Abstract
Advanced glycation endproducts (AGE) contribute to kidney disease due to diabetes or aging by means of mesangial cell (MC) receptors, such as the receptor for AGE (RAGE), which promote oxidant-stress-dependent NF-κB activation and inflammatory gene expression. MC also express scavenger receptors SR-I and SR-II and AGE receptors 1, 2, and 3 (AGE-R1, -R2, and -R3), some of which are linked to AGE turnover. Because AGE-R1 expression is found suppressed in severe diabetic kidney disease, as other receptors increase, we investigated whether his molecule has a protective role against AGE-induced MC injury. A stable murine MC line overexpressing AGE-R1 (R1-MC) was generated, exhibiting a 1.8- to 2.7-fold increase in 125I-AGE-specific binding, uptake, and degradation, compared with mock-MC. However, AGE-stimulated NF-κB activity and mitogen-activated protein kinase (MAPK) (p44/42) phosphorylation were found markedly suppressed in R1-MC. Additionally, AGE-stimulated macrophage chemotaxis protein 1 and RAGE overexpression were abolished in R1-MC. The effect of R1 on RAGE signaling was investigated after overexpressing RAGE in Chinese hamster ovary cells, which lack RAGE. AGE stimulation elicited NF-κB and MAPK activities in RAGE-Chinese hamster ovary cells; however, after cotransfection with R1, these responses were suppressed. Also, after silencing endogenous R1 in wild-type MC by R1 small interfering RNA, AGE-mediated MAPK/p44/42 activation exceeded by >2-fold that of mock-MC, consistent with loss of the activation-inhibitory properties of native AGE-R1. AGE-R1, although enhancing AGE removal, is also a distinct receptor in that it suppresses AGE-mediated MC inflammatory injury through negative regulation of RAGE, a previously uncharacterized pathway that may protect renal and other tissue injury due to diabetes and aging.
Keywords: oxidant stress, glycoxidation, NF-κB, extracellular signal-regulated kinase 1/2, nephropathy
The pathogenic effects of advanced glycation endproducts (AGE) include oxidant stress (OS) and tissue-specific inflammation, growth, or apoptosis (1–3) and are controlled by AGE receptors, antioxidant systems, and normal kidneys (4–6). The AGE-receptor system could be broadly divided into two arms: one is associated with increased OS, growth, and inflammatory effects, best represented by the receptor for AGE (RAGE) (7–9); and the other, involved in AGE detoxification, includes scavenger receptors class A, type II (MSR-AII), and class B, type I (MSR-BI, CD36) (10, 11), as well as AGE receptors 1, 2, and 3 (AGE-R1, -R2, and -R3) (12–15). Given the complex properties of AGE, a legitimate concern has been the apparent failure of AGE receptors as a bodywide defense system to prevent tissue-toxic AGE accumulation (16, 17).
Among the major organs, the kidney is a major regulator of AGE turnover but also a target for AGE toxicity. Mesangial cells (MC) occupy a central role in the maintenance of both architecture and function of the nephron and, with exposure to AGE, undergo phenotypic changes that contribute to the progression of glomerulosclerosis (18). MC AGE receptors, although contributing to AGE turnover (19), appear to be involved in AGE-induced cytokine, growth factor, and extracellular matrix overproduction (20–22). In high AGE states, as in diabetes or aging, tissue expression of RAGE and other receptors is enhanced (23, 24). An exception to this rule has been AGE-R1, a 48-kDa, AGE-specific, type I transmembrane receptor protein (13). The expression and function of this molecule was found to be suppressed in MC and in macrophages from nonobese diabetic mice, which are prone to severe kidney disease (25), and in circulating mononuclear cells from diabetic subjects with high AGE levels and severe nephropathy (15). This finding is evidence suggesting a possible inverse relationship between AGE toxicity and low AGE-R1 expression.
The present study was undertaken to determine whether MC AGE-R1 is involved in AGE turnover and/or in the regulation of AGE-mediated cell injury. By using murine MC overexpressing AGE-R1 (R1-MC), renal cell responses to AGE were investigated. Beyond its participation in AGE removal, AGE-R1 was found to negatively regulate AGE proinflammatory signal processing, by means of a previously uncharacterized potentially renal-protective mechanism.
Materials and Methods
Cell Culture. A murine MC line isolated from a single glomeruli was established, as described in refs. 19 and 20. Experiments were performed between passages 20 and 25 in medium containing 5 mM glucose to minimize phenotypic variation. Chinese hamster ovary (CHO) cells (ATCC CCL-61) were maintained in Ham's F12K medium containing 10% FBS.
Stable Expression of AGE-R1 and RAGE in MC and CHO Cells. The cDNA of human AGE-R1 and RAGE were amplified from human kidney mRNA (Clontech) by using the following primers: AGE-R1-F, 5′-GGC GAA GCT TAC CAT GGG GTA CTT CCG GTG TGC AGG TGC T-3′; AGE-R1-R, 5′-TTT TTC TAG ATC AGT CGG ACT TCT CCT TCT CCT TCA TGT G-3′; RAGE-F, 5′-GGC GAA GCT TAC CAT GGC AGC CGG AAC AGC AGT TGG AGC CT-3′; and RAGE-R, 5′-TTT TTC TAG ATC AAG GCC CTC CAG TAC TAC TCT CGC CT-3′. A HindIII site and Kozak consensus sequence were introduced in the forward primer and an XbaI site was introduced in the reverse primer, indicated in bold letters. Purified PCR products were subcloned into pcDNA3.1 (Invitrogen) vector by using HindIII and XbaI sites. Correct sequence and orientation of the constructs were confirmed by DNA sequencing. Stably overexpressing cells of AGE-R1 were created in MC and CHO cells. Plasmid pcDNA3.1-R1, comprising human AGE-R1 cDNA, was transfected into MC and CHO cells by the transfection reagent FuGENE6 (Roche Molecular Biochemicals), according to manufacturer's instructions. Two days after transfection, cells were selected by culturing in the presence of neomycin analogue G418 (1 mg/ml) (Invitrogen) for at least 3 weeks before use. G418-resistant cells transfected with pcDNA3.1-R1 were grown as polyclonal cell lines for further studies. Mock-transfected MC and CHO cells expressing neo alone also were established by using plasmid pcDNA3.1. CHO cells overexpressing RAGE also were established, as described above.
AGE-BSA Binding, Uptake, and Degradation Studies. Endotoxin-free AGE-BSA and native BSA were prepared and radiolabeled as described in refs. 12 and 25. 125I-AGE-BSA or 125I-BSA was added at 20 μg/ml in the presence or absence of 100-fold excess of unlabeled ligand (AGE-BSA, BSA) for 4 h at 4°C. After washing, 125I radioactivity was determined with a Packard Top-Count NXT Microplate Scintillation and Luminescence counter and cell protein by the Bradford method using a Dc Protein Assay kit (Bio-Rad).
The same procedure was repeated at 37°C to determine ligand uptake and degradation activity. Cell-associated radioligand was divided into two pools: surface-bound material eluted with heparin (10 mg/ml) and EDTA (5 mM)-containing buffer (pH 7.4) (5 min at 37°C) and internalized material eluted after solubilizing the cells in 0.1 M NaOH (30 min at 37°C). Ligand degradation was determined as trichloroacetic acid-soluble cpm in the aspirated media (12, 25, 26). AGE-specific binding, uptake, and degradation were defined as the radioactivity observed in wells incubated with 125I-AGE-BSA or 125I-BSA alone, subtracting the cpm found in the presence of 100-fold excess of unlabeled AGE-BSA or BSA (26). No specific binding was observed in wells without cells.
Transient Transfection Experiments and Luciferase Assay. Oxidant-sensitive NF-κB activation was assessed by transient transfection with a luciferase expression plasmid (pNF-κB-Luc, Stratagene) controlled by five tandem repeats of NF-κB consensus sequence, cloned in front of a minimal promoter. Near-confluent cells in six-well plates (Costar) were transfected with 500 ng of pNF-κB-Luc in serum-free complete medium, by using transfection reagent FuGENE6. After a 48-h incubation, fresh serum-free medium was added into the cells along with AGE-BSA or BSA, in variable concentrations or time intervals, to assess dose- and time-dependent responses. Luciferase activities were measured by using a LucLite Plus assay system (Packard), according to manufacturer protocol. To correct for transfection efficiency, pSV-β-gal plasmid (50 ng) was included. The ratio of luciferase activity to β-gal activity served to normalize luciferase activity.
Western Blotting. Cells were collected and solubilized in lysis buffer (New England Biolabs), pH 7.4. Western blotting was performed as described in refs. 12 and 25. The following primary antibodies were used: rabbit polyclonal anti-AGE-R1 (13), anti-RAGE (Santa Cruz Biotechnology), antiphospho-p44/42 (New England Biolabs), or antitotal p44/42 antibody (New England Biolabs).
Semiquantitative RT-PCR. Total RNA from MC was isolated with TRIzol reagent (Invitrogen). After RNase-free DNase (Invitrogen) treatment for 15 min at room temperature, RNA was further purified with the RNeasy RNA isolation kit (Qiagen, Valencia, CA). Total RNA (1 μg) was reverse transcribed in a 10-μl reaction system by using a smart PCR cDNA synthesis kit (Clontech) under conditions described by the supplier. Then, 1/20 reverse-transcribed cDNA was amplified by 30 cycles under standard condition in 50 μλ PCR system with primer monocyte chemoattractant protein 1 (MCP-1)-F, 5′-TCA CCT GCT GCT ACT CAT TC-3′, and primer MCP-1-R, 5′-ACT GGT CAC TGG TAC AGA AG-3′. The gene expression level of MCP-1 was normalized against β-actin.
MCP-1 Determination. To quantify the level of MCP-1 protein expression under different experimental conditions, cell culture supernatants were assessed by a commercial solid phase quantitative sandwich ELISA kit, specific for mouse MCP-1 and sensitive to 8 pg of MCP-1 per milliliter (BioSource International, Camarillo, CA).
AGE-R1 Small Interfering RNA (siRNA) Assay. MC endogenous AGE-R1 expression was silenced by the siRNA expression system (27). Four sequences, (i) CATCAACGTGGAGACCATC, (ii) CAGGCTCTTCAACCTCTTA, (iii) TGCCTACACTGTCACCGAC, and (iv) AGTGGATTACAACCGGCTA, were chosen and cloned in the pSilencer 3.1-H1 hygro vector (Ambion, Austin, TX). Both control vector and vector containing R1 siRNA were transfected into MC by using FuGENE6 (Roche Molecular Biomedicals). Transfected cells were selected by culture in hygromycin (250 μg/ml), and pure cell populations were used for experiments. The inhibitory expression efficiency of R1 siRNA was determined by Western blotting for AGE-R1. Sequence i, above, showing the highest efficacy (60% reduction of AGE-R1 expression), was selected for experiments.
Results
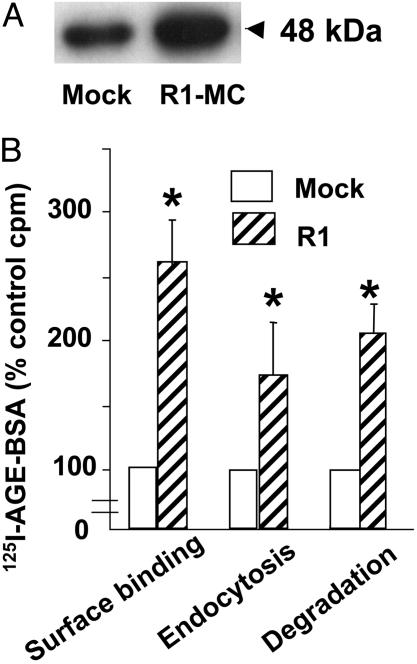
Increased AGE-BSA Turnover by R1-MC. Increased AGE-R1 protein expression (2- to 3-fold above the endogenous R1 in mock-MC) was confirmed in MC transfected with human AGE-R1 (R1-MC), based on immunoblotting (Fig. 1A). This finding was associated with a significant increase in the AGE-ligand binding activity at 4°C (>2.5-fold, P < 0.01) (Fig. 1B), internalization (>1.5-fold, P < 0.05), and degradation (>2.0-fold, P < 0.05) at 37°C in R1-MC compared with mock-MC (Fig. 1B), indicating that AGE-R1 is involved in AGE turnover in these cells. No binding of unmodified 125I-BSA was observed (data not shown).
Fig. 1.
Overexpression of AGE-R1 in MC increases the specific binding, endocytosis, and degradation of AGE-BSA. (A) Western blot for AGE-R1 in R1-MC or mock-MC cell lysates was performed with anti-R1. (B) 125I-AGE-BSA binding, uptake, and degradation assays were performed as described in Materials and Methods. Each experiment was performed in triplicate and repeated three to five times. The data are presented as percent cpm obtained in mock-MC (means ± SD). *, P < 0.01 vs. mock-MC.
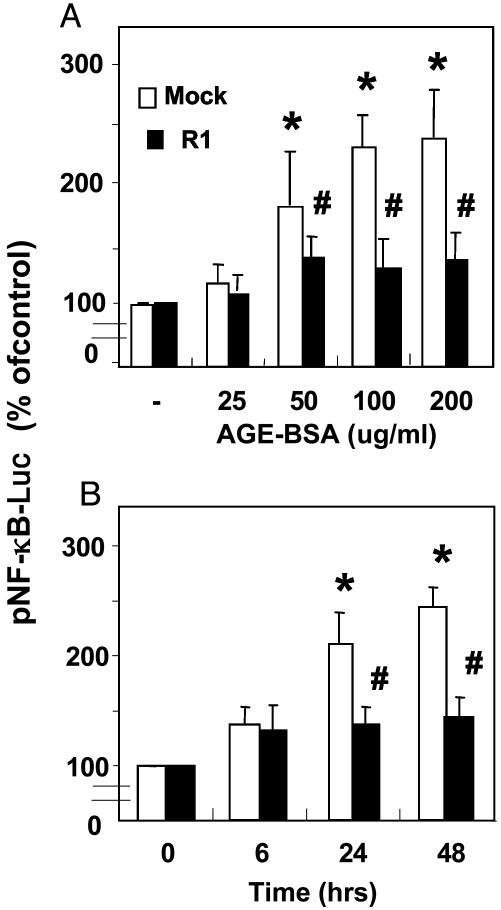
Reduction of AGE-Induced NF-κB Transcriptional Activity in R1-MC. The redox-sensitive NF-κB activity in response to AGE stimulation was studied after transient transfection of MC with pNF-κB-Luc. As expected, in mock-MC, NF-κB-dependent gene expression increased significantly (>2-fold, P < 0.01) after 24 h of stimulation with AGE-BSA (100 μg/ml). However, in R1-MC, AGE-BSA-induced NF-κB activity was reduced to ≈20% of mock-MC (P < 0.01) (Fig. 2A) and in a time-dependent manner (maximum at 48 h) (P < 0.01) (Fig. 2B).
Fig. 2.
Increased AGE-R1 expression blocks AGE-induced NF-κB activation in R1-MC. (A) Dose–response. (B) Time course. NF-κB activity was measured by using a luciferase expression plasmid and after incubation with different concentrations of AGE-BSA for different intervals. Luciferase expression in unstimulated cells served as control and as baseline for NF-κB activity (100%). Each experiment was performed in triplicate, and experiments were repeated at least three times. Data are means ± SD. *, P < 0.01 vs. unstimulated control; #, P < 0.01 vs. mock-MC.
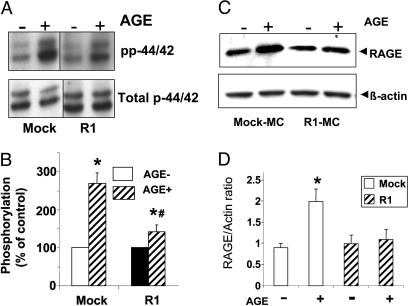
AGE-R1 Expression in MC Inhibits AGE-Mediated Mitogen-Activated Protein Kinase (MAPK)/p44/42 Phosphorylation. By using antibodies specific for total and phosphorylated p44/42, the activation of MAPK pathway was assessed (Fig. 3A). After stimulation of mock-MC with AGE-BSA (100 μg/ml, for 10 min), a strong induction of p44/42 phosphorylation was observed (2.75-fold from baseline). In contrast, in R1-MC, AGE-mediated p44/42 phosphorylation was significantly lower (1.3-fold above baseline or of unmodified BSA, P < 0.01) (Fig. 3 A and B). No activation of p38 or JNK was observed after AGE stimulation in either mock-MC or R1-MC (data not shown).
Fig. 3.
AGE-R1 inhibits AGE-induced p42/44 phosphorylation and RAGE stimulation in R1-MC. (A) Western blot. Synchronized quiescent MC were incubated with AGE-BSA (100 μg/ml, for 10 min), cells were lysed, and Western blot was performed with a polyclonal antibody specific for phosphorylated p42/44 and normalized with total p42/44. (B) Densitometric analysis. The densities of phosphorylated p42/44 were corrected for total p42/44. Phosphorylated p42/44 in unstimulated MC serves as control and was considered as a baseline. Data are means ± SD of three independent experiments. *, P < 0.01 vs. unstimulated condition; #, P < 0.01 vs. mock-MC. (C) RAGE protein. Lysates from mock-MC and R1-MC, treated as in A and B, were collected for Western blot and analyzed by using anti-RAGE antibody and β-actin as internal control. (D) Densitometry of data shown in C. Data represent four identical experiments (mean ± SD). *, P < 0.05.
AGE-R1 Suppresses RAGE-Signaling in AGE-Stimulated CHO Cells. We then assessed the effect of R1 overexpression on RAGE in R1-MC after AGE stimulation by using Western blot analysis. As expected, AGE increased RAGE expression in mock-MC (≈2-fold above background level) (P < 0.05). However, this response was abolished in R1-MC (Fig. 3 C and D), indicating that R1 can block RAGE expression in this expression system.
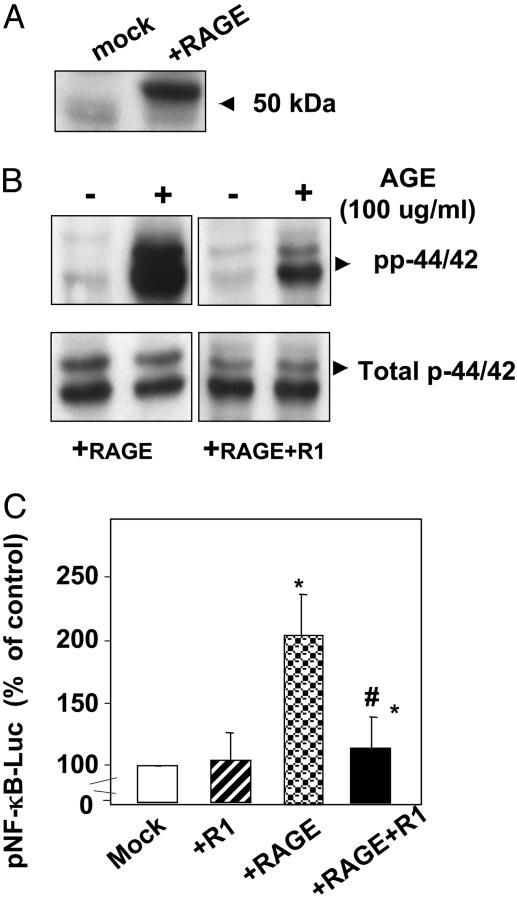
Because the RAGE response could be an indirect effect, we proceeded to test whether R1 inhibition of AGE-signaling occurred in the presence of active RAGE gene expression. Human RAGE was thus overexpressed in CHO cells. Although undetectable in wild-type CHO cells, RAGE expression clearly increased in RAGE-CHO transfectants (Fig. 4A), and upon stimulation with AGE (100 μg/ml for 10 min), these cells exhibited a strong p44/42 phosphorylation signal (Fig. 4B Left). In contrast, when RAGE-CHO cells were cotransfected with AGE-R1 (RAGE+R1-CHO), AGE-induced p44/42 phosphorylation was almost completely blocked (Fig. 4B Right). Similarly, in RAGE-CHO cells, NF-κB activity increased significantly (≈2-fold above baseline, P < 0.01) after stimulation with AGE; in RAGE+R1-CHO cells, however, this response was virtually abolished (P < 0.05) (Fig. 4C). Neither mock-CHO nor R1-CHO cells exhibited AGE-induced phosphorylation of p42/44 or NF-κB activation. These data indicated that AGE-R1 intercepts RAGE-induced signals initiated by AGE.
Fig. 4.
AGE-R1 expressed in CHO cells counteracts RAGE-induced signal transduction pathway induced by AGE. (A) Immunoblot analysis of RAGE-transfected CHO or mock-CHO cell lysates was performed by anti-RAGE antibody. (B) Quiescent and AGE-stimulated RAGE-CHO and double-transfected RAGE+R1 CHO cell lysates were analyzed with antiphosphorylated p42/44 or antitotal p42/44. (C) NF-κB activity was studied by transient transfection with pNF-κB-Luc, as described in Materials and Methods. Luciferase expression in unstimulated cells served as controls for NF-κB activity. Each experiment was performed in triplicate and repeated at least three times. Data are shown as means ± SD. *, P < 0.01 vs. unstimulated cells; #, P < 0.01 vs. mock-MC.
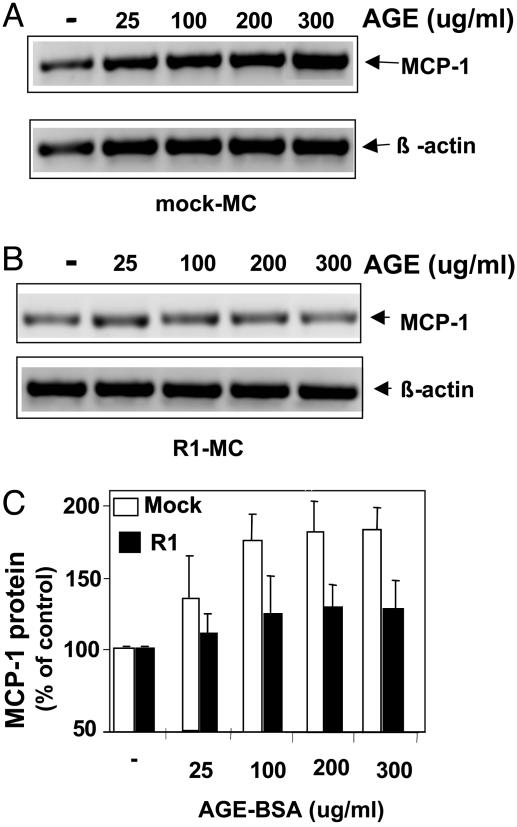
In R1-Expressing MC, AGE-Mediated MCP-1 Synthesis Is Suppressed. RAGE/AGE-mediated MCP-1 induction is considered important in the pathogenesis of kidney disease (28, 29). Thus, the effect of R1 overexpression on AGE-mediated MCP-1 production by MC was investigated. Whereas in mock-MC, AGE stimulation led to a significant increase of MCP-1 mRNA expression in a dose-dependent manner (up to 3-fold from baseline) (Fig. 5A), in R1-MC, it produced only a minimal change (up to 10% of mock-MC) (P < 0.01) (Fig. 5B). Similarly, the AGE-induced MCP-1 protein secretion was markedly attenuated in R1-MC, compared with mock-MC (Fig. 5C).
Fig. 5.
MCP-1 in R1-expressing MC is suppressed after AGE stimulation. Synchronized quiescent mock (A) or R1-MC (B) were incubated with different concentrations of AGE-BSA. After 8 h, total RNA was isolated and subjected to RT-PCR with specific primers for murine MCP-1 and β-actin. After gel electrophoresis, PCR products were visualized with ethidium bromide. (C) MCP-1 protein secretion. After 48-h MC stimulation with AGE-BSA, MCP-1 protein was measured in cell supernatants with ELISA. Data are shown as percentage of those obtained in unstimulated cells and represent the means ± SD of three independent experiments. *, P < 0.01 vs. unstimulated condition; #, P < 0.01 vs. mock-MC.
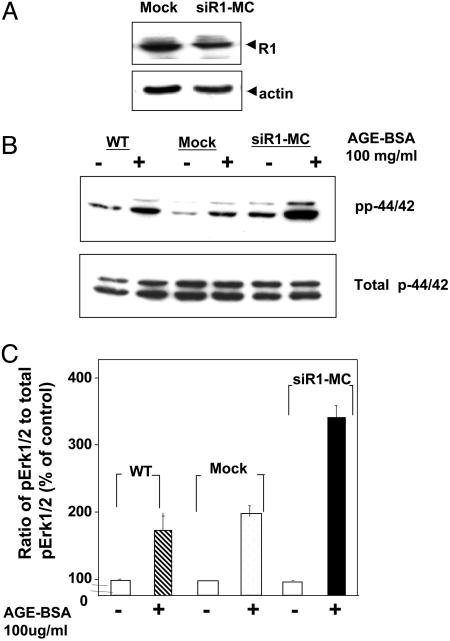
Silencing of AGE-R1 Gene Expression Leads to Enhanced AGE-Dependent p44/42 Phosphorylation. To rule out possible nonspecific effects of the transfection, we used MC expressing siRNA specific for AGE-R1. As shown in Fig. 6A, in such cells, AGE-R1 protein synthesis was markedly reduced. In addition, AGE stimulation elicited greater p44/42 phosphorylation signal in MC R1-silenced cells compared with control cells (Fig. 6 B and C). This finding was consistent with the observation that reduced R1 expression may lead to an MC phenotype prone to inflammatory activation.
Fig. 6.
Reduction of MC AGE-R1 expression by siRNA leads to enhanced AGE-induced phospho-extracellular signal-regulated kinase (ERK) 1/2. R1-siRNA MC were prepared as described in Materials and Methods. After exposure to AGE (200 μg/ml) for 15 min, cell lysates from both R1-siRNA-expressing or control cells were prepared for Western blot. (A) Western blot of AGE-R1 in cells expressing R1-siRNA and in control cells. (B) Western blot for phospho-ERK1/2 and total ERK1/2 in siRNA-expressing and control cells with or without AGE stimulation. Experiments were done in triplicate. Data are representative of three identical experiments. *, P < 0.05.
Discussion
The findings described here identify AGE-R1 as a receptor actively contributing to AGE turnover by MC (19) and, more importantly, in the counterregulation of potent proinflammatory signals triggered by AGE substances by means of RAGE, leading to renal and other tissue injury (7, 30). These previously uncharacterized properties of R1 are potentially beneficial because they stand in direct opposition to the predominantly proinflammatory MC response to AGE (28, 31, 32) (Fig. 7 Right).
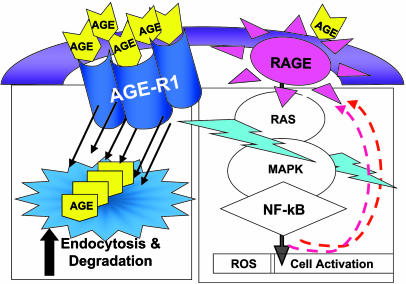
Fig. 7.
Diagram of the interaction between AGE-R1 and RAGE. Overexpression of AGE-R1 enhances AGE binding and degradation, inhibits RAGE-mediated RAS-MAPK-NF-κB-MCP1 activation, and thereby prevents diabetic nephropathy.
MC were selected because of their pivotal role in the normal glomerulus. Numerous studies on MC AGE-ligand–receptor interactions have confirmed the role of RAGE in the progression of renal and other tissue injury by means of OS (33–35). Such information underscores the paucity of findings on receptor-based mechanisms that serve to ameliorate the cumulative effects of AGE.
In this context, the MC expression system used here has helped identify AGE-R1 as such a receptor. Indeed, R1-expressing MC exhibited enhanced AGE uptake and degradation, concurring with earlier studies on nonobese diabetic mice and human subjects with diabetic nephropathy, which first raised the possibility of a link between suppressed R1 and impaired AGE turnover (15, 25). Further supportive evidence was provided by CHO cells, a different cell line, which, although lacking AGE-receptors, after R1 transfection acquired significant AGE endocytotic and degradative efficiency (data not shown). Together, these findings provided the basis for the speculation that AGE-R1 may mediate AGE clearance to prevent AGE deposition and to control unwanted OS-dependent signal pathway activation.
At the cellular level, excess AGE are known stimulants of OS-sensitive MAPK, p42/44 and NF-κB activity (36–39). In MC, as in many cell types, NF-κB regulates the transcription of genes involved in inflammatory response and cell growth (40–43). Abnormal activation of this chain of signals by AGE has been attributed largely to RAGE, a molecule that promotes and is promoted by cellular OS (8, 44). RAGE expression, unlike AGE-R1, is found to be elevated in diabetes and other high-OS states (23, 24, 45), which implies a sustained reactive oxygen species and AGE generation, resulting in RAGE activation, and so on. This cycle could conceivably alter the cell's phenotype, obscuring other receptor properties or depleting cellular anti-oxidant systems (46, 47). By engineering an intracellular environment of sustained high AGE-R1 availability, as in the present system of R1-MC, it was possible to disengage the perpetual cycle of RAGE → OS → NF-κB → RAGE,, revealing a previously unrecognized mechanism, whereby excessive activation of MAPK/p42/44 and NF-κB activation could be restricted, a key switch being AGE-R1.
A related question was whether the activation-inhibitory effects of AGE-R1 were due to a direct interference with RAGE. Although in control MC, AGE induced the expected RAGE overexpression, in R1-MC this same response was aborted, a result that pointed strongly toward a negative cooperativity between AGE-R1 and RAGE. Such a relationship could be intended under physiological conditions for purposes of AGE homeostasis. It also could involve the RAGE gene or protein regulation directly or indirectly via alternate routes of AGE-induced NF-κB activation (36).
To decipher this question, first we opted for the CHO cell expression system: the transfer of RAGE gene in these cells rendered them AGE-responsive, capable of a rise in MAPK/p42/44 phosphorylation and NF-κB activity. Cotransfection of RAGE-CHO cells with AGE-R1 gene completely abolished the newly acquired NF-κB activity, in response to AGE. These findings argue strongly that the activation-inhibitory action of R1 in MC is due to the interception of the RAGE/OS-sensitive signal pathway. To confirm R1 specificity for these findings, constitutive expression of AGE-R1 in primary MC was silenced by using R1-targeted siRNA (27), which caused the release of strong p44/42 signals exceeding the levels seen in wild-type and mock-MC. These results indicated that interfering with the normal expression of AGE-R1 can lead to a change in the cell's quiescent phenotype to an activated one with proinflammatory tendencies.
Recent evidence suggests that in the kidney, up-regulation of the MC-derived chemokine MCP-1 is associated with monocytic infiltration of the mesangium in the early phases of the disease (28, 48). Here, MCP-1 was selected as a downstream functional endpoint of the AGE/RAGE-signaling pathway linking MC dysfunction to kidney pathology. Thus, the complete suppression of MCP-1 production in R1-overexpressing MC was a compelling finding that supported the speculation that, in principle, AGE-R1 acts to neutralize inappropriate signal generation; this unique property, however, could be rendered ineffective under chronically elevated ambient AGE levels.
Several lines of evidence suggest that diabetes and aging processes may be shared to an extent. Both AGE and OS are significantly increased in diabetic and aging organisms (1–6), as antioxidant systems and AGE-R1 are suppressed (15, 25). Animal and human diets constitute major donors of AGE and reactive oxygen species (5, 6), as does tobacco smoking (29), and the total AGE pool may well exceed the receptor saturation limit, resulting in cytoplasmic sequestration or down-regulation of this system. Although the in vitro data presented in this study are based on observations made in human diabetes (15), given the limitations related to the use of mouse MC as a model, cautious extrapolation to the clinical setting is required. However, the findings provide a valid basis for subsequent in vivo studies. In this context, a new AGE-R1-transgenic mouse model may help confirm the inverse relationship between AGE-R1 expression and diabetic complications (15, 25) and assess whether maintaining glycemic control can preserve AGE-R1 function in diabetic patients.
Here, AGE-R1 is shown to override a well established AGE/RAGE-mediated signal pathway and effector molecules by negatively regulating signals activated by AGE by means of RAGE and reactive oxygen species in MC (32, 35). Thus, AGE-R1 may represent a unique candidate for AGE-defense element, intended for tissue protection against AGE toxicity. Further studies should elucidate the molecular basis of the properties engendered in this switchlike receptor system, to be explored as a potential therapeutic target.
Acknowledgments
We thank Ina Katz for excellent editorial assistance. This work was funded by National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases Grant DK54788 (to H.V.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AGE, advanced glycation endproducts; AGE-R1, AGE receptor 1; CHO, Chinese hamster ovary; MAPK, mitogen-activated protein kinase; MC, mesangial cell(s); MCP-1, monocyte chemoattractant protein 1; OS, oxidant stress; RAGE, receptor for AGE; R1-MC, MC overexpressing AGE-R1; siRNA, small interfering RNA.
References
- 1.Vlassara, H. (2001) Diabetes Metab. Res. Rev. 17, 436–443. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt, A. M., Yan, S. D., Yan, S. F. & Stern, D. M. (2001) J. Clin. Invest. 108, 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel, T. & Holbrook, N. J. (2000) Nature 408, 239–247. [DOI] [PubMed] [Google Scholar]
- 4.Kislinger, T., Tanji, N., Wendt, T., Qu, W., Lu, Y., Ferran, L. J., Jr., Taguchi, A., Olson, K., Bucciarelli, L., Goova, M., et al. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 905–910. [DOI] [PubMed] [Google Scholar]
- 5.Koschinsky, T., He, C. J., Mitsuhashi, T., Bucala, R., Liu, C., Buenting, C., Heitmann, K. & Vlassara, H. (1997) Proc. Natl. Acad. Sci. USA 94, 6474–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He, C., Sabol, J., Mitsuhashi, T. & Vlassara, H. (1999) Diabetes 48, 1308–1315. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann, M. A., Drury, S., Fu, C., Qu, W., Taguchi, A., Lu, Y., Avila, C., Kambham, N., Bierhaus, A., Nawroth, P., et al. (1999) Cell 97, 889–901. [DOI] [PubMed] [Google Scholar]
- 8.Wautier, M. P., Chappey, O., Corda, S., Stern, D. M., Schmidt, A. M. & Wautier, J. L. (2001) Am. J. Physiol. 280, E685–E694. [DOI] [PubMed] [Google Scholar]
- 9.Basta, G., Lazzerini, G., Massaro, M., Simoncini, T., Tanganelli, P., Fu, C., Kislinger, T., Stern, D. M., Schmidt, A. M. & De Caterina, R. (2002) Circulation 105, 816–822. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki, H., Kurihara, Y., Takeya, M., Kamada, N., Kataoka, M., Jishage, K., Ueda, O., Sakaguchi, H., Higashi, T., Suzuki, T., et al. (1997) Nature 386, 292–296. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki, A., Nakayama, H. & Horiuchi, S. (2002) Trends Cardiovasc. Med. 12, 258–262. [DOI] [PubMed] [Google Scholar]
- 12.Vlassara, H., Li, Y. M., Imani, F., Wojciechowicz, D., Yang, Z., Liu, F. T. & Cerami, A. (1995) Mol. Med. 1, 634–646. [PMC free article] [PubMed] [Google Scholar]
- 13.Li, Y. M., Mitsuhashi, T., Wojciechowicz, D., Shimizu, N., Li, J., Stitt, A., He, C., Banerjee, D. & Vlassara, H. (1996) Proc. Natl. Acad. Sci. USA 93, 11047–11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugliese, G., Pricci, F., Iacobini, C., Leto, G., Amadio, L., Barsotti, P., Frigeri, L., Hsu, D. K., Vlassara, H., Liu, F. T. & Di Mario, U. (2001) FASEB J. 15, 2471–2479. [DOI] [PubMed] [Google Scholar]
- 15.He, C. J., Koschinsky, T., Buenting, C. & Vlassara, H. (2001) Mol. Med. 7, 159–168. [PMC free article] [PubMed] [Google Scholar]
- 16.Vlassara, H., Striker, L. J., Teichberg, S., Fuh, H., Li, Y. M. & Steffes, M. (1994) Proc. Natl. Acad. Sci. USA 91, 11704–11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, Y. M., Steffes, M., Donnelly, T., Liu, C., Fuh, H., Basgen, J., Bucala, R. & Vlassara, H. (1996) Proc. Natl. Acad. Sci. USA 93, 3902–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fioretto, P., Steffes, M. W., Sutherland, D. E. & Mauer, M. (1995) Kidney Int. 48, 1929–1935. [DOI] [PubMed] [Google Scholar]
- 19.Skolnik, E. Y., Yang, Z., Makita, Z., Radoff, S., Kirstein, M. & Vlassara, H. (1991) J. Exp. Med. 174, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi, T., Vlassara, H., Kirstein, M., Yamada, Y., Striker, G. E. & Striker, L. J. (1992) Proc. Natl. Acad. Sci. USA 89, 2873–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang, C. W., Vlassara, H., Peten, E. P., He, C. J., Striker, G. E. & Striker, L. J. (1994) Proc. Natl. Acad. Sci. USA 91, 9436–9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, C. W., Vlassara, H., Striker, G. E. & Striker, L. J. (1995) Kidney Int. Suppl. 49, S55–S58. [PubMed] [Google Scholar]
- 23.Brett, J., Schmidt, A. M., Yan, S. D., Zou, Y. S., Weidman, E., Pinsky, D., Nowygrod, R., Neeper, M., Przysiecki, C., Shaw, A., et al. (1993) Am. J. Pathol. 143, 1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 24.Tanji, N., Markowitz, G. S., Fu, C., Kislinger, T., Taguchi, A., Pischetsrieder, M., Stern, D., Schmidt, A. M. & D'Agati, V. D. (2000) J. Am. Soc. Nephrol. 11, 1656–1666. [DOI] [PubMed] [Google Scholar]
- 25.He, C. J., Zheng, F., Stitt, A., Striker, L., Hattori, M. & Vlassara, H. (2000) Kidney Int. 58, 1931–1940. [DOI] [PubMed] [Google Scholar]
- 26.Vlassara, H., Brownlee, M. & Cerami, A. (1985) Proc. Natl. Acad. Sci. USA 82, 5588–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sioud, M. (2004) Trends Pharmacol. Sci. 25, 22–28. [DOI] [PubMed] [Google Scholar]
- 28.Banba, N., Nakamura, T., Matsumura, M., Kuroda, H., Hattori, Y. & Kasai, K. (2000) Kidney Int. 58, 684–690. [DOI] [PubMed] [Google Scholar]
- 29.Cerami, C., Founds, H., Nicholl, I., Mitsuhashi, T., Giordano, D., Vanpatten, S., Lee, A., Al-Abed, Y., Vlassara, H., Bucala, R. & Cerami, A. (1997) Proc. Natl. Acad. Sci. USA 94, 13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kislinger, T., Fu, C., Huber, B., Qu, W., Taguchi, A., Du Yan, S., Hofmann, M., Yan, S. F., Pischetsrieder, M., Stern, D. & Schmidt, A. M. (1999) J. Biol. Chem. 274, 31740–31749. [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi, S., Inagaki, Y., Okamoto, T., Amano, S., Koga, K., Takeuchi, M. & Makita, Z. (2002) J. Biol. Chem. 277, 20309–20315. [DOI] [PubMed] [Google Scholar]
- 32.Lal, M. A., Brismar, H., Eklof, A. C. & Aperia, A. (2002) Kidney Int. 61, 2006–2014. [DOI] [PubMed] [Google Scholar]
- 33.Tsuji, H., Iehara, N., Masegi, T., Imura, M., Ohkawa, J., Arai, H., Ishii, K., Kita, T. & Doi, T. (1998) Biochem. Biophys. Res. Commun. 245, 583–588. [DOI] [PubMed] [Google Scholar]
- 34.Iwashima, Y., Eto, M., Horiuchi, S. & Sano, H. (1999) Biochem. Biophys. Res. Commun. 264, 441–448. [DOI] [PubMed] [Google Scholar]
- 35.Scivittaro, V., Ganz, M. B. & Weiss, M. F. (2000) Am. J. Physiol. 278, F676–F683. [DOI] [PubMed] [Google Scholar]
- 36.Lander, H. M., Tauras, J. M., Ogiste, J. S., Hori, O., Moss, R. A. & Schmidt, A. M. (1997) J. Biol. Chem. 272, 17810–17814. [DOI] [PubMed] [Google Scholar]
- 37.Yeh, C. H., Sturgis, L., Haidacher, J., Zhang, X. N., Sherwood, S. J., Bjercke, R. J., Juhasz, O., Crow, M. T., Tilton, R. G. & Denner, L. (2001) Diabetes 50, 1495–1504. [DOI] [PubMed] [Google Scholar]
- 38.Huang, J. S., Guh, J. Y., Chen, H. C., Hung, W. C., Lai, Y. H. & Chuang, L. Y. (2001) J. Cell Biochem. 81, 102–113. [DOI] [PubMed] [Google Scholar]
- 39.Satoh, H., Togo, M., Hara, M., Miyata, T., Han, K., Maekawa, H., Ohno, M., Hashimoto, Y., Kurokawa, K. & Watanabe, T. (1997) Biochem. Biophys. Res. Commun. 239, 111–115. [DOI] [PubMed] [Google Scholar]
- 40.Rovin, B. H., Dickerson, J. A., Tan, L. C. & Hebert, C. A. (1995) Kidney Int. 48, 1263–1271. [DOI] [PubMed] [Google Scholar]
- 41.Hisada, Y., Sakurai, H. & Sugaya, T. (2000) Biochem. Biophys. Res. Commun. 269, 309–316. [DOI] [PubMed] [Google Scholar]
- 42.Lynn, E. G., Siow, Y. L., Frohlich, J., Cheung, G. T. & O, K. (2001) Kidney Int. 60, 520–532. [DOI] [PubMed] [Google Scholar]
- 43.Ha, H., Yu, M. R., Choi, Y. J., Kitamura, M. & Lee, H. B. (2002) J. Am. Soc. Nephrol. 13, 894–902. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka, N., Yonekura, H., Yamagishi, S., Fujimori, H., Yamamoto, Y. & Yamamoto, H. (2000) J. Biol. Chem. 275, 25781–25790. [DOI] [PubMed] [Google Scholar]
- 45.Soulis, T., Thallas, V., Youssef, S., Gilbert, R. E., McWilliam, B. G., Murray-McIntosh, R. P. & Cooper, M. E. (1997) Diabetologia 40, 619–628. [DOI] [PubMed] [Google Scholar]
- 46.Arnalich, F., Hernanz, A., Lopez-Maderuelo, D., De la Fuente, M., Arnalich, F. M., Andres-Mateos, E., Fernandez-Capitan, C. & Montiel, C. (2001) Free Radical Res. 35, 873–884. [DOI] [PubMed] [Google Scholar]
- 47.Cai, W., Gao, Q. D., Zhu, L., Peppa, M., He, C. & Vlassara, H. (2002) Mol. Med. 8, 337–346. [PMC free article] [PubMed] [Google Scholar]
- 48.Furuta, T., Saito, T., Ootaka, T., Soma, J., Obara, K., Abe, K. & Yoshinaga, K. (1993) Am. J. Kidney Dis. 21, 480–485. [DOI] [PubMed] [Google Scholar]