Abstract
The central melanocortin system is critical in the regulation of appetite and body weight, and leptin exerts its anorexigenic actions partly by increasing hypothalamic proopiomelanocortin (POMC) expression. The POMC-derived peptide α-melanocyte-stimulating hormone (αMSH) is a melanocortin 4 receptor agonist, and its potency in reducing energy intake is strongly increased by N-acetylation. The reason for the higher biological activity of N-acetylated αMSH (Act-αMSH) compared with that of N-desacetylated αMSH (Des-αMSH) is unclear, and regulation of acetylation by leptin has not been investigated. We show here that total hypothalamic αMSH levels are decreased in leptin-deficient ob/ob mice and increased in leptin-treated ob/ob and C57BL/6J mice. The increase in total αMSH occurred as soon as 3 h after leptin injection and was entirely due to an increase in Act-αMSH. Consistent with this observation, leptin rapidly induced the enzymatic activity of a N-acetyltransferase in the hypothalamus of mice. In 293T cells expressing the melanocortin 4 receptor, Act-αMSH is far more potent than Des-αMSH in stimulating cAMP accumulation, an effect caused by a dramatically increased stability of Act-αMSH. Moreover, Des-αMSH is rapidly degraded in the hypothalamus after intracerebroventricular injection in rats and was less potent in inhibiting energy intake. The results suggest that leptin activates a N-acetyltransferase in POMC neurons, leading to increased hypothalamic levels of Act-αMSH. Due to its increased stability, this posttranslational modification of αMSH may play a critical role in leptin action via the central melanocortin pathway.
The adipocyte-derived hormone leptin is expressed in fat tissue and acts on the central nervous system to inform key regulatory centers about energy stores (1–3). Leptin receptors (ObRs) are highly expressed within the hypothalamus, including in proopiomelanocortin (POMC) neurons located in the arcuate nucleus (4, 5). POMC-derived neuropeptides, primarily α-melanocyte-stimulating hormone (αMSH), activate the melanocortin 4 receptor (MC4R) and function downstream of leptin signaling to regulate energy balance, although other pathways are also important (6–10). Supporting the role of the melanocortin pathway in leptin action are data showing that central injection of synthetic melanocortin receptor antagonists inhibits the effect of leptin to reduce food intake (10). Furthermore, mutations in the pomc and mc4r genes result in severe obesity in mice (6, 11) and humans (7, 12).
Studies investigating the regulation of POMC neurons by leptin have focused mainly on gene expression analyses. It is known that fasting, which is a state of low serum leptin levels, results in reduced amounts of POMC mRNA levels. This reduction is also seen in the hypothalamus of the leptin-deficient ob/ob mice (13–16). In addition, hypothalamic POMC mRNA is stimulated by the administration of recombinant leptin to rodents (16). However, few studies have examined regulation of hypothalamic POMC-derived peptides, such as αMSH, in response to leptin. Generation of αMSH from POMC involves extensive posttranslational processing by prohormone convertases 1 and 2 (PC1 and PC2) and carboxypeptidase E (CPE) and amidation on the C terminus by peptidyl α-amidating monooxygenase (PAM) (17). Moreover, mature αMSH [N-acetylated αMSH (Act-αMSH)] is generated from desacetylated αMSH (Des-αMSH) by a N-acetyltransferase that has yet to be identified (17). The amount of Act-αMSH in the rodent brain is controversial, because some studies report undetectable levels of the N-acetylated form in the hypothalamus (18–20). On the other hand, it is well established that synthetic Act-αMSH is far more potent than Des-αMSH in reducing food intake when administered centrally in rats (21, 22). It is presently unclear whether N-acetylation of hypothalamic αMSH is regulated by leptin, and the mechanism underlying the different potencies of the two forms of αMSH is poorly understood.
The purpose of this study was therefore fourfold: (i) to determine total αMSH levels in hypothalamic extracts in response to leptin, (ii) to investigate the potential role of leptin to influence N-acetylation of αMSH, (iii) to determine whether leptin can regulate hypothalamic αMSH N-acetyltransferase activity, and (iv) to understand the mechanism by which Act-αMSH is more potent than Des-αMSH in activating MC4R signaling and inhibiting feeding.
Materials and Methods
Animals. Male Wistar rats and C57BL/6J mice were purchased from Charles River Laboratories. Leptin-deficient ob/ob mice and WT littermates on the C57BL/6J background were purchased from The Jackson Laboratory. Animals were individually housed and maintained at 25°C with a 14-h/10-h light/dark cycle. Animal procedures were performed in accordance with the guidelines and approval of the Harvard Medical School and Beth Israel Deaconess Medical Center institutional animal care and use committees.
Peptide Extraction. Hypothalamic explants were excised with razor blades at the optical chiasm rostrally, the hypothalamic sulcus laterally, the mammillary bodies caudally, and the ventral surface of the thalamus dorsally. Similar amounts of tissue from the cerebral cortex were also taken. Tissues were immediately immersed in 2 M acetic acid (200 μl for mouse and 400 μl for rat explants) and boiled for 10 min. The samples were then sonicated and centrifuged at 23,000 × g and 4°C for 30 min. Finally, supernatants were collected, and protein concentration was adjusted to 1.0 mg/ml for each tissue extract. Wet tissue weights were ≈36 and ≈52 mg per hypothalamic explant from mice and rats, respectively.
RIA. Rabbit anti-αMSH antiserum was generated by BioSource International (Camarillo, CA) using a customized Act-αMSH (acetyl-SYSMEHFRWGKPVC-amide). Purified Des-αMSH from Bachem was iodinated with 125I, purified by HPLC, and used as tracer. The RIA was performed in a volume of 0.5 ml of PBS (pH 7.4)/500 mg/liter sodium azide/2.5 g/liter BSA (Sigma-Aldrich) by using the anti-αMSH serum (1:10,000) and 5,000 cpm of 125I-αMSH tracer.
Separation of Hypothalamic Act-αMSH and Des-αMSH by HPLC. αMSH peptides were separated by HPLC analysis using a Symmetry C18 (5 μm) column (4.6 × 150 mm) (Waters). The mobile phase consisted of a gradient between 0.1% trifluoroacetic acid in water and acetonitrile/methanol (80:20). The flow rate was 1 ml/min, and 1-ml fractions were collected and concentrated by a SpeedVac system (Appropriate Technical Resources, Laurel, MD).
Immunohistochemistry. αMSH peptides were detected in rat brain sections by immunohistochemistry as described previously (23).
Leptin Treatments in Mice for Measurement of Hypothalamic αMSH. Recombinant murine leptin was from A. F. Parlow (National Hormone and Pituitary Program, Torrance, CA). One experiment involved three groups of C57BL/6J mice that were individually housed. One group was treated for 3 days with two daily i.p. injections of leptin (50 μg per injection) and fed ad libitum. The second group (control) was injected with PBS and fed ad libitum. The third group (pair-fed) was injected with PBS and pair-fed to the leptin-treatment group. Pair-feeding was done by measuring the food intake of the ad libitum-fed leptin-treated mice every 24 h. The following day, the PBS-treated pair-fed mice were given the average amount of food consumed by the leptin-treated mice on the previous day. In a separate experiment, ob/ob mice and control littermates were injected i.p. with 100 μg of leptin or PBS and killed 3 h after injection. Food was removed during the latter experiment.
Cell Culture, Transient Transfection, and DNA Constructs. HEK 293T cells were grown in DMEM with 10% FCS, penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2. Reagents for tissue culture were from Invitrogen Life Technologies. Cells were transfected by using Lipofectamine and OPTI-MEM medium (Invitrogen), and treatments were done ≈18 h posttransfection. The MC4R expression vector and CRE-luciferase reporter construct were kindly provided by J. S. Flier and A. N. Hollenberg (Beth Israel Deaconess Medical Center). The CMV-lacZ reporter construct was from Clontech.
Luciferase and β-Galactosidase Assays. Luciferase and β-galactosidase activities were measured as described in ref. 24.
Measurement of Intracellular cAMP. HEK 293T cells were transfected as described above. In some experiments, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX, Sigma-Aldrich) was added 30 min before the addition of peptides. Cellular cAMP was measured according to the manufacturer's instructions (RPN 225, Amersham Pharmacia).
Determination of αMSH Peptide Stability. Fifty nanomolar Des-αMSH or Act-αMSH was incubated in 0.5 ml of medium with or without cells for different periods of time, and the residual amount of peptides was measured by RIA. For degradation studies in brain lysates, 50 nM Des-αMSH or Act-αMSH was incubated in 0.4 ml of fresh mouse brain lysates at 37°C. The reaction was terminated by the addition of 0.4 ml of 0.2 M HCl and residual peptides determined by RIA. The brain lysate was generated by homogenizing the brain from one C57BL/6J mouse in 2.5 ml of PBS buffer. The final clarified lysate was obtained by centrifugation of the homogenate and dilution of the supernatant to a protein concentration of 1 mg/ml in PBS buffer.
Intracerebroventricular Injections. Permanent 22-gauge cannulae were preimplanted into the third ventricle of male Wistar rats by Charles River Laboratories. For measurement of 2-h food intake, saline or 3 nmol of Des-αMSH or Act-αMSH (5 μl each) was administered into the third ventricle before the onset of the dark segment of the light/dark cycle. Food was removed 6 h before injection and returned after injection. For peptide degradation analyses, residual amounts of Des-αMSH and Act-αMSH in hypothalamic extracts were measured by RIA after intracerebroventricular administration. The injection rate was 1 μl/min in all experiments.
Measurement of αMSH N-Acetyltransferase Enzyme Activity. Hypothalamic explants from leptin-injected (i.p., 100 μg, 3 h) or PBS-injected C57BL/6J or ob/ob mice were homogenized in a 50 mM Tris·HCl buffer (pH 7.4) containing 50 mM KCl, 3 mM MgCl2, and 300 mM sucrose in a total volume of 200 μl. The homogenate was centrifuged, and 10 μl of the supernatant was incubated with 10 μl of 60-pmol Des-αMSH and 2.5 μlof30 μM acetyl CoA for 10 min at 37°C. The reaction was terminated by the addition of 25 μl of 2 M acetic acid. Samples were injected into the HPLC system, and fractions were analyzed by RIA.
Results
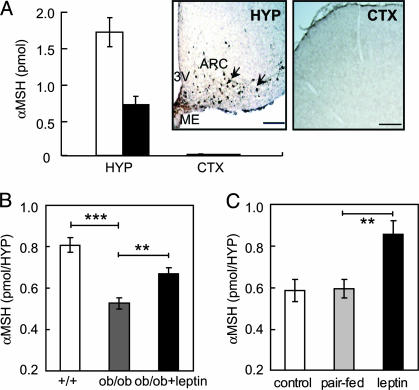
Hypothalamic Content of αMSH Peptides in C57BL/6J Mice and Wistar Rats. To examine posttranslational modification and regulation of hypothalamic αMSH in rodents, we first developed an RIA. The assay was equally sensitive in detecting purified peptides of Des-αMSH and Act-αMSH, and the sensitivity was 5 fmol per sample. To further test the specificity of the assay on biological samples, levels of total αMSH were determined in tissues known to contain high or low levels. We found 0.75 ± 0.2 pmol per hypothalamus in C57BL/6J mice and 1.8 ± 0.2 pmol per hypothalamus in Wistar rats. αMSH was not detected in the cortex (Fig. 1A). This observation is consistent with immunohistochemical analyses of brain sections from rats using a different αMSH antibody than that used in the RIA, showing the expected presence of αMSH-producing (POMC) neurons in the hypothalamus, but no staining in the cortex (Fig. 1 A Insets). The hypothalamic levels of total αMSH are consistent with previous reports (19, 20).
Fig. 1.
Leptin stimulates the hypothalamic content of αMSH in mice. (A) Total αMSH levels in the hypothalamus (HYP) and cortex (CTX) of C57BL/6J mice (black bars) and Wistar rats (white bars). (Inset) Immunohistochemistry demonstrates αMSH-producing POMC neurons in the arcuate nucleus (ARC) of rat hypothalamus (arrows) and the lack of αMSH in the cortex. ME, median eminence; 3V, third ventricle. (Scale bars = 200 μm.) (B) Hypothalamic (HYP) αMSH levels in ob/ob mice with or without 3 h of leptin treatment. (C) Hypothalamic (HYP) αMSH in C57BL/6J mice after 3 days of leptin and PBS treatment and in pair-fed control mice. Leptin reduced food intake with 0.9 ± 0.2 g/day per mouse. Data in B and C are means ± SE of five animals. Significant differences were determined by Student's t test. **, P < 0.005; ***, P < 0.0005.
Total Hypothalamic αMSH Levels Are Reduced in ob/ob Mice and Elevated by Leptin Treatment. To examine regulation of total hypothalamic αMSH by leptin, hypothalami were isolated from leptin-deficient ob/ob mice and from leptin-treated mice. In ob/ob mice, αMSH levels were decreased by ≈35%, compared with those in WT littermates, and a single injection of leptin increased the amounts 3 h after administration (Fig. 1B). Leptin also increased steady-state levels of hypothalamic αMSH in C57BL/6J mice after 3 days of leptin treatment, compared with those in control and pair-fed mice (Fig. 1C). These results show that leptin can positively regulate total hypothalamic αMSH levels in mice, consistent with data showing that leptin can increase hypothalamic POMC mRNA (13, 16, 25).
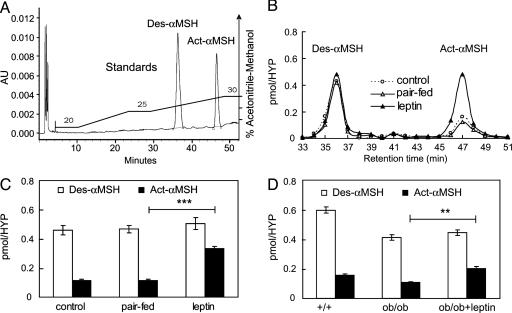
Leptin Stimulates N-Acetylation of αMSH in the Hypothalamus. To measure N-acetylation of αMSH, we developed conditions to separate Des-αMSH and Act-αMSH by HPLC. The retention times of Des-αMSH and Act-αMSH were obtained by loading 6 nmol of Des-αMSH and Act-αMSH standards on the HPLC system followed by UV detection (OD220). As shown in Fig. 2A, the two peptides separated as individual peaks with Des-αMSH eluting at 36 min and Act-αMSH at 47 min. Because hypothalamic αMSH levels in mice are much lower (≈1 pmol; see Fig. 1), we also demonstrated that 0.15 pmol of the peptide standards could be detected in the expected fractions by HPLC followed by RIA (data not shown). Analysis of hypothalamic extracts showed ≈0.15 pmol of Act-αMSH and ≈0.58 pmol of Des-αMSH in fed C57BL/6J mice, and ≈0.35 pmol of Act-αMSH and ≈1.32 pmol of Des-αMSH in fed rats. This result demonstrates that both Des-αMSH and Act-αMSH exist in the hypothalamus of rats and mice and that Des-αMSH is the predominant form (≈80%). Earlier studies have reported presence of diacetyl-αMSH in the rat hypothalamus, although the levels were extremely low (19). Our data show that the identified acetylated MSH in the hypothalamus is monoacetylated, because the retention time of endogenous hypothalamic Act-αMSH is identical to that of the synthetic mono-N-acetylated peptide.
Fig. 2.
Leptin stimulates N-acetylation of αMSH in the hypothalamus. (A) HPLC elution profile of Des-αMSH and Act-αMSH peptide standards detected by UV (OD220). AU, absorbance units. (B) Representative HPLC/RIA profiles of Des-αMSH and Act-αMSH in hypothalamic (HYP) extracts from PBS-treated, pair-fed, and leptin-treated C57BL/6J mice. (C) Hypothalamic (HYP) amounts of Des-αMSH and Act-αMSH in C57BL/6J mice treated with leptin and PBS or pair-fed for 3 days. (D) Hypothalamic (HYP) levels of Des-αMSH and Act-αMSH 3 h after leptin or PBS injection in ob/ob mice. Data in C and D are means ± SE of five animals. Significant differences were determined by Student's t test. **, P < 0.05; ***, P < 0.0005.
Hypothalamic extracts from leptin-treated C57BL/6J mice and PBS-injected control and pair-fed groups were next analyzed by HPLC coupled with RIA; representative elution profiles are shown in Fig. 2B. Three days of leptin treatment of C57BL/6J mice caused an ≈300% increase in Act-αMSH, compared with that in control and pair-fed groups, whereas there was no significant change in steady-state levels of Des-αMSH (Fig. 2C). The amounts of Act-αMSH and Des-αMSH were both decreased in the hypothalamus of ob/ob mice, compared with those in the hypothalamus of WT littermates (Fig. 2D). Three hours after leptin administration, the levels of Act-αMSH, but not Des-αMSH, were significantly increased in ob/ob mice. These data show that recombinant leptin can specifically increase the hypothalamic content of Act-αMSH in C57BL/6J and ob/ob mice.
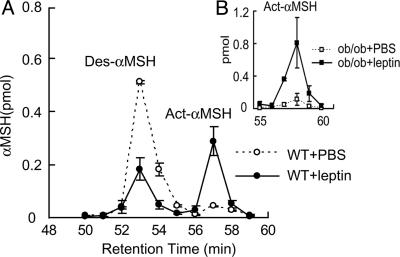
Leptin Rapidly Stimulates the Activity of an αMSH N-Acetyltransferase in the Hypothalamus. The rapid and specific increase of Act-αMSH after leptin administration suggests activation of an αMSH N-acetyltransferase in the hypothalamus. To examine this possibility, we injected C57BL/6J mice with either leptin or vehicle. Three hours later, hypothalamic protein extracts were obtained and mixed with purified Des-αMSH peptides and acetyl CoA for 10 min at 37°C and subjected to HPLC and RIA. As shown in Fig. 3A, little Act-αMSH was generated in hypothalamic extracts from vehicle-treated mice. In contrast, leptin injection resulted in a dramatic increase in formation of Act-αMSH in the assay. Similar results were obtained from analysis of leptin-treated ob/ob mice (Fig. 3B). These data demonstrate that leptin can rapidly stimulate the activity of a hypothalamic N-acetyltransferase with the capacity to acetylate αMSH at the N terminus.
Fig. 3.
Leptin stimulates the activity of an αMSH N-acetyltransferase in the hypothalamus of C57BL/6J and ob/ob mice. Hypothalamic protein extracts from mice injected with either leptin or PBS (3 h) were incubated with purified Des-αMSH and acetyl CoA for 10 min. Samples were applied to an HPLC system, and fractions were measured by RIA. The condition of the HPLC analysis was changed so that elution times were different, compared with those shown in Fig. 2. (A) Results from C57BL/6J mice. (B) Results from ob/ob mice. Data are means ± SE of three to four animals in each group.
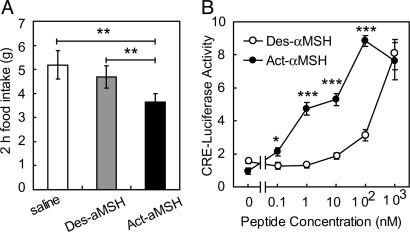
Act-αMSH Is More Potent than Des-αMSH in Reducing Food Intake in Rats and in Activating MC4R Signaling in Transfected Cells. To investigate the different potencies of the two forms of αMSH (26), we first measured food intake after intracerebroventricular injection of purified peptides into rats. As shown in Fig. 4A, Act-αMSH caused an ≈30% decrease in 2-h energy intake, whereas Des-αMSH was without effect, confirming earlier data (21). To examine the mechanism underlying this difference, the bioactivity of Act-αMSH and Des-αMSH was studied in a transfection system. Because Des-αMSH and Act-αMSH mediate their central effects through the melanocortin G protein-coupled receptors (27), we generated a heterologous cell system consisting of 293T cells transfected with MC4R plasmids and a cAMP-responsive luciferase reporter construct. Cells were stimulated with different doses of Des-αMSH or Act-αMSH for 5 h, and luciferase activities were measured. We found that Act-αMSH was far more potent than Des-αMSH in activating MC4R signaling (Fig. 4B), a finding thus consistent with the differential in vivo effects on food intake. EC50 values of Des-αMSH and Act-αMSH were 316 and 1.1 nM, respectively.
Fig. 4.
Increased activity of Act-αMSH in transfected cells and in vivo.(A) Rats with cannulae preimplanted into the third ventricle were injected with saline or 3 nmol of Des-αMSH or Act-αMSH. Shown is 2 h of food intake. Data are means ± SE of six animals. Significant differences were determined by Student's t test. **, P < 0.005. (B) 293T cells were transiently transfected with MC4R and pCRE-LUC plasmids. Cells were incubated with different concentrations of Des-αMSH or Act-αMSH for 5 h, and luciferase activity was measured. Data are means ± SE of results obtained in triplicate. Significant differences were determined by ANOVA and Student's t test for post hoc analysis. *, P < 0.05; ***, P < 0.0005.
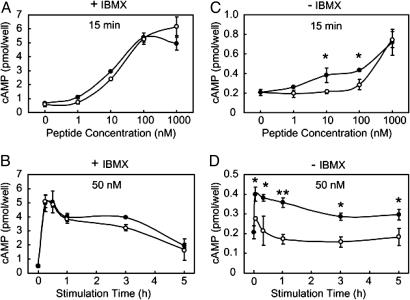
It has been reported that Act-αMSH and Des-αMSH bind to the MC4R with similar affinities (21) and that the peptides have equal capacity to activate intracellular signaling as measured by cAMP assays (21, 28). To understand the reason for the apparent discrepancies between these published results (21, 28) and the above data, we first examined cAMP activation by Des-αMSH and Act-αMSH. Dose responses and time courses of intracellular cAMP accumulation after stimulation of 293T cells expressing MC4R are shown in Fig. 5. In the presence of IBMX, a commonly used inhibitor of cellular phosphodiesterases, Act-αMSH and Des-αMSH dose-dependently stimulated cAMP production with similar potencies (Fig. 5A) and time courses (Fig. 5B). In contrast, in the absence of IBMX pretreatment, the dose responses diverged between the peptides, showing that Act-αMSH was more potent in stimulating intracellular cAMP (Fig. 5C). This difference was more pronounced when examined further at a submaximum concentration (Fig. 5D). We conclude that IBMX pretreatment, which results in ≈10-fold higher cAMP responses, is masking signaling differences between the peptides, which may explain why earlier studies in which IBMX pretreatment was used (21, 28) did not detect increased activity of Act-αMSH. Therefore, our cAMP signaling data are consistent with the transcriptional signaling results and altogether demonstrate that Act-αMSH is more potent than Des-αMSH both in vitro and in vivo.
Fig. 5.
Act-αMSH is more potent than Des-αMSH in stimulating cAMP in 293T cells expressing MC4R. Shown are dose responses (A and C) and time courses (B and D) of Des-αMSH (open circles) or Act-αMSH (filled circles) used to induce cAMP. (A and B) IBMX pretreatment (30 min). (C and D) Absence of IBMX. Significant differences were determined by ANOVA and Student's t test for post hoc analysis. *, P < 0.05; **, P < 0.005.
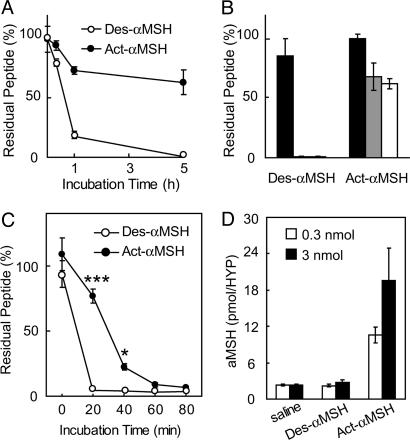
Act-αMSH Is More Stable than Des-αMSH in a Cell System and in the Rodent Brain. To examine whether peptide stability might explain the differential activity of the two peptides, 50 nM of each peptide was incubated in the medium with 293T cells for different periods of time. As shown in Fig. 6A, ≈75% of Act-αMSH remained in the medium after 5 h of incubation. In striking contrast, Des-αMSH disappeared to undetectable levels. Both peptides were equally stable after5hin pure medium (Fig. 6B, black bars), but Des-αMSH was fully degraded in the presence of cells (Fig. 6B, gray bar), demonstrating a role of a cell-derived factor. Moreover, the disappearance of Des-αMSH and partial degradation of Act-αMSH occurred independently of expression of the MC4R (Fig. 6B, white bar). Combined, the data suggest that the decreased potency of Des-αMSH in activating MC4R signaling is due to rapid degradation of the peptide, resulting in loss of its biological activity.
Fig. 6.
Act-αMSH is more stable than Des-αMSH. (A) Fifty nanomolar Des-αMSH or Act-αMSH was incubated with cells for up to 5 h, and residual peptide amounts were measured by RIA. (B) Fifty nanomolar Des-αMSH or Act-αMSH was incubated in pure medium (black bars), in medium with 293T cells (gray bar), or in medium with 293T cells expressing MC4R (white bar) for 5 h, and residual peptides were measured by RIA. Data are means ± SE of results obtained in triplicate. (C) Fifty nanomolar Des-αMSH or Act-αMSH was incubated with brain lysates from C57BL/6J mice at 37°C, and residual amounts of peptides were measured by RIA. Data are means ± SE of results obtained in triplicate. Significant differences were determined by ANOVA and Student's t test for post hoc analysis. *, P < 0.05; ***, P < 0.0005. (D) Saline or 0.3 or 3 nmol of Des-αMSH or Act-αMSH was injected into the third ventricle of Wistar rats, and residual amounts in the hypothalamus were measured by RIA 30 min postinjection of 3 nmol and 15 min postinjection of 0.3 nmol.
To further support these results, we tested the stability of the two peptides in brain lysates from C57BL/6J mice and after central injection into cannulated rats. As shown in Fig. 6C, Act-αMSH was more stable than Des-αMSH when incubated with mouse brain lysates. After 20 min, ≈80% of Act-αMSH remained, whereas Des-αMSH was undetectable. Moreover, when 3 nmol of Des-αMSH and Act-αMSH was administered into the third ventricle of rats, Des-αMSH was degraded to basal levels 30 min postinjection, whereas significant amounts of Act-αMSH remained in the hypothalamic extract (Fig. 6D, black bars). Similar results were observed with 10 times less peptide (Fig. 6D, white bars). We conclude that the higher potency of Act-αMSH in activating MC4R signaling in transfected cells and in inhibiting feeding in rats is due to a dramatically increased stability of the peptide. The data suggest that central proteases/peptidases may exist with relative higher specificity toward the nonacetylated form of αMSH.
Discussion
Our data demonstrate that leptin specifically and rapidly stimulates generation of Act-αMSH in the hypothalamus of mice. This is likely caused by a leptin-dependent increase in the activity of a N-acetyltransferase in the hypothalamus, presumably in POMC neurons. We also show that the N-acetylated form of the peptide is more potent in activating MC4R signaling and in reducing food intake, effects that are likely due to rapid degradation of the nonacetylated form of αMSH. Combined, these data suggest that increased N-acetylation of αMSH is a critical step in the central leptin–melanocortin pathway to inhibit appetite and body weight.
We also show that hypothalamic levels of total αMSH peptides are regulated by leptin. Specifically, the chronic lack of leptin in ob/ob mice leads to a decrease in αMSH, and leptin treatment rapidly increases the hypothalamic content. These results are clearly consistent with studies showing low levels of hypothalamic POMC mRNA in ob/ob mice and in food-deprived animals and that leptin can stimulate POMC gene expression (13, 16, 25). The generation of mature αMSH from POMC requires a complex pathway of posttranslational modifications involving numerous enzymes such as PC1 and PC2. Interestingly, Renz et al. (29) have suggested that leptin may regulate PC2 expression in the pituitary, raising the possibility that this regulation may also occur in POMC neurons. We therefore cannot rule out that the increase in total αMSH in response to leptin is a result of both a stimulatory effect on pomc gene expression (13, 16, 25) and enhanced posttranslational processing of the POMC precursor.
The mechanism whereby leptin specifically enhances steady-state levels of Act-αMSH without significantly affecting hypothalamic amounts of Des-αMSH remains unclear. An intriguing possibility that may account for this selectivity is that leptin acts on only a subpopulation of POMC neurons, consistent with our earlier data showing that leptin activates ≈40% of POMC neurons (23). We therefore hypothesize that leptin may coordinately stimulate POMC expression and activate the αMSH N-acetylating enzyme in this subpopulation of POMC neurons without affecting the amounts or acetylation of αMSH in POMC cells that do not express the leptin receptor. We also show that both forms of αMSH are significantly decreased in hypothalamus of leptin-deficient ob/ob mice. The fact that only Act-αMSH is increased in leptin-treated ob/ob mice may suggest that factors other than leptin regulate the content of Des-αMSH in these mice. More specifically, it is possible that the lack of leptin in ob/ob mice may reduce the amount of Act-αMSH in leptin-responsive POMC neurons, whereas other factors that are altered in ob/ob mice may specifically reduce levels of Des-αMSH in non-leptin-responsive cells. Further anatomical or genetic studies are required to test these hypotheses.
It has been suggested that the N-acetyltransferase responsible for N-acetylation of αMSH in the pituitary is expressed in secretory vesicles near the cell membrane (30). Analysis of αMSH peptides in rat hypothalami ex vivo shows that the majority (>90%) of αMSH released under basal conditions is the nonacetylated form (20). Interestingly, treatment of these hypothalamic explants with the secretagogue KCl resulted in a 3- to 4-fold higher proportion of Act-αMSH in the medium. Because it has been shown that leptin rapidly depolarizes POMC neurons (31), leading to the release of αMSH (32), one possibility is that N-acetylation of αMSH occurs in association with the release process. Determination of the relative levels of the two forms of αMSH released from nerve terminals of leptin-responsive POMC neurons and elucidation of how leptin regulates N-acetyltransferase activity remain open questions.
The mechanism explaining the higher biological potency of Act-αMSH has remained elusive. We applied a heterologous transfection system to study the activity of the two forms of αMSH under well defined conditions. We found that Act-αMSH is far more potent than Des-αMSH in activating MC4R-dependent transcriptional activation of a cAMP-inducible reporter gene. Consistent with this finding, in the absence of IBMX, Act-αMSH is more active than Des-αMSH in stimulating intracellular levels of cAMP. This indicates that measurement of cAMP after pretreatment of cells with IBMX does not truly reflect melanocortin receptor signaling and may explain why differences in potencies were not detected in earlier studies (21). Furthermore, Des-αMSH is rapidly subjected to degradation in a mammalian cell line. Although it was reported that MC4R undergoes αMSH-dependent internalization (33), our data show that the disappearance of αMSH occurs independently of the MC4R. Des-αMSH was also rapidly degraded in brain lysates from mice and after central injection in rats. Combined, these data suggest that the increased biological activity of Act-αMSH in inhibiting feeding is due to rapid proteolysis of the nonacetylated form of the peptide and that acetylation of αMSH may serve to protect the peptide against enzymatic degradation.
In conclusion, our data suggest that central leptin action via the melanocortin pathway to affect energy balance has additional levels of complexity, namely regulation of N-acetylation of αMSH and enzymatic degradation of αMSH peptides. The identities of the enzymes responsible for these processes are unknown. Cloning of the genes and examination of their potential regulation requires further studies that may provide identification of novel antiobesity drug targets. Finally, it is possible that mutations in the genes encoding these enzymes may explain some cases of human obesity.
Acknowledgments
We thank Dr. J. S. Flier (Beth Israel Deaconess Medical Center) for supporting this work by providing access to the HPLC system and Dr. A. N. Hollenberg (Beth Israel Deaconess Medical Center) for helpful advice. This work was supported by National Institutes of Health Grants RO1 DK60673 (to C.B.) and RO1 DK58148 (to E.A.N.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: POMC, proopiomelanocortin; αMSH, α-melanocyte-stimulating hormone; MC4R, melanocortin 4 receptor; Act-αMSH, N-acetylated αMSH; Des-αMSH, desacetylated αMSH; IBMX, 3-isobutyl-1-methylxanthine; PC, prohormone convertase.
References
- 1.Friedman, J. M. & Halaas, J. L. (1998) Nature 395, 763–770. [DOI] [PubMed] [Google Scholar]
- 2.Elmquist, J. K. (2001) Int. J. Obes. Relat. Metab. Disord. 25, Suppl. 5, S78–S82. [DOI] [PubMed] [Google Scholar]
- 3.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J. M. (1994) Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]
- 4.Mercer, J. G., Hoggard, N., Williams, L. M., Lawrence, C. B., Hannah, L. T. & Trayhurn, P. (1996) FEBS Lett. 387, 113–116. [DOI] [PubMed] [Google Scholar]
- 5.Cheung, C. C., Clifton, D. K. & Steiner, R. A. (1997) Endocrinology 138, 4489–4492. [DOI] [PubMed] [Google Scholar]
- 6.Huszar, D., Lynch, C. A., Fairchild-Huntress, V., Dunmore, J. H., Fang, Q., Berkemeier, L. R., Gu, W., Kesterson, R. A., Boston, B. A., Cone, R. D., et al. (1997) Cell 88, 131–141. [DOI] [PubMed] [Google Scholar]
- 7.Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. (1998) Nat. Genet. 20, 113–114. [DOI] [PubMed] [Google Scholar]
- 8.Cone, R. D. (1999) Trends Endocrinol. Metab. 10, 211–216. [DOI] [PubMed] [Google Scholar]
- 9.Marsh, D. J., Hollopeter, G., Huszar, D., Laufer, R., Yagaloff, K. A., Fisher, S. L., Burn, P. & Palmiter, R. D. (1999) Nat. Genet. 21, 119–122. [DOI] [PubMed] [Google Scholar]
- 10.Kask, A., Rago, L., Wikberg, J. E. & Schioth, H. B. (1998) Eur. J. Pharmacol. 360, 15–19. [DOI] [PubMed] [Google Scholar]
- 11.Yaswen, L., Diehl, N., Brennan, M. B. & Hochgeschwender, U. (1999) Nat. Med. 5, 1066–1070. [DOI] [PubMed] [Google Scholar]
- 12.Krude, H., Biebermann, H., Luck, W., Horn, R., Brabant, G. & Gruters, A. (1998) Nat. Genet. 19, 155–157. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz, M. W., Seeley, R. J., Woods, S. C., Weigle, D. S., Campfield, L. A., Burn, P. & Baskin, D. G. (1997) Diabetes 46, 2119–2123. [DOI] [PubMed] [Google Scholar]
- 14.Korner, J., Chua, S. C., Jr., Williams, J. A., Leibel, R. L. & Wardlaw, S. L. (1999) Neuroendocrinology 70, 377–383. [DOI] [PubMed] [Google Scholar]
- 15.Bergendahl, M., Wiemann, J. N., Clifton, D. K., Huhtaniemi, I. & Steiner, R. A. (1992) Neuroendocrinology 56, 913–920. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno, T. M., Kleopoulos, S. P., Bergen, H. T., Roberts, J. L., Priest, C. A. & Mobbs, C. V. (1998) Diabetes 47, 294–297. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard, L. E., Turnbull, A. V. & White, A. (2002) J. Endocrinol. 172, 411–421. [DOI] [PubMed] [Google Scholar]
- 18.Evans, C. J., Lorenz, R., Weber, E. & Barchas, J. D. (1982) Biochem. Biophys. Res. Commun. 106, 910–919. [DOI] [PubMed] [Google Scholar]
- 19.Emeson, R. B. & Eipper, B. A. (1986) J. Neurosci. 6, 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jegou, S., Tranchand-Bunel, D., Delbende, C., Blasquez, C. & Vaudry, H. (1989) Brain Res. Mol. Brain Res. 5, 219–226. [DOI] [PubMed] [Google Scholar]
- 21.Abbott, C. R., Rossi, M., Kim, M., AlAhmed, S. H., Taylor, G. M., Ghatei, M. A., Smith, D. M. & Bloom, S. R. (2000) Brain Res. 869, 203–210. [DOI] [PubMed] [Google Scholar]
- 22.Tsujii, S. & Bray, G. A. (1989) Brain Res. Bull. 23, 165–169. [DOI] [PubMed] [Google Scholar]
- 23.Munzberg, H., Huo, L., Nillni, E. A., Hollenberg, A. N. & Bjorbaek, C. (2003) Endocrinology 144, 2121–2131. [DOI] [PubMed] [Google Scholar]
- 24.Bjorbaek, C., Elmquist, J. K., Frantz, J. D., Shoelson, S. E. & Flier, J. S. (1998) Mol. Cell 1, 619–625. [DOI] [PubMed] [Google Scholar]
- 25.Thornton, J. E., Cheung, C. C., Clifton, D. K. & Steiner, R. A. (1997) Endocrinology 138, 5063–5066. [DOI] [PubMed] [Google Scholar]
- 26.O'Donohue, T. L., Handelmann, G. E., Chaconas, T., Miller, R. L. & Jacobowitz, D. M. (1981) Peptides 2, 333–344. [DOI] [PubMed] [Google Scholar]
- 27.Vergoni, A. V. & Bertolini, A. (2000) Eur. J. Pharmacol. 405, 25–32. [DOI] [PubMed] [Google Scholar]
- 28.Mountjoy, K. G., Willard, D. H. & Wilkison, W. O. (1999) Endocrinology 140, 2167–2172. [DOI] [PubMed] [Google Scholar]
- 29.Renz, M., Tomlinson, E., Hultgren, B., Levin, N., Gu, Q., Shimkets, R. A., Lewin, D. A. & Stewart, T. A. (2000) J. Biol. Chem. 275, 10429–10436. [DOI] [PubMed] [Google Scholar]
- 30.Jenks, B. G., Verburg van Kemenade, B. M., Tonon, M. C. & Vaudry, H. (1985) Peptides 6, 913–921. [DOI] [PubMed] [Google Scholar]
- 31.Cowley, M. A., Smart, J. L., Rubinstein, M., Cerdan, M. G., Diano, S., Horvath, T. L., Cone, R. D. & Low, M. J. (2001) Nature 411, 480–484. [DOI] [PubMed] [Google Scholar]
- 32.Watanobe, H. & Habu, S. (2002) J. Neurosci. 22, 6265–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinyama, H., Masuzaki, H., Fang, H. & Flier, J. S. (2003) Endocrinology 144, 1301–1314. [DOI] [PubMed] [Google Scholar]