Abstract
Approximately 1 million people in the United States suffer from interstitial cystitis, a chronic painful urinary bladder disorder characterized by thinning or ulceration of the bladder epithelial lining; its etiology is unknown. We have identified a glycosylated frizzled-related peptide inhibitor of cell proliferation that is secreted specifically by bladder epithelial cells from patients with this disorder. This antiproliferative factor (APF) profoundly inhibits bladder cell proliferation by means of regulation of cell adhesion protein and growth factor production. The structure of APF was deduced by using ion trap mass spectrometry (MS), enzymatic digestion, lectin affinity chromatography, and total synthesis, and confirmed by coelution of native and synthetic APF derivatives on microcapillary reversed-phase liquid chromatography (μRPLC)/MS. APF was determined to be an acidic, heat-stable sialoglycopeptide whose peptide chain has 100% homology to the putative sixth transmembrane domain of frizzled 8. Both synthetic and native APF had identical biological activity in normal bladder epithelial cells and T24 bladder cancer cells. Northern blot analysis indicated binding of a probe containing the sequence for the frizzled 8 segment with mRNA extracted from cells of patients with interstitial cystitis but not controls. APF is therefore a frizzled-related peptide growth inhibitor shown to contain exclusively a transmembrane segment of a frizzled protein and is a potential biomarker for interstitial cystitis.
Keywords: frizzled-related peptide, growth inhibitor, bladder epithelium
Interstitial cystitis is a chronic painful bladder disorder that affects approximately 1 million Americans (1). Cystoscopic abnormalities seen in the bladder of patients with this disorder include petechial hemorrhages called “glomerulations” and ulcers that extend into the lamina propria (Hunner's ulcers) (2, 3). The most consistent histologic abnormalities include denudation or thinning of the bladder epithelium to 1–2 cell layers (2–4). These findings suggest that interstitial cystitis (IC) may be caused by an inhibition of normal bladder epithelial cell proliferation, resulting in a loss of epithelial barrier integrity with subsequent exposure of sensory nerve cells in the bladder wall to urinary constituents. However, the pathogenesis of IC is currently unknown.
We previously reported the discovery of an antiproliferative factor (APF) peptide (5) that is made uniquely by bladder epithelial cells from IC patients (6) and profoundly inhibits normal bladder epithelial cell growth (7). Picomolar quantities of HPLC-purified APF were able to induce several changes in normal bladder epithelial cells in vitro, including significantly decreased rates of proliferation (7) and decreased production of a growth factor required for log-phase growth of bladder epithelial cells (heparin-binding epidermal growth factor-like growth factor, or HB-EGF) (6, 7). Microarray analysis indicated that APF can also induce changes in the pattern of cellular gene expression toward a more differentiated phenotype (8). Identification of this factor is therefore important for determining its potential role in the pathogenesis of IC and establishing its utility as a biomarker for this disease.
Preliminary characterization of APF indicated that it was a low-molecular-weight, relatively heat-stable peptide (6). APF is found in minute quantities in both patient urine specimens and explanted patient bladder cell supernatants, making conventional methods of structural analysis, such as NMR spectroscopy, unfeasible. We therefore proceeded to completely characterize this potent growth inhibitor by using a combination of techniques including mass spectrometry (MS), lectin affinity chromatography, enzymatic analysis, and total synthesis. Confirmation of APF's structure was provided by using microcapillary reversed-phase liquid chromatography (μRPLC) of native and synthetic APF derivatives, as well as by demonstration of synthetic APF's ability to regulate growth factor production and bladder epithelial cell proliferation. Additional evidence of APF's identity was provided by identification of mRNA that bound to a probe for the frizzled 8 protein segment in cells from IC patients but not controls.
Methods
Cell Cultures. Cystoscopy was performed under general anesthesia, and 4-mm2 pieces of transitional epithelium with submucosa bladder tissue were obtained by using rigid cold-cup biopsy forceps from six patients who had previously undergone cystoscopy and fulfilled the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health diagnostic criteria for IC, and six age-, race-, and gender-matched controls who were asymptomatic for urinary tract disease, as described (5–9). All patients were at least 18 years old and enrolled in accordance with guidelines of the Institutional Review Board of the University of Maryland School of Medicine.
Explanted epithelial cells were propagated from these biopsy specimens in DMEM-F12 (Mediatech, Herndon, VA) with 10% heat-inactivated FBS, 1% antibiotic/antimycotic solution, 1% l-glutamine, 1.0 unit/ml insulin (all from Sigma), and 5 μg/ml human epidermal growth factor (EGF) (R & D Systems) at 37°C in a 5% CO2 atmosphere, and characterized by binding of AE-1/AE-3 pancytokeratin antibodies (Signet Laboratories, Dedham, MA), as described (5–9).
T24 bladder carcinoma cells (ATCC HTB 4) were purchased from the American Type Culture Collection and cultured in McCoy's 5A medium (GIBCO–Invitrogen) containing 10% FBS, 1% glutamine, and 1% antibiotic/antimycotic solution (all from Sigma).
APF Purification. APF was harvested from the supernatant of explanted patient bladder epithelial cells and purified by using molecular weight fractionation, ion exchange chromatography, hydrophobic interaction chromatography, and reversed-phase high-performance liquid chromatography (HPLC), as described (6). Mock APF was prepared by using the supernatant of normal control bladder epithelial cells and the same purification procedure.
μRPLC-MS/MS Analysis. μRPLC was performed by using an Agilent 1100 capillary LC system (Agilent Technologies, Palo Alto, CA) coupled online to an ion trap mass spectrometer (Finnigan LCQ Deca XP, Thermo Electron, San Jose, CA). Reversed-phase separations of each sample were performed by using 75-μm i.d. × 10-cm-long fused silica electrospray ionization capillary columns (Polymicro Technologies, Phoenix, AZ) that were slurry packed in house with 3-μm, 300-Å-pore-size C-18 stationary phase (Vydac Hesperia, CA). After sample injection, the column was washed for 20 min with 98% solvent A (0.1% vol/vol formic acid in water), and gradient elution was conducted by using a linear step gradient from 2% solvent B (0.1% vol/vol formic acid in acetonitrile) to 42% solvent B in 40 min, then from 42% to 95% solvent B in 15 min, at a constant flow rate of 0.5 μl/min.
The ion trap mass spectrometer was operated in a data-dependent mode in which each full MS scan was followed by three tandem MS scans where the three most abundant molecular ions were dynamically selected for collision-induced dissociation (CID) by using a normalized collision energy of 38%. The temperatures of the heated capillary and the electrospray voltage were 180°C and 1.8 kV, respectively.
[3H]Thymidine Incorporation. Cell proliferation was measured by [3H]thymidine incorporation into explanted normal human bladder epithelial cells, as described (5–9). Briefly, purified native or synthetic APF (or the appropriate mock preparation) was diluted in serum-free MEM (containing only glutamine and antibiotics/antimycotics) and applied to the cells; cell controls received serum-free MEM alone. Cells were then incubated at 37°C in a 5% CO2 atmosphere for 48 h. The cells were then labeled with 1 μCi per well [3H]thymidine (1 Ci = 37 GBq) for 4 h and trypsinized; insoluble cell contents were harvested and methanol-fixed onto glass fiber filter paper; and the amount of radioactivity incorporated was determined. Significant inhibition of [3H]thymidine incorporation was defined as a mean decrease in cpm of >2 SD from the mean of control cells for each plate.
Cell-Count Determination. For cell-count experiments, explanted normal human bladder epithelial cells or T24 bladder carcinoma cells were plated onto Corning 24-well tissue culture plates (VWR Scientific) in MEM (primary bladder cells) or McCoy's 5A medium (T24 cells) containing 10% FBS, 1% antibiotic/antimycotic solution, 1% l-glutamine at a density of 1 × 104 cells per well and incubated at 37°C in a 5% CO2 atmosphere overnight. On the next day, the medium was removed and replaced with serum-free MEM containing 1% antibiotic/antimycotic solution and 1% l-glutamine (both cell types). HPLC-purified or synthetic APF (or their mock preparations) were then added to the medium, and cells were further incubated at 37°C with 5% CO2. Forty-eight hours later, the culture supernatant was removed for growth factor analysis by ELISA, and cells were trypsinized, stained with trypan blue (Sigma), and counted by using a hemacytometer.
ELISAs. HB-EGF. ELISAs were performed as described (5, 10), coating 96-well Immulon II plates (Dynatech) with 200 λ culture supernatant at 4°C overnight. Plates were subsequently rinsed and blocked, and an anti-HB-EGF antibody (1 μg/ml) (R & D Systems) was applied. After additional incubation and rinses, biotinylated anti-goat IgG/avidin D horseradish peroxidase was added. Binding was detected by development with ABTS [2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)]; absorbance was read at 405 nm.
EGF. Culture supernatant (200 λ) was pipetted into wells precoated with monoclonal anti-EGF antibody, according to the manufacturer's instructions (R & D Systems). After incubation and rinses, horseradish peroxidase-linked polyclonal anti-EGF was added, and binding was detected by development with tetramethylbenzidine (TMB) substrate; absorbance was read at 450 nm.
Linear absorbance vs. concentration curves were prepared from results with known concentrations of EGF or HB-EGF, and sample concentrations were determined by plotting absorbance values.
Enzymatic Cleavage. Partially purified APF was incubated with any of three positionally specific neuraminidases (2-3; 2-3,6; 2-3,6,8,9; all from Sigma) at 37°C for 2 h; control APF was incubated under the same conditions but without enzyme. APF was then purified from enzyme by ultrafiltration using a 3,000-MW cut-off Centricon filter (Amicon), and antiproliferative activity and lectin-binding of each ultrafiltrate were determined.
Lectin Binding. HPLC-purified APF or enzymatically treated APF samples were applied to the following agarose-conjugated lectins and eluted according to the manufacturer's instructions: wheat germ agglutinin, Con A, lentil lectin (all from Amersham Pharmacia), Vicia villosa (Sigma), Erythrina cristagalli, Griffonia simplicifolia I and II, peanut agglutinin, Jacalin lectin (all from Vector Laboratories), or Tritrichomonas mobilensis lectin (Calbiochem-Novobiochem). Eluates were tested for antiproliferative activity by the [3H]thymidine incorporation assay.
Sucrose Density Gradient Isoelectric Focusing. HPLC-purified APF was fractionated by high-speed electrofocusing in a pH 2–10 sucrose density gradient formed at 15 W for 18 h with an LKB 8100-1 column. The pH of the fractions was determined at 4°C, and the antiproliferative activity of the neutralized (pH 7) fractions was determined in normal bladder cells by using the [3H]thymidine incorporation assay.
APF Synthesis. The synthesis of the peptides was carried out by solid-phase methods on the Nautilus 2400 synthesizer (Argonaut Technologies, Foster City, CA) by using standard 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry on alanyl 2-chlorotrityl resin (Calbiochem-Novobiochem). Fmoc-protected L amino acids (Anaspec, San Jose, CA) were coupled by using N-{(dimethylamino)-1H-1,2,3-triazolo[4,5-b]pyridin-1-ylmethylene}-N-methylmethanaminium hexafluorophosphate N-oxide (HATU) (Sigma–Aldrich) and 1-hydroxy-7-azabenzotriazole (HOAt) (Anaspec) reagents. All other reagents were purchased from Sigma–Aldrich. All intermediates and the final products were verified by MS.
Fmoc-protected N-acetyllactosamine-α-O-threonine. Fmoc-l-Thr (Calbiochem-Novabiochem) was converted to its phenacyl ester and glycosylated with 2-azido-1-α-bromo-hexa-O-acetyl-2-deoxylactose in the presence of silver triflate according to a slight modification of the procedure by Leuck and Kunz (11). The reaction was carried out at –40°C that ensured >98% selectivity for the α-anomer. The anomeric purity was determined by proton NMR spectroscopy. The phenacyl ester was deprotected by zinc/acetic acid/acetic anhydride, which also resulted in the simultaneous reduction of the azido group and acetylation of the resulting amino group (12). The final product was purified by preparative, reverse-phase (C8 column) HPLC.
Fmoc-protected N-acetyllactosamine-β-O-threonine. The procedure for production of the β anomer was identical to that for production of the α anomer, except that the glycosylation of threonine by 2-azido-1-α-bromo-hexa-O-acetyl-2-deoxylactose was carried out at –20°C. The product generated by this procedure was a mixture of the α (90%) and β (10%) anomers, which were readily separated by silica gel flash chromatography by using an ethyl acetate/hexane gradient.
Fmoc-protected Galβ(1→3)GalNAcα-O-threonine. The synthetic procedure was similar to the method used to produce the Fmoc-protected N-acetyllactosamine-α-O-threonine. Fmoc-l-threonine phenacyl ester was glycosylated by the trichloroacetimidate-disaccharide donor in the presence of boron trifluoride diethyl etherate, following the procedure published by Qiu et al. (13) with slight modifications. The conversion of the azido group and the deprotection of the phenacyl ester were identical to the procedures used in the Fmoc-protected N-acetyllactosamine-α-O-threonine synthesis.
General method for glycopeptide synthesis. The glycosylated Fmoc-protected threonine was activated by HATU/HOAt and added to the growing peptide chain in the presence of Hunig's base for a prolonged coupling time (16 h). The glycopeptide was cleaved from the resin with a mixture of trifluoroacetic acid, water, and triisopropylsilane (90:5:5 vol/vol/vol); the solvent was removed in vacuo; and the residue was dried under high vacuum. The crude, dry glycopeptide was dissolved in anhydrous methanol and treated with sodium methoxide powder for 30 min. When HPLC-MS indicated the complete removal of the acetyl groups, the reaction was quenched with acetic acid and evaporated to dryness. The crude deacetylated product was purified by preparative HPLC by using a C8 reverse-phase column.
Sialylation of N-terminal threonine disaccharide residue. The N-acetyllactosamine derivative of the peptide was sialylated enzymatically by using recombinant rat α-2,3(N)sialyltransferase (EMD Biosciences, La Jolla, CA) and CMP-N-acetyl neuraminic acid substrate (Sigma) in 250 mM Mops buffer (pH 7.4). All crude glycopeptides were purified by reverse-phase HPLC on a C8 column, and the purified peptides were analyzed by MS.
Northern Blot Analysis. Total RNA was extracted from explanted bladder epithelial cells from six patients with IC and their age-, race-, and gender-matched asymptomatic controls by using TRIzol/chloroform (GIBCO/BRL) extraction. Equivalent amounts of RNA from each sample were loaded onto a 1% agarose/2% formaldehyde gel, separated by standard gel electrophoresis, and transferred to a nylon membrane. Digoxigenin (DIG)-labeled probe for APF mRNA was prepared by random labeling using the known sequence of nucleotides 1626–1652 (accgtgcccgccgcggtggtggtcgcc) of human frizzled 8 protein (EMBL NM_031866) and the DIG Northern Starter Kit with T7 RNA polymerase (Roche Applied Science, Indianapolis, IN); DIG-labeled probe for β actin mRNA was purchased from Roche Applied Science (prepared by using nucleotides 69–618 of human β actin) (EMBL HSAC07). Blots were developed by chemiluminescence detection with CDP-Star (Roche Applied Science).
Results
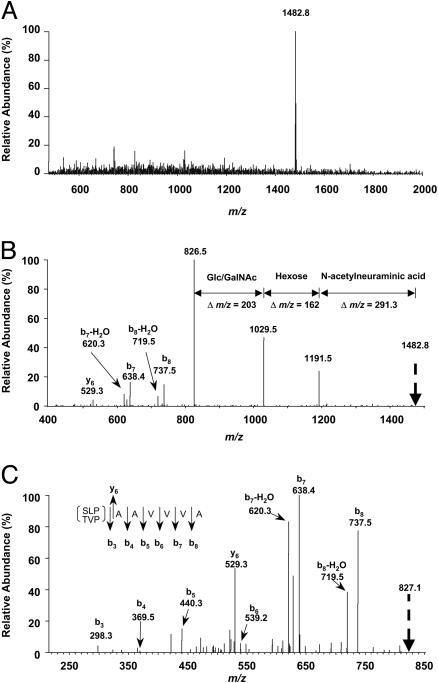
Identification of Amino Acids by MS Analysis. μRPLC was used to obtain extremely pure preparations of APF peptide for MS. Analysis of three preparations of HPLC-purified APF using the microcapillary technique indicated the presence of three peptide peaks in approximately equal proportions in each preparation, only one of which had antiproliferative activity against primary bladder epithelial cells in vitro. Ion trap MS analysis of the active peak indicated a molecular mass of 1,482.8 Da (Fig. 1A). Analysis of peaks generated by collision-induced dissociation of the active substance indicated a terminal sialic acid linked to a hexose moiety, which in turn was linked to an N-acetylhexose moiety (Fig. 1B); detectable amino acid moieties present from the N terminus after further dissociation of the 827-Da peptide moiety included alanine-alanine-valine-valine-valine-alanine (Fig. 1C). The remaining N-terminal amino acids present had a combined molecular mass of 298.3 Da. Search for a gene encoding a homologous peptide indicated 100% homology between a sequence with the appropriate total molecular weight (T-V-P-A-A-V-V-V-A) and amino acids 541–549 in the sixth transmembrane region of frizzled 8, a Wnt ligand receptor (13).
Fig. 1.
Ion trap MS analysis of APF. (A) Molecular mass analysis of the active peak from microcapillary fractionation of HPLC-purified APF. (B and C) Analysis after successive fragmentation of predominant species by collision-induced dissociation.
Identification of Sugar Moieties by Lectin Binding Analysis. To determine the identity and linkage of the hexose and hexosamine moieties, HPLC-purified APF was incubated in its native state with various agarose-conjugated lectins, and the eluates were tested for antiproliferative activity (Table 1). Native APF bound to wheat germ agglutinin and T. mobilensis lectins, but not to a variety of other lectins, confirming the likely presence of a terminal sialic acid residue. Treatment of native APF with a neuraminidase that cleaves sialic acid linked by any of four known linkages (2,3; 2,6; 2,8; or 2,9) did not decrease its biological activity, but did allow subsequent binding of APF to G. simplicifolia I, peanut agglutinin, Jacalin lectin, and E. cristagalli with elution of biologically active toxin. These results indicate the presence of terminal β-galactose, attached either to the 3 position of galactosamine (Galβ1–3GalNAc, core 1 mucin type) or to the 4 position of glucosamine (Galβ1–4GlcNAc, N-acetyllactosamine), with the peanut agglutinin and Jacalin binding favoring the former linkage. Apparent lack of binding of the desialylated APF to V. villosa lectin indicated that the disaccharide moiety remaining did not consist of Galα1–3GalNAc, and lack of binding to G. simplicifolia II confirmed that the GlcNAc moiety was not terminal after removal of sialic acid. The sialic acid was subsequently proven to be linked by means of a 2,3 bond to the galactose moiety by demonstration of binding to G. simplicifolia I and E. cristagalli lectins after digestion with neuraminidase specific for 2,3 bond cleavage. The deduced structure of APF was therefore sialic acid α-2,3 linked to either galactose β1–4 GlcNAc or galactose β1–3 GalNAc, which was in turn O-linked to threonine at the N terminus of the peptide. Isoelectric focusing of the complete sialylated native APF indicated an acidic peptide with an isoelectric point of ≈2.3.
Table 1. Lectin-binding analysis.
| APF binding
|
|||
|---|---|---|---|
| Lectin | Reported specificity | Native | Neuraminidase-treated |
| Wheat germ agglutinin | Sialic acid; terminal GlcNAc | + | ND |
| Tritrichomonas mobilensis | Sialic acid | + | ND |
| Peanut agglutinin | Galβ1-3GalNAc; galactose | - | + |
| Concanavalin A | Mannose, glucose | - | - |
| Lentil lectin | Mannose, glucose; terminal GlcNAc | - | - |
| Vicia villosa | Terminal GalNAc; Galα1-3GalNAc | - | - |
| Griffonia simplicifolia I | Galactose; GalNAc | - | + |
| Griffonia simplicifolia II | Terminal GlcNAc | - | - |
| Jacalin | GalNAc; galactose | - | + |
| Erythrina cristagalli | Galβ1-4GlcNAc; galactose | - | + |
Total Synthesis of APF. Because APF bound to E. cristagalli, and because it is a secreted rather than membrane-bound peptide, we first synthesized GlcNAc linked to threonine by using both α- and β-glycosylated amino acid building blocks for solid phase synthesis of the nonaglycopeptide. A chemoenzymatic approach was taken to construct the complete molecule by means of synthesis of appropriately Fmoc-protected N-acetyllactosamine-threonine glycoamino acids in both configurations. These glycoamino acids were then incorporated into a peptide chain containing the other eight amino acids. Because it was known from the lectin-binding studies that the sialic acid moiety was not necessary for APF activity, both α- and β-N-acetyllactosamine-modified peptides were then assayed for their antiproliferative activity, as well as their ability to regulate specific growth factor production by explanted primary normal bladder epithelial cells.
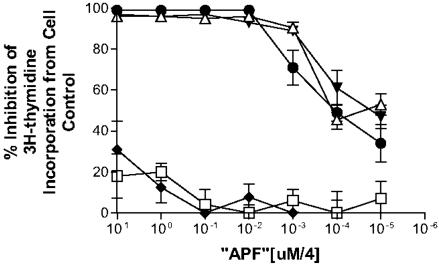
Like native APF, the nonsialylated α anomer of the N-acetyllactosamine derivative was a potent inhibitor of cell proliferation, having an IC50 of ≈0.4 nM; maximal inhibition was possible by using as little as 1 nM of the synthetic toxin (Fig. 2). In comparison, the β anomer of the N-acetyllactosamine derivative and the nonglycosylated peptide had almost no measurable activity. The neuraminic acid unit was therefore then added to the α anomer by using recombinant 2,3-sialyltransferase, and the antiproliferative activity of the sialylated derivative was determined to be similar to the nonsialylated glycopeptide (Fig. 2). The same 1-nM concentration of sialylated or nonsialylated α anomeric synthetic APF that maximally inhibited [3H]thymidine incorporation was also shown to significantly decrease cell counts (data not shown), as well as significantly decrease HB-EGF production and increase EGF production by primary bladder cells (Table 2). APF, as determined by all of these analyses, was therefore an α anomeric sialoglycopeptide.
Fig. 2.
Antiproliferative activity of APF peptide and glycosylated derivatives. Inhibition of primary normal bladder epithelial cell [3H]thymidine incorporation by synthetic APF and its derivatives. Equimolar quantities of the peptide backbone alone (□), N-acetyllactosamine-α-O-Thr derivative (▾), sialylated N-acetyllactosamine-α-O-Thr derivative (▵), N-acetyllactosamine-β-O-Thr derivative (♦), and Galβ1–3GalNAcα-O-Thr derivative (•) were applied to normal bladder epithelial cells, and [3H]thymidine incorporation was determined and compared with incorporation in cells grown in medium containing buffer alone. All specimens were assayed in triplicate in two to three repeated experiments. Data are expressed as the mean inhibition of incorporation, and vertical lines indicate SEM.
Table 2. Effect of native APF vs. synthetic APF and its derivatives on bladder epithelial cell growth factor production.
| HB-EGF, (ng/ml) | EGF, ng/ml | |
|---|---|---|
| Native APF | 0.18 ± 0.25* | 0.49 ± 0.14* |
| Mock APF | 5.24 ± 1.8 | 0.02 ± 0.02 |
| SynthAPF (hexNAc/NANA) | 0.13 ± 0.1† | 0.54 ± 0.12† |
| SynthAPF (hexNAc) | 0.05 ± 0.1† | 0.38 ± 0.01† |
| SynthAPF (peptide alone) | 4.65 ± 0.5 | 0.001 ± 0.002 |
NANA, N-acetylneuraminic acid. *, P < 0.0001 compared with Mock APF; †, P < 0.0001 compared with peptide alone.
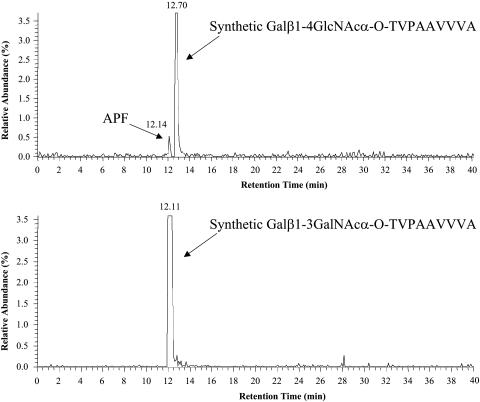
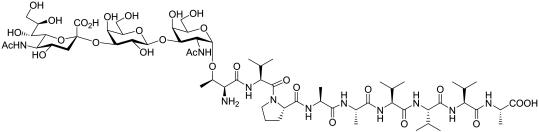
However, whereas the β anomeric form of O-linked GlcNAc linked to threonine has been described in eukaryotic cells, the α anomeric form has not although O-α-GalNAc is commonly found in eukaryotic (primarily mucin-type) cell glycoproteins. We therefore proceeded to synthesize APF containing the truncated core 1-O-linked disaccharide Galβ1–3GalNAc (commonly referred to as the Thomsen Friedenreich or T-antigen) α-O-linked to the N-terminal threonine. As also shown in Fig. 2, this synthetic APF had activity similar to the synthetic compound containing α GlcNAc. μRPLC-MS/MS, in which desialylated native APF was spiked with either the GalNAc-containing or GlcNAc-containing synthetic glycopeptide (Fig. 3), was therefore used to establish the correct structure for APF. Both the native and synthetic GalNAc-containing APF derivatives had an identical retention time, which was readily distinguishable from that of the GlcNAc-containing compound. The correct structure of APF is therefore the GalNAc-containing sialoglycopeptide shown in Fig. 4.
Fig. 3.
μRPLC of native APF and synthetic GlcNAc- and GalNAc-containing derivatives. Neuraminidase-treated APF was injected along with either synthetic Galβ1–4GlcNAcα-O-Thr-containing APF or synthetic Galβ1–3GalNAcα-O-Thr-containing APF, and their relative mobilities on C18 were determined.
Fig. 4.
The structure of APF.
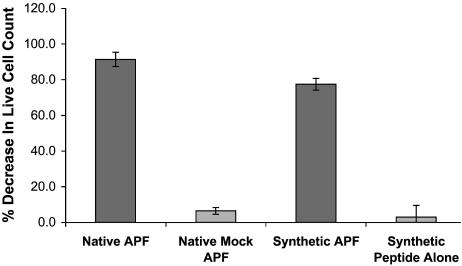
Inhibition of Bladder Cancer Cell Proliferation by Native and Synthetic APF. Additional evidence that the synthetic and native APF were the same was provided by measurement of their antiproliferative activities against a bladder carcinoma T24 cell line, by using live cell count as determined by trypan blue exclusion. As shown in Fig. 5, these cells were sensitive to both native and synthetic APF species at approximately the same concentration.
Fig. 5.
Inhibition of T24 cell proliferation by native and synthetic APF. Native APF (estimated concentration 3–5 μg/ml based on absorbance at 260 nm), an equivalent volume of mock APF, synthetic Galβ1–3GalNAcα-O-Thr-containing APF (3.0 μg/ml glycopeptide), or an equimolar amount of peptide backbone alone were compared for their ability to inhibit cell proliferation by determining live cell count after 48 h by using trypan blue exclusion. All specimens were assayed in triplicate. Data are expressed as the mean decrease in cell count, and vertical lines indicate SEM.
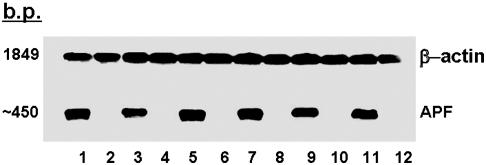
Identification of APF mRNA by Northern Blot Analysis. Northern blot analysis of mRNA extracted from the explanted bladder epithelial cells of six IC patients and six normal controls was performed to provide additional evidence that APF was a frizzled 8-related peptide. As shown in Fig. 6, a small (≈450 bp) mRNA species that bound to a probe encoding the nonapeptide was present only in the extracts of all six cell explants from patients with IC previously shown to produce APF, but not in extracts of any explanted cells from age-, race-, and gender-matched controls that did not produce APF. Although a faint band was also seen at ≈3,100 bp in IC but not control specimens on three of five experiments, it was not detectable in the other two experiments, and the predominant band was at 450 bp each time. However, no band was seen at the size of full-length mRNA for human frizzled 8 (4 kb), and no bands were seen for control cells in any experiment. In comparison, all cells from both groups seemed to produce similar amounts of β-actin mRNA.
Fig. 6.
Northern blot analysis of mRNA encoding the APF nonapeptide. Total RNA was extracted from explanted bladder epithelial cells from six patients with IC (lanes 1, 3, 5, 7, 9, and 11) and their age-, race-, and gender-matched asymptomatic controls (lanes 2, 4, 6, 8, 10, and 12). The membrane was then incubated with digoxigenin-labeled probes for APF mRNA and β-actin mRNA and developed by using a chemiluminescent substrate.
Discussion
The reported results demonstrate that the APF made specifically by bladder epithelial cells explanted from patients with IC is a uniquely modified frizzled 8-related sialoglycopeptide with a peptide structure that bears 100% homology to the sixth transmembrane segment of this G protein-coupled Wnt ligand receptor (14). Whether this small secreted frizzled-related peptide is normally expressed in human bladder epithelial cells during embryogenesis, or whether it is an abnormal variant of a frizzled protein that is produced only by bladder epithelial cells from these patients, is unknown. However, its specificity for this disorder, as well as its identification as a secreted frizzled-related peptide growth inhibitor, lends credence to its potential role in the pathogenesis of this disease. Identification of APF also allows for the possible development of treatment for IC based on inhibition of APF activity. In addition, exclusive expression of APF in adults by bladder epithelial cells from IC patients should allow for development of the first direct noninvasive diagnostic test for this disorder.
To date, two disease states have been noted to be associated with abnormally increased expression of secreted frizzled-related proteins other than APF: overload-induced heart failure, in which mRNA for two such proteins have been shown to be elevated in failing ventricles as compared with control hearts (15), and degenerative retinal disease, in which a secreted frizzled-related protein and its mRNA expression are greatly elevated (16). The previously identified secreted frizzled-related proteins contain a cysteine-rich extracellular domain, allowing them to inhibit Wnt signaling by one of two mechanisms: by binding to the Wnt ligand, or by forming nonsignaling dimers with frizzled receptors (17). APF is therefore the first frizzled-related growth inhibitor that bears homology only to a transmembrane portion of a frizzled receptor, suggesting that growth inhibition by frizzled-related proteins may also occur by means of additional mechanisms. APF is also the smallest secreted frizzled-related peptide identified to date, with previously described frizzled-related peptides having molecular masses of 33.5–39.9 kDa (18).
Microarray analysis previously indicated that APF decreases the expression of several genes, including Jun N-terminal kinase in bladder epithelial cells (8), suggesting the possibility that APF may interfere with noncanonical Wnt signaling mediated by means of a frizzled receptor protein (19). However, linkage of the cytoskeleton to its substratum includes binding of the actin–catenin complex to E-cadherin, a protein whose expression has also been shown to be significantly up-regulated by APF in bladder epithelial cells (8), and which is known to inhibit canonical Wnt signaling in both urothelial carcinoma and normal urothelial cells in vitro (20). Further studies are needed to determine whether APF mediates its antiproliferative effects by means of inhibition of canonical or noncanonical Wnt pathway(s) in either bladder carcinoma or normal bladder cells. The extent to which the proliferation of various bladder tumor cells can be negatively regulated by the APF, both in vitro and in vivo, also needs to be determined to understand its utility as a potential therapeutic agent against bladder carcinoma.
Although other potent sialylated small glycopeptide growth inhibitors have been isolated from serum or brain of normal mammals and shown to inhibit proliferation of both normal and malignant cells from a variety of tissues (21, 22), no structural data are available for any of these natural growth inhibitors, and their relationship to frizzled-related proteins or any specific signaling pathway(s) has not been determined. APF is therefore the first of this class of growth inhibitors to be completely characterized, as well as the first to be synthesized. The requirement of the α-O-linked N-acetylhexosamine structure for anti-proliferative activity and regulation of growth factor production by APF suggests that APF may bind to a specific cellular receptor(s), as suggested for another sialoglycopeptide growth inhibitor (23). The extremely hydrophobic nature of the peptide moiety of APF further suggests the possibility that it may interact with the plasma membrane and expose a distinct conformation of the sugar moiety at the cell surface. However, the specific cell component(s) with which APF interacts to mediate its potent inhibition of cell proliferation remains to be elucidated.
Acknowledgments
We thank Toby Chai (University of Maryland School of Medicine) for providing bladder biopsy specimens, Arnold Kreger for assistance with isoelectric point determination, David Grkovic for technical assistance, and Nadya Tarasova and Terry Copeland for helpful discussions. This work was supported in part by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK52596), Merit Review Funding from the Department of Veterans Affairs, and the Interstitial Cystitis Association/Fishbein Foundation. This project was also funded in part by the National Cancer Institute, National Institutes of Health, under Contract NO1-CO-12400. We acknowledge the assistance of the Biophysics Resource, Structural Biophysics Laboratory, Center for Cancer Research, National Cancer Institute.
Abbreviations: APF, antiproliferative factor; EGF, epidermal growth factor; HB-EGF, heparin-binding EGF-like growth factor; μRPLC, microcapillary reversed-phase liquid chromatography; Fmoc, 9-fluorenylmethoxycarbonyl.
References
- 1.Curhan, G. C., Speizer, F. E., Hunter, D. J., Curhan, S. G. & Stampfer, M. J. (1999) J. Urol. 161, 549–552. [PubMed] [Google Scholar]
- 2.Johansson, S. L. & Fall, M. (1990) J. Urol. 143, 1118–1124. [DOI] [PubMed] [Google Scholar]
- 3.Skoluda, D., Wegner, K. & Lemmel, E.-M. (1974) Urologe 13, 15–23. [PubMed] [Google Scholar]
- 4.Tomaszewski, J. E., Landis, J. R., Russack, V., Williams, T. M., Wang, L. P., Hardy, C., Brensinger, C., Matthews, Y. L., Abele, S. T., Kusek, J. W., et al. (2001) Urology 57, 67–81. [DOI] [PubMed] [Google Scholar]
- 5.Keay, S., Zhang, C.-O., Shoenfelt, J., Erickson, D. R., Whitmore, K., Warren, J. W., Marvel, R. & Chai, T. (2001) Urology 57, 9–14. [DOI] [PubMed] [Google Scholar]
- 6.Keay, S., Kleinberg, M., Zhang, C.-O., Hise, M. K. & Warren, J. W. (2000) J. Urol. 64, 2112–2118. [PubMed] [Google Scholar]
- 7.Keay, S., Zhang, C.-O., Shoenfelt, J. L. & Chai, T. C. (2003) Urology 61, 1278–1284. [DOI] [PubMed] [Google Scholar]
- 8.Keay, S., Seillier-Moiseiwitsch, F., Zhang, C.-O., Chai, T. C. & Zhang, J. (2003) Physiol. Genomics 14, 107–115. [DOI] [PubMed] [Google Scholar]
- 9.Keay, S., Zhang, C.-O., Hise, M., Trifillis, A. L., Hebel, J. R., Jacobs, S. C. & Warren J. W. (1996) J. Urol. 156, 2073–2078. [PubMed] [Google Scholar]
- 10.Keay, S., Zhang, C.-O., Hise, M. K., Hebel, J. R., Jacobs, S. C., Gordon, D., Whitmore, K., Bodison, S., Gordon, N. & Warren, J. W. (1998) Urology 52, 974–978. [DOI] [PubMed] [Google Scholar]
- 11.Leuck, M. & Kunz, H. (1997) J. Prakt. Chem./Chem.-Ztg. 339, 322–334. [Google Scholar]
- 12.Svarovsky, S. A. & Barchi, J. J., Jr. (2003) Carbohydr. Res. 338, 1925–1935. [DOI] [PubMed] [Google Scholar]
- 13.Qiu, D., Gandhi, S. S. & Koganty, R. R. (1996) Tetrahedron Lett. 37, 595–598. [Google Scholar]
- 14.Saitoh, T., Hirai, M. & Katoh, M. (2001) Int. J. Oncol. 18, 991–996. [DOI] [PubMed] [Google Scholar]
- 15.Schumann, H., Holtz, J., Zerkowski, H. R. & Hatzfield, M. (2000) Cardiovasc. Res. 45, 720–728. [DOI] [PubMed] [Google Scholar]
- 16.Jones, S. E., Jomarcy, C., Grist, J., Stewart, H. J. & Neal, M. J. (2000) NeuroReport 11, 3963–3967. [DOI] [PubMed] [Google Scholar]
- 17.Bafico, A., Gazit, A., Pramila, T., Finch, P. W., Yaniv, A. & Aaronson, S. A. (1999) J. Biol. Chem. 274, 16180–16187. [DOI] [PubMed] [Google Scholar]
- 18.Jones, S. E., Jomacy, C. (2002) BioEssays 24, 811–820. [DOI] [PubMed] [Google Scholar]
- 19.Pandur, P., Maurus, D., Kuhl, M. (2002) BioEssays 24, 881–884. [DOI] [PubMed] [Google Scholar]
- 20.Thievessen, J., Seifert, H. H., Swiatkowski, S., Florl, A. R. & Schulz, W. A. (2003) Br. J. Cancer 88, 1932–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auger, G., Blanot, D., van Heijenoort, J., Nadal, C., Gournay, M. F., Winchenne, J. J., Boffa, G. A., Lambin, P., Maes, P. & Tartar, A. (1989) J. Cell Biochem. 40, 439–451. [DOI] [PubMed] [Google Scholar]
- 22.Moos, P. J., Fattaey, H. K. & Johnson, T. C. (1995) J. Cell. Biochem. 59, 79–90. [DOI] [PubMed] [Google Scholar]
- 23.Sharifi, B. G. & Johnson, T. C. (1987) J. Biol. Chem. 262, 15752–15755. [PubMed] [Google Scholar]