Abstract
A humanized mouse bearing the HLA-DR2 (DRA/DRB1*1501) protein associated with multiple sclerosis (MS) and the myelin basic protein (MBP) 85–99-specific HLA-DR2-restricted T cell receptor from an MS patient has been used to examine the effectiveness of modified amino acid copolymers poly(F,Y,A,K)n and poly-(V,W,A,K)n in therapy of MBP 85–99-induced experimental autoimmune encephalomyelitis (EAE) in comparison to Copolymer 1 [Copaxone, poly(Y,E,A,K)n]. The copolymers were designed to optimize binding to HLA-DR2. Vaccination, prevention, and treatment of MBP-induced EAE in the humanized mice with copolymers FYAK and VWAK ameliorated EAE more effectively than Copolymer 1, reduced the number of pathological lesions, and prevented the up-regulation of human HLA-DR on CNS microglia. Moreover, VWAK inhibited MBP 85–99-specific T cell proliferation more efficiently than either FYAK or Copolymer 1 and induced anergy of HLA-DR2-restricted transgenic T cells as its principle mechanism. In contrast, FYAK induced proliferation and a pronounced production of the antiinflammatory T helper 2 cytokines IL-4 and IL-10 from nontransgenic T cells as its principle mechanism of immunosuppression. Thus, copolymers generated by using different amino acids inhibited disease using different mechanisms to regulate T cell responses.
Keywords: multiple sclerosis, DRB1*1501, microglia, CNS, autoimmunity
Multiple sclerosis (MS) is an autoimmune disease mediated by CD4+ T cells reactive to antigens of the myelin sheath, resulting in inflammation and demyelination in the CNS (1). Myelin basic protein (MBP), one of the major myelin antigens, has long been presumed to be an important autoantigen, based on the animal model of MS, experimental autoimmune encephalomyelitis (EAE). Several lines of evidence suggest that MBP-specific T cells are involved in the pathogenesis of MS: (i) The frequency of MBP-specific T cells is increased in the peripheral blood of patients with MS (2). (ii) A number of studies have shown persistent clonal expansion of MBP-specific T cells either in the peripheral blood or in the cerebrospinal fluid of MS patients (3–6). (iii) T cell infiltrates of MS plaques in the CNS and MBP-specific T cell clones share significant homologies in their T cell receptor (TCR) CDR3 sequences, implying that MBP-specific T cells contribute to the neuropathology of MS (7). (iv) An increase in the frequency and affinity of MBP-reactive T cells in the peripheral blood was shown to exacerbate disease and increase the number of plaques in the CNS of MS patients (8).
Human HLA-DR2, and specifically that encoded by the DRA/DRB1*1501 alleles, is thought to be important in the presentation of the immunodominant epitope MBP 85–99 (9, 10). Although the association of the human MHC class II (MHC II) locus with MS has consistently been observed in genome-wide scans (11), an association of the HLA-DR2, DQ6 haplotype, including the DRB1*1501 allele, with susceptibility to MS has been suggested in several population- and family-based studies (12). The importance of the DRB1*1501-associated MBP 85–99 epitope in the pathogenesis of MS has been particularly proposed based on data that, in MS lesions, the microglia/macrophages were positive for the HLA-DR2/MBP 85–99 complex detected by a monoclonal antibody specific for this complex (13). Moreover, mice transgenic (tg) for HLA-DR2 and the TCR from an MS patient develop EAE spontaneously in 6–12 months and the disease appearance can be accelerated by immunization with MBP 85–99 (14).
Several clinical attempts have been made to interfere with the MHC II/MBP 85–99/TCR trimolecular complex with varying effectiveness (8, 15–19). Currently, Copolymer 1 (Cop1, Copaxone, glatiramer acetate) is used in MS patients as an immunomodulator (20–22). Cop1 is a random polypeptide synthesized from four amino acids, l-tyrosine, l-glutamic acid, l-alanine, and l-lysine [poly(Y,E,A,K)n], in a specific molar ratio of 1.0, 1.4, 4.2, and 3.4, respectively. Although the mechanism of action of Cop1 is not fully understood, several immunologic effects have been suggested (23, 24). These include competition of Cop1 with the immunodominant MBP 85–99 peptide for binding to DRB1*1501 and competition of MHC II/Cop1 and MHC II/MBP complexes for binding to the TCR (23, 25–28). Cop1 has also recently been shown to polarize T cells into a T helper 2 (Th2) phenotype. Splenocytes from Cop1-immunized mice transfer protection against EAE, and Cop1-specific Th2 cells capable of secreting IL-4 and IL-10 dominate in the CNS of protected mice (29). Similarly, MS patients treated with Cop1 have Cop1-specific T cells with a Th2 phenotype (30).
Although widely used in the treatment of MS, Cop1 reduces the frequency of relapses by only ≈30% (21). We hypothesized that by improving the binding capacity of copolymers to HLA-DR2 relative to MBP 85–99, it may be possible to develop a more effective immunomodulator. Several random 4-aa copolymers were designed based on the knowledge of anchor residues used by the immunodominant MBP 85–99 epitope that bind to the DRB1*1501-encoded molecule. To determine the in vivo and in vitro effect of these copolymers, a humanized double-tg mouse expressing the human MHC II molecule encoded by DRA/DRB1*1501 and an MS patient-derived MBP 85–99-specific TCR, similar to that recently described (14), was used.
Here we show that two copolymers, namely poly(F,Y,A,K)n and poly(V,W,A,K)n, significantly reduced the severity of MBP 85–99-induced EAE in the humanized mice more effectively than Cop1 [poly(Y,E,A,K)n] in three different modalities of administration–vaccination, prevention, and treatment. In addition we show that several different mechanisms are involved in immunomodulation by these copolymers and that distinct copolymers may inhibit the disease by different mechanisms.
Materials and Methods
Copolymers, MBP 85–99 Peptide, and HLA-DR2. Copolymers are described in the companion paper (31). Peptides were synthesized on an Applied Biosystems Peptide Synthesizer and purified by reverse-phase HPLC. Peptide sequences were MBP 85–99, ENPVVHFFKNIVTPR, either unlabeled or with biotin linked to the N terminus by the spacer SGSG and free acid at the C terminus. Peptide binding to HLA-DR2 (DRA/DRB1*1501) isolated from S2 insect cells by affinity chromatography was competed by copolymers or MBP 85–99 as described (22, 32). The effects of copolymers on T cell proliferation and cytokine measurements were carried out as described (22, 31).
Humanized tg Mice. Humanized double tg mice expressing both HLA-DR2 (DRA/DRB1*1501) and the TCR from the MS patient Ob in an Aβ0 background were generated by standard techniques described in more detail in Supporting Text, which is published as supporting information on the PNAS web site.
Prepulse Assay and Induction of Anergy. Assays were performed as described with a few modifications (23, 26). To induce anergy, CD3+ T cells were enriched from spleens of humanized tg mice by using Mouse T Cell Enrichment kit (R & D Systems). A total of 1 × 106 T cells and 2 × 106 irradiated splenic cells of humanized mice were incubated for 48 h in 24-well culture plates in the presence of copolymers (0.01, 0.1, or 1 μg/ml). After resting for 2 days, viable cells were isolated by centrifugation over Ficoll-Hypaque, and 4 × 104 cells were restimulated with 20 μg/ml MBP 85–99 peptide in the presence of 5 × 105 irradiated antigen-presenting cells (APCs). The cells were pulsed with 3H 2 days later, and the proliferative response was measured after 16 h by [3H]thymidine incorporation.
To examine competition for the MBP 85–99-specific TCR, 3 × 106 per ml irradiated splenocytes from the humanized mice were incubated with 2 μg/ml MBP 85–99 peptide at 37°C for 3 h. Cells were washed, and 3 × 105 cells per well were cultured in 96-well microculture plates with 2 × 104 per well fluorescence-activated cell sorted tg hVβ2+CD4+ or non-tg hVβ2–CD4+ T cells. Copolymers FYAK or VWAK were added at 5 μg and 20 μg/ml concentration with the T cells.
Antibodies Used for Surface Marker Staining and Sorting by Flow Cytometry of tg and Endogenous (Non-tg) T Cells. A total of 5 × 105 lymphocytes from humanized mice were stained with anti-CD69-phycoerythrin (PE) (Pharmingen), anti-CD4-allophycocyanin (Pharmingen), anti-human HLA-DR-PE (Pharmingen), or anti-human Vβ2-FITC/PE (Immunotech) for 30 min on ice in 1× PBS containing 2% FCS. Cells were washed and acquired by flow cytometry using FACSFlow cytometer (Becton Dickinson) and analyzed by flowjo software (Treestar, Amersham Pharmacia). Labeled T cells were sorted by FACSVantage SE (Becton Dickinson). The purity of sorted T cells was usually 98–99%.
Immunization Procedures, EAE Induction, and Treatment of Mice with Copolymers. Female humanized tg mice were immunized with 150 μg of MBP 85–99 peptide or 500 μg of FYAK, VWAK, or Cop1 in complete Freund's adjuvant (CFA) s.c. in a volume of 200 μl in the inguinal and axillary areas, and mice were monitored as described (22, 31).
Results
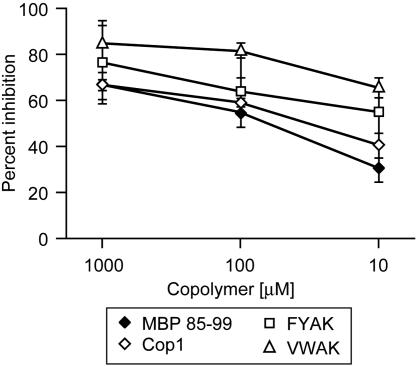
Synthetic Copolymers FYAK and VWAK Compete with MBP 85–99 for Binding to HLA-DR2 (DRB1*1501). Based on the knowledge of anchor residues used by the immunodominant MBP 85–99 epitope to bind to HLA-DR2, two random copolymers, FYAK and VWAK, were designed. To investigate whether these copolymers compete with MBP 85–99 peptide for binding to HLA-DR2 (encoded by HLA-DRA/DRB1*1501), competitive binding assays were performed by using biotinylated MBP 85–99 and unlabeled copolymers at varying concentrations. Both copolymers were more efficient than Cop1 in competing with MBP 85–99 for binding to HLA-DR2, and VWAK competed even better than FYAK (Fig. 1).
Fig. 1.
Recently developed copolymers compete for binding to human HLA-DR2 (DRB1*1501). Inhibition of biotinylated MBP 85–99 binding to HLA-DR2 molecules by these copolymers. Recombinant HLA-DR2 molecules were incubated with 0.13 μM biotinylated MBP 85–99 alone or in the presence of excess unlabeled competitors at the indicated concentrations.
Copolymers Inhibit the in Vitro HLA-DR2-Restricted MBP 85–99-Specific T Cell Response. To determine whether the copolymers could also inhibit presentation of the MBP 85–99 peptide to HLA-DR2-restricted T cells, TCR transfectants (Ob8073 and Hy1B) were used. The TCRs introduced into the transfectants were derived from MBP 85–99-specific HLA-DR2-restricted T cell lines established from patients Ob (DRB1*1501) and Hy (DRB1*1602). FYAK and VWAK inhibited IL-2 production from both MBP 85–99-specific Hy1B and Ob 8073 TCR transfectants much better than Cop1, when MGAR (human lymphoblastoid B cell line that expresses DRB1*1501) was used as the APC (Fig. 6, which is published as supporting information on the PNAS web site). FYAK and VWAK also inhibited proliferation of both transfectants. As a control, when L cell transfectants expressing HLA-DR2a (encoded by DRA/DRB5*0101) were used as APCs, no proliferative response was observed, demonstrating that both Ob8073 and Hy1B transfectants were restricted by HLA-DR2b (encoded by HLA-DRA/DRB1*1501) (data not shown). Thus, the copolymers were able to reduce the in vitro MBP 85–99-specific T cell response in a HLA-DR2-restricted fashion.
Recently Developed Copolymers FYAK and VWAK Ameliorate MBP 85–99 Peptide-Induced EAE in Humanized tg Mice. Next, the effect of the copolymers was examined in vivo on the development of EAE. A double tg humanized mouse model generated by using human TCR and the HLA-DR2 (DRB1*1501) molecule was used. In this model of MS, the tg mice express the rearranged TCR α and β chains of an MBP 85–99-specific T cell clone (ObA1.12) derived from an MS patient (Ob) and the DRB1*1501 gene together with the DRA gene in a mouse strain lacking expression of endogenous mouse MHC II molecules.
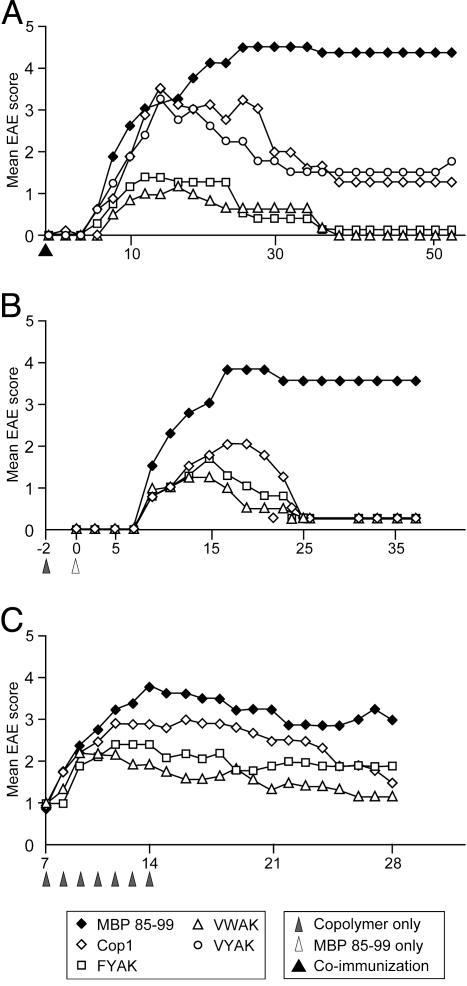
EAE was induced by immunizing the humanized tg mice with MBP 85–99 in CFA. The majority of mice showed the clinical signs of EAE as early as day 7. The disease was manifested by severe paralysis (score 3–4) and was fatal in one-third of the mice. The mice that survived developed a chronic unremitting paralysis. In contrast, in mice coimmunized with copolymers and MBP 85–99, the severity of EAE was significantly reduced. The inhibition of disease was most pronounced in groups of mice coimmunized with copolymers FYAK or VWAK. None of the animals died in these two groups. Cop1 and a third copolymer, VYAK, which were less effective in the in vitro assays, also reduced the signs of EAE, but not as effectively as VWAK or FYAK (Fig. 2A and Table 1, which is published as supporting information on the PNAS web site). VYAK has not been studied further. Similar results were obtained when mice were preimmunized with FYAK or VWAK 2 days before immunization with the encephalitogenic MBP 85–99 peptide (Fig. 2B).
Fig. 2.
Recently developed copolymers ameliorate MBP 85–99-induced EAE in humanized tg mice. (A) Humanized tg mice were immunized with 150 μg/ml MBP 85–99 in CFA or coimmunized with 500 μg/ml FYAK, VWAK, VYAK, or Cop1 and 150 μg/ml MBP 85–99 in CFA. (B) Humanized tg mice were immunized with 500 μg/ml copolymers FYAK, VWAK, or Cop1 2 days before disease was induced with 150 μg/ml MBP 85–99 in CFA. (C) EAE was induced by immunization with 150 μg/ml MBP 85–99 followed by administration of 150 μg FYAK or VWAK s.c. for 7 consecutive days beginning at the first sign of disease. Data shown represent one of two to three separate experiments of four to six mice per group.
Next the effect of FYAK and VWAK on already established EAE was investigated in comparison to a group of mice treated with Cop1. The disease was induced with MBP 85–99, and at the first sign of disease, the animals were randomized and treated s.c. with 150 μg of soluble FYAK, VWAK, or Cop1 in PBS/mannitol. The treatment was then repeated daily for 1 week. As compared to untreated mice, which developed significant EAE with peak disease around days 15–20, FYAK and VWAK significantly reduced the severity of disease in both the acute and chronic phases. VWAK was slightly more effective than FYAK, and both were more efficient than Cop1 (Fig. 2C and Table 1).
Recently Developed Copolymers FYAK and VWAK Reduce Inflammation and Demyelination. Analysis of CNS tissues from animals immunized with MBP 85–99 alone showed perivascular mononuclear infiltrates at all levels of the brain and spinal cord. In animals coimmunized with FYAK or VWAK and MBP 85–99, the perivascular cuffs were smaller, the infiltration into the parenchyma was less marked, and the number of inflammatory foci was significantly reduced compared to the MBP 85–99-induced disease (MBP 85–99, 28 ± 3; FYAK, 13.3 ± 2.2; VWAK, 16.3 ± 0.9) (Fig. 7, which is published as supporting information on the PNAS web site). Despite extensive inflammation, the demyelination was mild in the MBP 85–99-induced disease, and was further reduced in the copolymer-treated mice (data not shown).
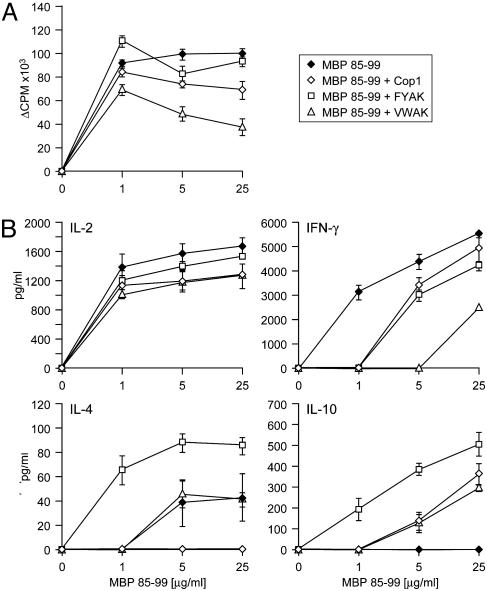
Coimmunization with MBP 85–99 and FYAK or VWAK Differentially Alters the Recall T Cell Response to MBP 85–99 in Vitro. To test the possibility that a reduced or altered MBP 85–99-specific T cell response in vivo might be responsible for the amelioration of EAE in humanized tg mice, the tg mice were coimmunized with MBP 85–99 and FYAK, VWAK, or Cop1, and the recall response to MBP 85–99 peptide was examined 7 days later in splenocyte cultures (Fig. 3). Coimmunization with VWAK markedly reduced the proliferative response to MBP 85–99. On the other hand, FYAK and Cop1 barely altered the proliferative response to MBP 85–99 in tg splenocytes (Fig. 3A).
Fig. 3.
Coimmunization with copolymers alters the recall response to MBP 85–99 peptide in vitro. (A) Humanized tg mice were immunized with 150 μg/ml MBP 85–99 or coimmunized with 500 μg/ml Cop1, FYAK, or VWAK and 150 μg/ml MBP peptide in CFA. In vitro proliferative response of splenocytes to MBP 85–99 is shown at day 7. (B) Splenocytes from mice immunized with MBP 85–99 or coimmunized with MBP 85–99 and copolymers were incubated with different concentration of MBP 85–99 peptide, and cytokine production was analyzed by ELISA.
Cytokine secretion was measured by ELISA in the above cultures restimulated with 25 μg/ml MBP 85–99 peptide. In splenocyte cultures established from mice immunized with MBP 85–99 peptide alone, IL-2, IFN-γ, and a small amount of IL-4 were produced, but no IL-10 was produced. Splenocytes of mice coimmunized with FYAK produced large amounts of IL-4 and IL-10 in response to MBP 85–99, whereas IFN-γ production was moderately reduced. In contrast, in splenocyte cultures of mice coimmunized with VWAK, IFN-γ production was markedly suppressed, but the amount of IL-4 and IL-10 was lower in comparison to FYAK. In cultures derived from Cop1-coimmunized mice, no IL-4 and only a low level of IL-10 were produced, whereas IFN-γ secretion was only moderately reduced (Fig. 3B).
Thus, although VWAK reduced proliferation and IFN-γ production of splenocytes, FYAK induced IL-4 and IL-10 production more efficiently. The two copolymers appear to act through different mechanisms. These data raised several questions: Are other mechanisms in addition to MHC II blockade also responsible for the reduced MBP-specific proliferative response and reduced IFN-γ production in the case of VWAK? In the case of FYAK particularly, are the cytokines IL-4 and IL-10 derived from the tg MBP-specific T cells or from endogenous copolymer-specific T cells?
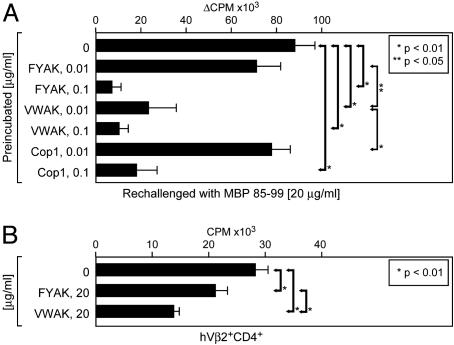
Copolymers VWAK and FYAK Induce Anergy and/or Have an Antagonistic Effect on the Humanized MBP 85–99-Specific tg TCR. Cop1 was able to induce unresponsiveness of MBP 85–99-specific and copolymer-reactive T cells (23, 26, 33). To examine whether all the copolymers are able to induce unresponsiveness, enriched CD3+ T cells from humanized tg mice were cultured for 48 h with low-dose FYAK, VWAK, or Cop1 in the presence of irradiated splenocytes from the tg mice. After 2 days of resting, viable cells were restimulated with fresh, MBP 85–99-pulsed irradiated splenocytes. The MBP 85–99-specific proliferative response was effectively reduced at 0.01 μg/ml concentration only by VWAK, although all of the copolymers were able to significantly reduce it at 0.1 μg/ml (Fig. 4A). The percentage of CD4+ T cells was unaltered and, thus, apoptosis of T cells at these concentrations was unlikely (data not shown). Preincubation with 0.1 and 0.01 μg/ml MBP 85–99 peptide did not induce anergy of tg T cells. Neither were copolymers able to induce anergy of tg T cells of another specificity, indicating that this action required the MBP 85–99-specific TCR (data not shown).
Fig. 4.
Antagonistic effects of copolymers FYAK and VWAK on tg MBP 85–99-specific T cells. (A) Anergy. Enriched CD3+ T cells and irradiated splenocytes of the humanized mice were incubated in the presence of copolymers at the indicated concentrations. Cells were rested for 48 h, and viable cells were restimulated with 20 μg/ml MBP 85–99 peptide. Data are shown as Δcpm of triplicates. (B) TCR antagonism. Irradiated splenocytes of humanized tg mice were incubated with 2 μg/ml MBP 85–99 peptide. Cells were then washed and subsequently cultured with sorted hVβ2+CD4+ cells in the presence of FYAK or VWAK at the indicated concentrations. Data are shown as mean cpm of triplicates.
Antagonistic effects of the copolymers for the MBP 85–99-specific TCR was tested in a prepulse assay (26). First, irradiated tg splenocytes were incubated with 2 μg/ml MBP 85–99 for 3 h at 37°C to saturate HLA-DR2 (DRB1*1501). Then, sorted tg hVβ2+CD4+ T cells were added to the splenocytes in the absence or presence of FYAK or VWAK. When incubated with copolymers, tg T cells proliferated significantly less. VWAK showed strongest inhibition whereas FYAK showed only modest inhibition of T cell proliferation (Fig. 4B). Endogenous, non-tg Vβ2–CD4+ T cells from naïve mice did not proliferate to either MBP or copolymers (data not shown).
Thus, in addition to competition for binding to HLA-DR2, both induction of T cell unresponsiveness and TCR antagonism may also contribute to the in vivo effect of these copolymers. Moreover, different effects on T cell responses of the individual copolymers were again noted. VWAK was most effective in inducing both T cell anergy and antagonism.
Immunization with Recently Developed Copolymers FYAK and VWAK Differentially Induces Copolymer-Reactive Endogenous Th2 Cells. Although the reduced T cell response in the case of coimmunization with VWAK could be explained by the combined effects of MHC II competition, induction of anergy, and competition for TCR, it was not clear whether these mechanisms were responsible for the inhibiting effects of FYAK. Because Cop1 has been shown to induce responding Th2 cells (30), cytokine production induced by the copolymers in the tg and non-tg T cells was examined. The tg T cells contribute about half of the T cell repertoire in the humanized mice, so the effect of copolymers on the non-tg T cell repertoire had to be considered.
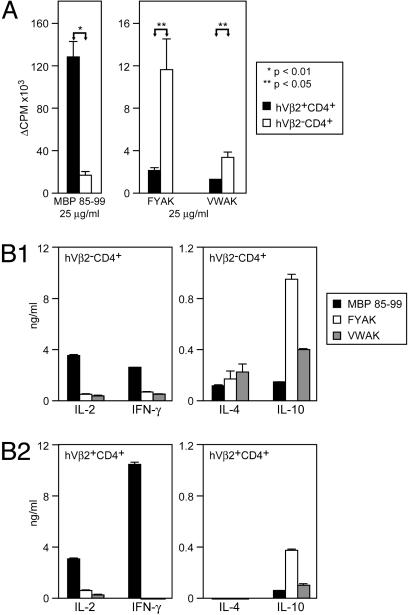
First, to test whether copolymers can prime copolymer-reactive T cells in this model, humanized tg mice were immunized with either FYAK or VWAK, and the T cell response to the corresponding copolymer was examined in splenic cultures 7 days later. The proliferative response was higher in spleen cells from FYAK-than from VWAK-immunized mice, and FYAK also induced more IL-4 and IL-10 (data not shown). However, it was not clear whether the copolymers primed endogenous T cells to produce Th2 cytokines or cross-reacted with the MBP 85–99-specific tg TCR and induced cytokine deviation as partial TCR agonists. To address this question, non-tg (hVβ2–CD4+) and tg (hVβ2+CD4+) T cells were separated by flow cytometry from mice immunized with FYAK or VWAK alone. Both fractions were then restimulated in vitro by the corresponding copolymer or with MBP 85–99 peptide. MBP 85–99 induced significantly higher proliferation of tg T cells as expected. However, FYAK and VWAK primed the non-tg T cells (Fig. 5A). The proliferative response of non-tg T cells was significantly higher to FYAK than to VWAK. In addition, endogenous non-tg hVβ2– T cells produced more IL-4 and IL-10 in response to copolymers particularly notable in IL-10 production after immunization with FYAK (Fig. 5B1). By contrast, the tg hVβ2+ T cells produced a large amount of IL-2, and particularly IFN-γ, in response to MBP 85–99 (Fig. 5B2). Thus, although VWAK was more effective in competing for MHC II and for the MBP-specific tg TCR in which it induced anergy, FYAK primed non-tg copolymer-specific T cells to produce the Th2 cytokine IL-10 much more effectively.
Fig. 5.
Recently developed copolymers induce a copolymer-reactive non-tg T cell response and immune deviation of tg MBP 85–99-specific T cells. (A) Humanized mice were immunized with 500 μg/ml FYAK or VWAK in CFA. Lymph nodes and spleen cells were isolated 7 days later, and hVβ2+CD4+ and hVβ2–CD4+ were sorted. Fractionated T cells were restimulated with 25 μg/ml MBP 85–99 peptide (Left) or 25 μg/ml of the corresponding copolymer (Right). (B) Non-tg hVβ2–CD4+ (B1) and tg hVβ2+CD4+ T cells (B2) from mice immunized with FYAK or VWAK were stimulated with MBP 85–99 or the corresponding copolymer, and cytokine production was analyzed by ELISA.
FYAK Induces Cytokine Deviation of tg MBP 85–99-Specific T Cells. Although both FYAK and VWAK copolymers primed essentially non-tg T cells, a low-grade proliferation of tg T cells was observed in response to FYAK and less to VWAK in immunized mice (Fig. 5A). Because the purity of sorted fractions was 98–99%, this response might suggest cross-reactivity of FYAK with the MBP-specific TCR. Therefore, the question whether this cross-reactive response was characterized by altered cytokine production as compared to the MBP 85–99-induced response was examined. Cytokine production of tg T cells to MBP 85–99 was characterized by high levels of IFN-γ. In contrast, tg T cells from mice immunized with FYAK or VWAK produced low amounts of IL-10 but no IFN-γ in response to copolymers. This IL-10 production of tg T cells was significantly higher in FYAK-immunized mice (Fig. 5B2). Although both tg and non-tg subsets appeared to contribute to the IL-10 production, IL-4 was produced predominantly by endogenous non-tg T cells.
Discussion
The availability of a humanized mouse model of MS has made it possible to study two recently developed copolymers, VWAK and FYAK, in comparison to Cop1 (a drug currently in use for treatment of MS) in an EAE animal model that is as close to MS as is presently possible. This mouse is tg for the major MS susceptibility gene HLA-DR2 (DRB1*1501) as well as for the TCR from an MBP 85–99-specific, HLA-DR2-restricted T cell from an MS patient. In three different modalities of administration of copolymers in this mouse, termed vaccination, prevention, and treatment, the order of effectiveness of the copolymers was VWAK > FYAK > Cop1 (Fig. 2).
Studies of the mechanism by which these copolymers modulate the EAE in this model have revealed the following. First, not surprisingly because the two copolymers, VWAK and FYAK, were designed to optimize binding to HLA-DR2, both bound more effectively than Cop1 (Fig. 1) and both inhibited the proliferation of and IL-2 secretion by MBP-specific TCR transfectants (Fig. 6). The amino acid composition of Cop1 (YEAK) is not optimal to block binding of MBP 85–99 to MHC II, because Y (tyrosine) has a side chain too large to bind in the small P1 pocket of HLA-DR2 (encoded by HLA-A/DRB1*1501); A (alanine) is too small, whereas E (glutamate) and K (lysine) are too hydrophilic to bind in the hydrophobic P1 pocket (9, 22, 32, 34). Thus, replacing the Y and/or E was hypothesized to improve binding to HLA-DR2. Indeed, the strongest effect was seen when E was replaced with F (phenylalanine) (FYAK), which hypothetically fits better into the small P1 pocket, or when both Y and E were replaced by V (valine) and W (tryptophane) (VWAK), which may possibly improve binding to both P1 (V) and P4 pockets (W). K was retained for solubility and because it is a principal stimulating TCR contact residue at P5 of MBP 85–99.
Other studies of mechanism focused on splenocytes derived from immunized tg mice. Initially, splenocytes of mice immunized with MBP 85–99 or coimmunized with this same peptide together with a copolymer were examined. The marked enhancement of proliferation induced by MBP 85–99 was reduced by VWAK, but only slightly affected by FYAK or Cop1. Measurement of cytokine production in the supernatant of these same cultures showed that splenocytes from tg mice immunized with MBP 85–99 alone produced IL-2 and INF-γ, but little IL-4 and no IL-10. By contrast, splenocytes of animals coimmunized with FYAK produced, in response to MBP 85–99, large amounts of IL-4 and IL-10 and a reduced amount INF-γ, particularly at low doses of antigen. Coimmunization with VWAK resulted in a more striking reduction of INF-γ production, but secretion of only modest amounts of IL-4 and IL-10. Cop1 reduced INF-γ production similarly to FYAK, but no IL-4 and only modest amounts of IL-10 were produced. Thus, the three copolymers tested have distinct effects on cytokine production by splenocytes, with FYAK clearly resulting in production of the largest amounts of Th2 cytokines. Further studies of anergy induction and TCR antagonism again revealed difference among the copolymers, with VWAK clearly more effective than either FYAK or Cop1 in both types of assays (Fig. 4).
Finally, this model permitted separation of two types of splenocytes from mice immunized with FYAK or VWAK alone, the tg hVβ 2+CD4+ and endogenous non-tg hVβ 2–CD4+ T cells. This separation made it possible to separately analyze the effects of copolymers on the MBP 85–99-specific tg T cell response and on the non-tg, endogenous T cell repertoire in the same model. As expected, tg T cells that carried a TCR with specificity for the HLA-DR2/MBP 85–99 complex proliferated vigorously to MBP 85–99 presented by an HLA-DR2-bearing APC (Fig. 5A). By contrast, the non-tg T cells were the cells that proliferated to FYAK or VWAK, but the response to the latter was quite small, as had already been seen in studies of splenocytes. In parallel, production of IFN-γ on stimulation with MBP 85–99 was mainly due to the tg T cells, whereas production of IL-4 and IL-10 by cells from FYAK- or VWAK-treated mice, particularly the former, was derived mainly from the non-tg T cells (Fig. 5B). In addition, as with splenocytes, FYAK was much more effective in stimulating cells that produce IL-10 than VWAK. Furthermore, FYAK induced some IL-10 production also by the tg T cells (Fig. 5B), suggesting that FYAK may cross-react with the MBP 85–99-specific TCR, and alter the cytokine production of tg T cells (immune deviation). Alternatively, FYAK-specific Th2 cells could influence activation of the tg T cells. The properties of FYAK- and VWAK-specific T cells as regulatory T cells were clearly demonstrated in the companion paper by using adoptive transfer experiments (31). Furthermore, expression of HLA-DR2 on microglia in the CNS of humanized mice coimmunized with copolymers and MBP 85–99 was not observed by immunohistochemistry, whereas it was detected in the CNS of mice immunized only with MBP 85–99. These results were also complemented by in vitro studies showing that copolymers alone had no effect on the expression of HLA-DR2 by microglia in culture (data not shown). Thus, the possibility that this modulation of expression in vivo may be due to an altered inflammatory environment in the CNS induced by coimmunization with copolymers requires further investigation.
Thus, two important points are derived from these studies of mechanisms. First, a variety of mechanisms operate to ameliorate EAE in humanized tg mice immunized with MBP 85–99 and various copolymers. First of all, binding of the copolymers to the HLA-DR2 protein on the tg APC has been greatly enhanced. The other effects observed are all necessarily derived from this primary binding event, and thus, enhanced binding may contribute importantly to the protective effects in the EAE model. FYAK and VWAK are more efficient binders than Cop1, as expected, because they were designed for this purpose. Second, the three different copolymers studied differ in the extent to which they stimulated the other mechanisms. FYAK was clearly most effective in inducing non-tg T cells that proliferated and secreted, particularly, the Th2 cytokine IL-10, which is known to have a suppressive effect on autoimmune diseases (35). However, VWAK was the most effective copolymer in inducing T cell anergy and in reducing IFN-γ production by MBP 85–99-stimulated splenocytes. Both of the copolymers were superior to Cop1 in ameliorating disease and in stimulating those mechanisms that may be important in this effect.
Finally, it may be useful to compare the two EAE models studied in this and the accompanying paper (31). The humanized mouse model allowed examination of the effect of copolymers on the MBP-specific and nonspecific T cell responses in the context of a human trimolecular complex. However, using SJL/J mice, the effect of copolymers on T cells specific to another major myelin protein, PLP, presented by a different MHC II (H-2s) could be studied. Although the T cell repertoire is restricted in the humanized tg mouse, the SJL/J mice encompass a broad T cell repertoire. This fact facilitated establishment of T cell lines, and performing of adoptive transfer experiments to test the efficacy of copolymer-specific T cells in vivo. Interestingly, although VWAK and FYAK were designed to optimize binding to HLA-DR2 present in the double tg mouse, they also bound more effectively to the I-AS protein in the SJL/J mouse than Cop1. The amelioration of disease in the two different mouse models was similar in the three types of studies used, vaccination, prevention, and treatment, in the same order of effectiveness, VWAK > FYAK > Cop1. A single notable difference in the mechanism in the two models was observed. Although VWAK effectively induced anergy of MBP 85–99-specific HLA-DR2-restricted T cells in the double tg humanized mouse, it did not do so in the PLP139–151-specific I-AS-restricted T cells of the SJL/J mouse. Both studies suggest the possibility that the copolymers VWAK and FYAK may be effective in the treatment of MS.
Acknowledgments
We thank Robert McGilp and Deneen Kozoriz for the excellent fluorescence-activated cell sorting at the Center for Neurologic Diseases, Harvard Medical School. This research was supported by National Institutes of Health Grants AI-49524 (to J.L.S.), R01 NS30843 (to V.K.K.), and P01 NS38037 (to H. L. Weiner) and by National Multiple Sclerosis Society (NMSS) Grant RG2571-D-9 (to V.K.K.). The generation of tg humanized mice was partly supported by the Multiple Sclerosis Society of Great Britain. The NMSS provided funds for the synthesis of amino acid copolymers. J.R. is a recipient of advanced postdoctoral fellowship award from the NMSS.
Abbreviations: MS, multiple sclerosis; MHC II, MHC class II; MBP, myelin basic protein; EAE, experimental autoimmune encephalomyelitis; TCR, T cell receptor; tg, transgenic; Cop1, Copolymer 1; Th2, T helper 2; CFA, complete Freund's adjuvant; APC, antigen-presenting cell.
References
- 1.Compton, A., Evers, G., Lassman, H., McDonald, I., Matthews, B. & Wekerle, H. (1998) McAlpine's Multiple Sclerosis (Churchill-Livingstone, London).
- 2.Bieganowska, K. D., Ausubel, L. J., Modabber, Y., Slovik, E., Messersmith, W. & Hafler, D. A. (1997) J. Exp. Med. 185, 1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang, J., Markovic-Plese, S., Lacet, B., Raus, J., Weiner, H. L. & Hafler, D. A. (1994) J. Exp. Med. 179, 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wucherpfennig, K. W., Zhang, J., Witek, C., Matsui, M., Modabber, Y., Ota, K. & Hafler, D. A. (1994) J. Immunol. 152, 5581–5592. [PubMed] [Google Scholar]
- 5.Illes, Z., Kondo, T., Yokoyama, K., Ohashi, T., Tabira, T. & Yamamura, T. (1999) J. Immunol. 162, 1811–1817. [PubMed] [Google Scholar]
- 6.Allegretta, M., Nicklas, J. A., Sriram, S. & Albertini, R. J. (1990) Science 247, 718–721. [DOI] [PubMed] [Google Scholar]
- 7.Allegretta, M., Albertini, R. J., Howell, M. D., Smith, L. R., Martin, R., McFarland, H. F., Sriram, S., Brostoff, S. & Steinman, L. (1994) J. Clin. Invest. 94, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielekova, B., Goodwin, B., Richert, N., Cortese, I., Kondo, T., Afshar, G., Gran, B., Eaton, J., Antel, J., Frank, J. A., et al. (2000) Nat. Med. 6, 1167–1175. [DOI] [PubMed] [Google Scholar]
- 9.Wucherpfennig, K. W., Sette, A., Southwood, S., Oseroff, C., Matsui, M., Strominger, J. L. & Hafler, D. A. (1994) J. Exp. Med. 179, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin, R., Howell, M. D., Jaraquemada, D., Flerlage, M., Richert, J., Brostoff, S., Long, E. O., McFarlin, D. E. & McFarland, H. F. (1991) J. Exp. Med. 173, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liblau, R. & Gautam, A. M. (2000) Rev. Immunogenet. 2, 95–104. [PubMed] [Google Scholar]
- 12.Oksenberg, J. R., Barcellos, L. F. & Hauser, S. L. (1999) Semin. Neurol. 19, 281–288. [DOI] [PubMed] [Google Scholar]
- 13.Krogsgaard, M., Wucherpfennig, K. W., Cannella, B., Hansen, B. E., Svejgaard, A., Pyrdol, J., Ditzel, H., Raine, C., Engberg, J., Fugger, L. & Canella, B. (2000) J. Exp. Med. 191, 1395–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsen, L. S., Andersson, E. C., Jansson, L., Krogsgaard, M., Andersen, C. B., Engberg, J., Strominger, J. L., Svejgaard, A., Hjorth, J. P., Holmdahl, R., et al. (1999) Nat. Genet. 23, 343–347. [DOI] [PubMed] [Google Scholar]
- 15.Weiner, H. L. (1997) Immunol. Today 18, 335–343. [DOI] [PubMed] [Google Scholar]
- 16.Warren, K. G. & Catz, I. (2000) Mult. Scler. 6, 300–311. [DOI] [PubMed] [Google Scholar]
- 17.Vandenbark, A. A., Chou, Y. K., Whitham, R., Mass, M., Buenafe, A., Liefeld, D., Kavanagh, D., Cooper, S., Hashim, G. A. & Offner, H. (1996) Nat. Med. 2, 1109–1115. [DOI] [PubMed] [Google Scholar]
- 18.Kappos, L., Comi, G., Panitch, H., Oger, J., Antel, J., Conlon, P. & Steinman, L. (2000) Nat. Med. 6, 1176–1182. [DOI] [PubMed] [Google Scholar]
- 19.Goodkin, D. E., Shulman, M., Winkelhake, J., Waubant, E., Andersson, P., Stewart, T., Nelson, S., Fischbein, N., Coyle, P. K., Frohman, E., et al. (2000) Neurology 54, 1414–1420. [DOI] [PubMed] [Google Scholar]
- 20.Goodin, D. S., Frohman, E. M., Garmany, G. P., Jr., Halper, J., Likosky, W. H., Lublin, F. D., Silberberg, D. H., Stuart, W. H. & van den Noort, S. (2002) Neurology 58, 169–178. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, K. P., Brooks, B. R., Cohen, J. A., Ford, C. C., Goldstein, J., Lisak, R. P., Myers, L. W., Panitch, H. S., Rose, J. W. & Schiffer, R. B. (1995) Neurology 45, 1268–1276. [DOI] [PubMed] [Google Scholar]
- 22.Fridkis-Hareli, M., Santambrogio, L., Stern, J. N., Fugger, L., Brosnan, C. & Strominger, J. L. (2002) J. Clin. Invest. 109, 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gran, B., Tranquill, L. R., Chen, M., Bielekova, B., Zhou, W., Dhib-Jalbut, S. & Martin, R. (2000) Neurology 55, 1704–1714. [DOI] [PubMed] [Google Scholar]
- 24.Neuhaus, O., Farina, C., Wekerle, H. & Hohlfeld, R. (2001) Neurology 56, 702–708. [DOI] [PubMed] [Google Scholar]
- 25.Fridkis-Hareli, M., Teitelbaum, D., Gurevich, E., Pecht, I., Brautbar, C., Kwon, O. J., Brenner, T., Arnon, R. & Sela, M. (1994) Proc. Natl. Acad. Sci. USA 91, 4872–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aharoni, R., Teitelbaum, D., Arnon, R. & Sela, M. (1999) Proc. Natl. Acad. Sci. USA 96, 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teitelbaum, D., Aharoni, R., Arnon, R. & Sela, M. (1988) Proc. Natl. Acad. Sci. USA 85, 9724–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fridkis-Hareli, M. & Strominger, J. L. (1998) J. Immunol. 160, 4386–4397. [PubMed] [Google Scholar]
- 29.Aharoni, R., Teitelbaum, D., Leitner, O., Meshorer, A., Sela, M. & Arnon, R. (2000) Proc. Natl. Acad. Sci. USA 97, 11472–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duda, P. W., Schmied, M. C., Cook, S. L., Krieger, J. I. & Hafler, D. A. (2000) J. Clin. Invest. 105, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern, J. N. H., Illés, Z., Reddy, J., Keskin, D. B., Sheu, E., Fridkis-Hareli, M., Nishimura, H., Brosnan, C. F., Santambrogio, L., Kuchroo, V. K. & Strominger, J. L. (2004) Proc. Natl. Acad. Sci. USA 101, 11743–11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, K. J., Pyrdol, J., Gauthier, L., Wiley, D. C. & Wucherpfennig, K. W. (1998) J. Exp. Med. 188, 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmied, M., Duda, P. W., Krieger, J. I., Trollmo, C. & Hafler, D. A. (2003) Clin. Immunol. 106, 163–174. [DOI] [PubMed] [Google Scholar]
- 34.Fridkis-Hareli, M., Neveu, J. M., Robinson, R. A., Lane, W. S., Gauthier, L., Wucherpfennig, K. W., Sela, M. & Strominger, J. L. (1999) J. Immunol. 162, 4697–4704. [PubMed] [Google Scholar]
- 35.Bettelli, E., Das, M. P., Howard, E. D., Weiner, H. L., Sobel, R. A. & Kuchroo, V. K. (1998) J. Immunol. 161, 3299–3306. [PubMed] [Google Scholar]