows CVB3 replication to increase (32, 33). One mechanism by which NO inhibits viral replication is by reversible S-nitrosylation of viral proteases (13, 14). Our current study shows that another antiviral mechanism of NO is the formation of peroxynitrite, which blocks viral entry into the host cell.
Peroxynitrite is produced by the host during viral infection and inhibits viral replication. Because phylogenetically divergent pathogens express peroxiredoxins that detoxify peroxynitrite (27), peroxynitrite may thus be beneficial to the host during infection caused by specific pathogens. Peroxynitrite may be an endogenous effector of the immune system.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL63706-04, R01 HL074061, P01 HL65608 (to C.J.L.), and P01 HL56091 (to W.M.B.); American Heart Association Grant EIG 0140210N; the Ciccarone Center; the John and Cora H. Davis Foundation (to C.J.L.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CVB3, Coxsackievirus serotype B3; NOS, NO synthase; GED, guanidinoethyl disulfide; NAME, nitroarginine methyl ester, MOI, multiplicity of infection; pfu, plaque-forming units.
References
- 1.Bogdan, C. (2001) Nat. Immunol. 2, 907–916. [DOI] [PubMed] [Google Scholar]
- 2.Nathan, C. (2003) J. Clin. Invest. 111, 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang, F. C. (1997) J. Clin. Invest. 99, 2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karupiah, G., Xie, Q. W., Buller, R. M., Nathan, C., Duarte, C. & MacMicking, J. D. (1993) Science 261, 1445–1448. [DOI] [PubMed] [Google Scholar]
- 5.Mannick, J. B. (1995) Res. Immunol. 146, 693–697. [DOI] [PubMed] [Google Scholar]
- 6.Reiss, C. S. & Komatsu, T. (1998) J. Virol. 72, 4547–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMicking, J., Xie, Q. W. & Nathan, C. (1997) Annu. Rev. Immunol. 15, 323–350. [DOI] [PubMed] [Google Scholar]
- 8.Mannick, J. B., Stamler, J. S., Teng, E., Simpson, N., Lawrence, J., Jordan, J. & Finberg, R. W. (1999) J. Acquired Immune Defic. Syndr. 22, 1–9. [DOI] [PubMed] [Google Scholar]
- 9.Sehajpal, P. K., Basu, A., Ogiste, J. S. & Lander, H. M. (1999) Biochemistry 38, 13407–13413. [DOI] [PubMed] [Google Scholar]
- 10.Karupiah, G. & Harris, N. (1995) J. Exp. Med. 181, 2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esteban, M. & Patino, C. (2000) J. Interferon Cytokine Res. 20, 867–877. [DOI] [PubMed] [Google Scholar]
- 12.Mannick, J. B., Asano, K., Izumi, K., Kieff, E. & Stamler, J. S. (1994) Cell 79, 1137–1146. [DOI] [PubMed] [Google Scholar]
- 13.Zaragoza, C., Ocampo, C. J., Saura, M., McMillan, A. & Lowenstein, C. J. (1997) J. Clin. Invest. 100, 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saura, M., Zaragoza, C., McMillan, A., Quick, R. A., Hohenadl, C., Lowenstein, J. M. & Lowenstein, C. J. (1999) Immunity 10, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckman, J. S., Beckman, T. W., Chen, J., Marshall, P. A. & Freeman, B. A. (1990) Proc. Natl. Acad. Sci. USA 87, 1620–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espey, M. G., Miranda, K. M., Thomas, D. D., Xavier, S., Citrin, D., Vitek, M. P. & Wink, D. A. (2002) Ann. N.Y. Acad. Sci. 962, 195–206. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer, S., Gorren, A. C., Schmidt, K., Werner, E. R., Hansert, B., Bohle, D. S. & Mayer, B. (1997) J. Biol. Chem. 272, 3465–3470. [DOI] [PubMed] [Google Scholar]
- 18.Niles, J. C., Burney, S., Singh, S. P., Wishnok, J. S. & Tannenbaum, S. R. (1999) Proc. Natl. Acad. Sci. USA 96, 11729–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merenyi, G., Lind, J., Goldstein, S. & Czapski, G. (1998) Chem. Res. Toxicol. 11, 712–713. [DOI] [PubMed] [Google Scholar]
- 20.Wink, D. A., Hanbauer, I., Krishna, M. C., DeGraff, W., Gamson, J. & Mitchell, J. B. (1993) Proc. Natl. Acad. Sci. USA 90, 9813–9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckman, J. S. & Koppenol, W. H. (1996) Am. J. Physiol. 271, C1424–C1437. [DOI] [PubMed] [Google Scholar]
- 22.Ischiropoulos, H., Zhu, L., Chen, J., Tsai, M., Martin, J. C., Smith, C. D. & Beckman, J. S. (1992) Arch. Biochem. Biophys. 298, 431–437. [DOI] [PubMed] [Google Scholar]
- 23.Akaike, T., Noguchi, Y., Ijiri, S., Setoguchi, K., Suga, M., Zheng, Y. M., Dietzschold, B. & Maeda, H. (1996) Proc. Natl. Acad. Sci. USA 93, 2448–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boven, L. A., Gomes, L., Hery, C., Gray, F., Verhoef, J., Portegies, P., Tardieu, M. & Nottet, H. S. (1999) J. Immunol. 162, 4319–4327. [PubMed] [Google Scholar]
- 25.Akaike, T., Fujii, S., Kato, A., Yoshitake, J., Miyamoto, Y., Sawa, T., Okamoto, S., Suga, M., Asakawa, M., Nagai, Y. & Maeda, H. (2000) FASEB J. 14, 1447–1454. [DOI] [PubMed] [Google Scholar]
- 26.Radi, R., Beckman, J. S., Bush, K. M. & Freeman, B. A. (1991) Arch. Biochem. Biophys. 288, 481–487. [DOI] [PubMed] [Google Scholar]
- 27.Bryk, R., Griffin, P. & Nathan, C. (2000) Nature 407, 211–215. [DOI] [PubMed] [Google Scholar]
- 28.Szabo, C., Bryk, R., Zingarelli, B., Southan, G. J., Gahman, T. C., Bhat, V., Salzman, A. L. & Wolff, D. J. (1996) Br. J. Pharmacol. 118, 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suarez-Pinzon, W. L., Mabley, J. G., Strynadka, K., Power, R. F., Szabo, C. & Rabinovitch, A. (2001) J. Autoimmun. 16, 449–455. [DOI] [PubMed] [Google Scholar]
- 30.Scott, G. S., Kean, R. B., Southan, G. J., Szabo, C. & Hooper, D. C. (2001) Neurosci. Lett. 311, 125–128. [DOI] [PubMed] [Google Scholar]
- 31.Lakey, J. R., Suarez-Pinzon, W. L., Strynadka, K., Korbutt, G. S., Rajotte, R. V., Mabley, J. G., Szabo, C. & Rabinovitch, A. (2001) Lab. Invest. 81, 1683–1692. [DOI] [PubMed] [Google Scholar]
- 32.Zaragoza, C., Ocampo, C., Saura, M., Leppo, M., Wei, X. Q., Quick, R., Moncada, S., Liew, F. Y. & Lowenstein, C. J. (1998) Proc. Natl. Acad. Sci. USA 95, 2469–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaragoza, C., Ocampo, C. J., Saura, M., Bao, C., Leppo, M., Lafond-Walker, A., Thiemann, D. R., Hruban, R. & Lowenstein, C. J. (1999) J. Immunol. 163, 5497–5504. [PubMed] [Google Scholar]
- 34.Lowenstein, C. J., Hill, S. L., Lafond-Walker, A., Wu, J., Allen, G., Landavere, M., Rose, N. R. & Herskowitz, A. (1996) J. Clin. Invest. 97, 1837–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feron, O., Belhassen, L., Kobzik, L., Smith, T. W., Kelly, R. A. & Michel, T. (1996) J. Biol. Chem. 271, 22810–22814. [DOI] [PubMed] [Google Scholar]
- 36.Babior, B. M. (2000) Am. J. Med. 109, 33–44. [DOI] [PubMed] [Google Scholar]
- 37.Suh, Y. A., Arnold, R. S., Lassegue, B., Shi, J., Xu, X., Sorescu, D., Chung, A. B., Griendling, K. K. & Lambeth, J. D. (1999) Nature 401, 79–82. [DOI] [PubMed] [Google Scholar]