Abstract
Copolymer 1 [Cop1, glatiramer acetate, Copaxone, poly(Y,E,A,K)n] is widely used in the treatment of relapsing/remitting multiple sclerosis in which it reduces the frequency of relapses by ≈30%. In the present study, copolymers with modified amino acid compositions (based on the binding motif of myelin basic protein 85–99 to HLA-DR2) have been developed with the aim of suppressing multiple sclerosis more effectively. The enhanced efficacy of these copolymers in experimental autoimmune encephalomyelitis (EAE) induced in SJL/J mice with proteolipid protein 139–151 was demonstrated by using three protocols: (i) simultaneous administration of autoantigen and copolymer (termed prevention), (ii) pretreatment with copolymers (vaccination), or (iii) administration of copolymers after disease onset (treatment). Strikingly, in the treatment protocol administration of soluble VWAK and FYAK after onset of disease led to stasis of its progression and suppression of histopathological evidence of EAE. The mechanisms by which these effects are achieved have been examined in several types of assays: binding of copolymers to I-As in competition with proteolipid protein 139–151 (blocking), cytokine production by T cells (T helper 2 polarization), and transfer of protection by CD3+ splenocytes or, notably, by copolymer-specific T cell lines (induction of regulatory T cells). The generation of these copolymer-specific regulatory T cells that secrete IL-4 and IL-10 and are independent of the immunizing autoantigen is very prominent among the multiple mechanisms that account for the observed suppressive effect of copolymers in EAE.
Keywords: multiple sclerosis, cytokines, peptides, T cells, autoimmunity
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system affecting young adults. In Northern European Caucasian MS patients, the disease is strongly associated with the HLA-DR2 (DRA/DRB1*1501) haplotype (1–3). Experimental autoimmune encephalomyelitis (EAE), an animal model for MS, can be induced in mice by administration of peptides derived from myelin proteins, i.e., proteolipid protein (PLP) 139–151 (4), myelin oligodendrocyte glycoprotein 35–55 (5), or myelin basic protein (MBP) 85–99 (6–8). In this model, self-reactive CD4+ T cells produce IFN-γ, a T helper (Th)-1 cytokine, that is believed to mediate the disease (9), whereas Th2 cells and cytokines, namely IL-4 and IL-10, have been shown to reduce its severity (10, 11).
Various therapeutic strategies involving agents that compete with the process of recognition of HLA-DR2 (DRA/DRB1*1501)/MBP85–99 complexes by autoreactive T cells have been attempted in MS. Agents such as copolymers, peptides, oligomers, altered peptide ligands, and peptide antigens have been used for this purpose, and of these Copolymer 1 (Cop1) is currently widely used in the treatment of MS (12–21). Cop1 is a random amino acid copolymer of l-tyrosine (Y), l-glutamic acid (E), l-alanine (A), and l-lysine (K), in a molar ratio of ≈1.0:1.4:4.2:3.4, synthesized in solution by using N-carboxyamino acid anhydrides (20). Cop1 was originally designed to induce EAE, but instead was found to be effective in suppressing EAE (20–23) and is in current use in the treatment of relapsing-remitting forms of MS (24–26). A recent clinical study demonstrated its sustained efficacy in MS patients over a period of 6 years (27). Nevertheless, Cop1 only has a modest effect on the course of the disease. Much like IFN-β (28), it reduces the relapse rate by ≈30% in MS patients. Novel compounds with higher efficacy in the treatment of MS that take into consideration the fact that ≈60% of patients with MS are HLA-DR2 positive are needed. Additionally, the mechanism(s) by which Cop1 performs its suppressive function remains incompletely known (29).
Structural studies obtained from HLA-DR2/peptide complexes revealed that the P1 pocket of DRB1*1501 includes β86Val, which results in a small pocket that can accommodate relatively small hydrophobic amino acids such as V or F but not Y or W (30, 31). Additionally, the residues that form the P4 pocket include β71Ala. The resulting large hydrophobic pocket can accommodate F, Y, or W, whereas the P9 pocket is promiscuous. In so far as the binding of MBP 85–99 to HLA-DR2 is concerned, the P1 amino acid is V and the P4 amino acid is F, although Y or W at P4 would provide a tighter fit (30, 31). Judging from the components of Cop1, the amino acids YEAK do not appear to fit HLA-DR2 optimally.
For the present study several random 4-aa copolymers that were designed to bind more tightly in the key binding pockets of HLA-DR2 were generated. Replacing Y and E with a variety of amino acids was found to facilitate the interaction between the resulting copolymer and the DRB1*1501 pockets and improved binding. Among the copolymers generated, both VWAK and FYAK [initial studies of the latter as reported (15)] had a pronounced effect in suppressing the PLP 139–151-specific T cell response and the severity of EAE. These effects are mediated, at least in part, by copolymer-specific, antigen-nonspecific regulatory T cells.
Materials and Methods
Mice. SJL/J female mice (8–10 weeks of age, The Jackson Laboratory) were maintained according to the Guidelines of the Committee on Animals of Harvard University and the Committee on Care and Use of Laboratory Animal Resources, National Research Council (Department of Health and Human Services Publication 85-23, revised 1987). The procedures for induction and suppression of EAE and T cell proliferative responses in SJL/J mice have been described (15). Mice were scored in a double-blind manner.
Copolymers and Peptides. Copolymers and peptides were synthesized as described (15). Peptide sequences were PLP 139–151, HSLGKWLGHPDKF; hemagglutinin 306–318, PKYVKQNTLKLAT, either unlabeled or with biotin linked to the N terminus by the spacer SGSG and free acid at the C terminus; and Nase 101–120, EALVRQGLAKVAYVYKPNNT.
Peptide Binding to I-As Protein. These studies were performed as described (15) by using mAb Y3P to isolate I-As from LS 102.9 B cell lines by affinity chromatography (32). Binding of biotinylated PLP 139–151 to I-As was competed by copolymers or PLP 139–151.
Western Blot Analysis. Samples containing MHC or MHC-biotin labeled copolymer complexes were separated on 15% SDS/PAGE. The gel was blotted onto poly(vinylidene difluoride) (Schleicher & Schuell) membrane by using transblot apparatus (Bio-Rad). Membranes were blocked with 5% nonfat dry milk in TBS (0.05% Tween 20) buffer. MHC–biotin copolymer complexes were detected by using Streptavidin POD reagent (Roche Diagnostics). I-As was detected by using mAb Y3P and anti-mouse F(ab′)2-peroxidase (Roche Diagnostics).
Cytokine Measurement by ELISA. Lymphocytes from SJL mice immunized with PLP 139–151 with or without copolymers (FYAK, VWAK, and Cop1) were restimulated with the corresponding peptides or copolymers in the presence of antigen-presenting cells in 24-well plates for 3 days. Briefly, cytokine mAbs for IL-2, IL-4, IL-10, and IFN-γ were coated to 96-well plates at a concentration of 1 μg/ml overnight. The plates were washed and treated with blocking solution (Kirkegaard & Perry Laboratories), followed by incubation of cytokine standards and culture supernatants overnight at 4°C. The plates were washed and incubated with their corresponding biotinylated anticytokine-detecting mAb (1 μg/ml) for 2 h. They were developed after adding avidin peroxidase and its substrate. The mAb pairs used were from the following clones: IL-2, JES6-A112 and JES6-5H4; IL-4, 11B11 and BVD6-24G2; IL-10, JES5-16E3 and SXC-1; and IFN-γ, R4-6A2 and XMG 1.2 (Pharmingen).
Adoptive Transfer of CD3+ Spleen Cells. Splenocytes were isolated from SJL/J mice immunized with either PLP139–151 and copolymers (VWAK, FYAK, or Cop1) or PLP 139–151 alone. After 24 days, a mouse T cell enrichment column (R & D Systems) was used to isolate CD3+ cells. T cells (5 × 106) were injected i.v. into naïve 6- to 8-week-old SJL/J mice. The next day, mice were immunized with PLP 139–151 peptide (50 μg per mouse) as described (15) and scored daily for signs of EAE.
Adoptive Transfer of PLP 139–151 and Copolymer-Specific T Cell Lines. SJL/J mice were immunized with either 50 μg of PLP 139–151 peptide or 500 μg of copolymers (VWAK, FYAK, or Cop1). Stimulator cells were prepared by loading naïve SJL/J splenocytes in vitro for 12 h with 10 μg/ml PLP or copolymers (VWAK, FYAK, or Cop1). Ten days postimmunization splenocytes were cocultured with irradiated stimulators (3,000 rad) in 1:1 ratio for 5 days in T 25 flasks (1 × 108 total cells in media containing 20 units of IL-2) and restimulated weekly for 3 weeks with fresh antigen-loaded splenocytes to obtain cell lines. T cells (5 × 106) from these lines were injected i.v. into naïve 6- to 8-week-old SJL/J mice and the protocol described above was followed.
Results
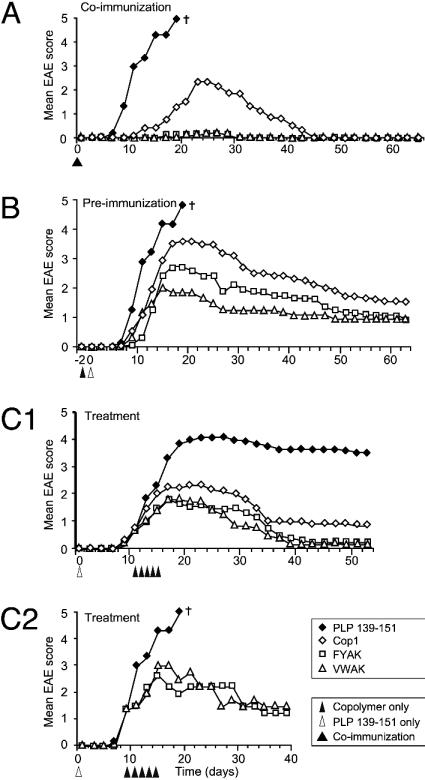
Amelioration of EAE by Synthetic Amino Acid Copolymers. Coimmunization of mice with PLP 139–151 and copolymers protects against EAE. SJL/J mice were immunized s.c. with 50 μg of PLP 139–151 and/or 500 μg of copolymer in CFA. In the PLP 139–151-immunized group, the first sign of EAE appeared at day 8 with a mortality of 100% (mean score, 5) by day 16 (Fig. 1A). Mice coimmunized with Cop1 (YEAK) developed EAE beginning at day 12 with a maximal mean score of 2.3 around day 23 followed by slow recovery by day 45. On the other hand, in mice coinjected with two copolymers, VWAK or FYAK, essentially no disease was evident, with only one mouse in each treatment group developing a mild disease (score of 1–2) for 10–12 days. However, two of six mice treated with two other copolymers, FWAK and VYAK, developed severe signs of EAE (score of 3) with no mortality and the remainder developed a mild disease (score of 1 or 2) (data not shown); no further experiments have been carried out with these latter two copolymers. All scoring in this and the following experiments was done in a double-blind manner.
Fig. 1.
Suppression of EAE induced with the PLP 139–151 by different random copolymers. Statistical analysis is shown in Table 1, which is published as supporting information on the PNAS web site. (A) SJL/J mice were coinjected s.c. with 50 μg of PLP 139–151 peptide and 500 μg of FYAK, VWAK, or Cop1 in CFA, or the PLP 139–151 peptide alone, with six to eight mice in each group. The progression of clinical signs of the disease was monitored daily. Results represent the mean daily score. This experiment is representative of four identical experiments with similar results. (B) SJL/J mice were immunized s.c. with 500 μg of copolymers in CFA 2 days before administration of 50 μgof PLP 139–151 in CFA and were observed daily for the appearance of EAE. Results represent the mean daily score. Six to 10 mice per group were used in each independent experiment. This experiment was carried out three times by using preimmunization with copolymers in CFA twice and in PBS/mannitol once, again with similar results in all three experiments. (C) Treatment of SJL/J mice after induction of PLP 139–151-induced EAE. SJL/J mice were injected s.c. with 50 μg of PLP 139–151 in CFA s.c. After onset of disease at a mean EAE score of 1, 12 mice in each group (C1) or at a mean score of 1.5, 4 mice in each group (C2) were injected s.c. on 5 consecutive days with 150 μg of copolymers in PBS/mannitol and observed daily for the appearance of signs of EAE. Data shown in C1 represent two separate trials of 12 mice per group. Note that experiment (C1) was carried out with a second batch of PLP 139–151 that was less pure and that only 6 of 10 mice in this study died, hence the plateau in EAE score. †, All mice died.
Preimmunization with copolymers protects mice against PLP 139–151-induced EAE. SJL/J mice were immunized s.c. with 500 μg of copolymer either in complete Freund's adjuvant (CFA) or PBS, 2 days before administration of 50 μg of PLP 139–151 in CFA (Fig. 1B). Copolymer in PBS or CFA yielded essentially identical data. All control mice immunized with PLP 139–151 developed severe EAE with a mortality of 100%. On the contrary, preinjection on day 2 with VWAK, FYAK, or Cop1 reduced the clinical signs of PLP 139–151-induced EAE. VWAK and FYAK reduced the intensity and duration of EAE with maximal mean scores of 1.9 and 2.8 on day 16, respectively, whereas Cop1-treated mice had a maximal mean score of 3.8 (Fig. 1B). Thus, in this assay VWAK was significantly more effective than the other copolymers. Residual disease was also significantly greater at day 64 with Cop1 than with either VWAK or FYAK. Preimmunization with copolymers 4 days before inducing the disease gave similar results (data not shown). Treatment of PLP 139–151-induced EAE with copolymers ameliorates already established disease. PLP 139–151 (50 μg in CFA) was injected s.c. into SJL/J mice. On day 11, at which time all mice had developed mild EAE (score: 1, limp tail), 150 μg of VWAK, FYAK, or Cop1 in PBS/mannitol per mouse was administered s.c. for 5 consecutive days. All of the copolymers suppressed further progression of EAE. VWAK- and FYAK-treated mice peaked on days 14 and 18 (maximal mean score 2, including one mortality in each group), whereas Cop1 was less effective (score 2.5 on day 16, including two mortalities) (Fig. 1C1). Again residual disease was evident at 54 days after treatment with Cop1 but not with either VWAK or FYAK. Six of nine untreated PLP 139–151-immunized mice died but three survived, resulting in a score of 3.9.
A second treatment using only four animals per group was initiated at a slightly later stage of EAE (score: 1.5 on day 9) (Fig. 1C2). In this experiment treatment with VWAK or FYAK (150 μg per mouse s.c. in PBS/mannitol for 5 successive days) was less effective (score: 2.5 or 3.0, two to three limbs paralyzed at day 14), with only one death in the copolymer-treated group, whereas all of the mice in the PLP 139–151-immunized group died by day 16. Histology. Histological analysis of tissues from animals sensitized with PLP alone showed perivascular mononuclear infiltrates typical of EAE at all levels of the brain and spinal cord. The appearance of these lesions in white matter tracts of the cerebellum is shown in Fig. 5, which is published as supporting information on the PNAS web site, and demonstrates the extensive infiltration of inflammatory cells into the CNS parenchyma, resulting in disruption of the normal tissue architecture. In animals treated with Cop1, the perivascular cuffs were smaller and infiltration of inflammatory cells into the surrounding parenchyma was less marked. In animals treated with FYAK, the lesions were even smaller, and lesions were detected only rarely at this site in animals treated with VWAK. Analysis of myelin loss, using immunhistochemical staining for MBP, showed that the extent of demyelination was well correlated with the extent of the inflammatory infiltrates, with large demyelinated plaques centered around blood vessels evident in the PLP controls, medium plaques in Cop1-treated mice, much smaller plaques in animals treated with FYAK, and well-preserved myelin in animals treated with VWAK.
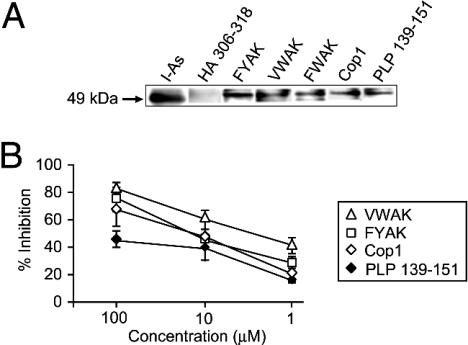
Mechanism of Suppression of PLP 139–151-Induced EAE by Copolymers. Blocking of binding of PLP 139–151 to I-As molecules by copolymers. To investigate whether various copolymers inhibited binding of PLP 139–151 to purified I-As, binding assays and confocal microscopy were performed. With biotinylated PLP 139–151 all of the copolymers were shown to compete for its binding to I-As (Fig. 2A). However, VWAK was significantly better than Cop1 or FYAK in competing with the biotinylated peptide (Fig. 2B), particularly at low concentrations. Confocal microscopy was also used to show that I-As and copolymers colocalized on the surface of bone marrow-derived murine dendritic cells (Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 2.
Inhibition of binding of biotinylated PLP 139–151 to I-As molecules by different competitors. (A) I-As (100 μM) isolated from LS102.9 cells was incubated with biotinylated peptides or copolymers (1 mM) for 48 h at 37C in PBS (30 μl total volume). Samples containing 15 μl of the mixture were separated on 15% SDS/PAGE. The gel was blotted onto a poly(vinylidene difluoride) membrane. I-As protein was detected by using mAb 10.3.6 and horseradish peroxidase (HRP)-conjugated anti-mouse IgG (lane 1). Peptide and copolymer complexes were detected with streptavidin HRP by using standard blotting and ECL detection techniques (lanes 2–7). (B) I-As molecules were incubated with biotinylated PLP 139–151 (0.13 μM, ♦) and the unlabeled copolymers (FYAK, □; Cop-1, ⋄; and VWAK, ▵) at a range of concentrations. All incubations were carried out in triplicate at pH 7.0 for 40 h at 37°C. Results represent one of three independent experiments. Specific binding is expressed as percentage of inhibition by using the formula: percentage of inhibition = 100% – [(absorbance at 450 nm with competitor – background)/(absorbance without competitor – background) × 100]. The signals at 450 nm without competitor was from 0.910 to 1.04, and the background was 0.08.
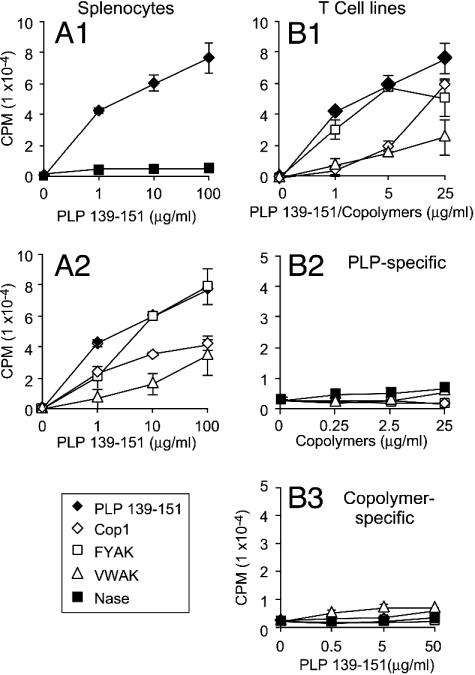
Suppression by copolymers of PLP-specific T cell proliferation. SJL/Jmice were immunized with 50 μg of PLP 139–151 in CFA alone or coimmunized with PLP and 500 μg of copolymers s.c. On day 10 splenocytes (that contain both T cells and antigen-presenting cells) from mice immunized with PLP 139–151 responded vigorously to the antigen in vitro, as expected, whereas little proliferation to PLP 139–151 was seen after immunization with the irrelevant peptide Nase (Fig. 3A1). However, when splenocytes from mice coimmunized with copolymers were restimulated with PLP 139–151, expansion of PLP 139–151-reactive T cells in splenocytes was suppressed; VWAK had the most pronounced effect followed by Cop1 and FYAK (Fig. 3A2).
Fig. 3.
Stimulation of splenocytes and T cell lines by PLP 139–151 or copolymers. (A) SJL mice were immunized with either PLP 139–151 in CFA (A1) or coimmunized with copolymers (A2). After 10 days, splenocytes were cultured for 3 days with PLP 139–151 alone (♦) or Nase peptide (used as a control) (▪). Sixteen hours after pulsing with 3H-thymidine, the proliferative response was measured as radioactivity incorporated. (B) SJL/J mice were immunized with either PLP 139–151 (♦) or copolymers, VWAK (▵, FYAK (□), and Cop1, (⋄) in CFA. After 10 days, splenocytes were restimulated with either PLP 139–151 or copolymers biweekly three times, and the proliferative response was determined as above to each of the four antigens of the homologous cell lines (B1), each of the copolymers or Nase of the PLP-specific cell line (B2), or PLP 139–151 of each of the copolymer-specific cell lines (B3).
Cell lines responsive to either PLP 139–151 or individual copolymers were then established by restimulation of splenocytes pulsed with the individual antigens alone over a period of 3 weeks (Fig. 3B1). The PLP 139–151-specific cell line did not react to any of the copolymers (Fig. 3B2). Likewise, PLP 139–151 did not stimulate any of the copolymer-specific T cell lines (Fig. 3B3), and no cross-stimulation by copolymers was observed (data not shown). Thus, no cross-reactivity between PLP 139–151 and copolymer-specific cell lines was observed and each of the copolymer-specific cell lines was specific for the copolymer used in immunization and restimulation.
Blocking by copolymers of PLP-specific T cell expansion in vitro. To determine the frequency of PLP-responsive T cells in spleen, I-As/PLP 139–151 tetramers were used. The percent PLP 139–151-reactive CD4 T cells in splenocytes was determined by flow cytometric analysis using Theiler's murine encephalomyelitis virus (TMEV) 70–86 tetramers as negative controls (32–34). Splenic lymphocytes from PLP 139–151-immunized mice were cultured with PLP 139–151 with or without copolymers for 4 days. The CD4+CD25+-activated T cell population was gated after eliminating dead cells. The I-As tetramer-positive cells within this CD4 subset were then determined (Fig. 7A, which is published as supporting information on the PNAS web site). After restimulation, splenocytes from mice immunized with PLP 139–151 alone had 4.61% PLP 139–151 tetramer+ CD4+ cells. No PLP 139–151-reactive CD4+ T cells were detected in mice immunized with only CFA. When cells from PLP 139–151-immunized mice were restimulated in vitro with both PLP 139–151 and copolymers, the frequency of PLP 139–151-reactive T cells was decreased in all three groups, Cop1 (4.01%), FYAK (3.34%), and VWAK (2.92%; P < 0.05). TMEV 70–86 tetramer-stained cells were negligible in all groups. The suppressive effect on the antigen-specific response to PLP 139–151 by copolymers in vitro was maximum for VWAK. Although this decrease may not seem large, it amounts to a 37% decrease in total number of PLP 139–151-reactive cells. Thus, coactivation with copolymers can suppress expansion of antigen-specific T cells.
The question of whether coimmunization of mice with copolymers and PLP 139–151 altered the expansion of PLP-specific T cells in vivo was examined after in vitro expansion (because their frequency ex vivo is too low to measure by tetramer staining without in vitro expansion). The effect of coimmunization with copolymers was remarkably similar to the effect observed on addition of copolymers in vitro. The PLP 139–151-reactive T cell expansion in cultures stimulated with PLP 139–151 was consistently reduced in all of the groups as compared with the mice immunized with only PLP 139–151 (maximum reduction, 37%) (Fig. 7). In contrast, no expansion of PLP 139–151-reactive T cells was observed in cultures treated only with copolymers, again supporting the lack of cross-reactivity.
Cytokine production by splenocytes after coimmunization with copolymers and PLP 139–151. Cytokine profiles were determined by ELISA in splenocyte cultures derived from SJL/J mice immunized with PLP 139–151 with or without copolymers and restimulated in vitro with PLP 139–151 or copolymers. CD4 T cells from PLP 139–151-immunized mice produce IFN-γ, but not IL-4 or IL-10, upon in vitro activation with PLP 139–151. Splenocytes from mice coimmunized with PLP 139–151 and copolymers continued to produced INF-γ. In addition, IL-4 and IL-10 both were produced by splenocytes from coimmunized mice on copolymer stimulation (presumably by copolymer-specific T cells, see below) (Fig. 8, which is published as supporting information on the PNAS web site).
Lack of anergy induction by copolymers. See Fig. 9, which is published as supporting information on the PNAS web site.
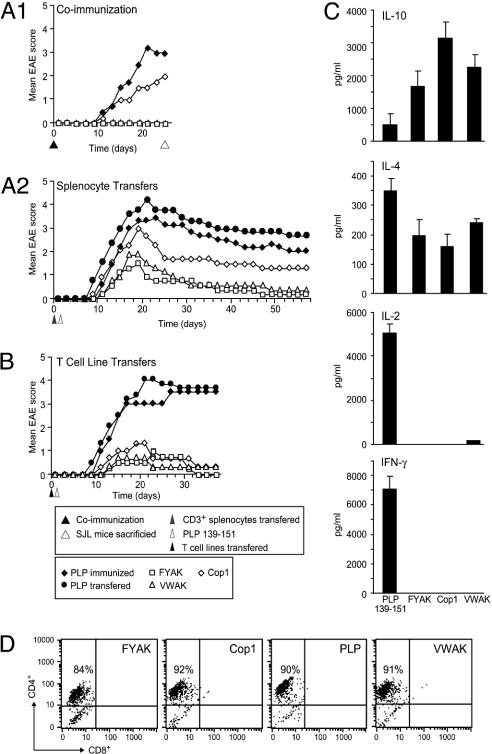
Suppression by adoptive transfer of CD3+ T splenocytes from mice coimmunized with PLP 139–151 and copolymers. SJL/J mice were immunized with PLP 139–151 in CFA alone or coimmunized with Cop1, VWAK, or FYAK together with PLP 139–151. Only mice immunized with PLP 139–151 with or without Cop1 developed mild or severe EAE symptoms with scores of 1.7 and 2.8, respectively on day 24. As expected, no disease appeared in mice coimmunized with PLP 139–151 and either VWAK or FYAK (Fig. 4A1). On day 24, CD3+ splenocytes (5 × 106) from each of the coimmunized mice were transferred into naïve SJL mice. The next day they were immunized s.c. with 50 μg of PLP 139–151 in CFA.
Fig. 4.
Suppression of EAE upon adoptive transfer of either CD3+ T cells from coimmunized SJL/J mice or copolymer-specific T cell lines from copolymer-immunized SJL/J mice. Statistical analysis is shown in Table 1. (A1) SJL/J mice were injected with 50 μg of PLP 139–151 in CFA with or without 500 μg of copolymer s.c. After disease induction, mice were observed daily for the appearance of signs of EAE. (A2) Splenocytes (5 × 106) from mice immunized s.c. with 50 μg of PLP 139–151 in CFA only (♦), without prior immunization for comparison (•), or mice coimmunized s.c. with PLP 139–151 and 500 μg of copolymer (VWAK, □; FYAK, □; or Cop1, ⋄) were transferred on day 24 of the experiment in A1 into naïve SJL/J mice. The next day, the mice were immunized s.c. with 50 μg of PLP 139–151 in CFA. Mice were observed daily for the appearance of signs of EAE. This is one of two nearly identical independent experiments. (B) Copolymer-specific or PLP 139–151-specific T cells (5 × 106) from lines established after immunization with either copolymers or PLP 139–151 were transferred into naïve SJL/J mice, and the next day the mice were immunized s.c. with 50 μg of PLP 139–151 in CFA. (C) Cytokine secretion. The culture supernatants of the above lines were examined for cytokine production by ELISA. (D) Fluorescence-activated cell sorting analysis of copolymer-specific T cell lines.
Mice into which CD3+ splenocytes from PLP 139–151-immunized mice had been transferred developed disease with onset on day 8 and a maximal mean score of 4, about the same as PLP 139–151-immunized mice that had not received any adoptive splenocytes. Mice into which splenocytes from VWAK- or FYAK-coimmunized animals were transferred had scores of 1.6 and 2.2, respectively, both with an onset on day 10, and essentially no disease by day 40 (Fig. 4A2). By contrast CD3+ splenocytes from Cop1-treated mice had a score of 3 on day 18 and appeared to stabilize with a score of 1.5 by day 30. Thus, CD3+ splenocytes generated after VWAK or FYAK, and to a lesser extent after Cop1 treatment, had significantly suppressed PLP 139–151-induced EAE. Residual disease at day 58 was present when using CD3+ splenocytes from Cop1-treated mice but not from YFAK- or VWAK-treated mice.
Suppression by adoptive transfer of copolymer-specific T cell lines. SJL/J mice were immunized s.c. with either 50 μg of PLP 139–151 or 500 μg of copolymer. On day 10, splenocytes were restimulated three times in vitro every other week with peptide- or copolymer-pulsed irradiated splenocytes from naïve mice. After the third round of stimulation, 5 × 106 T cells of each copolymer- or PLP 139–151-stimulated population were administered i.v. into each mouse. The disease was induced the next day by using PLP 139–151 in CFA as described in Materials and Methods. Compared to PLP 139–151 controls all of the mice that received copolymer-specific T cells had a delayed onset of a much milder form of EAE (Fig. 4B).
Cytokine profiles were determined by ELISA in splenocyte cultures derived from SJL/J mice immunized with either PLP 139–151 or copolymers and restimulated in vitro with the respective antigen. Previously, splenocytes from PLP 139–151-immunized mice produced IFN-γ but not IL-4 or IL-10 upon in vitro activation with PLP 139–151 (Fig. 8). Splenocytes from these PLP 139–151-immunized mice were restimulated three times biweekly to establish lines. These PLP 139–151-specific lines produced all of these cytokines (Fig. 4C). Interestingly, similar lines established with copolymers did not produce the Th1 cytokines IFN-γ and IL-2 although they produced the Th2 cytokines IL-4 and IL-10 (Fig. 4C). The T cell lines used for transfer were in all cases ≈90% CD4+ T cells (Fig. 4D).
Discussion
In this study, the copolymers FYAK and VWAK, which were designed based on the amino acid residues responsible for binding of MBP 85–99 and the peptide binding pockets of HLA-DR2 (30, 31), effectively suppressed the severity of PLP 139–151-induced EAE in SJL/J (I-As) mice (Fig. 1) that was paralleled by evidence of reduction of disease severity or disease elimination as seen by histochemical staining (Fig. 5). MBP 85–99 interacts with the HLA-DR2 (DRB1*1501) molecule to which MS is linked, and for this reason the copolymers were primarily designed to bind to HLA-DR2 molecules. Importantly, these copolymers were shown to bind also to I-As, the only class II MHC protein expressed in SJL/J mice and to cluster with and compete with PLP 139–151 for binding to I-As (Figs. 2 and 6). Previously, aggregates (clusters) of I-As molecules after Cop1 binding were detected on the surface of antigen-presenting cells from SJL/J mice (35). FYAK and VWAK were more potent than Cop1 in binding to mouse I-As molecules and in competing for PLP 139–151 binding. Their efficacy in vivo in SJL/J mice with PLP 139–151-induced EAE was examined by using three protocols: (i) preimmunization of mice with copolymers before the induction of EAE by PLP 139–151 (vaccination); (ii) coimmunization of mice with copolymers and PLP 139–151 together (prevention); and (iii) treatment of mice with copolymers after onset of EAE induced by PLP 139–151 (therapy). In all of these protocols, the copolymers showed a pronounced suppressive effect on PLP 139–151-induced EAE in the order VWAK > FYAK » Cop1.
The mechanism by which the copolymers exert their effects was examined in several protocols in addition to the binding assay. First, the copolymers were inhibitors of the expansion of PLP 139–151-specific T cells (proliferation assays), both in vitro and in vivo, again in the order VWAK > FYAK > Cop1 (Figs. 3 and 7).
Second, copolymers shifted the T cell immune response from a classical Th1 phenotype toward a Th2 response (immune deviation). EAE induced by myelin antigens such as MBP and PLP 139–151 are regarded as Th1 cell-mediated diseases, although Th2 cells have been shown to induce EAE under certain conditions (36). In SJL/J mice, restimulation of splenocytes from PLP 139–151-immunized animals with PLP 139–151 in vitro induced the production of IFN-γ but not IL-4 or IL-10 (Fig. 8). However, splenocytes from mice coimmunized with PLP 139–151 and copolymers when restimulated with their corresponding copolymers also produced IL-4 and IL-10 without much alteration in the production of IFN-γ. The Th2 cytokines, IL-4 and IL-10, have antiinflammatory properties (10, 11, 37). Furthermore, B6 mice transgenic for IL-4 or IL-10 are resistant to myelin oligodendrocyte glycoprotein 35–55-induced EAE (10, 11) and hence these cytokines may play a critical role in reducing the severity of inflammatory diseases such as EAE. These cytokines may be produced by copolymer-specific T cells (see below) with a negligible contribution, if any, from PLP 139–151-reactive T cells.
Third, copolymers may mediate their effects by inducing copolymer-specific T cells with the Th2 phenotype (38). The copolymers upon immunization of SJL/J mice induced a copolymer-specific T cell response (Fig. 3), i.e., the copolymers are immunogenic. Moreover, adoptive transfer of copolymer-specific T cells reduced markedly the severity of EAE (Fig. 4), suggesting that they produce Th2 cytokines without copolymer restimulation. How then do the copolymer-specific T cells regulate autoantigen-reactive T cells in vivo? Do the copolymer-specific T cells work in a manner similar to CD4+CD25+ T cells, which have been shown to prevent the occurrence of several autoimmune diseases including EAE (39, 40), or by secretion of Th2 cytokines? Further clarification is required. Moreover, the copolymer-specific T cell lines are antigen nonspecific, i.e., they can be generated and they respond to copolymers in the absence of antigen (Figs. 3 and 4). Thus, they may be useful in the treatment of other autoimmune diseases or in those where several autoantigens are involved, as is likely to be the case in MS.
However, whatever the mechanism, the first step must be binding to a class II MHC protein (41, 42). The copolymers were optimized for binding to HLA-DR2 but they are likely to bind promiscuously to class II MHC proteins with varying affinities (41). They obviously bind to I-As with high affinity (Fig. 3B). A number of mechanisms in addition to blocking and immune deviation resulting from the generation of copolymer-specific T cells, such as T cell antigen receptor competition (43, 44) or induction of anergy (45), may be operative. Induction of hyporesponsive T cells (anergy) in MS patients after continuous administration of Cop1 has been observed (45). The generation of copolymer-specific CD4+ Th2 cell lines that secrete IL-4 and IL-10 and can adoptively transfer resistance to EAE appears very prominent among these mechanisms. Copolymers might also suppress disease through modulating CNS antigen-presenting cells i.e., microglia.
Different copolymers may have different mechanisms of suppression. VWAK appears to be less able to generate T cell lines (Fig. 3 B1) and also generates larger amounts of IL-4 and lower amounts of IFN-γ (Fig. 8). Yet it suppresses EAE somewhat more effectively (Fig. 1). However, VWAK binds more tightly to I-As and may be a better blocking agent. In an accompanying paper (46), the efficacy of these copolymers has also been tested in a humanized double-transgenic mouse model expressing human HLA-DR2 (DRB1*1501) and a human T cell antigen receptor specific for MBP 85–99 from an MS patient and their mechanisms were compared. Although the mechanisms are similar, some differences were observed.
What accounts for the slightly greater effectiveness of VWAK than FYAK? FYAK is much more effective in stimulating copolymer-specific T cell lines and production of antiinflammatory cytokines IL-4 and IL-10, and thus should be much more effective in disease reduction if immune deviation is the mechanism. On the other hand, in an accompanying paper (48), VWAK is shown to induce T cell anergy much more efficiently than FYAK in the humanized double-transgenic mouse model, although in the present work in H-2s mice no anergy induction by either copolymer was observed (Fig. 9). Conversely VWAK induces IL-4 and IL-10 production only relatively weakly and, like FYAK, also induces T cell anergy relatively poorly. Thus, a combination of mechanisms may be involved in the reduction of severity of EAE and perhaps a combination of copolymers would be the most effective treatment of EAE and by extension of MS.
Acknowledgments
We thank P. Klimovitsky for isolation of I-As; M. L. Wong for expert technical assistance; N. Reppas, H. Ploegh, J. Orange, and R. Strom for helpful discussions; and T. Aldridge and the Bauer Center for Genomics Research for use of equipment and facilities. This work was supported by National Institutes of Health Grants AI 49524 (to J.L.S.), R01 NS30843 (to V.K.K.), and P01 NS38037 (to H. L. Weiner) and National Multiple Sclerosis Society Grant RG2571-D-9 (to V.K.K.). The National Multiple Sclerosis Society provided funds for the synthesis of amino acid copolymers. J.R. is a recipient of an advanced postdoctoral fellowship award from the National Multiple Sclerosis Society.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; Cop1, Copolymer 1; MBP, myelin basic protein; MS, multiple sclerosis; PLP, proteolipid protein; Th, T helper; CFA, complete Freund's adjuvant.
References
- 1.Jersild, C., Fog, T., Hansen, G. S., Thomsen, M., Svejgaard, A. & Dupont, B. (1973) Lancet 2, 1221–1225. [DOI] [PubMed] [Google Scholar]
- 2.Spielman, R. S. & Nathanson, N. (1982) Epidemiol. Rev. 4, 45–65. [DOI] [PubMed] [Google Scholar]
- 3.Olerup, O. & Hillert, J. (1991) Tissue Antigens 38, 1–15. [DOI] [PubMed] [Google Scholar]
- 4.Tuohy, V. K., Lu, Z., Sobel, R. A., Laursen, R. A. & Lees, M. B. (1989) J. Immunol. 142, 1523–1527. [PubMed] [Google Scholar]
- 5.Mendel, I., Kerlero de Rosbo, N. & Ben-Nun, A. (1995) Eur. J. Immunol. 25, 1951–1959. [DOI] [PubMed] [Google Scholar]
- 6.Zamvil, S. S., Mitchell, D. J., Moore, A. C., Kitamura, K., Steinman, L. & Rothbard, J. B. (1986) Nature 324, 258–260. [DOI] [PubMed] [Google Scholar]
- 7.Kono, D. H., Urban, J. L., Horvath, S. J., Ando, D. G., Saavedra, R. A. & Hood, L. (1988) J. Exp. Med. 168, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen, L. S., Andersson, E. C., Jansson, L., Krogsgaard, M., Andersen, C. B., Engberg, J., Strominger, J. L., Svejgaard, A., Hjorth, J. P., Holmdahl, R., et al. (1999) Nat. Genet. 23, 343–347. [DOI] [PubMed] [Google Scholar]
- 9.Windhagen, A., Nicholson, L. B., Weiner, H. L., Kuchroo, V. K. & Hafler, D. A. (1996) Chem. Immunol. 63, 171–186. [PubMed] [Google Scholar]
- 10.Falcone, M., Rajan, A. J., Bloom, B. R. & Brosnan, C. F. (1998) J. Immunol. 160, 4822–4830. [PubMed] [Google Scholar]
- 11.Bettelli, E., Das, M. P., Howard, E. D., Weiner, H. L., Sobel, R. A. & Kuchroo, V. K. (1998) J. Immunol. 161, 3299–3306. [PubMed] [Google Scholar]
- 12.Whitacre, C. C., Gienapp, I. E., Meyer, A., Cox, K. L. & Javed, N. (1996) Clin. Immunol. Immunopathol. 80, S31–S39. [DOI] [PubMed] [Google Scholar]
- 13.Falk, K., Rotzschke, O. & Strominger, J. L. (2000) Eur. J. Immunol. 30, 3012–3020. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz, P. J., DeVoss, J. J., Nguyen, L. V., Fontoura, P. P., Hirschberg, D. L., Mitchell, D. J., Garcia, K. C. & Steinman, L. (2001) J. Immunol. 167, 2688–2693. [DOI] [PubMed] [Google Scholar]
- 15.Fridkis-Hareli, M., Santambrogio, L., Stern, J. N., Fugger, L., Brosnan, C. & Strominger, J. L. (2002) J. Clin. Invest. 109, 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karin, N., Mitchell, D. J., Brocke, S., Ling, N. & Steinman, L. (1994) J. Exp. Med. 180, 2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fridkis-Hareli, M., Stern, J. N., Fugger, L. & Strominger, J. L. (2001) Hum. Immunol. 62, 753–763. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson, L. B., Murtaza, A., Hafler, B. P., Sette, A. & Kuchroo, V. K. (1997) Proc. Natl. Acad. Sci. USA 94, 9279–9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaur, A., Wiers, B., Liu, A., Rothbard, J. & Fathman, C. G. (1992) Science 258, 1491–1494. [DOI] [PubMed] [Google Scholar]
- 20.Teitelbaum, D., Meshorer, A., Hirshfeld, T., Arnon, R. & Sela, M. (1971) Eur. J. Immunol. 1, 242–248. [DOI] [PubMed] [Google Scholar]
- 21.Teitelbaum, D., Webb, C., Meshorer, A., Arnon, R. & Sela, M. (1973) Eur. J. Immunol. 3, 273–279. [DOI] [PubMed] [Google Scholar]
- 22.Teitelbaum, D., Webb, C., Bree, M., Meshorer, A., Arnon, R. & Sela, M. (1974) Clin. Immunol. Immunopathol. 3, 256–262. [DOI] [PubMed] [Google Scholar]
- 23.Teitelbaum, D., Fridkis-Hareli, M., Arnon, R. & Sela, M. (1996) J. Neuroimmunol. 64, 209–217. [DOI] [PubMed] [Google Scholar]
- 24.Aharoni, R., Teitelbaum, D. & Arnon, R. (1993) Eur. J. Immunol. 23, 17–25. [DOI] [PubMed] [Google Scholar]
- 25.Bornstein, M. B., Miller, A., Slagle, S., Weitzman, M., Crystal, H., Drexler, E., Keilson, M., Merriam, A., Wassertheil-Smoller, S., Spada, V., et al. (1987) N. Engl. J. Med. 317, 408–414. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, K. P., Brooks, B. R., Cohen, J. A., Ford, C. C., Goldstein, J., Lisak, R. P., Myers, L. W., Panitch, H. S., Rose, J. W., Schiffer, R. B., et al. (1998) Neurology 50, 701–708. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, K. P., Brooks, B. R., Ford, C. C., Goodman, A., Guarnaccia, J., Lisak, R. P., Myers, L. W., Panitch, H. S., Pruitt, A., Rose, J. W., et al. (2000) Multiple Sclerosis 6, 255–266. [DOI] [PubMed] [Google Scholar]
- 28.Kelley, C. L. (1996) J. Neurosci. Nurs. 28, 114–120. [DOI] [PubMed] [Google Scholar]
- 29.Aharoni, R., Kayhan, B., Eilam, R., Sela, M. & Arnon, R. (2003) Proc. Natl. Acad. Sci. USA 100, 14157–14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wucherpfennig, K. W., Sette, A., Southwood, S., Oseroff, C., Matsui, M., Strominger, J. L. & Hafler, D. A. (1994) J. Exp. Med. 179, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, K. J., Pyrdol, J., Gauthier, L., Wiley, D. C. & Wucherpfennig, K. W. (1998) J. Exp. Med. 188, 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy, J., Bettelli, E., Nicholson, L., Waldner, H., Jang, M. H., Wucherpfennig, K. W. & Kuchroo, V. K. (2003) J. Immunol. 170, 870–877. [DOI] [PubMed] [Google Scholar]
- 33.Gerety, S. J., Clatch, R. J., Lipton, H. L., Goswami, R. G., Rundell, M. K. & Miller, S. D. (1991) J. Immunol. 146, 2401–2408. [PubMed] [Google Scholar]
- 34.Gerety, S. J., Rundell, M. K., Dal Canto, M. C. & Miller, S. D. (1994) J. Immunol. 152, 919–929. [PubMed] [Google Scholar]
- 35.Fridkis-Hareli, M., Teitelbaum, D., Pecht, I., Arnon, R. & Sela, M. (1997) Int. Immunol. 9, 925–934. [DOI] [PubMed] [Google Scholar]
- 36.Lafaille, J. J., Keere, F. V., Hsu, A. L., Baron, J. L., Haas, W., Raine, C. S. & Tonegawa, S. (1997) J. Exp. Med. 186, 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aharoni, R., Teitelbaum, D., Sela, M. & Arnon, R. (1998) J. Neuroimmunol. 91, 135–146. [DOI] [PubMed] [Google Scholar]
- 38.Duda, P. W., Schmied, M. C., Cook, S. L., Krieger, J. I. & Hafler, D. A. (2000) J. Clin. Invest. 105, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151–1164. [PubMed] [Google Scholar]
- 40.Illes, Z., Kondo, T., Yokoyama, K., Ohashi, T., Tabira, T. & Yamamura, T. (1999) J. Immunol. 162, 1811–1817. [PubMed] [Google Scholar]
- 41.Fridkis-Hareli, M. & Strominger, J. L. (1998) J. Immunol. 160, 4386–4397. [PubMed] [Google Scholar]
- 42.Fridkis-Hareli, M., Teitelbaum, D., Gurevich, E., Pecht, I., Brautbar, C., Kwon, O. J., Brenner, T., Arnon, R. & Sela, M. (1994) Proc. Natl. Acad. Sci. USA 91, 4872–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aharoni, R., Teitelbaum, D., Arnon, R. & Sela, M. (1999) Proc. Natl. Acad. Sci. USA 96, 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wraith, D. C., McDevitt, H. O., Steinman, L. & Acha-Orbea, H. (1989) Cell 57, 709–715. [DOI] [PubMed] [Google Scholar]
- 45.Schmied, M., Duda, P. W., Krieger, J. I., Trollmo, C. & Hafler, D. A. (2003) Clin. Immunol. 106, 163–174. [DOI] [PubMed] [Google Scholar]
- 46.Illés, Z., Stern, J. N. H., Reddy, J., Waldner, H., Mycko, M. P., Brosnan, C. F., Ellmerich, S., Altmann, D. M., Santambrogio, L., Strominger, J. L. & Kuchroo, V. K. (2004) Proc. Natl. Acad. Sci. USA 101, 11749–11754. [DOI] [PMC free article] [PubMed] [Google Scholar]