Abstract
Some monocytes normally take up residence in tissues as sessile macrophages, but others differentiate into migratory cells resembling dendritic cells that emigrate to lymph nodes. In an in vitro model of a vessel wall, lipid mediators lysophosphatidic acid and platelet-activating factor, whose signals are implicated in promoting atherosclerosis, blocked conversion of monocytes into migratory cells and favored their retention in the subendothelium. In vivo studies revealed trafficking of monocyte-derived cells from atherosclerotic plaques during lesion regression, but little emigration was detected from progressive plaques. Thus, progression of atherosclerotic plaques may result not only from robust monocyte recruitment into arterial walls but also from reduced emigration of these cells from lesions.
Recruitment of monocytes into atherogenic foci is required for the onset and progression of atherosclerosis. In lesions, monocyte-derived cells take up modified lipoprotein and produce secretory factors that attract and activate smooth muscle cells, other immune cells, and more monocytes (1). Mechanisms that attract monocytes to lesions are partly defined (1, 2), but relatively little is known about the fate of monocytes once they migrate into lesions. Many begin to differentiate into lipid-laden macrophage foam cells, but it has also been recently appreciated that a substantial population of lesional foam cells in humans and animals bears features of dendritic cells (DCs) (3, 4), rather than macrophages.
Monocytes exit the blood continuously in the steady state and appear to do so at a higher rate than needed to replenish relatively long-lived macrophages (5). We have, therefore, wondered whether the conversion of some monocytes into migratory cells with the capacity to emigrate from tissue, either via lymphatics or reentry into blood, might partly explain how macrophage homeostasis in tissues is maintained and why a progressive accumulation of resident macrophages does not result from continuous trafficking of monocytes into tissues. Because DCs are known to migrate especially efficiently into lymph (6, 7), it might be anticipated that any monocyte-derived cells that emigrate out of tissues would bear at least some features of DCs, perhaps especially the gene products that confer to DCs their migratory characteristics. Indeed, many monocyte-derived cells emigrate to the T cell zone of lymph nodes via lymphatic vessels, and thereby exit peripheral tissues like skin (8). These migratory monocyte-derived cells express higher levels of MHC II than those that remain resident in the skin, and they express several, but possibly not all, DC-restricted markers that are not observed on macrophages (8). Thus, conversion of monocytes to a migratory DC-like cell is associated with their capacity to emigrate from tissues (8, 9). Besides potentially participating in the regulation of tissue macrophage homeostasis, conversion of some monocytes to migratory DCs may also contribute to a continuous source of antigen-presenting cells arriving in lymph nodes.
Because tissue macrophage homeostasis is not maintained in atherosclerotic lesions, but instead macrophages accumulate abundantly in plaques along with cells resembling DCs (3, 4), we examined herein whether the increase of monocyte-derived cells in atherosclerotic plaques might result not only from robust recruitment of monocytes into lesions, but also from a depressed rate of their migratory clearance from the lesions. Thus, we examined rates of emigration of human monocyte-derived cells out of a model of a vessel wall in the presence or absence of proatherogenic lipids and then compared migratory clearance of monocyte-derived cells from murine atherosclerotic lesions under conditions in which plaques regressed or progressed.
Methods
In Vitro Model of Transendothelial Trafficking. Blood from healthy donors was drawn according to guidelines approved by the Internal Review Board of the Mount Sinai School of Medicine. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation on Ficoll. Reverse-transmigrated and monocyte-derived cells were studied as described (9, 10). In brief, second-passage human umbilical vein endothelial cells were cultured on bovine type I collagen gels in microtiter wells and used for experiments ≈3 days after reaching confluence. PBMCs were applied to the endothelium, incubated for 1.5 h, washed thoroughly in medium to remove nonmigrated cells from above the endothelium, and continued in culture for 48 h in Medium 199 containing 20% human serum. Monocytes, but few lymphocytes, migrate across the endothelium during the 1.5-h incubation. Carbamyl platelet-activating factor (PAF) (Biomol, Plymouth Meeting, PA), a stable and close structural analog of PAF that manifests a similar biological potency and receptor binding profile as PAF (11), 18:1 lysophosphatidic acid (LPA) (Avanti Polar Lipids), sphingosine 1-phosphate (Biomol), bovine brain sphingomyelin (Matreya, Pleasant Gap, PA), or prostaglandin E2 (Biomol) were included in the medium and applied to the cultures after the wash at 1.5 h.
For quantification of reverse transmigration, samples were fixed for microscopic analysis after washing to remove nonmigrated cells at 1.5 or 48 h. Differential interference contrast optics was used to quantify the number of monocyte-derived cells that were beneath the endothelial monolayer in at least five high-power fields per sample. Each experiment included six replicates per parameter tested. Percent of reverse transmigrated monocyte-derived cells was calculated as the percentage decrease in the number of monocyte-derived cells beneath the endothelial monolayer at 48 h relative to the number present at 1.5 h.
Oil red O staining was conducted after permeabilizing the cultures with 60% isopropanol, following by application of oil red O dissolved in absolute isopropanol, with clarifying rinses in 60% isopropanol. Hematoxylin was added for counterstaining.
Mice. C57BL/6 mice expressing Ly5.1 (CD45.1) or Ly5.2 (CD45.2) were obtained from The Jackson Laboratory. Apolipoprotein E (ApoE)–/– mice (The Jackson Laboratory) were crossed on the Ly5.1 background. At 4 weeks of age, cohorts of apoE–/– mice were changed to a Western diet (Harlan Teklad; 21% milk fat, 0.15% cholesterol) to accelerate lesion development. All experiments with mice were approved by the Institutional Animal Care and Use Committee at the Mount Sinai School of Medicine.
Surgical Transfer of the Aortic Arch. Previous studies detailed the method of surgery (12, 13). CD45.2+ or CD45.1+ ApoE–/– mice on the Western diet for 15 weeks were used as aortic arch donors. Recipients were age- and sex-matched and included either apoE+/+ CD45.2+ C56BL/6 mice or CD45.2+ apoE–/– mice. The latter were also maintained on the Western diet before and after surgery to match the disease progression of the donors. Two to four surgeries were conducted on a given day, and the recipients on each day of surgery always included matched representatives of both genotypes, apoE+/+ and apoE–/–, for comparison between genotypes in side-by-side processing.
Lesion Analysis. Mean cross-sectional lesion area was calculated for individual mice from ≈25 measurements at regular intervals along the entire transferred arch. Area measurements were made by tracing the circumference of the lesions that protruded luminal to the internal elastic lamina.
Identification of CD68 staining was made by using anti-CD68 mAb (Serotec) followed by FITC-conjugated anti-rat IgG (Jackson ImmunoResearch). Polyclonal anti-VEGFR3 antibody was from R & D Systems and was detected by anti-goat Cy3-conjugated IgG (Jackson ImmunoResearch). Staining with biotinylated anti-CD45.1, anti-CD45.2 mAbs (Southern Biotechnology Associates), or nonspecific isotype-matched controls (BD Pharmingen) was conducted in acetone-fixed sections by using the peroxidase-mediated tyramide amplification kit (Molecular Probes) and Cy3-conjugated streptavidin (Sigma). Nonspecific antibody binding was blocked by using FcBlock (BD Pharmingen) and 1% goat serum before addition of primary mAbs. Endogenous peroxidase was blocked by using 0.1 M sodium azide and 0.3% peroxide for 45 min.
Measurements of lesion area and area covered by CD45.2 staining were made by using the Morphology Bundle component of Improvision (Lexington, MA) openlab software.
Flow Cytometry. Brachial, iliac, hepatic, and occasionally other lymph nodes were removed from mice after death. Single-cell suspensions were prepared by digestion of teased lymph nodes with collagenase D (Roche Molecular Biochemicals) followed by gentle pressing through a cell strainer. Cells were resuspended in buffer containing 0.1% BSA and FcBlock (BD Pharmingen). After preincubation in this solution, biotinylated anti-CD45.1 or anti-CD45.2 mAb (Southern Biotechnology Associates) was added, along with other surface markers, including FITC-conjugated CD11c (BD Pharmingen), R-phycoerythrin (PE)-conjugated I-Ab (BD Pharmingen), PE-conjugated anti-CD3 (BD Pharmingen), and/or PE-conjugated anti-CD115 (eBioscience, San Diego). Quantification of migration was assessed by using flow cytometry by running the whole suspension from each type of lymph node for every animal studied.
Results and Discussion
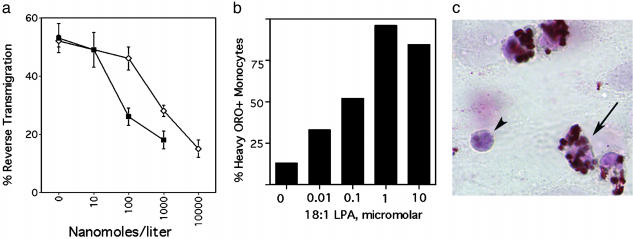
In an in vitro model of a vessel wall, the migratory behavior of human monocytes can be readily traced (9, 10). After transendothelial passage of monocytes into a subendothelial collagenous matrix, a substantial fraction of monocytes later retraverse the overlying endothelium (reverse transmigration) (9). These emigrating cells bear features of veiled DCs (9), whereas monocyte-derived cells that remain in the matrix resemble macrophages. This model is also predictive of molecular events that facilitate trafficking of DCs through lymphatic vessels (9, 10), where DCs are abundant (6). Using this model, we observed that two lipids expected to be relevant mediators of atherosclerosis impaired the ability of human monocytes to differentiate into cells that could emigrate from the cultured vessel wall constructs. One of the lipids studied was the stable PAF analog carbamyl PAF (Fig. 1a), included in these experiments to model PAF-like signals that are major products generated during oxidation of phosphatidylcholine in low density lipoprotein particles (14, 15). These products of oxidation mimic PAF closely enough to bind to the PAF receptor (14). Evidence indicates that PAF-related signals promote atherosclerotic disease, because inactivation of these signals via PAF acetylhydrolase (16), treatment with PAF receptor antagonists (15), or amplification of naturally occurring neutralizing antibodies that recognize the PAF-like lipids in oxidized low density lipoprotein (17) protect against advance of disease. Furthermore, individuals with an inactivating PAF acetlyhydrolase mutation are at increased risk for atherosclerosis (18).
Fig. 1.
Effect of PAF and LPA signals on the migratory fate of monocyte-derived cells in a model of a vessel wall. Carbamyl PAF (filled symbols) or 18:1 LPA (open symbols) was added to the culture medium at the concentrations shown. Reverse transmigration (a) and neutral lipid uptake (b) were quantified. (c) Arrows indicate a monocyte just beneath the endothelium that accumulated neutral lipid heavily, as assessed by staining with oil red O (ORO). Arrowhead shows a leukocyte that did not accumulate lipid. Shown is a representative experiment of three conducted, all with similar results.
We also investigated the effect of another phospholipid-derived mediator, LPA, on reverse transmigration (Fig. 1a). LPA has been recently identified as a potent natural ligand for the transcription factor peroxisome-proliferator-activated receptor γ (19) that promotes lipid accumulation in macrophages in vitro (19) and atherogenesis in vivo (20). Concentrations of LPA that caused maximal neutral lipid accumulation in most monocyte-derived cells (Fig. 1 b and c) were similar to those that impaired the capacity of monocyte-derived cells to emigrate from the vessel wall (Fig. 1a). This result parallels the role of LPA in neointima formation (20). The inhibition of emigration by these mediators could not be attributed to induction of cell death, because although fewer monocyte-derived cells were found to emigrate back across the endothelium, elevated numbers of live monocyte-derived cells were recovered in the subendothelial matrix. Other lipid mediators tested over a wide range of concentrations in the same assay, including sphingosine 1-phosphate, sphingomyelin, and prostaglandin E2 did not affect the magnitude of reverse transmigration (data not shown). Together, these findings imply that at least some proatherogenic factors may impede monocyte egress from the vessel wall. Thus, we hypothesized that emigration of monocyte-derived cells from the vessel wall may be reduced during atherogenesis in vivo, thereby possibly contributing to plaque progression.
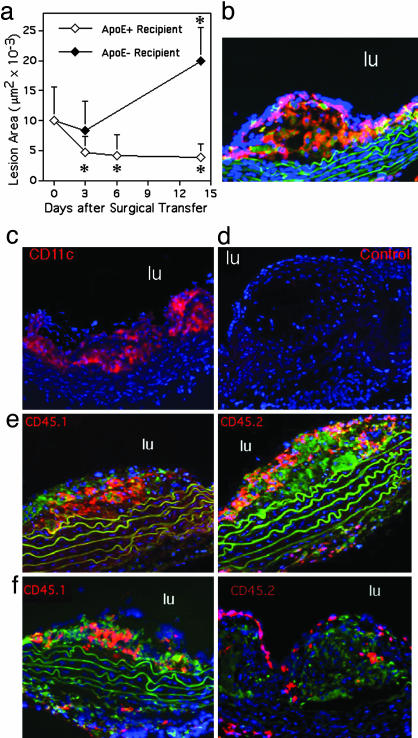
To investigate this hypothesis in vivo, experiments were carried out by using apoE-deficient mice (21). A technical hurdle to readily quantifying migration of cells out of lesions is the difficulty in specifically labeling or identifying cells that accumulated within lesions so that they can be distinguished from cells that continuously migrate into lesions. Recently, we demonstrated a useful approach to study regression of atherosclerotic lesions. In this model, a region of the aorta enriched in atherosclerotic plaques from an apoE-deficient donor is transferred to a recipient mouse such that the surgically transferred segment becomes a functional segment of the recipient's aorta (12, 13). If the recipient is likewise apoE deficient, disease progression in the lesions within the transferred segment of aorta continues, consistent with our previous studies (12, 22). Cholesterol levels do not drop in apoE–/– recipients (12). Transfer of these lesion-rich aortic arches into WT recipients (C57BL/6 apoE+/+) results in an immediate conversion to normal lipid profiles, which in turn promotes lesion regression (12), in agreement with nonsurgical methods that restore normal blood lipid profiles and/or apoE (23, 24). Significant changes in lesion area were observed by or before 2 weeks after surgical transfer (Fig. 2a).
Fig. 2.
Assessment of methods and kinetics to study migration from lesions in vivo. (a) ApoE–/– male and female mice on a C57BL/6 Ly5.1 (CD45.1) congenic background were fed a Western-type diet from 4 to 19 weeks of age. Aortic arches from these donor mice were surgically implanted on the abdominal aorta of recipient mice (17). Each surgery included matched representatives of both genotypes, apoE+/+ and apoE–/–, for comparison between genotypes in side-by-side processing. Lesion area was quantified over a 2-week time course in an experimental design to compare outcomes in lesion size between apoE–/– and apoE+/+ recipients (five animals per time point) and to delineate the kinetics of regression in apoE+/+ recipients. * denotes significant change in lesion area from time 0, P < 0.02. Lesions examined at time 0 were stained with CD45.1 (b, red staining), CD68 (b, green staining that is in addition to autofluorescent green elastic lamina), and CD11c (c, red). Isotypematched control mAb did not stain the lesions (d). (e and f) Monocyte-derived cells from CD45.1 donor lesions (red staining, Left) or CD45.2 circulating monocytes of the recipient that were recruited to the lesion after surgical transfer (red staining, Right) were respectively identified in plaques 3 days after they were transferred to apoE+/+ or apoE–/– recipients. Counterstaining was with anti-CD68 mAb (green) and 4′,6-diamidino-2-phenylindole (blue). Green fluorescence was not recorded in c and d. lu denotes lumen of aorta.
With this surgical model, we studied congenic Ly5.1 and Ly5.2 C57BL/6 donors and recipients, respectively, that can be distinguished by the singular difference in an allele for CD45 (Ly5), a pan-hematopoietic marker. This approach permitted definitive tracing of the fate of hematopoietic cells that may leave lesions. To focus on the fate of recruited lesional monocytes, we chose to analyze lesions that contained numerous CD68+ cells without signs of advanced lesions such as smooth cell infiltration or necrotic cores. We selected the time point of 19 weeks of age (15 weeks of feeding a high-fat diet). At this time point, lesions in the aortic arch contain near-maximal numbers of monocyte-derived cells per lesional area and nearing transition to late-stage lesions.
The initial rate of regression in WT recipients was very rapid, such that 3 days after surgery a 50% drop in lesional area was observed (Fig. 2a). Rapid decreases in lesion size and macrophage content have also been seen in apoE-deficient mice administered artificial high density lipoprotein-like preparation (21). After 3 days, further decreases in lesion size occurred at a much slower rate. In apoE–/– recipients, there was neither a significant reduction nor increase in lesion area 3 days after surgery (Fig. 2a), but there was a significant increase in lesion area by 2 weeks (Fig. 2a).
Before surgery, CD45 was observed throughout lesions, where it colocalized with CD68+ cells, as expected (Fig. 2b). Most of these cells were also CD11c+ (Fig. 2c). Lack of nonspecific staining by these mAbs was verified by using isotype-matched controls (Fig. 2d). Visualization of CD45.1 and CD45.2 markers, respectively, in lesions after surgery showed robust recruitment of recipient monocytes in both regressive (Fig. 2e) and progressive lesions (Fig. 2f). Even as lesions regressed in WT recipients, newly recruited monocytes (CD45.2+) infiltrated the lesions significantly enough to account for more than half of the CD45+ hematopoietic cells in the lesion. Newly recruited CD45.2+ cells tended to layer over the donor CD45.1+ monocyte-derived cells that were present in the transferred lesion (Fig. 2 e and f). Area of lesion covered by the newly recruited CD45.2+ cells was not different between apoE+/+ (2.0 ± 1.2 × 103 μm2 at day 3) and apoE–/– (2.0 ± 0.8 × 103 μm2 at day 3) recipients. Given these findings, the possibility that regressing lesions differed from progressing lesions because of a rapid cessation in monocyte recruitment after surgical transfer to a normal recipient was untenable. Therefore, we explored whether the extent of migration out of lesions was different.
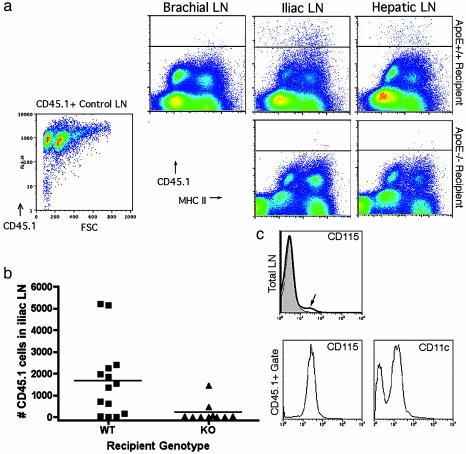
In CD45.2/Ly5.2 recipients that were either of apoE+/+ or apoE–/– genotype, we quantified lesional cell migration to iliac lymph nodes, which drained the transferred segment of CD45.1/Ly5.1 apoE–/– aorta. For quantification, we focused on the time point of 3 days, because this early time point reflected the period of greatest change in lesion size in WT recipients and particularly because early time points would increase our chance of detecting the emigration from lesions of hematopoietic cells, such as DCs, that might be relatively short-lived (25). Staining for lymphatic vessels around the transplant was very robust, suggesting rapid repair of lymphatic pathways for migration (Fig. 4, which is published as supporting information on the PNAS web site). The number of migrated CD45.1+ cells that accumulated in the iliac lymph node was significantly greater (P <0.03) in WT recipients (1,457 ± 422) than in paired apoE–/– recipients (209 ± 146) (Fig. 3 a and b). Emigrating CD45.1+ cells were only rarely detected at days 6, 9, 12, and 14 in either type of recipient, suggesting that CD45.1+ cells may turn over completely after several days. Additional control experiments included mock transfers of WT nondiseased aortas into WT recipients. No CD45.1+ cells were found in lymph nodes after such transfers (data not shown).
Fig. 3.
Tracing emigration from lesions to lymph nodes. (a) Flow cytometric evaluation was carried out on apoE–/– and apoE+/+ recipients 3 days after transplant. CD45.2 nontranplanted mouse lymph nodes were routinely stained with biotinylated anti-CD45.1 mAb and streptavidin-allophycocyanin to determine the extent of mAb cross-reactivity. This measure was used to delineate the position of the negative control line in each plot as shown. Staining of CD45.1+ control lymph nodes (LN) with anti-CD45.1 mAb established that the positively stained cells were uniformly detectable above the cross-reactive threshold (lower left flow plot). Staining was routinely conducted in single suspensions of iliac lymph nodes that drain the transferred aortic segments, the brachial lymph nodes as a representative distant nondraining lymph nodes, and the hepatic lymph node. Representative plots are shown, and each depict flow cytometric evaluation of the entire lymph node population. (b) Plot charts the total number of CD45.1+ cells observed in the iliac lymph nodes of individuals (each symbol, one individual) 3 days after aortic transfer into apoE+/+ (WT) or apoE–/– (KO) recipients. Differences in the number of migrated cells are statistically significant, P <0.03. (c) Evaluation of CD115 staining is shown in the iliac lymph nodes of a representative apoE+/+ recipient (bold line in Total LN histogram) compared with iliac lymph node of nontransplanted mouse (filled histogram). CD115 and CD11c staining in gated CD45.1 cells (lower histogram pair) is shown. Negative staining is observed in peaks that fall between 100 and 101 log.
Cells that emigrated from lesions expressed high levels of I-Ab (Fig. 3a), CD11b (data not shown), the macrophage colony-stimulating factor receptor (M-CSF) CD115 (Fig. 3c), and over half were CD11c+ (Fig. 3c), consistent with an origin of these cells from M-CSF-dependent monocytes. The influx of these cells into the iliac lymph nodes correlated with a substantial increase in the fraction of total lymph node cells that expressed CD115, which rose to nearly 2% of the lymph node fraction from a typical content of 0.4% (Fig. 3c, arrow). Among these CD115+ cells, 57% were also CD45.1+. None of the CD45.1+ cells expressed CD3 (data not shown). CD11c is considered a selective marker for mouse DCs. It is rarely coexpressed with CD115 in normal tissues, suggesting that the emigrating cells, and likely many of the lesional cells (Fig. 2c), exhibited an unusual phenotype bearing classical features of both DCs and macrophages. In contrast, monocyte-derived cells that emigrate to the T cell zone of lymph nodes after engulfing phagocytic tracers in skin (8) completely down-regulate CD115 by the time of arrival in the lymph node (data not shown).
In many settings, migration of DCs is limited to the draining local lymph node, because DCs do not readily migrate out of tissues via routes that would permit their reentry into blood. However, it has been suggested that monocyte-derived cells may migrate from lesions by retraversing the arterial endothelium in the ablumenal-to-lumenal direction (26). Thus, we wondered whether migrating DCs could be found in locations that would be consistent with dissemination through blood as well as lymphatics. Both spleen and hepatic lymph nodes are lymphoid compartments that can accumulate cells that have accessed blood (27). Because the number of cells that would be expected to emigrate from lesions was relatively small (we estimate a lesional content of ≈20,000 CD45.1+ cells in each surgical transfer), evaluating the large number of spleen cells needed to detect the relatively few CD45.1+ cells within this organ was unreliable, due to interference by endogenous autofluorescent cells in spleen (data not shown). Therefore, we analyzed the hepatic lymph node, which drains the sinusoids of the liver where blood borne cells can readily cross into afferent lymphatic channels (27). This lymph node then serves as an unusual anatomic read-out of cells that enter blood with the capacity to transmigrate into afferent lymphatics, but which otherwise lack the capacity to traverse high endothelial venules to enter other lymph nodes directly from blood.
Migration of CD45.1+ cells to hepatic lymph nodes was observed in the same mice that also contained CD45.1+ cells in the iliac lymph node (Fig. 3a). Iliac lymph nodes lacking CD45.1+ cells also lacked CD45.1+ cells in hepatic lymph nodes. All other distal lymph nodes, such as brachial lymph nodes (Fig. 3a), in all animals were negative for CD45.1.
This study reveals that clearance of monocytes from tissues by conversion into migratory cells that can traffic to lymphoid organs occurs under conditions when atherosclerotic disease, and the dyslipidemia with which it is associated, are rapidly reversed. Because different results with regard to migratory fate were observed in apoE+/+ vs. apoE–/– recipients, the data argue that the phenotype of the recipients dominates over possible artifactual effects of the surgical procedure. Because conversion of monocytes into lymph-trafficking cells occurs efficiently under normal conditions (8) as well as during regression of atherosclerosis as shown here, failure to detect significant migratory clearance when the disease progresses and dyslipidemia persists suggests that progression of atherosclerosis results not only from robust monocyte recruitment into arterial walls but also from reduced emigration from lesions.
Sluggish migratory clearance may directly increase plaque cellularity and may indirectly contribute to plaque progression if activated, nonmigratory monocyte-derived cells produce cytokines and interact locally to promote the activation in situ of T cells, natural killer T cells, smooth muscle, and other cells to generally exacerbate innate immune reactions (28). In strong agreement with the present studies, recent additional data in our laboratory have revealed that migration of skin DCs to skin-draining lymph nodes in apoE-deficient and low density lipoprotein receptor-deficient mice is, as in lesions, impaired, surprisingly even as signs of active autoimmunity are present (V.A., J.L., E.A.F., and G.J.R., unpublished work). These studies show that skin DCs become activated in atherosclerotic mice but eventually fail to emigrate to lymph nodes. Restoration of migration is achieved through the action of PAF acetylhydrolase (AH) and PAF AH-replete HDL, confirming a role for PAF-like lipids in inhibiting migration from tissues (V.A., J.L., E.A.F., and G.J.R., unpublished work). In summary, these findings underscore a so-far unknown alteration in the cellular trafficking that distinguishes atherosclerotic lesions that regress compared with those that continue to progress and suggest that interventions to maintain functional pathways of emigration out of lesions may favor disease regression and quell plaque progression.
Acknowledgments
We thank Sehba Khan and Jacob Yount for technical assistance, Drs. Hayes Dansky and Peter Boros for helpful advice, and Dr. Igor Chereshnev for conducting surgeries that generated preliminary data for this study. This work was supported by National Institutes of Health Grants HL69446 and AI49653 (to G.J.R.) and HL61814 (to E.A.F.). G.J.R. was the recipient of an Investigator Award from the Cancer Research Institute. V.A. was supported by a postdoctoral fellowship from the European Molecular Biology Organization.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DC, dendritic cell; PAF, platelet-activating factor; LPA, lysophosphatidic acid; apoE, apolipoprotein E.
See Commentary on page 11529.
References
- 1.Glass, C. K. & Witztum, J. L. (2001) Cell 104, 503–516. [DOI] [PubMed] [Google Scholar]
- 2.Osterud, B. & Bjorklid, E. (2003) Physiol. Rev. 83, 1069–1112. [DOI] [PubMed] [Google Scholar]
- 3.Spanbroek, R., Grabner, R., Lotzer, K., Hildner, M., Urbach, A., Ruhling, K., Moos, M. P., Kaiser, B., Cohnert, T. U., Wahlers, T., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobryshev, Y. V. (2000) Curr. Opin. Lipidol. 11, 511–517. [DOI] [PubMed] [Google Scholar]
- 5.Van Furth, R. (1988) in Inflammation: Basic Principles and Clinical Correlates, eds. Gallin, J. I., Goldstein, I. M. & Snyderman, R. (Raven, New York), pp. 281–295.
- 6.Pugh, C. W., MacPherson, G. G. & Steer, H. W. (1983) J. Exp. Med. 157, 1758–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanagh, L. L. & Von Andrian, U. H. (2002) Immunol. Cell Biol. 80, 448–462. [DOI] [PubMed] [Google Scholar]
- 8.Randolph, G. J., Inaba, K., Robbiani, D. F., Steinman, R. M. & Muller, W. A. (1999) Immunity 11, 753–761. [DOI] [PubMed] [Google Scholar]
- 9.Randolph, G. J., Beaulieu, S., Lebecque, S., Steinman, R. M. & Muller, W. A. (1998) Science 282, 480–483. [DOI] [PubMed] [Google Scholar]
- 10.Randolph, G. J., Beaulieu, S., Pope, M., Sugawara, I., Hoffman, L., Steinman, R. M. & Muller, W. A. (1998) Proc. Natl. Acad. Sci. USA 95, 6924–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Flaherty, J. T., Redman, J. F., Schmitt, J. D., Ellis, J. M., Surles, J. R., Marx, M. H., Piantadosi, C. & Wykle, R. L. (1987) Biochem. Biophys. Res. Commun. 147, 18–24. [DOI] [PubMed] [Google Scholar]
- 12.Reis, E. D., Lie, J., Fayad, Z. A., Rong, J. X., Hansoty, D., Aguinaldo, J.-G., Fallon, J. T. & Fisher, E. A. (2001) Vasc. Surg. 34, 541–547. [DOI] [PubMed] [Google Scholar]
- 13.Chereshnev, I., Trogan, E., Omerhodzic, S., Itskovich, V., Aguinaldo, J. G., Fayad, Z. A., Fisher, E. A. & Reis, E. D. (2003) J. Surg. Res. 111, 171–176. [DOI] [PubMed] [Google Scholar]
- 14.Marathe, G. K., Davies, S. S., Harrison, K. A., Silva, A. R., Murphy, R. C., Castro-Faria-Neto, H., Prescott, S. M., Zimmerman, G. A. & McIntyre, T. M. (1999) J. Biol. Chem. 274, 28395–28404. [DOI] [PubMed] [Google Scholar]
- 15.Subbanagounder, G., Leitinger, N., Shih, P. T., Faull, K. F. & Berliner, J. A. (1999) Circ. Res. 85, 311–318. [DOI] [PubMed] [Google Scholar]
- 16.Quarck, R., De Geest, B., Stengel, D., Mertens, A., Lox, M., Theilmeier, G., Michiels, C., Raes, M., Bult, H., Collen, D., et al. (2001) Circulation 103, 2495–2500. [DOI] [PubMed] [Google Scholar]
- 17.Binder, C. J., Horkko, S., Dewan, A., Chang, M. K., Kieu, E. P., Goodyear, C. S., Shaw, P. X., Palinski, W., Witztum, J. L. & Silverman, G. J. (2003) Nat. Med. 9, 736–743. [DOI] [PubMed] [Google Scholar]
- 18.Yamada, Y., Ichihara, S., Fujimura, T. & Yokota, M. (1998) Metabolism 47, 177–181. [DOI] [PubMed] [Google Scholar]
- 19.McIntyre, T. M., Pontsler, A. V., Silva, A. R., St. Hilaire, A., Xu, Y., Hinshaw, J. C., Zimmerman, G. A., Hama, K., Aoki, J., Arai, H. & Prestwich, G. D. (2003) Proc. Natl. Acad. Sci. USA 100, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, C., Baker, D. L., Yasuda, S., Makarova, N., Balazs, L., Johnson, L. R., Marathe, G. K., McIntyre, T. M., Xu, Y., Prestwich, G. D., et al. (2004) J. Exp. Med. 199, 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breslow, J. L. (1996) Science 272, 685–688. [DOI] [PubMed] [Google Scholar]
- 22.Rong, J. X., Li, J., Reis, E. D., Choudhury, R. P., Dansky, H. M., Elmalem, V. I., Fallon, J. T., Breslow, J. L. & Fisher, E. A. (2001) Circulation 104, 2447–2452. [DOI] [PubMed] [Google Scholar]
- 23.Lieu, H. D., Withycombe, S. K., Walker, Q., Rong, J. X., Walzem, R. L., Wong, J. S., Hamilton, R. L., Fisher, E. A. & Young, S. G. (2003) Circulation 107, 1315–1321. [DOI] [PubMed] [Google Scholar]
- 24.Tangirala, R. K., Pratico, D., FitzGerald, G. A., Chun, S., Tsukamoto, K., Maugeais, C., Usher, D. C., Pure, E. & Rader, D. J. (2001) J. Biol. Chem. 276, 261–266. [DOI] [PubMed] [Google Scholar]
- 25.Kamath, A. T., Henri, S., Battye, F., Tough, D. F. & Shortman, K. (2002) Blood 100, 1734–1741. [PubMed] [Google Scholar]
- 26.Gerrity, R. G. (1981) Am. J. Pathol. 103, 191–200. [PMC free article] [PubMed] [Google Scholar]
- 27.Saiki, T., Ezaki, T., Ogawa, M. & Matsuno, K. (2001) Transplantation 71, 1806–1815. [DOI] [PubMed] [Google Scholar]
- 28.Bjorkbacka, H., Kunjathoor, V. V., Moore, K. J., Koehn, S., Ordija, C. M., Lee, M. A., Means, T., Halmen, K., Luster, A. D., Golenbock, D. T. & Freeman, M. W. (2004) Nat. Med. 10, 416–421. [DOI] [PubMed] [Google Scholar]