Abstract
Bone and bone marrow are closely aligned physiologic compartments, suggesting that these tissues may represent a single functional unit with a common bone marrow progenitor that gives rise to both osteoblasts and hematopoietic cells. Although reports of multilineage engraftment by a single marrow-derived stem cell support this idea, more recent evidence has challenged claims of stem cell transdifferentiation and therefore the existence of a multipotent hematopoietic/osteogenic progenitor cell. Using a repopulation assay in mice, we show here that gene-marked, transplantable marrow cells from the plastic-nonadherent population can generate both functional osteoblasts/osteocytes and hematopoietic cells. Fluorescent in situ hybridization for the X and Y chromosomes and karyotype analysis of cultured osteoblasts confirmed the donor origin of these cells and excluded their generation by a fusion process. Molecular analysis demonstrated a common retroviral integration site in clonogenic hematopoietic cells and osteoprogenitors from each of seven animals studied, establishing a shared clonal origin for these ostensibly independent cell types. Our findings indicate that the bone marrow contains a primitive cell able to generate both the hematopoietic and osteocytic lineages. Its isolation and characterization may suggest novel treatments for genetic bone diseases and bone injuries.
Bone marrow cells contribute to many diverse tissues after systemic transplantation in both mice and humans (1, 2). This capacity may solely reflect the activities of multiple discrete stem cells with restricted genetic programs, such as hematopoietic stem cells able to differentiate to leukocytes, erythrocytes, or megakaryocytes and mesenchymal stem cells that can differentiate to bone, cartilage, or adipose tissue (3). Alternatively, the bone marrow may contain rare “marrow stem cells” with the potential to differentiate to a spectrum of tissues, possibly in response to specific environmental cues. This paradigm is supported by the findings of Krause et al. (4), which indicate that a single marrow stem cell can generate both hematopoietic and nonhematopoietic lineages. However, that study lacked a unique molecular marker for confirming the multipotentiality of single transplanted stem cells; further, recent evidence shows that many examples of so-called stem cell developmental plasticity may represent fusions of hematopoietic stem cells with committed progenitors of nonhematopoietic tissues, generating cell hybrids that possess both donor and host genetic information (5–8).
Bone marrow and bone are anatomically contiguous tissues that show parallel age-related changes and share several genetic features (9–11), suggesting a close developmental relationship. Thus, a reasonable hypothesis is that the hematopoietic marrow harbors a stem cell with osteogenic potential. Consistent with this idea is the observation, reported over a decade ago, that nonadherent CD34 bone marrow cells can differentiate to osteoblasts (12) and the quite recent report that murine bone marrow side population (SP) cells can engraft in bone after transplantation (13). Moreover, in our human cell therapy trials, donor osteoblast engraftment was demonstrated after transplantation of unmanipulated bone marrow (14), but the percentage of such engraftment could not be improved by transplanting as many as 5 × 106 isolated plastic-adherent marrow stromal cells per kg of body weight, a cell number that greatly exceeds the marrow stromal cell content of unmanipulated marrow (15). One interpretation of these observations is that cells other than those in the adherent population, where mesenchymal stem cells are thought to reside (3), are potent transplantable progenitors of osteoblasts, consistent with laboratory studies showing that nonadherent cells can give rise to bone (12, 13, 16). We tested this prediction in a murine transplantation model by using gene-marked bone marrow cells and retroviral integration site-specific PCR analysis.
Methods
Transduction and Transplantation of Marrow Cells. Bone marrow was flushed from the dissected femurs and tibias of FVB/N mice (The Jackson Laboratory), and the isolated adherent marrow cells were transduced with a GFP-expressing retroviral vector (multiplicity of infection, ≈5) as described (17). In separate studies, nonadherent marrow cells, isolated from a 5-day ex vivo culture in which adherent cells stuck to the plastic dish, were transduced with the same GFP-expressing retroviral vector (70–80% efficiency) and transplanted into 4- to 6-week-old lethally irradiated (1,100 cGy) FVB/N mice (18). In this phase of the study, 106 nonadherent cells in 500 μl of PBS were infused into the tail veins of recipient mice at ≈4 h postirradiation.
Southern Blot Analysis. Genomic DNA was isolated (PureGene kit, Gentra Systems) from bone fragments that had been rigorously flushed to remove marrow cells, repeatedly minced and washed, and treated with collagenase to remove any blood cell remnants, by using the same procedure applied in the preparation of osteoblasts (14). DNA was also isolated from murine hematopoietic colony-forming unit-spleen (CFU-S) colonies and from culture-expanded osteoblasts and stromal cells. The resultant Southern blots were hybridized with a GFP-specific probe and visualized with the Storm 860 PhosphorImager (Molecular Dynamics).
Proviral Integration Analysis. Inverse PCR was performed as described in detail elsewhere (19), except that primers B and C were modified to complete complementarity to the murine stem cell virus (MSCV) sequence, and the genomic DNA was digested with the CpG methylation-insensitive TaqI restriction endonuclease. Primer sequences were as follows: VirA, 5′-TCCATGCCTTGCAAAATGGC-3′; VirB-MSCV, 5′-AGGACCTGAAAATGACCCTGTGCCTTATT-3′; VirC-MSCV, 5′-TTACTTAAGCTAGCTTGCCAAACCTACAGGT-3′; and VirD, 5′-CAACCCCTCACTCGGCGCGCCAGTC-3′. For integration site analysis, 100 ng of DNA was amplified with site-specific primers (forward, VirB-MSCV as above; reverse, 5′-AAAGCAAAAACAAAAATGGTTCCCTTTC-3′) for 25 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. PCR products containing 32P-labeled nucleotides, were analyzed by polyacrylamide gel electrophoresis and visualized with the Storm 860 PhosphorImager.
RT-PCR. RT-PCR was performed with the Titan One Tube RT-PCR System (Roche Applied Sciences) according to the manufacturer's directions. Primer sequences were as follows: β-actin, 5′-CATTGTGATGGACTCCGGAGACGG-3′ and 5′-CATCTCCTGCTCGAAGTCTAGAGC-3′; CD45, 5′-CTTCGACGGAGAGTTAATGC-3′ and 5′-GTCGCCTTAGCTTGACAACA-3′; collagen I, 5′-GCAATCGGGATCAGTACGAA-3′ and 5′-CTTTCACGCCTTTGAAGCCA-3′; osteopontin, 5′-TCACCATTCGGATGAGTCTG-3′ and 5′-ACTTGTGGCTCTGATGTTCC-3′; and osteocalcin 5′-CTCTGTCTCTCTGACCTCACAG-3′ and 5′-GGAGCTGCTGTGACATCCATAC-3′. Conditions for amplification were 27 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Products containing 32P-labeled nucleotides were analyzed and visualized as above.
Immunohistologic Staining. Immunohistochemical staining of cells was performed according to standard protocols. Briefly, GFP expression in sections of bone and cartilage was identified by incubating formalin-fixed, decalcified, paraffin-embedded sections overnight at 4°C with a rabbit anti-GFP antibody (1:300) (Molecular Probes). The primary antibody was visualized with a biotinylated goat anti-rabbit secondary antibody (1-h incubation, room temperature, dilution of 1:200) and peroxidase-conjugated avidin (ABC kit, Vector Laboratories) by using NovaRED (Vector Laboratories) as a substrate; counterstaining was with Harris hematoxylin (Surgipath Medical Industries, Richmond, IL). Immunohistochemical staining of collagen I and collagen II was performed with rabbit anticollagen primary antibodies (Chemicon) and visualized by use of a biotinylated goat anti-rabbit secondary antibody followed by peroxidase-conjugated avidin with NovaRED as a substrate. Osteocalcin staining was performed with a goat anti-mouse osteocalcin primary antibody (Biomedical Technologies, Stoughton, MA) and visualized with a biotinylated donkey anti-goat secondary antibody, as above. For double immunohistochemical staining, GFP was visualized with nickel DAB (Vector Laboratories) as a substrate. Negative control specimens were serial sections of bone and cartilage incubated with isotypic rabbit IgG primary antibody (Vector Laboratories) and visualized in the same manner as the experimental specimens. As an additional negative control, bone sections from animals transplanted with freshly harvested (non-transduced) nonadherent bone marrow cells were studied with the rabbit anti-GFP primary antibody and visualized as above. Background staining was not apparent.
Osteoblast Isolation. Osteoblasts were isolated from mouse bone as described for human osteoblasts (14).
Stromal Cell Isolation. Stromal cells were isolated and expanded in culture as described for human stromal cells (14). Fibroblastic adherent cells that were CD45–/CD11b– by flow cytometry were analyzed.
Results
Adherent Marrow Cell Transplantation. To establish a suitable assay for transplantable osteoprogenitor cells and to evaluate the osteoprogenitor capacity of mesenchymal stem cells, we isolated plastic-adherent marrow stromal cells from FVB/N mice and transduced them (82–93% efficiency) with a murine stem cell virus (MSCV) retroviral vector encoding GFP (17, 18). The cells (106 per mouse) were then infused into lethally irradiated (1,100 cGy, n = 3) or sublethally irradiated (400 cGy, n = 3) mice. Flow cytometric and PCR analyses of peripheral blood demonstrated the absence of GFP-transduced cells (data not shown), indicating that these transplanted adherent cells did not contribute to hematopoietic reconstitution. The fraction of GFP-positive osteoblasts and osteocytes identified by immunohistochemical staining at 3 months posttransplantation ranged from 0% to 2% (median, 1.5%; no difference between groups; data not shown), indicating that the systemically infused adherent marrow stromal cells lack robust repopulating activity in this murine system.
Transplanted Nonadherent Marrow Cells Engraft in Bone. To test the hypothesis that transplantable stem cells capable of generating committed osteoprogenitor cells after systemic infusion reside within the nonadherent marrow cell population, we isolated nonadherent marrow cells and transduced them with a GFP-encoding retroviral vector (20). Transduced GFP-expressing cells were isolated by fluorescence-activated cell sorting (FACS; >98% purity) and transplanted into lethally irradiated recipient mice. Two to 6 months after transplantation, flow cytometric analysis for GFP expression showed that bone marrow mononuclear cells (mean percentage with the fluorescent marker, >99%), blood leukocytes (mean, 96%), erythrocytes (mean, 92%), and platelets (mean, 95%) from seven mice were all predominantly derived from transduced marrow cells.
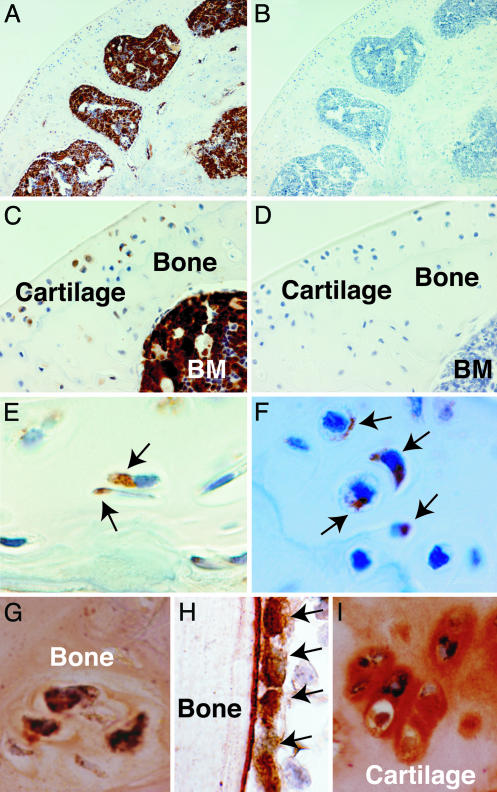
Microscopic evaluation of bone sections from these mice demonstrated GFP-expressing osteocytes and chondrocytes in the metaphysis/epiphysis and articular cartilage, respectively (Fig. 1 A–F). Double staining for GFP and collagen I, osteocalcin, or collagen II (Fig. 1 G–I) indicated osteogenic and chondrogenic differentiation and function, as well as engraftment, by the transplanted marrow cells. Osteoclasts, large multinucleated cells derived from the hematopoietic stem cell, were not observed in the specimens shown but could be identified by examining numerous sections [<1% of all bone cells were osteoclasts, consistent with published findings (21)]. Scanning of several histologic sections revealed a median of 18% gene-marked cells per 20× field (range, 0–50%; n = 60 fields). Quite strikingly, the gene-marked cells were observed in clusters accounting for up to 50% of the total cellular content of some regions of bone and cartilage.
Fig. 1.
Engraftment and differentiation of transplanted nonadherent cells to osteocytes and chondrocytes. Immunohistochemical staining with horseradish peroxidase demonstrates GFP expression in bone, cartilage, and marrow stroma. (A and B) The epiphysis and articular cartilage of a femur obtained 6 months after transplantation (A) and a negative control specimen (B). Original magnification, ×100. (C and D) Enlarged view to demonstrate the GFP-expressing cells within cartilage and bone (C), compared with the negative control (D). BM, bone marrow. Original magnification, ×400. (E and F) High-power views demonstrating individual GFP-expressing osteocytes (E) and chondroctyes (F). Arrows indicate positive cells. Original magnification, ×1,000. (G–I) Double immunohistochemical staining to demonstrate coexpression of GFP (black) and collagen I (orange) in osteocytes (G), osteocalcin (orange) in metaphyseal osteoblasts (H), and collagen II (orange) in chondrocytes (I). Original magnification, ×1,000.
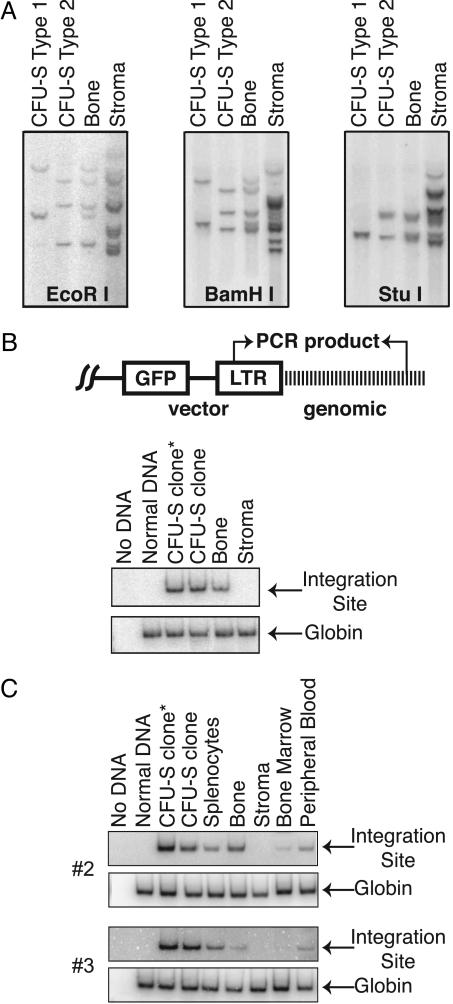
A Unique Bone Marrow Cell Gives Rise to Both Blood and Bone. To address whether the same gene-marked, nonadherent marrow cells that reconstituted hematopoiesis were also progenitors for the differentiated mesenchymal tissues, we isolated 47 secondary CFU-S clones derived from the bone marrow of a primary transplant recipient and killed at 6 months posttransplantation. Southern blot analysis of DNA isolated from these clones after digestion with restriction enzymes revealed three unique patterns designated type 1 (3 of 47 clones; Fig. 2A), type 2 (43 of 47 clones; Fig. 2 A), and type 3 (1 of 47 clones; data not shown). Similar analysis of DNA isolated from bone in the same mouse revealed a restriction pattern that was a composite of the patterns of two CFU-S colonies (Fig. 2 A), providing evidence for a common progenitor of the osteoblastic and hematopoietic lineages. The complex restriction pattern in stromal cell DNA suggests that many transduced, nonadherent progenitors had contributed to the reconstitution of stroma over the first 6 months posttransplantation. Although none of the cells seem to have been generated by the specific CFU-S colonies we identified, the possibility of their origin from a progenitor cell with both hematopoietic and osteogenic potential cannot be excluded.
Fig. 2.
Retroviral integration site analysis of CFU-S cells and nonhematopoietic tissue. (A) DNA isolated from representative colonies of CFU-S clones types 1 and 2 were compared with those from bone and stroma. Isolated DNA (10 μg) was digested with the indicated restriction enzymes, and the resulting blots were hybridized with a GFP-specific probe. (B) PCR analysis using integration site-specific primers of DNA isolated from two colonies of CFU-S clone type 2, bone, and stroma, as above. The asterisk indicates the CFU-S clone from which the integration site was isolated. No DNA and normal DNA are negative controls. PCR amplification of the globin β-major sequences was used as a control for the quality and quantity of DNA. (Upper) The integration site-specific PCR target sequence is depicted schematically. (C) Integration site-specific PCR, as in B, for two additional animals. As above, the asterisk indicates the CFU-S clone from which the integration site was isolated; another CFU-S clone of the same origin (by Southern blot analysis) is also shown. Analysis of DNA from bone marrow, peripheral blood, and splenocytes provides a comparison with other hematopoietic cells.
To confirm the Southern blot analysis indicating a common progenitor for hematopoietic cells and bone cells (Fig. 2 A), we used inverse PCR to isolate a proviral integration site on chromosome 3 in clone 16, a member of the predominant group of CFU-S clones (type 2). Using site-specific primers (Fig. 2B), we detected the predicted 616-bp PCR product from this clone and then verified its presence in another CFU-S clone of the type 2 pattern (clone 17). By PCR analysis, the clonal integration site in the hematopoietic CFU-S colonies was also present in bone.
These findings were extended by isolating an integration site from the predominant CFU-S clones, identified by Southern blot analysis, in two additional mice (Fig. 2C, animals 2 and 3). In animal 2, site-specific PCR amplified the expected 361-bp product from chromosome 9 in the DNA of bone, splenocytes, bone marrow, and peripheral blood. In animal 3, a 578-bp product was amplified from chromosome 1 in splenocytes, bone, and peripheral blood. Each of four additional transplanted animals also had a common hematopoietic/osteoblastic integration site, as demonstrated by Southern blot analysis (Figs. 3 and 4 and data not shown)
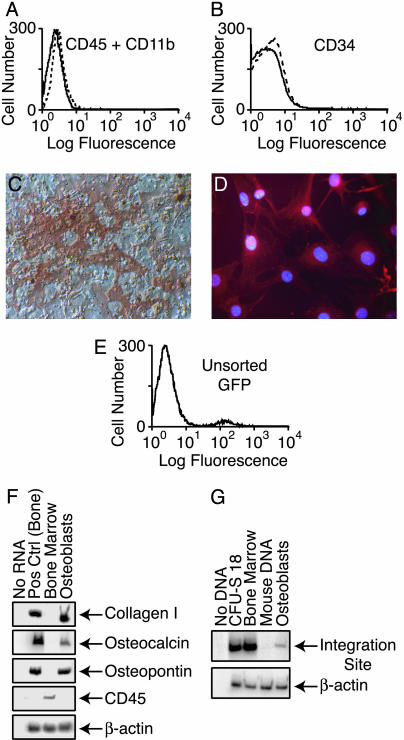
Fig. 3.
Clonal analysis of cultured osteoblasts by site-specific PCR. (A and B) Graphs of cells sorted by FACS demonstrating the absence of hematopoietic (CD45+/CD11b+) cells (A) and marrow progenitor (CD34+) cells (B). Straight lines represent cultured osteoblasts; dashed lines represent isotype control. (C) Alizarin red staining revealing mineral deposition within the extracellular matrix secreted by the cultured osteoblasts. (D) Immunofluorescence staining of the cultured osteoblasts for collagen I. (E) Graph of CD45–/CD11b–/CD34– cells showing ≈2% GFP fluorescence. (F) RT-PCR analysis of RNA isolated from the cultured osteoblasts. (G) PCR analysis of the cultured osteoblasts with site-specific primers.
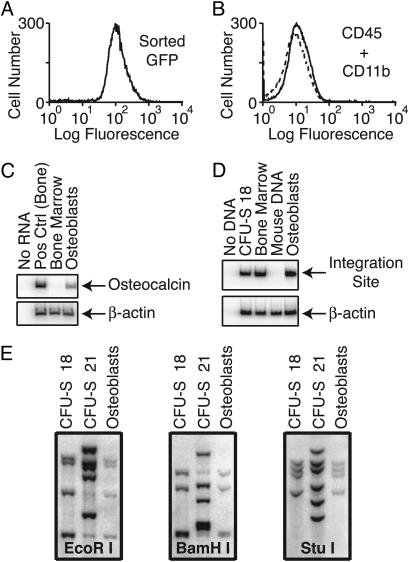
Fig. 4.
Clonal analysis of GFP-enriched osteoblasts by site-specific PCR and Southern blotting. (A) Graph of CD45–/CD11b–/CD34– cells analyzed for GFP fluorescence after FACS to enrich the population for GFP expression, demonstrating the homogeneity of GFP expression. (B) Graph of cells sorted by FACS demonstrating the lack of CD45+/CD11b+ (hematopoietic) cells. (C) RT-PCR analysis for osteocalcin expression by the pure GFP-positive cultured osteoblasts. (D) PCR analysis with site-specific primers of DNA from the same sorted osteoblasts in C, demonstrating a common, unique integration site. (E) Integration site analysis of CFU-S and osteoblasts by Southern blotting after digestion with the indicated restriction enzymes and hybridization with a GFP-specific probe. DNA (5 μg) was isolated from CFU-S clone 18, the colony from which the common integration site was originally identified; CFU-S clone 21, a different colony from the same animal; and osteoblasts sorted by FACS. The common integration pattern between CFU-S clone 18 and the osteoblasts confirms that these cells originated from a single hematopoietic/osteoblastic progenitor.
Lack of Hematopoietic Contamination. To further exclude hematopoietic contamination as a major source of bias in these assays, we obtained cultured mitotically active osteoblasts from bone of a mouse killed at 2 months posttransplantation and sorted them by FACS to eliminate possible contamination by CD45+/CD11b+ (hematopoietic) cells. Subsequent flow cytometric analysis demonstrated the lack of CD45+/CD11b+ (Fig. 3A) and CD34+ cells (Fig. 3B). The cell population secreted an extracellular matrix with mineralizing capacity as shown by alizarin red staining (Fig. 3C) and expressed collagen I as shown by immunocytochemical staining (Fig. 3D). One to 2% of the sorted cells expressed GFP (donor marker; Fig. 3E), consistent with our findings in previous clinical studies (14, 22). RT-PCR analysis showed that the cells expressed collagen I, osteopontin, and osteocalcin but not CD45 (Fig. 3F), indicative of the osteoblast phenotype without blood cell contamination. Site-specific PCR analysis demonstrated a common retroviral integration site in the hematopoietic and osteoblast DNA of this mouse (Fig. 3G).
To enrich for donor cells, we next sorted the osteoblasts by FACS for GFP expression, obtaining a population that was >99% GFP-positive (Fig. 4A). Further analysis of the cells confirmed the lack of CD45 and CD11b expression (Fig. 4B). When the CD45–/CD11b–/CD34– GFP-positive cells were expanded in culture and analyzed by RT-PCR for osteocalcin expression, the results confirmed the osteoblast phenotype of the highly enriched cells (Fig. 4C). Site-specific PCR analysis again demonstrated a common retroviral integration site in DNA from both hematopoietic and osteoblastic cells (Fig. 4D). Finally, Southern blot analysis after digestion with three different restriction enzymes showed a common proviral integration pattern (four integrants per cell) for CFU-S clone 18 and the GFP-positive osteoblasts (Fig. 4E), confirming a common clonal origin and excluding low-level contamination.
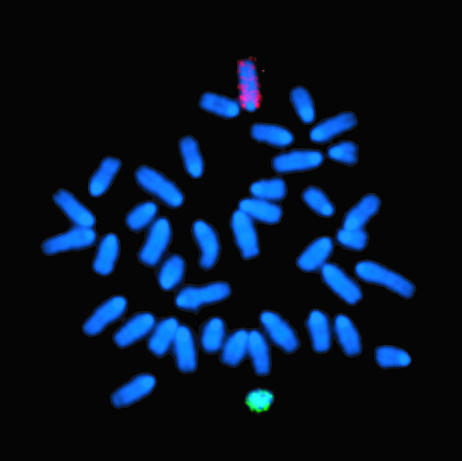
Lack of Cellular Fusion. Finally, to exclude the possibility that fusion of hematopoietic stem cells with osteoprogenitors might have been responsible for these observations, we performed fluorescence in situ hybridization for the X and Y chromosomes on cultured osteoblasts from three mice killed at 2–6 months posttransplantation (Fig. 5). All osteoblasts (n = 200 per mouse), including all donor cells (denoted by the Y chromosome), contained 40 chromosomes (normal mouse), indicating that cell fusion was not the source of the clonal bilineage differentiation seen after bone marrow transplantation.
Fig. 5.
Representative cytogenetic analysis of cultured osteoblasts. The 40 chromosomes (normal mouse) were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). The X (red) and Y (green) chromosomes were identified by fluorescence in situ hybridization, using chromosome-specific probes.
Discussion
Our results provide compelling evidence that a unique progenitor cell with both hematopoietic and osteocytic differentiation potential resides in the nonadherent subset of bone marrow cells. This conclusion is reinforced by previous studies showing that both hematopoietic and osteogenic cells express the AML/Cbfa/Runx family of transcription factors (11), by in vitro data suggesting the existence of a transforming growth factor β1 (TGF-β1)-responsive premesenchymal/prehematopoietic marrow progenitor (23), and by evidence that endoglin (CD105), a TGF-β receptor, may serve as a marker of both mesenchymal stem cells (24) and long-term repopulating hematopoietic stem cells (25, 26). Furthermore, sca-1-null mice have recently been reported to have age-related osteoporosis as well as hematopoietic deficiencies (9, 10).
The major strength of our analysis is the use of gene marking of the normal cellular constituents of bone marrow. Retroviral integration site analysis unequivocally identifies a single transduced cell and all subsequent progeny, allowing the fate of individual cells to be tracked within a population of transplanted cells (27–29). This strategy allows in vivo differentiation to be critically evaluated against results obtained in vitro, which often do not represent normal cellular function. Moreover, the use of isolated bone marrow cells in our experiments permitted insights into normal marrow cell transplantation biology that would not have been possible had the cells been subjected to extensive processing or ex vivo cultivation. The theoretical possibility that integration of the proviral sequence into the genome may have altered the normal biology of the progenitor cells is highly unlikely because animals with >99% transduced marrow mononuclear cells maintained functional engraftment in both marrow and bone with normal blood and marrow cellularity. Additionally, the animals were free of opportunistic infections and hematologic or nonhematologic malignancies. In short, our findings clearly implicate a distinct, physiologic bone marrow progenitor cell as the source of both hematopoietic and osteocytic lineages after transplantation of whole bone marrow.
Our immunohistochemical data showed uniform engraftment of transplanted adherent cells throughout the histologic fields, representing only 1.5% of osteocytes and osteoblasts. Transplantation of nonadherent marrow cells, in contrast, yielded clusters of donor cells that accounted for 18% of such bone cells. These data indicate that nonadherent marrow cells have more robust (1 log greater) bone-repopulating activity than do adherent cells after systemic infusion and, quite importantly, that there are two, presumably distinct, populations of marrow cells with the capacity to generate osteoprogenitors. One source may be more important for bone homeostasis, whereas the other may contribute to the osteogenic compartment in response to physiologic stress or to demands for the repair of injured bone.
Experimental data indicating the differentiation of a bone marrow progenitor cell to hematopoietic and nonhematopoietic cells must be interpreted cautiously. Fusion of marrow-derived cells with mature nonhematopoietic tissue cells could have lent the appearance of stem/progenitor cell differentiation, much in the manner that KTLS cells, highly purified murine hematopoietic stem cells (30), were shown to fuse with hepatocytes (7, 8, 31), rather than differentiate into them, as first reported (32). Similarly, transplanted marrow cells can fuse with neural elements to generate Purkinje cells (31), in contrast to transdifferentiation (33). In our analyses, we excluded fusion of hematopoietic and osteocytic progenitors by demonstrating the presence of donor (Y chromosome) osteoblasts with a diploid genome (Fig. 5), substantiating our contention that a single bone marrow progenitor cell gave rise to mature cells in blood and bone.
Although retroviral integration site analysis affords an extremely specific means of assessing clonality (27–29), low-level contamination by extraneous cells could confound the assay. Several lines of evidence indicate that such interference was not a factor in the present study. First of all, in animal 3 (Fig. 2C), DNA isolated from the stroma and hematopoietic marrow, both potential sources of contaminating cells, did not contain the retroviral sequences by PCR analysis and therefore could not have contributed to the bone DNA signals. Second, two experimental approaches demonstrated that the FACS-purified population of GFP-expressing osteoblasts (Fig. 4 A–C) contained cells derived from a progenitor that also produced hematopoietic cells. PCR analysis demonstrated a very strong site-specific signal indicating that the target sequence was represented in the vast majority of osteoblasts (Fig. 4D), whereas Southern blot analysis revealed a single GFP-positive clone, the most prevalent in the presumably polyclonal GFP-expressing osteoblast population, that was derived from the same marrow progenitor as the hematopoietic CFU-S clone 18 (Fig. 4E).
Olmsted-Davis et al. (13) recently described mouse long bone engraftment by a population of transplanted marrow-derived SP cells, which contains hematopoietic repopulating cells (34). However, primitive progenitor cells with an SP-like phenotype (ability to efflux fluorescent DNA-binding dye) have been identified in a variety of tissues (35), and the marrow population of SP cells is quite heterogeneous with respect to dye-exclusion capacity, progenitor activity, and surface antigen expression (34, 36). This raises the possibility that multiple, disparate SP cells, which may include the previously described CD34-nonadherent marrow cell (12), contributed to the bone engraftment reported by Olmsted-Davis et al. Our data, by contrast, demonstrate that a single gene-marked marrow cell can engraft and differentiate to both blood and bone. Although the marrow progenitor cell we identified could well reside in the SP cell population, it may also represent other populations of hematopoietic progenitor cells (26, 30) or perhaps is a rare and previously unrecognized marrow constituent.
Although providing proof of principle that a common progenitor for the hematopoietic and osteocytic lineages resides in bone marrow, we were unable to ascertain the frequency or the potential clinical utility of this shared progenitor cell. However, the robust regeneration of both functional hematopoietic and osteocytic target tissues (up to 50% of bone cells in some sections) suggests a physiologically important progenitor cell response rather than a rare stochastic event that typifies most instances of “stem cell plasticity” (37). This striking engraftment of marked progenitor cells might be explained by observations that murine osteoblasts can be recruited from progenitor cells in only a few days in response to changes in stressful stimuli (38), such as chemotherapy or irradiation. Indeed, the robust osteopoietic engraftment we observed may well depend on the marrow-ablative effects of radiation (39), although this relationship will need to be assessed in carefully controlled competitive repopulation experiments. We would emphasize, however, that marrow cell engraftment in patients with genetic disorders of bone may be adequate without the use of a preparative regimen (15).
How durable is the regenerative contribution of this hematopoietic/osteocytic progenitor cell? In the present study, a significant fraction of osteoblasts from mice killed at 2–6 months posttransplantation were of donor origin, as were osteoblast samples collected at 3 months posttransplantation from patients (14, 22). However, in long-term follow-up studies of transplant patients, samples of osteoblasts were exclusively of host origin (40). Thus, bone repair and regeneration during the early posttransplantation period seems to be driven by transplanted, donor-derived marrow progenitor cells that either engraft directly in bone or are recruited from the marrow to bone. For routine homeostasis, the integrity of bone seems to be maintained by repopulating cells normally present in the bone microenvironment. This distinction between bone repair/regeneration and maintenance will be important in developing widely applicable cellular therapies for injured or genetically disordered bone.
Acknowledgments
We thank Dr. Richard Ashmun for flow cytometric analyses, Virginia Valentine for cytogenetic analyses, John Gilbert for editorial review, and Ms. Angie Williams for assistance in preparation of this manuscript. This work was supported by Doris Duke Charitable Foundation Clinical Scientist Development Award T99102B; National Heart, Lung, and Blood Institute Clinical Scientist Award K08-HL0420; National Cancer Institute Cancer Center Support CORE Grant P30-CA-21765; and the American Lebanese Syrian Associated Charities.
Abbreviations: SP, side population; CFU-S, hematopoietic colony-forming unit-spleen; FACS, fluorescence-activated cell sorting.
References
- 1.Herzog, E. L., Chai, L. & Krause, D. S. (2003) Blood 102, 3483–3493. [DOI] [PubMed] [Google Scholar]
- 2.Korbling, M., Katz, R. L., Khanna, A., Ruifrok, A. C., Rondon, G., Albitar, M., Champlin, R. E. & Estrov, Z. (2002) N. Engl. J. Med. 346, 738–746. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Moorman, M. A., Simonetti, D. W., Craig, S. & Marshak, D. R. (1999) Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- 4.Krause, D. S., Theise, N. D., Collector, M. I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S. & Sharkis, S. J. (2001) Cell 105, 369–377. [DOI] [PubMed] [Google Scholar]
- 5.Ying, Q. L., Nichols, J., Evans, E. P. & Smith, A. G. (2002) Nature 416, 545–548. [DOI] [PubMed] [Google Scholar]
- 6.Terada, N., Hamazaki, T., Oka, M., Hoki, M., Mastalerz, D. M., Nakano, Y., Meyer, E. M., Morel, L., Petersen, B. E. & Scott, E. W. (2002) Nature 416, 542–545. [DOI] [PubMed] [Google Scholar]
- 7.Wang, X., Willenbring, H., Akkari, Y., Torimaru, Y., Foster, M., Al Dhalimy, M., Lagasse, E., Finegold, M., Olson, S. & Grompe, M. (2003) Nature 422, 897–901. [DOI] [PubMed] [Google Scholar]
- 8.Vassilopoulos, G., Wang, P. R. & Russell, D. W. (2003) Nature 422, 901–904. [DOI] [PubMed] [Google Scholar]
- 9.Ito, C. Y., Li, C. Y., Bernstein, A., Dick, J. E. & Stanford, W. L. (2003) Blood 101, 517–523. [DOI] [PubMed] [Google Scholar]
- 10.Bonyadi, M., Waldman, S. D., Liu, D., Aubin, J. E., Grynpas, M. D. & Stanford, W. L. (2003) Proc. Natl. Acad. Sci. USA 100, 5840–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian, J. B., Balint, E., Javed, A., Drissi, H., Vitti, R., Quinlan, E. J., Zhang, L., van Wijnen, A. J., Stein, J. L., Speck, N., et al. (2003) J. Cell Physiol. 196, 301–311. [DOI] [PubMed] [Google Scholar]
- 12.Long, M. W., Williams, J. L. & Mann, K. G. (1990) J. Clin. Invest. 86, 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olmsted-Davis, E. A., Gugala, Z., Camargo, F., Gannon, F. H., Jackson, K., Kienstra, K. A., Shine, H. D., Lindsey, R. W., Hirschi, K. K., Goodell, M. A., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 15877–15882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz, E. M., Prockop, D. J., Fitzpatrick, L. A., Koo, W. W., Gordon, P. L., Neel, M., Sussman, M., Orchard, P., Marx, J. C., Pyeritz, R. E., et al. (1999) Nat. Med. 5, 309–313. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz, E. M., Gordon, P. L., Koo, W. K., Marx, J. C., Neel, M. D., McNall, R. Y., Muul, L. & Hofmann, T. (2002) Proc. Natl. Acad. Sci. USA 99, 8932–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long, M. W. (2001) Blood Cells Mol. Dis. 27, 677–690. [DOI] [PubMed] [Google Scholar]
- 17.Marx, J. C., Allay, J. A., Persons, D. A., Nooner, S. A., Hargrove, P. W., Kelly, P. F., Vanin, E. F. & Horwitz, E. M. (1999) Hum. Gene Ther. 10, 1163–1173. [DOI] [PubMed] [Google Scholar]
- 18.Persons, D. A., Allay, J. A., Allay, E. R., Smeyne, R. J., Ashmun, R. A., Sorrentino, B. P. & Nienhuis, A. W. (1997) Blood 90, 1777–1786. [PubMed] [Google Scholar]
- 19.van Lohuizen, M., Verbeek, S., Scheijen, B., Wientjens, E., van der Gulden, H. & Berns, A. (1991) Cell 65, 737–752. [DOI] [PubMed] [Google Scholar]
- 20.Persons, D. A., Allay, J. A., Allay, E. R., Ashmun, R. A., Orlic, D., Jane, S. M., Cunningham, J. M. & Nienhuis, A. W. (1999) Blood 93, 488–499. [PubMed] [Google Scholar]
- 21.Roodman, G. D. (1996) Endocr. Rev. 17, 308–332. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz, E. M., Prockop, D. J., Gordon, P. L., Koo, W. W., Fitzpatrick, L. A., Neel, M. D., McCarville, M. E., Orchard, P. J., Pyeritz, R. E. & Brenner, M. K. (2001) Blood 97, 1227–1231. [DOI] [PubMed] [Google Scholar]
- 23.Hall, F. L., Han, B., Kundu, R. K., Yee, A., Nimni, M. E. & Gordon, E. M. (2001) J. Hematother. Stem Cell Res. 10, 261–271. [DOI] [PubMed] [Google Scholar]
- 24.Barry, F. P., Boynton, R. E., Haynesworth, S., Murphy, J. M. & Zaia, J. (1999) Biochem. Biophys. Res. Commun. 265, 134–139. [DOI] [PubMed] [Google Scholar]
- 25.Chen, C. Z., Li, M., De Graaf, D., Monti, S., Gottgens, B., Sanchez, M. J., Lander, E. S., Golub, T. R., Green, A. R. & Lodish, H. F. (2002) Proc. Natl. Acad. Sci. USA 99, 15468–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, C. Z., Li, L., Li, M. & Lodish, H. F. (2003) Immunity 19, 525–533. [DOI] [PubMed] [Google Scholar]
- 27.Kim, H. J., Tisdale, J. F., Wu, T., Takatoku, M., Sellers, S. E., Zickler, P., Metzger, M. E., Agricola, B. A., Malley, J. D., Kato, I., et al. (2000) Blood 96, 1–8. [PubMed] [Google Scholar]
- 28.Nolta, J. A., Dao, M. A., Wells, S., Smogorzewska, E. M. & Kohn, D. B. (1996) Proc. Natl. Acad. Sci. USA 93, 2414–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt, M., Carbonaro, D. A., Speckmann, C., Wissler, M., Bohnsack, J., Elder, M., Aronow, B. J., Nolta, J. A., Kohn, D. B. & von Kalle, C. (2003) Nat. Med. 9, 463–468. [DOI] [PubMed] [Google Scholar]
- 30.Spangrude, G. J., Heimfeld, S. & Weissman, I. L. (1988) Science 241, 58–62. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez-Dolado, M., Pardal, R., Garcia-Verdugo, J. M., Fike, J. R., Lee, H. O., Pfeffer, K., Lois, C., Morrison, S. J. & Alvarez-Buylia, A. (2003) Nature 425, 968–973. [DOI] [PubMed] [Google Scholar]
- 32.Lagasse, E., Connors, H., Al Dhalimy, M., Reitsma, M., Dohse, M., Osborne, L., Wang, X., Finegold, M., Weissman, I. L. & Grompe, M. (2000) Nat. Med. 6, 1229–1234. [DOI] [PubMed] [Google Scholar]
- 33.Brazelton, T. R., Rossi, F. M., Keshet, G. I. & Blau, H. M. (2000) Science 290, 1775–1779. [DOI] [PubMed] [Google Scholar]
- 34.Goodell, M. A., Rosenzweig, M., Kim, H., Marks, D. F., DeMaria, M., Paradis, G., Grupp, S. A., Sieff, C. A., Mulligan, R. C. & Johnson, R. P. (1997) Nat. Med. 3, 1337–1345. [DOI] [PubMed] [Google Scholar]
- 35.Abbott, B. L. (2003) Hematol. Oncol. 21, 115–130. [DOI] [PubMed] [Google Scholar]
- 36.Storms, R. W., Goodell, M. A., Fisher, A., Mulligan, R. C. & Smith, C. (2000) Blood 96, 2125–2133. [PubMed] [Google Scholar]
- 37.Orkin, S. H. & Zon, L. I. (2002) Nat. Immunol. 3, 323–328. [DOI] [PubMed] [Google Scholar]
- 38.Turner, C. H., Owan, I., Alvey, T., Hulman, J. & Hock, J. M. (1998) Bone 22, 463–469. [DOI] [PubMed] [Google Scholar]
- 39.Pateder, D. B., Sheu, T. J., O'Keefe, R. J., Puzas, J. E., Schwarz, E. M., Constine, L. S., Okunieff, P. & Rosier, R. N. (2002) Radiat. Res. 157, 62–68. [DOI] [PubMed] [Google Scholar]
- 40.Koc, O. N., Peters, C., Aubourg, P., Raghavan, S., Dyhouse, S., DeGasperi, R., Kolodny, E. H., Yoseph, Y. B., Gerson, S. L., Lazarus, H. M., et al. (1999) Exp. Hematol. 27, 1675–1681. [DOI] [PubMed] [Google Scholar]