Abstract
We characterize the survival, migration, and differentiation of human neurospheres derived from CNS stem cells transplanted into the ischemic cortex of rats 7 days after distal middle cerebral artery occlusion. Transplanted neurospheres survived robustly in naive and ischemic brains 4 wk posttransplant. Survival was influenced by proximity of the graft to the stroke lesion and was negatively correlated with the number of IB4-positive inflammatory cells. Targeted migration of the human cells was seen in ischemic animals, with many human cells migrating long distances (≈1.2 mm) predominantly toward the lesion; in naive rats, cells migrated radially from the injection site in smaller number and over shorter distances (0.2 mm). The majority of migrating cells in ischemic rats had a neuronal phenotype. Migrating cells between the graft and the lesion expressed the neuroblast marker doublecortin, whereas human cells at the lesion border expressed the immature neuronal marker β-tubulin, although a small percentage of cells at the lesion border also expressed glial fibrillary acid protein (GFAP). Thus, transplanted human CNS (hCNS)-derived neurospheres survived robustly in naive and ischemic brains, and the microenvironment influenced their migration and fate.
Stroke is a leading cause of death and disability (1), but, despite intensive research, few treatment options exist. Here, we present our investigation of the potential for human stem cell transplantation to repair the ischemic brain. Fetal brain tissue transplants have been shown to produce some recovery in animal models of stroke (2–4), but ethical considerations and a short supply of human fetal tissue limited this approach. More recently, a host of cell types have been tested in stroke models, including human bone marrow cells (5–7), human umbilical cord blood cells (5, 8), rat trophic factor-secreting kidney cells (2, 5), and immortalized cell lines such as the human neuron-like NT2N (hNT) cells (9, 10) and MHP36, an embryonic murine immortalized neuroepithelial cell line (11, 12). In most cases some index of behavioral function has been improved. hNT cells have also been used in clinical trials, and preliminary observations suggest that cell transplantation may be a viable stroke therapy (2, 13).
Few studies have assessed the behavior of normal human CNS stem cells after transplantation. Studies in animal models of Parkinson's disease (PD) and Huntington's disease (HD) show that transplanted human neural precursor cells survive and differentiate into neurons and glia (14–16). Functional studies with such human cells have not been reported in these animal models, but primary fetal human tissue is known to ameliorate the symptoms of PD (17, 18), findings that have been replicated to some extent in clinical trials (19). Recently, Jeong et al. (20) reported that human neural precursors delivered intravenously were beneficial in an animal model of stroke. In our study, human neurospheres derived from fetal CNS were transplanted directly into the cortex after cerebral ischemia. These human CNS (hCNS)-derived cells are purified by antibody-based cell sorting and expanded through several passages, resulting in a well defined, renewable cell bank, an essential prerequisite for any cell replacement therapy to be practical. These sorted/expanded human cells survive in nonobese diabetic (NOD)-severe combined immunodeficient (SCID) mice, and migrate and differentiate in a site-appropriate fashion into neurons, astrocytes, and oligodendrocytes (21, 22). The extensive cell death and massive inflammatory response of the ischemic brain make it a more hostile environment for cell grafts than uninjured brains. It is important to determine how hCNS-derived neurospheres respond to such an environment to assess their therapeutic potential for stroke. We examined the ability of these hCNS-derived neurospheres to survive, migrate, and differentiate after transplantation into the cortex 7 days after focal cortical ischemia.
Experimental Procedures
Generation of hCNS-Derived Neurospheres. hCNS-stem cells were isolated by flow cytometry from fetal brain tissue (16–20 wk) as described (21, 22). CD133+CD24-/lo cells were plated at 105 cells/ml in human neurosphere culture media (X-VIVO 15 medium, BioWhittaker), N-2 supplement (GIBCO), and 0.2 mg/ml heparin supplemented with basic fibroblast growth factor (b-FGF, 20 ng/ml), epidermal growth factor (EGF, 20 ng/ml), and leukemia inhibitory factor (LIF, 10 ng/ml). Neurospheres form after 10–14 days in culture. They were passaged every 10–14 days by enzymatic dissociation into single cells by using collagenase (0.5 mg/ml in PBS containing 0.1% human serum albumin) for 5–10 min. Cells were taken from frozen stocks, thawed, and passaged at least once before transplantation (between passage six and nine). One or 2 days before transplantation, neurospheres were enzymatically dissociated into single-cell suspensions and cultured in fresh neurosphere media. On the day of transplantation, cells were harvested and counted, their viability was determined, and they were resuspended at a density of 105 cells per μl. The cells were enumerated by collagenasing a small aliquot of the harvested cells on the day of transplantation; transplanted cells were a combination of single cells and small clusters.
Distal Middle Cerebral Artery Occlusion (dMCAo). All animal procedures were approved by the Stanford University Administrative Panel on Laboratory Animal Care. Adult male Sprague–Dawley rats (320 ± 10 g) were anesthetized with isoflurane, and the distal MCA was exposed through a burr hole between the left eye and ear, cauterized, and cut just above the rhinal fissure (see ref. 23). Common carotid arteries were exposed by means of a ventral midline incision and occluded for 1 h. The suture was removed, the wound was closed, and the animals were allowed to recover. Physiological parameters [electrocardiogram (ECG), core temperature, respiratory rate] were monitored and recorded throughout.
Cell Transplantation. Cells were transplanted under anesthesia 7 days post-dMCAo when lesion sizes were relatively stable, allowing targeting of the peri-infarct by using predetermined coordinates. Also, the acute release of products of tissue damage and death should have subsided at 7 days, providing a potentially less hostile graft environment (24). Rats were given three 1.0-μl deposits of suspended cells (1 × 105 cells per μl, n = 13, or 1.0 μl of buffer vehicle, n = 12) along the anterior-posterior axis into the cortex at these coordinates: (i) anterior–posterior (A–P), +1; medial–lateral (M–L), -1.2; dorsal–ventral (D–V), -2.7; (ii) A–P, -0.26; M–L, -1.2; D–V, -2.6; (iii) A–P, -1.8; M–L, -1.0; D–V, -1.8. Deposits were delivered at 0.5 μl/min by an infusion pump. The needle was left in situ for 2 min postinjection before being slowly removed.
Cyclosporine A Treatment. Rats were immunosuppressed by daily i.p. injection of cyclosporine A (CsA, 10 mg/kg) from 6 to 35 days post-dMCAo.
Tissue Processing. Four weeks posttransplantation, rats were transcardially perfused with heparinized saline (0.5%) and fixed with 4°C paraformaldehyde (PFA, 3%). Brains were left in the skull overnight in 3% PFA at 4°C, then transferred to 20% sucrose/3% PFA. Five equidistant sets of coronal sections (40 μm thick) were prepared on a cryostat and stored in cryoprotectant at -20°C until required.
Lesion Size. Because very little ischemic tissue remains 5 wk after dMCAo, we estimated lesion size as a percentage of the whole brain by using the following: [(area of contralateral hemisphere) - (area of remaining ipsilateral hemisphere)/(area of contralateral hemisphere) × 100/2]. The area of both hemispheres was measured on eight serial coronal sections per brain (200 μm apart) stained with hematoxylin and eosin, and the area of the infarct was averaged over these eight levels.
Immunohistochemical Staining. Free-f loating sections were blocked in PBS/1% BSA containing 5% normal serum of the species in which the secondary antibody was raised. Primary antibodies were diluted in PBS containing 1% BSA and the same serum used in the block (5%). Human cells were labeled with an antibody specific to human cytoplasm (SC121, StemCells) that has been shown to react with >90% of human neural cells and does not crossreact with rat cells (unpublished results). Endogenous peroxidase activity was quenched with H2O2 (0.3%), and sections were blocked for 30 min and incubated overnight at 4°C with SC121 (1:3,000). Sections were washed and incubated with biotinylated secondary antibody (Vector Laboratories) for 30 min, washed, incubated with an avidin-biotin-peroxidase complex (30 min, ABC, Vectastain Elite; Vector Laboratories), and visualized with the chromogen Vector NovaRED (Vector Laboratories). Isolectin B4 (IB4; Sigma) was used to detect inflammatory cells of monocytic lineage (macrophages, microglia, and monocytes). This staining differed slightly; sections were treated with H2O2, blocked in serum, and incubated with peroxidase-labeled IB4 (10 μg/ml) for 3 h at room temperature and colorized with diaminobenzidine (DAB).
Immunofluorescence Staining. Triple-label immunofluorescence staining was carried out on coronal sections adjacent to those used for immunohistochemistry. Sections were labeled with biotinylated SC121, Hoechst dye to label nuclei (1:1,000, Molecular Probes), and either anti-β-tubulin III (Tuj-1, 1:2,000, Covance Research Products, Berkeley, CA), anti-glial fibrillary acid protein (GFAP, 1:800, Advanced ImmunoChemicals, Long Beach, CA), anti-doublecortin (Dcx, 1:100, Santa Cruz Biotechnology), anti-NG2 chondroitin sulfate proteoglycan (NG2, 1:30, Chemicon), or anti-CXCR4 (CD184, 1:100, BD Pharmingen). Sections were blocked as above and incubated overnight at 4°C with primary antibodies. After washes in PBS, sections were incubated with fluorescence-conjugated secondary antibodies for 1 h (goat-anti-rabbit Cy3, 1:2000, Jackson ImmunoResearch, or Alexa Fluor 568 or streptavidin-488, 1:500, Molecular Probes). Fluorescence-labeled sections were viewed under a confocal microscope.
Unbiased Stereology and Cell Counting. Transplanted human and IB4-positive cells were counted by using unbiased computational stereology [fractionator method, using stereoinvestigator software (MicroBrightfield, Brattleboro, VT)]. Human cells were counted in the well defined bolus and in the migrating population outside the bolus. The bolus population was estimated from hematoxylin and eosin-stained sections spaced 200 μM apart. Purple hematoxylin-stained nuclei were counted; large, yellow/brown inflammatory or blood cells were not counted. We confirmed that the counts were representative by counting several adjacent sections stained with an antibody to human nuclei. Migrating cells were counted on every tenth SC121-stained section (400 μm apart). The densely packed cells in the bolus could not be discerned from one another when stained with SC121. The density of cells migrating from the graft toward or away from the lesion was measured by counting the cells found in a 150-μm wide strip lying 60 μm either side of the graft and running the graft's length. IB4-positive cells in the ischemic cortex were estimated over eight coronal sections (200 μm apart, sections adjacent to those used to measure lesion size). Human cell counts were corrected by using the Abercrombie correction factor (nuclear diameter was 4.49 μm, determined from measurements of >30 human cell nuclei in our stained sample). The proportion of SC121-labeled cells also stained with lineage-specific phenotype markers was determined by confocal microscopy. Split panel and z axis analysis were used for all counting. One hundred or more SC121-positive cells were scored for each marker (Tuj-1 and GFAP) per animal.
Statistics. All means are presented ± SEM. Correlations were computed with the Pearson correlation test by using instat and prism (GraphPad, San Diego). A Poisson regression analysis by using “proc genmod” in sas (SAS Institute, Cary, NC) was performed on the cell migration data.
Results
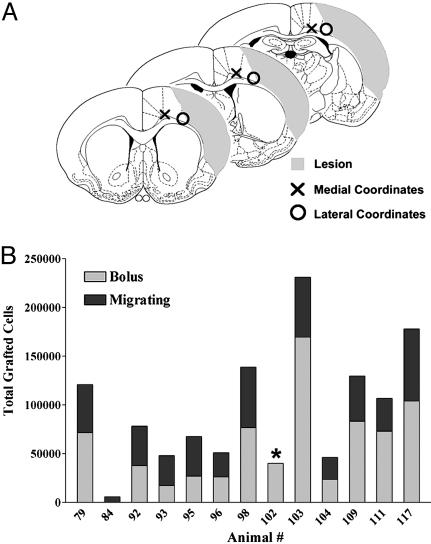
hCNS-Derived Neurospheres Survive in Ischemic Cortex. Rats received three deposits of hCNS-derived neurospheres along the anterior-posterior axis of the ipsilateral cortex 7 days after dMCAo (Fig. 1A). Four weeks later, brains were stained with a human-specific antibody (SC121) to identify the transplanted cells. Few cells survived when they were transplanted close to the lesion cavity (Fig. 1A, lateral coordinates); only one of nine rats had many cells survive at coordinates immediately adjacent to the lesion (data not shown). Cells survived robustly when transplanted more medially into nonischemic tissue adjacent to the lesion; on average 100,147 ± 28,944 (33.4 ± 6.1% of the initial 300,000) cells survived at these coordinates (Fig. 1). There was no correlation between the number of surviving cells and the distance from the lesion in these more medial transplants (data not shown; the distance between the cells and lesion varies due to variation in lesion size). Thus, cells were able to survive near the lesion as long as they were transplanted into nonischemic tissue.
Fig. 1.
Survival of hCNS-derived neurospheres in the ischemic brain. (A) Schematic of lesion (shaded area) and transplant coordinates. The three sections represent the three levels on the A-P axis where cells were transplanted. Cells transplanted close to the lesion core (○) showed reduced survival; cells transplanted more medially (×) further from the lesion showed robust survival. The medial coordinates were used for this study. (B) Quantitation of the number of surviving cells in rats subjected to dMCAo 4 wk posttransplant. The gray bar indicates the number of cells in the graft bolus; the black bar represents the number of cells migrating from the graft (the asterisk denotes that the migrating population was not counted in this animal). On average, 100,147 ± 28,944 cells survived.
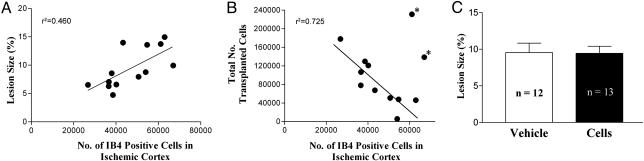
Lesion Size, Inflammatory Response, and Cell Survival. Adjacent sections were stained with hematoxylin and eosin to measure lesion size or IB4 to detect inflammatory cells of monocytic lineage. The number of IB4-positive cells in the ischemic cortex of transplanted rats directly correlated with lesion size (Fig. 2A; P = 0.011 by Pearson correlation test). In 10 of 12 rats, there was a strong negative correlation between the numbers of IB4-positive cells and surviving transplanted cells (Fig. 2B; P = 0.002 by Pearson correlation test). Lesion sizes did not differ significantly between transplanted and buffer-treated rats 4 wk after transplantation (Fig. 2C).
Fig. 2.
Lesion size and inflammatory response. (A) IB4-positive inflammatory cells in the cortex of cell-transplanted rats were counted in eight sections adjacent to those used to measure lesion size. Lesion size was directly correlated with the number of IB4+ cells. (B) In 10 of 12 cell-transplanted rats, the total number of surviving human cells (i.e., cells in the graft bolus plus migrating cells) was negatively correlated with the number of IB4-positive cells. An asterisk indicates animals excluded from the correlation. (C) There was no difference in lesion area in cell-treated and buffer-treated rats. Lesion area (mean ± SEM of 12 or 13 animals) was measured over eight levels per brain to get a total lesion area per animal.
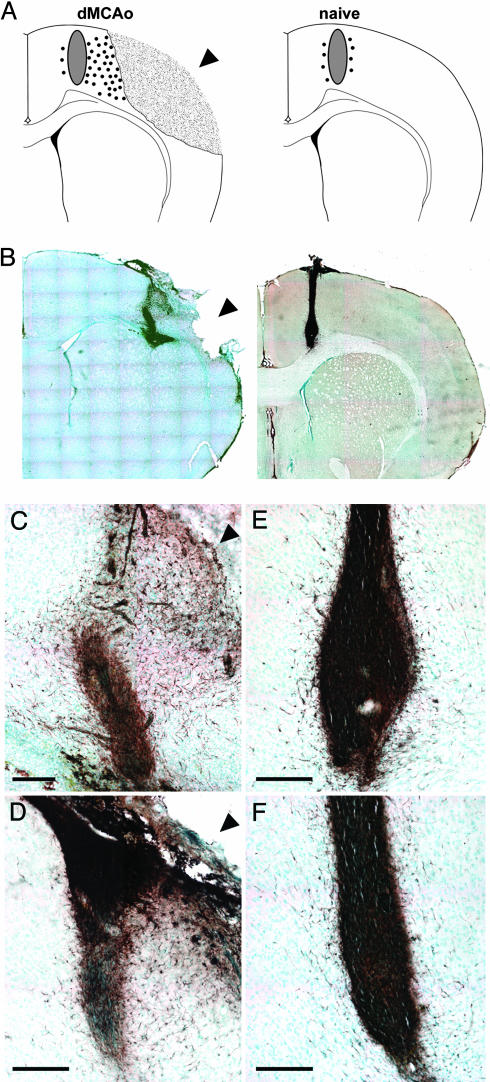
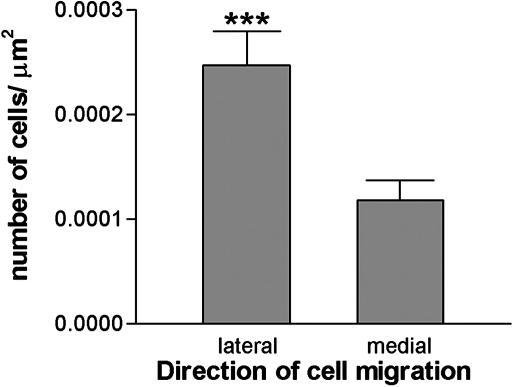
Human Cells Migrate Toward the Ischemic Lesion. Immunostaining with SC121 revealed extensive migration of human cells toward the lesion (up to 1.2 mm), mainly through the cortex (Fig. 3 A–D). Significantly more cells migrated from the graft toward the lesion than in the opposite direction, toward the midline (Fig. 4, P < 0.0001). In naive rats, cells migrated in smaller numbers over short distances (≈0.2 mm) in a radial fashion (Fig. 3).
Fig. 3.
Migration of transplanted human cells. (A) Schematic showing extensive migration of human cells (small circles) toward the lesion in dMCAo brains compared to very little migration away from the graft (gray oval) in naive brains. (B) 4wk posttransplant, sections were labeled with the human-specific antibody SC121, to identify human cells, and visualized with diaminobenzidine (DAB) (brown). Low magnification images depict the cells migrating long distances primarily toward the lesion in dMCAo brains. Cells transplanted into naive rats migrated little and equally in all directions. Shown are higher-magnification images of dMCAo (C and D) and naive (E and F) brains. Arrowhead indicates lesion. (Scale bar = 200 μM.)
Fig. 4.
Quantification of the human cell migration in dMCAo brains. Human cells stained with the human-specific antibody SC121 4 wk posttransplant in dMCAo brains were counted by using unbiased stereology. The number of cells migrating from the graft toward the lesion (laterally) was greater than the number migrating away from the lesion (medially) (***, P < 0.0001). Data are means ± SEM from 11 animals.
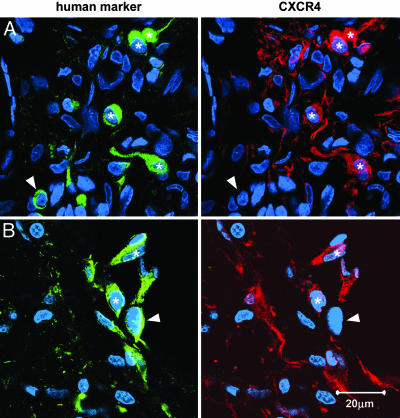
Human Cells Express the Chemokine Receptor CXCR4. Double labeling with human-specific SC121 and an anti-CXCR4 antibody revealed a subset of cells at the lesion border and some migrating cells that expressed the chemokine receptor CXCR4 (Fig. 5).
Fig. 5.
Transplanted human cells express the chemokine receptor CXCR4. Shown are confocal images from lesioned brains of human cells labeled with the human-specific antibody SC121 (green) and an anti-CXCR4 antibody (red); nuclei are stained with Hoechst (blue). (A) Representative image of migrating human cells. (B) Representative image of human cells at the lesion border. An asterisk indicates double-labeled cells; arrowhead indicates human cells that did not express CXCR4.
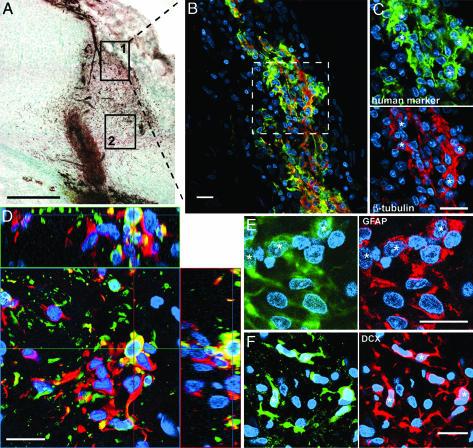
Migrating Human Cells Express Lineage-Specific Phenotype Markers. Triple labeling with SC121, a nuclear label (Hoechst) and lineage markers (below) were imaged by confocal microscopy. The marker profile of the cells depended on their location relative to the lesion. A large proportion (49 ± 5%) of human cells near the lesion border expressed the immature neuronal marker β-tubulin III (Fig. 6 A–D); a smaller proportion (14 ± 7%) expressed GFAP (Fig. 6E), a marker of astrocytes or undifferentiated precursors (25). Most of these cells were near the lesion (0–60 μm), but some were further away (150 μm). Cells between the graft and lesion border rarely expressed β-tubulin or GFAP but did express the migrating neuroblast marker Dcx (Fig. 6F). No human cells, not even those in white matter, labeled with the oligodendrocyte precursor marker NG2 (data not shown); this antibody did recognize rat cells and has also been shown to label the human cells in vitro. Thus, the cells' microenvironment seems to influence their differentiation, and the majority of cells migrating from the graft were neuronal, not astrocytic, precursors. Marker profiles in naive rats could not be assessed because cells were too densely clustered within the graft bolus to distinguish individual phenotypes.
Fig. 6.
Marker profile of human cells depends on proximity to the lesion. (A) Immunostaining of human cells (brown) with human-specific antibody SC121. Boxes show the location of the cells in the other panels. (Scale bar = 500 μm.) (B–F) Confocal images: human cells labeled with SC121 are green, nuclei stained with Hoechst are blue, and various phenotypic markers are in red. An asterisk denotes double-labeled cells. (Scale bar = 20 μM.) (B) Many human cells at the lesion border (box 1 in A) express β-tubulin III. (C) Higher magnification of demarked area in B.(D) Orthogonal view to confirm double labeling with the human marker and β-tubulin III. (E) Cells at the lesion border also express GFAP. (F) The migrating cells (box 2 in A) express doublecortin.
Discussion
Here, we demonstrate that intracerebral grafts of purified, unmodified, hCNS-derived neurospheres survive in the ischemic brain of adult rats. Targeted migration toward the lesion was observed, and most migrating cells had a neuronal phenotype. Similar human neural progenitor cells have been used in animal models of PD (15, 16) and HD (14), and delivered intravenously in animal models of stroke (20, 26). Compared with a PD or HD brain, the massive cell death and inflammatory response in the ischemic brain creates a more hostile environment for transplanted cells, an assertion supported by our finding of a negative correlation between the inflammatory response and cell survival. Showing that grafts remain viable in this model is an important step toward assessing the potential of these cells to ameliorate the damage caused by stroke in humans
Cell Survival. Graft placement relative to lesion was a critical determinant of cell survival. Cells deposited close to the lesion edge did not survive well, but transplants survived robustly when located more medially, further from the lesion. Cell survival within this more medial group did not correlate with the distance of the graft from the lesion. This finding suggests that there is no local gradient of factors affecting survival but rather that the hCNS-derived neurospheres can survive if they are transplanted into nonischemic tissue.
The negative correlation of cell survival to lesion size and IB4-positive cells suggests that inflammatory cytokines are detrimental to the transplanted cells. This suggestion is supported by work of Lindvall and colleagues (27), who show that newborn neurons in the adult rat are endangered by inflammatory cytokines and that their survival can be enhanced by blocking inflammation with minocycline. Given that the magnitude of the inflammatory response and the types of cytokines present change with time after ischemia (24), these data imply that the timing of the transplant could significantly influence graft survival, with greater survival predicted if cells are transplanted when inflammation has subsided.
Cell Migration. In naive brains, transplanted cells migrated little (≈0.2 mm) in 4 wk. In ischemic brains, cells migrated extensively (up to 1.2 mm) primarily toward the lesion through the cortex. Similar findings have been reported by others; human neural precursors injected into the tail vein homed to sites of damage in the stroke brain (20) and migration toward a site of injury or pathology was also reported with mouse neural and embryonic stem cells and with conditionally v-myc immortalized mouse neuroepithelial stem cells transplanted intracerebrally (11, 28, 29). Whether this targeted migration is due to a loss of repulsive cues normally found in the parenchyma or to chemoattractant signals such as cytokines is not known. Although several mechanisms may be involved, the inflammatory response may play an important role (30). Many migrating cells and cells at the lesion border expressed the chemokine receptor CXCR4 (31), suggesting that many transplanted cells have the potential to respond to a chemokine signal. It is of note that a CXCR4 antagonist was reported to inhibit the migration of SVZ-derived neurospheres in vitro (31). Not all of our migrating human cells expressed CXCR4, however. Perhaps the receptor was down-regulated on these CXCR4-negative cells, or these cells may be a neural subset that express different chemokine receptors, responding to other chemokines. Alternatively, these cells may never have expressed a chemokine receptor, which would imply a chemokine-independent mechanism of migration.
Human neural precursor cells did not migrate extensively in rodent models of PD or HD (14, 15). PD and HD lesions are much smaller than the present ischemic infarcts, so migratory signals may be weaker. However, migration potential may be, in part, an intrinsic property of the cell. Svendsen et al. (16) found that changing culture conditions or transplanting cells as neurospheres rather than dissociated cells enhanced their migration in a PD brain. Zhang et al. (32) also observed that how SVZ progenitors were grown influenced their ability to migrate through nonneurogenic regions. Interestingly, the migration observed by Svendsen et al. at 20 wk posttransplant was not targeted, but was a more radial migration similar to that seen when human neurospheres were transplanted into a nonneurogenic region of naive brains (33, 34). Together, these data suggest that there are two types of migration: a short-distance, radial diffusion-like migration influenced perhaps by intrinsic properties of the cells, and a targeted long-distance migration dependent on environmental signals emanating from injury.
Cell Differentiation. The marker profile of the transplanted cells seemed to be location-dependent. Human cells near the lesion border were more likely to express the immature neuronal marker β-tubulin than the astrocytic or undifferentiated progenitor marker GFAP, whereas migrating cells that had not reached the lesion expressed doublecortin (35). Modo et al. (11) obtained similar results with conditionally immortalized mouse neuroepithelial cells transplanted into the striatum of the ischemic brain. None of our cells expressed the oligodendrocyte marker NG2 at this time point; this finding is consistent with the results of Wennersten et al. (36), who transplanted human neural progenitors into a rat model of cortical trauma. However, we (N.U., unpublished data) and others (37) have shown that hCNS-derived neurospheres or other neural progenitors can become oligodendrocytes that myelinate axons of the myelin-deficient shiverer mouse. Our finding that the majority of the cells that migrated away from the graft were immature neurons is in contrast to studies in which human neurospheres transplanted into nonneurogenic regions (e.g., cortex or striatum) of naive or PD brains remained immature or became astrocytes (15, 16, 22, 33, 34). In these studies, some neurons were observed but close to the graft core; the migrating cells were mostly GFAP+. The tendency for our migrating cells to express neuronal markers may reflect the influence of the microenvironment of the ischemic brain, which may enhance neurogenesis in tissue that would normally give rise to glia. Alternatively, different cells in a heterogeneous hCNS-derived neurosphere graft such as ours may have different migration potential, and the ischemic lesion may attract cells that are more likely to become neurons. It is probably an interplay between cell microenvironment and intrinsic cell properties that determines differentiation fate (38); this finding would explain why some cells types transplanted into the striatum become mainly GFAP+ (15, 33, 34) whereas others give rise to mostly neurons (14, 39), and also why the same cells transplanted into different brain regions have different differentiation profiles (33, 34, 38, 40).
Conclusion
Clearly, the brain microenvironment has a profound influence on the survival, migration, and differentiation of hCNS-derived neurospheres. It will be interesting to characterize the factors that affect these parameters. That the brain can affect the cells is not disputed. The question that remains is: Can the cells influence the host brain? Grafted cells ultimately must aid functional recovery. Transplantation of other cell types has enhanced recovery of motor behavior after focal ischemia (7–11). The next step will be to test the effect of our hCNS-derived neurospheres on recovery of motor function after dMCAo in rats.
Acknowledgments
We thank Monika Dohse for help with immunostaining, Beth Hoyte and Dave Schaal for help preparing the manuscript, Pak Chan for scientific discussion, and Puja Kohli, Caleb Stokes, and Milton Little for technical assistance. This work was supported in part by National Institutes of Health National Institute of Neurological Disorders and Stroke Grant P01 NS37520 (to G.K.S.).
Abbreviations: hCNS, human CNS; dMCAo, distal middle cerebral artery occlusion; PD, Parkinson's disease; HD, Huntington's disease; GFAP, glial fibrillary acid protein.
References
- 1.American Heart Association (2002) Heart Disease and Stroke Statistics 2003 Update (American Heart Association, Dallas, TX).
- 2.Nishino, H. & Borlongan, C. V. (2000) Prog. Brain Res 127, 461-476. [DOI] [PubMed] [Google Scholar]
- 3.Mattsson, B., Sorensen, J. C., Zimmer, J. & Johansson, B. B. (1997) Stroke 28, 1225-1231; discussion 1231-1232. [DOI] [PubMed] [Google Scholar]
- 4.Riolobos, A. S., Heredia, M., de la Fuente, J. A., Criado, J. M., Yajeya, J., Campos, J. & Santacana, M. (2001) Neurobiol. Learn. Mem. 75, 274-292. [DOI] [PubMed] [Google Scholar]
- 5.Savitz, S. I., Rosenbaum, D. M., Dinsmore, J. H., Weschler, L. R. & Caplan, L. R. (2002) Ann. Neurol. 53, 266-275. [DOI] [PubMed] [Google Scholar]
- 6.Zhao, L. R., Duan, W. M., Reyes, M., Keene, C. D., Verfaillie, C. M. & Low, W. C. (2002) Exp. Neurol. 174, 11-20. [DOI] [PubMed] [Google Scholar]
- 7.Chen, J., Li, Y., Wang, L., Lu, M., Zhang, X. & Chopp, M. (2001) J. Neurol. Sci. 189, 49-57. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J., Sanberg, P. R., Li, Y., Wang, L., Lu, M., Willing, A. E., Sanchez-Ramos, J. & Chopp, M. (2001) Stroke 32, 2682-2688. [DOI] [PubMed] [Google Scholar]
- 9.Saporta, S., Borlongan, C. V. & Sanberg, P. R. (1999) Neuroscience 91, 519-525. [DOI] [PubMed] [Google Scholar]
- 10.Borlongan, C. V., Tajima, Y., Trojanowski, J. Q., Lee, V. M. & Sanberg, P. R. (1998) Exp. Neurol. 149, 310-321. [DOI] [PubMed] [Google Scholar]
- 11.Modo, M., Stroemer, R. P., Tang, E., Patel, S. & Hodges, H. (2002) Stroke 33, 2270-2278. [DOI] [PubMed] [Google Scholar]
- 12.Veizovic, T., Beech, J. S., Stroemer, R. P., Watson, W. P. & Hodges, H. (2001) Stroke 32, 1012-1019. [DOI] [PubMed] [Google Scholar]
- 13.Kondziolka, D., Wechsler, L., Goldstein, S., Meltzer, C., Thulborn, K. R., Gebel, J., Jannetta, P., DeCesare, S., Elder, E. M., McGrogan, M. & Reitman, M. A. (2000) Neurology 55, 565-569. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong, R. J., Watts, C., Svendsen, C. N., Dunnett, S. B. & Rosser, A. E. (2000) Cell Transplant. 9, 55-64. [DOI] [PubMed] [Google Scholar]
- 15.Svendsen, C. N., Clarke, D. J., Rosser, A. E. & Dunnett, S. B. (1996) Exp. Neurol. 137, 376-388. [DOI] [PubMed] [Google Scholar]
- 16.Svendsen, C. N., Caldwell, M. A., Shen, J., Borg, M. G., Rosser, A. E., Tyers, P., Karmiol, S. & Dunnett, S. B. (1997) Exp. Neurol. 148, 135-146. [DOI] [PubMed] [Google Scholar]
- 17.Brundin, P., Strecker, R. E., Widner, H., Clarke, D. J., Nilsson, O. G., Astedt, B., Lindvall, O. & Bjorklund, A. (1988) Exp. Brain Res. 70, 192-208. [DOI] [PubMed] [Google Scholar]
- 18.Clarke, D. J., Brundin, P., Strecker, R. E., Nilsson, O. G., Bjorklund, A. & Lindvall, O. (1988) Exp. Brain Res. 73, 115-126. [DOI] [PubMed] [Google Scholar]
- 19.Freed, C. R., Greene, P. E., Breeze, R. E., Tsai, W. Y., DuMouchel, W., Kao, R., Dillon, S., Winfield, H., Culver, S., Trojanowski, J. Q., Eidelberg, D. & Fahn, S. (2001) N. Engl. J. Med. 344, 710-719. [DOI] [PubMed] [Google Scholar]
- 20.Jeong, S. W., Chu, K., Jung, K. H., Kim, S. U., Kim, M. & Roh, J. K. (2003) Stroke 34, 2258-2263. [DOI] [PubMed] [Google Scholar]
- 21.Uchida, N., Buck, D. W., He, D., Reitsma, M. J., Masek, M., Phan, T. V., Tsukamoto, A. S., Gage, F. H. & Weissman, I. L. (2000) Proc. Natl. Acad. Sci. USA 97, 14720-14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamaki, S., Eckert, K., He, D., Sutton, R., Doshe, M., Jain, G., Tushinski, R., Reitsma, M., Harris, B., Tsukamoto, A., et al. (2002) J. Neurosci. Res. 69, 976-986. [DOI] [PubMed] [Google Scholar]
- 23.Zhao, H., Yenari, M. A., Cheng, D., Sapolsky, R. M. & Steinberg, G. K. (2003) J. Neurochem. 85, 1026-1036. [DOI] [PubMed] [Google Scholar]
- 24.Barone, F. C. & Feuerstein, G. Z. (1999) J. Cereb. Blood Flow Metab. 19, 819-834. [DOI] [PubMed] [Google Scholar]
- 25.Seri, B., Garcia-Verdugo, J. M., McEwen, B. S. & Alvarez-Buylla, A. (2001) J. Neurosci. 21, 7153-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu, K., Kim, M., Jeong, S. W., Kim, S. U. & Yoon, B. W. (2003) Neurosci. Lett. 343, 129-133. [DOI] [PubMed] [Google Scholar]
- 27.Ekdahl, C. T., Classen, J. H., Bonde, S., Kokaia, Z. & Lindvall, O. (2003) Proc. Natl. Acad. Sci. USA 100, 13632-13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoehn, M., Kustermann, E., Blunk, J., Wiedermann, D., Trapp, T., Wecker, S., Focking, M., Arnold, H., Hescheler, J., Fleischmann, B. K., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 16267-16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aboody, K., Brown, A., Rainov, N., Bower, K., Liu, S., Yang, W., Small, J., Herrlinger, U., Ourednik, V., Black, P., Breakfield, X. & Snyder, E. Y. (2000) Proc. Natl. Acad. Sci. USA 97, 12846-12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aarum, J., Sandberg, K., Haeberlein, S. L. & Persson, M. A. (2003) Proc. Natl. Acad. Sci. USA 100, 15983-15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran, P. B., Ren, D., Veldhouse, T. J. & Miller, R. J. (2004) J. Neurosci. Res. 76, 20-34. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, R. L., Zhang, L., Zhang, Z. G., Morris, D., Jiang, Q., Wang, L., Zhang, L. J. & Chopp, M. (2003) Neuroscience 116, 373-382. [DOI] [PubMed] [Google Scholar]
- 33.Englund, U., Bjorklund, A. & Wictorin, K. (2002) Brain Res. Dev. Brain Res. 134, 123-141. [DOI] [PubMed] [Google Scholar]
- 34.Fricker, R. A., Carpenter, M. K., Winkler, C., Greco, C., Gates, M. A. & Bjorklund, A. (1999) J. Neurosci. 19, 5990-5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleeson, J. G., Lin, P. T., Flanagan, L. A. & Walsh, C. A. (1999) Neuron 23, 257-271. [DOI] [PubMed] [Google Scholar]
- 36.Wennersten, A., Meier, X., Holmin, S., Wahlberg, L. & Mathiesen, T. (2004) J. Neurosurg. 100, 88-96. [DOI] [PubMed] [Google Scholar]
- 37.Windrem, M. S., Nunes, M. C., Rashbaum, W. K., Schwartz, T. H., Goodman, R. A., McKhann, G., 2nd, Roy, N. S. & Goldman, S. A. (2004) Nat. Med. 10, 93-97. [DOI] [PubMed] [Google Scholar]
- 38.Wu, P., Tarasenko, Y. I., Gu, Y., Huang, L. Y., Coggeshall, R. E. & Yu, Y. (2002) Nat. Neurosci. 5, 1271-1278. [DOI] [PubMed] [Google Scholar]
- 39.Hurelbrink, C. B., Armstrong, R. J., Dunnett, S. B., Rosser, A. E. & Barker, R. A. (2002) Eur. J. Neurosci. 15, 1255-1266. [DOI] [PubMed] [Google Scholar]
- 40.Shihabuddin, L. S., Horner, P. J., Ray, J. & Gage, F. H. (2000) Soc. Neurosci. 20, 8727-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]