Abstract
The molecular mechanisms that control the range and stability of emotions are unknown, yet this knowledge is critical for understanding mood disorders, especially bipolar illness. Here, we show that the glucocorticoid receptor (GR) modulates these features of emotional responsiveness. We generated transgenic mice overexpressing GR specifically in forebrain. These mice display a significant increase in anxiety-like and depressant-like behaviors relative to wild type. Yet, they are also supersensitive to antidepressants and show enhanced sensitization to cocaine. Thus, mice overexpressing GR in forebrain have a consistently wider than normal range of reactivity in both positive and negative emotionality tests. This phenotype is associated, in specific brain regions, with increased expression of genes relevant to emotionality: corticotropin-releasing hormone, serotonin, norepinephrine and dopamine transporters, and 5-hydroxytryptamine1A receptor. Thus, GR overexpression in forebrain causes higher “emotional lability” secondary to a unique pattern of molecular regulation. This finding suggests that natural variations in GR gene expression can contribute to the fine-tuning of emotional stability or lability and may play a role in bipolar disorder.
Whereas much attention has been devoted to defining genetic and neural mechanisms of anxiety and mood, we know less about the control of emotional range and emotional stability. It is evident that some individuals exhibit consistency in their mood, whereas others have a wider range of emotional intensity and/or a greater likelihood of switching from one emotional state to another. The latter pattern is termed “emotional instability” or “lability” and likely contributes to certain psychiatric disorders. Bipolar disorder (manic-depressive illness) is a prime example of uncontrolled emotional lability whereby patients exhibit extreme negative and positive emotions and can switch readily from one state to another (1). Moreover, bipolar patients can be highly sensitive to antidepressant drugs, such that the dose needed to reverse the negative affect can also trigger a switch into agitation and mania (2). By contrast, severe depression can be construed as the insufficient ability to emerge from a severe negative emotional state or to shift gears emotionally, a type of emotional rigidity. Thus, a better understanding of the mechanisms of emotional stability, lability, or rigidity could have important clinical implications.
The glucocorticoid receptor (GR) is a widely expressed ligand-dependent transcription factor that belongs to the nuclear hormone receptor superfamily and modulates a broad range of neural functions, including stress responsiveness and cognitive functions (3–8). Dysregulation of GR function has been associated with human depression and anxiety disorder (9, 10). Rats that show differences in anxiety and risk-taking behavior also have differences in GR expression in the hippocampus (11). Several mouse models with decreased GR activity have been created, including a model whereby a point mutation prevented receptor dimerization (12), a brain-specific GR knockout (13), and a GR-antisense model with reduced expression in brain and some peripheral tissues (14). The latter two models revealed decreased anxiety-like behavior accompanied by profound alterations in the neuroendocrine system (13, 15–17). Together, these findings led to the hypothesis that a sustained increase in GR activity in brain may be associated with increased anxiety-like emotional behavior. However, they did not suggest alterations in emotional lability. In this study, GR overexpression in forebrain (GRov) results in increased anxiety- and depressant-like behavior, coupled with surprisingly high responsiveness to several antidepressants. Moreover, these mice exhibit enhanced sensitization to cocaine challenge. In addition, we identify some of the molecular changes associated with this behavioral phenotype and show altered neural expression of the corticotropin-releasing hormone (CRH), the 5-hydroxytryptamine (5-HT)1A receptor, the serotonin transporter (SERT), the norepinephrine transporter (NET), and the dopamine transporter (DAT).
Materials and Methods
Generation of GRov Mice. The codons encoding the influenza hemagglutinin (HA) epitope were added to the 5′ end of the full-length mouse GR cDNA (18) by PCR. The fidelity of 2.6-kb HA-GR cDNA was verified by DNA sequencing. The transactivation property of HA-GR was verified by glucocorticoid response element-chloramphenicol acetyltransferase assay. The HA-GR cDNA was subcloned into the EcoRV site of pNN265. The HA-GR cDNA along with the attached 5′/3′ introns plus simian virus 40 polyadenylation signal was then inserted into the NotI site of pMM403, which contains 8.5 kb of the mouse calcium-calmodulin-dependent protein kinase II α promoter (19), resulting in transgene construct calcium-calmodulin-dependent protein kinase IIα-HA-GR. Mouse lines were established by breeding founders and their progeny to C57BL/6J mice. All experiments were performed with male mice. More detailed information is provided in Supporting Methods in Supporting Text, which is published as supporting information on the PNAS web site.
Behavioral Analysis. For antidepressant treatment in the elevated plus maze (EPM) test, mice were injected i.p. once daily for 10 days with vehicle (0.9% saline) or desipramine (DMI, 20 mg/kg), imipramine (IMI, 10 mg/kg), and fluoxetine (FLX, 10 mg/kg). An EPM test was performed 12 h after the last injection. In the forced swim test (FST), a 6-min test session was used 30 min after i.p. injection with vehicle, DMI (5, 10, and 20 mg/kg), IMI (10 mg/kg), or FLX (10 mg/kg) on the morning of day 1. The duration of immobility during the last 4 min of the test period was scored. The mice received the second injection 8–10 h after the first FST exposure. The final injection was administered 30 min before the second FST exposure 24 h after the first swim. The duration of immobility during the entire 6-min test session on day 2 was scored. In studies with cocaine (10 and 20 mg/kg; i.p.), mice were first habituated to the apparatus for 30 min, then given the drug, and were immediately placed back into the open field. For cocaine-induced behavioral sensitization, after daily injections of cocaine for 5 consecutive days, mice were challenged with the same dose of cocaine on day 14. More detailed information is provided in Supporting Methods.
Statistical Analysis. All statistical analyses were performed by unpaired two-tailed t test and ANOVA followed by post hoc testing as necessary. Two-factor ANOVA (genotype × day) with repeated measures was performed on general locomotor activity in the open field. One-factor ANOVAs (genotype) were performed on behaviors in EPM and the light-dark box. Two-factor ANOVAs (genotype × drug) were performed on behaviors in EPM and FST. Two-factor ANOVAs (genotype × drug or genotype × day) were performed on cocaine studies.
Results
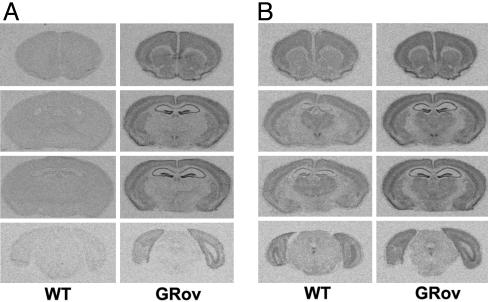
Generation of GRov Mice. To generate GRov mice, we used the previously characterized calcium-calmodulin-dependent protein kinase II α promoter to direct expression of the HA-tagged mouse GR (HA-GR) cDNA (Fig. 7A, which is published as supporting information on the PNAS web site). A transcriptional activation assay showed that addition of the HA epitope did not alter the transactivation properties of GR (Fig. 7B). In situ hybridization (ISH) experiments using a probe specific to the transgene showed that the distribution of transgene-specific GR mRNA in adult GRov mice was primarily forebrain-specific (Fig. 1A; more detailed information is provided in Supporting Results in Supporting Text). The signal was barely detectable in cerebellum (data not shown) and was undetectable in peripheral tissues, e.g., pituitary (data not shown). The most intense hybridization signals for transgene-specific GR mRNA were observed in the hippocampus (HC). There was no detectable transgene-specific signal in the WT littermates (Fig. 1 A). ISH experiments using a probe for GR mRNA showed that GRov mice exhibited significantly higher levels of total GR mRNA in forebrain regions (Fig. 1B). There was a particularly significant increase in GR mRNA levels in regions that are typically thought of as modulating emotional reactivity and stress responsiveness (Table 2, which is published as supporting information on the PNAS web site), including prefrontal cortex, nucleus accumbens, bed nucleus of the stria terminalis, central nucleus of the amygdala (CeA), paraventricular nucleus of the hypothalamus (PVN), and hippocampus. In contrast, basal mRNA expression of GR was unaffected in the anterior pituitary (Table 2) or in the midbrain catecholamine neurons (data not shown). Furthermore, transgene-specific HA-GR protein was expressed in the forebrain, as detected by Western blot with an antibody against the HA epitope (Fig. 7C left). Similarly, GRov mice expressed ≈78% more GR protein in the forebrain than WT controls, as detected by a specific antibody against GR (Fig. 7C Right). These results demonstrated higher expression of GR protein in the forebrain of GRov mice.
Fig. 1.
Generation of GRov mice. (A) ISH revealed transgene-specific GR mRNA was expressed in the forebrain. Representative images are shown ranging from Bregma 1.54 mm (top row) through Bregma -4.60 mm (bottom row). (B) Overexpression of GR in the forebrain of GRov mice.
Normal Hypothalamic-Pituitary-Adrenal (HPA) Axis Activity Basally and in Response to Mild Stress. To assess basal function of the HPA axis in GRov mice, plasma samples were collected at 2 h after lights on or 30 min before lights off. There were no significant differences in basal circulating corticosterone and adrenocorticotropic hormone (ACTH) levels between GRov and WT mice either in the morning or evening (Table 1). We also analyzed the endocrine responses to a mild stressor, 5 min free exploration in the EPM. GRov mice showed a tendency to have lower plasma ACTH levels than WT mice immediately or 10 min after the EPM test (Table 1, P = 0.09 and 0.12, respectively). However, there was clearly no difference in corticosterone levels between GRov and WT mice immediately or 10 min after that test (Table 1). These results suggest that, under this mild stress condition, GR overexpression in forebrain does not substantially alter the stress response. ISH experiments revealed no genotype difference in basal mRNA expression of proopiomelanocortin in the anterior pituitary or mineralocorticoid receptor in HC (Table 1). Furthermore, no genotype difference was observed in basal mRNA expression of CRH in PVN (Table 1).
Table 1. HPA axis activity.
| Component | WT | GRov |
|---|---|---|
| Corticosterone, μg/dl | ||
| Morning | 0.42 ± 0.20 | 0.64 ± 0.20 |
| Evening | 5.67 ± 0.70 | 5.04 ± 1.06 |
| EPM | 3.84 ± 0.22 | 4.07 ± 0.25 |
| Post | 6.70 ± 0.47 | 6.51 ± 0.57 |
| Plasma ACTH, pg/ml | ||
| Morning | 6.01 ± 1.14 | 7.64 ± 1.32 |
| Evening | 19.72 ± 5.21 | 22.51 ± 7.78 |
| EPM | 137.08 ± 14.63 | 94.27 ± 18.54 |
| Post | 123.46 ± 18.27 | 80.23 ± 17.39 |
| Basal proopiomelanocortin mRNA in the anterior pituitary | 24,527 ± 2,637 | 24,383 ± 2,532 |
| Basal CRH mRNA in the PVN | 2,184 ± 109 | 2,405 ± 147 |
| Basal mineralocorticoid receptor mRNA in the HC | 9,870 ± 259 | 10,015 ± 480 |
Proopiomelanocortin, CRH, and mineralocorticoid receptor mRNAs are given in integrated optical density. EPM values were obtained immediately after EPM exploration. Post values were obtained 10 min after EPM exploration. Results are expressed as mean ± SEM with n ≥ 5 per genotype.
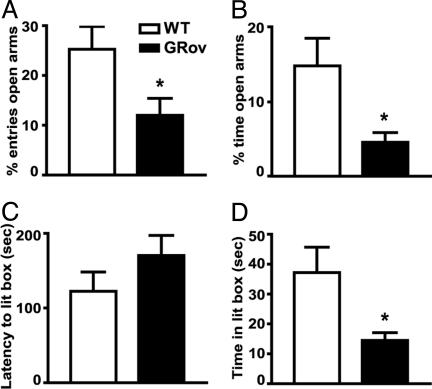
Increased Anxiety Normalized by Antidepressants. General locomotor activity was measured for 30 min in the open field with repeated measures for 3 days. The results did not reveal any changes in locomotor activity between GRov and WT mice (See information in Supporting Results). Moreover, there is no geno-type difference in body weight (data not shown). To determine the behavioral consequences of forebrain-specific GR overexpression in an anxiogenic environment, mice were examined by using the elevated plus maze. GRov mice entered the EPM open arms significantly fewer times than WT mice (Fig. 2A, P < 0.05). They also spent significantly less time than WT mice on the open arms (Fig. 2B, P < 0.05). Analysis of total arm entries indicated that GRov and WT mice exhibited equal locomotor activity in the EPM test (WT = 10.68 ± 1.58 entries, GRov = 11.38 ± 1.15 entries, P = 0.73). These results showed that GRov mice exhibit increased anxiety-like behavior in EPM. GRov mice also exhibited an increased anxiogenic response in the light-dark box. GRov mice demonstrated a tendency for a longer latency to enter the light box (Fig. 2C, P = 0.20), and a tendency to make fewer exits from the dark box (data not shown, P = 0.22). GRov mice spent significantly less time in the light box than WT mice (Fig. 2D, P < 0.05).
Fig. 2.
Increased anxiety-like behavior in GRov mice in the elevated plus maze (A and B) and in the light-dark box (C and D). Data are expressed as mean ± SEM. Elevated plus maze: WT, n = 19; GRov, n = 16. Light-dark box: WT, n = 20; GRov, n = 19. *, P < 0.05.
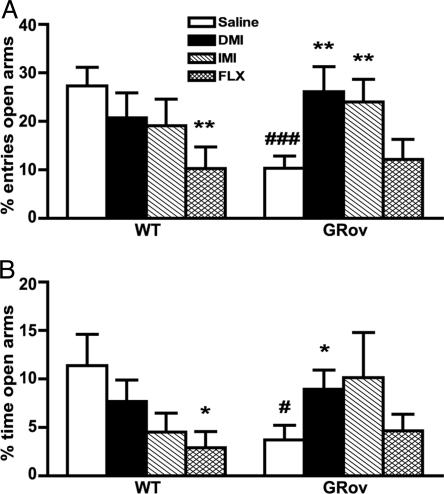
To examine whether antidepressants modulate these anxiety-like responses in GRov mice, the effects of three antidepressants were assessed in the EPM: IMI (a mixed norepinephrine/serotonin reuptake inhibitor), DMI (a selective norepinephrine reuptake inhibitor), and FLX (a selective serotonin reuptake inhibitor). After i.p. injection with either vehicle or antidepressant once daily for 10 days, saline-treated GRov mice entered the open arms significantly fewer times (Fig. 3A, P < 0.001) and spent significantly less time on the open arms (Fig. 3B, P < 0.05) than WT saline controls, demonstrating that the daily injection stress does not per se alter the enhancement in anxiety-like behavior exhibited by GRov mice. Neither DMI nor IMI treatment affected EPM behavior of WT mice. In contrast, these drugs had a profound effect on the transgenic mice (Fig. 3). DMI-treated GRov mice made more entries into the open arms (P < 0.01) and spent more time on the open arms (P < 0.05) than GRov saline controls; IMI-treated GRov mice made more entries into the open arms (P < 0.01) and spent marginally more time on the open arms (P = 0.14) than GRov saline controls. Thus GRov mice treated with DMI or IMI became indistinguishable from WT mice in the EPM test. Although treatment with FLX caused an anxiogenic effect in WT mice, as indicated by spending less time on the open arms (P < 0.05) and entering fewer times into the open arms (P < 0.01) in comparison with WT saline controls, the behavior of GRov mice in EPM was not affected by FLX treatment (Fig. 3). These results demonstrate that the increased anxiety-like behavior in GRov mice can be normalized by 10 days of treatment with DMI or IMI, but not FLX.
Fig. 3.
Increased anxiety-like behavior in GRov mice in EPM normalized by DMI and IMI, but not by FLX. (A and B) ANOVA revealed a significant geno-type × drug interaction, presented by the percentage of open arms entries (A, P < 0.05) and by the percentage of time spent on the open arms (B, P < 0.05). *, P < 0.05; **, P < 0.01 versus respective saline control. #, P < 0.05; ###, P < 0.001 versus WT saline control. The data are presented as mean ± SEM. WT mice: saline, n = 15; DMI, n = 10; IMI, n = 10; FLX, n = 12; GRov mice: saline, n = 15; DMI, n = 9; IMI, n = 10; FLX, n = 12.
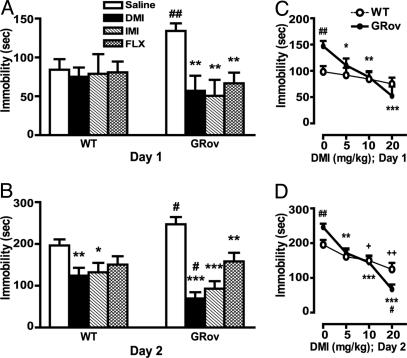
Hypersensitivity to Antidepressants in FST. To evaluate the role of forebrain-specific GR overexpression in antidepressant-mediated behavioral alterations in the FST, saline or antidepressant was administered i.p. to mice by using a three-dose regimen over 24 h, and immobility was measured in the morning (20). We tested three antidepressants: DMI, IMI, and FLX. On day 1, saline-treated GRov mice exhibited significantly increased baseline immobility in FST compared with WT saline controls (Fig. 4A, P < 0.01). After antidepressant treatment, WT mice did not show a decrease in immobility compared with saline-treated WT controls (Fig. 4A). However, treatment with DMI (20 mg/kg), IMI (10 mg/kg), or FLX (10 mg/kg) significantly reduced immobility in GRov mice compared with GRov saline controls (Fig. 4A, P < 0.001). Moreover, DMI decreased immobility scores in a dose-dependent manner in GRov mice in the FST (Fig. 4C).
Fig. 4.
Increased behavioral response in GRov mice to antidepressant in the FST. (A) On day 1, the duration of immobility during the last 4 min of the 6-min test session was scored. ANOVA revealed a significant genotype × drug interaction (P < 0.05). **, P < 0.001 versus GRov saline control. ##, P < 0.01 versus WT saline control. (B) The duration of immobility during the entire 6-min test session on day 2 was scored. ANOVA yielded a significant geno-type × drug interaction (P < 0.05). *, P < 0.05; **, P < 0.01; ***, P < 0.0001 versus respective saline control. #, P < 0.05 versus WT group under the same treatment condition. The data are presented as mean ± SEM. WT mice: saline, n = 15; DMI, n = 10; IMI, n = 10; FLX n = 12; GRov mice: saline, n = 14; DMI, n = 9; IMI, n = 10; FLX, n = 12. (C) DMI dose–response curve on day 1. ANOVA yielded a significant genotype × drug interaction (P < 0.05). *, P < 0.05; **, P < 0.001; ***, P < 0.0001 versus GRov saline control. ##, P < 0.01 versus WT saline control. (D) DMI dose–response curve on day 2. ANOVA revealed a significant genotype × drug interaction (P < 0.01). **, P < 0.001; ***, P < 0.0001 versus GRov saline control. +, P < 0.05; ++,P < 0.01 versus WT saline control. #, P < 0.05; ##, P < 0.01 versus WT group under the same treatment condition. The data are presented as mean ± SEM. Mice: saline, n = 15; 5 mg/kg, n = 12; 10 mg/kg, n = 10; 20 mg/kg, n = 10 per genotype.
The immobility in FST was also measured on day 2 by giving three i.p. injections over a 24-h period as described in Materials and Methods. Here again, saline-treated GRov mice exhibited significantly increased baseline immobility in FST compared with WT saline controls (Fig. 4B, P < 0.05). DMI (20 mg/kg) administered in this dosing regimen reduced immobility of GRov and WT mice compared with corresponding saline control groups (Fig. 4B; GRov, P < 0.0001; WT, P < 0.01). WT mice treated with saline or DMI were immobile for 196.40 ± 14.47 and 124.20 ± 18.63 sec, respectively, whereas GRov mice treated with saline or DMI were immobile for 247.07 ± 17.17 and 69.44 ± 14.98 sec, respectively. Although GRov mice had greater baseline immobility, DMI-treated GRov mice exhibited significantly decreased immobility compared with DMI-treated WT mice (P < 0.05). Thus, the differential effect induced by DMI on immobility scores was 2.4-fold higher in GRov mice compared with WT mice (Fig. 4B, 177.63 sec in GRov versus 72.2 sec in WT). IMI also reduced immobility of GRov and WT mice compared with corresponding saline control groups (Fig. 4B, GRov, P < 0.0001; WT, P < 0.05). IMI-treated GRov mice showed a tendency for decreased immobility in comparison with WT IMI mice (P = 0.19). After treatment with FLX, WT mice did not show a significant decrease in immobility compared with WT saline controls, whereas GRov mice showed dramatically decreased immobility compared with GRov saline controls (Fig. 4B, P < 0.01). Furthermore, DMI decreased immobility in a dose-dependent manner in both GRov and WT mice on day 2 (Fig. 4D). Immobility was reduced by a wider range of DMI in GRov mice relative to their saline controls (Fig. 4D). These results demonstrate that GRov mice have an increased baseline response in FST and increased response sensitivity to antidepressants, as assessed by IMI, DMI, and FLX.
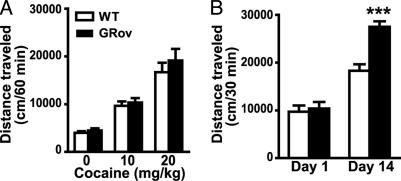
Enhancement of Cocaine-Induced Sensitization. To evaluate the role of forebrain-specific GR overexpression on the locomotor response produced by cocaine, we administered i.p. saline or cocaine to mice and measured locomotion in the open field. Acute cocaine administration produced a significant, dose-dependent increase in locomotion in both GRov and WT mice, and there was no genotype difference in the acute locomotor response to this drug (Fig. 5A). Moreover, there was no genotype difference in the locomotor response to repeated administration of 20 mg/kg cocaine over 5 days (data not shown). After 5 daily injections of cocaine, mice were given a challenge with the same dose of cocaine on day 14. Locomotor activity in response to the cocaine challenge was significantly enhanced relative to day 1 in both GRov and WT mice (Fig. 5B), indicating the development of behavioral sensitization. More importantly, GRov mice showed greater sensitization than WT mice, as indicated by a significant genotype × day interaction (P < 0.001). GRov mice also showed enhanced sensitization at the dose of 10 mg/kg cocaine under the same treatment regimen (data not shown).
Fig. 5.
Enhancement of cocaine-induced sensitization in GRov mice. (A) Dose-dependent increases in locomotor activity in the open field after acute treatment with cocaine. (B) Supersensitization to cocaine (20 mg/kg) challenge on day 14. ANOVA yielded a significant genotype × day interaction (P < 0.001). ***, P < 0.001 versus WT group on day 14. The data are presented as mean ± SEM. Mice: n = 10 per group per genotype.
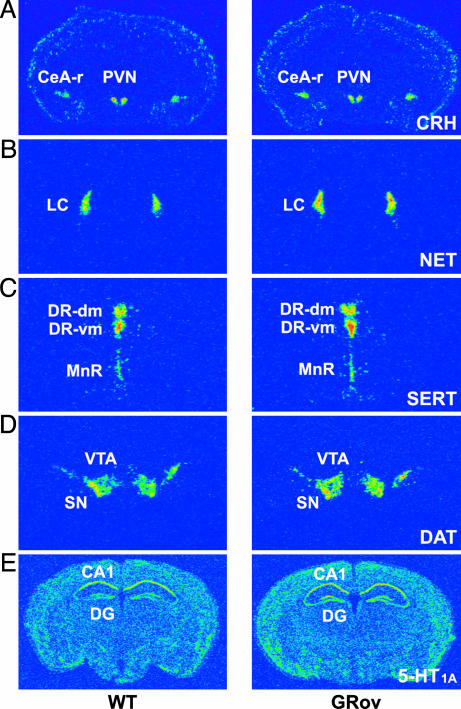
Altered Basal mRNA Expression of Emotionality-Related Genes. Given the above behavioral results, we asked whether the overexpression of GR in the forebrain leads to alteration in expression of target genes that may modulate emotional reactivity (e.g., CRH in the amygdala), responsiveness to antidepressants (NET, SERT, and 5-HT1A), and sensitization to cocaine (DAT). ISH experiments showed that overexpression of GR in forebrain significantly increased basal CRH expression in the rostral CeA (Fig. 6A and Table 3, which is published as supporting information on the PNAS web site). There was no genotype difference in basal CRH expression in the caudal CeA or in bed nucleus of the stria terminalis (Table 3). ISH studies showed that overexpression of GR in forebrain significantly increased basal NET expression in the locus coeruleus (LC) (Fig. 6B and Table 3), SERT expression in the ventromedial region of the dorsal raphe (DR) (Fig. 6C and Table 3), and DAT expression in the ventral tegmental area (VTA) (Fig. 6D and Table 3). There was no genotype difference in basal SERT expression in the dorsomedial DR or in the median raphe (MnR) (Table 3). Similarly, there was no genotype difference in basal DAT expression in the substantia nigra (Table 3). We also quantified the basal mRNA expression of the 5-HT1A receptor in raphe nuclei and hippocampus. ISH studies showed there was no genotype difference in basal expression of 5-HT1A autoreceptor mRNA in the DR or in the MnR (Table 3). However, there was a significant increase in basal expression of 5-HT1A postsynaptic receptor mRNA in the dentate gyrus of hippocampus in GRov mice (Fig. 6E and Table 3). These results suggest that overexpression of GR in the forebrain substantially alters basal mRNA expression of genes in CRH, noradrenergic, dopaminergic and serotonergic systems, but in a highly region-specific manner.
Fig. 6.
Basal expression of CRH, NET, SERT, DAT, and 5-HT1A receptor mRNAs in specific brain regions. (A) GRov mice expressed higher levels of CRH in rostral CeA (CeA-r) compared with WT mice (P < 0.05). There was no difference in CRH expression in PVN. (B) GRov mice expressed higher levels of NET in the LC (P < 0.05). (C) GRov mice expressed higher levels of SERT in the ventromedial DR (DR-vm; P < 0.05). There was no difference in SERT expression in either the dorsomedial DR (DR-dm) or in the MnR. (D) GRov mice expressed higher levels of DAT in the VTA (P < 0.05). There was no difference in DAT expression in the substantia nigra. (E) GRov mice expressed higher levels of 5-HT1A receptor in the DG of the hippocampus (P < 0.05). There was no difference in 5-HT1A receptor expression in CA1 of the hippocampus.
Discussion
This study represents an animal model of increased emotional lability. The GRov transgenic mice demonstrated a greater range in their responses to stimuli that trigger both negative and positive emotional responses. This finding would be exemplified by increased anxiety-like behavior in a novel environment, increased immobility in a test of “behavioral despair,” but also increased responses to antidepressants that alter emotional reactivity. Moreover, GRov mice showed greater behavioral sensitization to cocaine. These results demonstrate that the range of emotional responses is broader in GRov mice, and they are more prone to shift from one emotional state to another.
This work shows that an increase in GR expression in the forebrain leads to an increase in anxiety-like behavior as predicted by our hypothesis. There were no changes in basal plasma ACTH and corticosterone levels in GRov mice either in the morning or evening, and no changes in the basal mRNA expression of mineralocorticoid receptor in HC, CRH in PVN, or proopiomelanocortin in anterior pituitary. Moreover, GRov mice showed no differences from WT in corticosterone levels immediately and 10 min after the elevated plus maze test, a mild stressor. These findings reveal a dissociation between emotional responsiveness and HPA axis activity. It should be noted however that more severe and sustained stress does unveil changes in the dynamics of the HPA response in GRov mice (Q.W., unpublished work). The present results suggest that, even in the absence of peripheral neuroendocrine changes, there is a direct correlation between GR levels and anxiety, and that blockade of GR may prove to be a useful tool in altering affective tone.
The fact that increased anxiety in GRov mice in the elevated plus maze can be normalized by a 10-day treatment with DMI or IMI, but not FLX, suggests that GR overexpression in the forebrain may increase anxiety responses by means of alterations in the noradrenergic system, because the anxiety responses are blocked by norepinephrine-related antidepressants. However, this increased anxiety may also engage serotonergic mechanisms in more complex ways, because GR overexpression abrogates the anxiogenic effect of FLX seen in the WT animals.
The immobility in FST is thought to reflect either a failure of persistence in escape-directed behavior (i.e., behavioral despair) or the development of passive behavior that disengages the animal from active forms of coping with stressful stimuli (21). The increase in baseline immobility exhibited by GRov mice is indicative of a substantial depressant-like effect of the genetic manipulation. Alternative explanations involving learning capability and an energy conserving strategy remain a possibility (22). A notable finding of the present study is that the increase in GR expression in the forebrain leads to an increased response sensitivity to antidepressants in FST, including tricyclic antide-pressants and a selective serotonin reuptake inhibitor (Fig. 4). Despite the increased baseline immobility in GRov mice, DMI-treated GRov mice showed a significant decrease in immobility in comparison with DMI-treated WT mice at the dose of 20 mg/kg on day 2. This result suggests that the baseline immobility in GRov mice can be dissociated from the behavioral effect of the antidepressant. Previous studies (21) suggest that antiimmobility effects of selective serotonin reuptake inhibitors have been more difficult to demonstrate in FST. In this study, FLX treatment clearly decreased immobility in GRov mice, suggesting that GRov mice may represent a particularly useful model for testing a broad range of antidepressants.
Responsiveness to cocaine administration was used to assess reactivity to a strongly rewarding stimulus that is known to work in part by activating the dopaminergic system. It is well established that stress influences dopamine systems in the forebrain and modulates responsiveness to drugs of abuse (23). In this study, GRov mice are not overactive, or even overreactive to acute cocaine, but their increased susceptibility to sensitization indicates that they become increasingly hyperresponsive to potent emotional stimuli. This increase in salience may contribute to a variety of pathological states and a time-dependent process with different neuroadaptations at different points in time (24, 25). Future experiments will be needed to systematically examine the effect of GR overexpression on multiple aspects of rewarded behavior.
We then investigated the possible mechanisms whereby the increase in GR expression in the forebrain could lead to both increased emotional reactivity in positive and negative tests of emotionality, and enhanced responsiveness to antidepressants. We focused on the basal mRNA expression of target genes in CRH, noradrenergic, serotonergic, and dopaminergic systems. Whereas CRH in PVN is known to play a role in the activation of the stress response, its primary role in CeA is the modulation of fear and anxiety-like responses (26). We report here that increased GR expression in the forebrain is associated with a basal increase in CRH expression in the rostral CeA, which may contribute to increased anxiety-like behavior in GRov mice (10, 26). Another alteration in gene expression in the emotional circuits of GRov mice is the increased mRNA expression of NET in the LC, SERT in the ventromedial DR, and DAT in the VTA. Plasma membrane transporters are the molecular targets of antidepressants and psychostimulants (27). It has been hypothesized that clinical depression is accompanied by hypofunction of noradrenergic and/or serotonergic systems (28), because blocking reuptake of these monoamines by antidepressants represents an effective mode of antidepressant therapy. In GRov mice, the increase of NET expression in LC and SERT expression in the ventromedial DR may contribute to an increased responsiveness to both tricyclic antidepressants and a selective serotonin reuptake inhibitor in the FST. The alteration in DAT was also highly selective and shed light on the enhanced sensitization to cocaine challenge in GRov mice. DAT mRNA was up-regulated in GRov, but only in the VTA and not in the substantia nigra. This finding suggests that the nigrostriatal dopaminergic system was less affected than the mesocorticolimbic dopaminergic system that arises from VTA. In contrast to the nigrostriatal system that is more strongly implicated in motor control, the mesocorticolimbic dopaminergic system has been more clearly associated with incentive motivation and emotional processes (25, 29, 30). Given that many studies on the role of the monoamine transporters relied on genetic manipulations that produce profound alterations in expression (27), further studies of GRov mice will be required to determine how more subtle changes in basal mRNA expression of these transporters lead to increased responsiveness to antidepressants and sensitization to cocaine.
The pattern of alteration in 5-HT1A receptor expression is surprising because this receptor in the hippocampus is primarily responsive to the mineralocorticoid receptor rather than the GR (31, 32). Whereas the effect on 5-HT1A may be indirect, it is particularly conducive to increased emotional reactivity and responsiveness to antidepressants (33). There is evidence that the 5-HT1A autoreceptor in the raphe decreases neural firing in this nucleus and consequently the synthesis and release of serotonin in terminal areas, whereas the hippocampal postsynaptic 5-HT1A receptor facilitates the effects of antidepressants (34). Thus, the increase in hippocampal 5-HT1A gene expression in GRov is optimal for enhancing the neuronal effects of serotonin and the responsiveness of this system to antidepressants.
In summary, the selective overexpression of GR in the fore-brain leads to a distinct neural phenotype. This neural phenotype includes sustained increases in the expression of stress-specific genes (GR in the forebrain, CRH in the amygdala) that lead to enhanced negative affective responses in novel or stressful situations. It also leads to increases in monoaminergic genes that have been associated with increased coping and that mediate exquisite responsiveness to monoaminergic antidepressants and enhanced sensitization to cocaine. Together, these alterations in neural gene expression produce a behavioral phenotype whereby this mouse is poised between positive and negative affective responses, and therefore exhibits greater swings in reaction to the environment. It identifies GR not only as a regulator of stress responsiveness but also as a key controller of emotional lability, and is congruent with recent findings demonstrating that patients with either psychotic depression or bipolar disorder show improvement in mood after treatment with a GR antagonist (35, 36). Thus, this mouse model will likely prove important in investigating the molecular mechanisms that underlie human differences in emotional reactivity and vulnerability to mood disorders.
Acknowledgments
We thank Drs. E. Hebda-Bauer and C. Neal for helpful discussion; Dr. M. Danielsen (Georgetown University Medical Center, Washington, DC) for the gift of mouse GR cDNA; Dr. E. R. Kandel (Columbia University College of Physicians and Surgeons, New York) for the gift of plasmids pMM403 and pNN265; and the Transgenic Animal Core of the University of Michigan. This work was supported by National Institutes of Health Grant 5 P01 MH42251 (to S.J.W. and H.A.) and the Nancy Pritzker Network for Research on Depression.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DMI, desipramine; EPM, elevated plus maze; FLX, fluoxetine; FST, forced swim test; GR, glucocorticoid receptor; GRov, overexpressing GR in forebrain; IMI, imipramine; ACTH, adrenocorticotropic hormone; CRH, corticoptropin-releasing hormone; 5-HT, 5-hydroxytryptamine; SERT, serotonin transporter; NET, norepinephrine transporter; DAT, dopamine transporter; HA, hemagglutinin; HC, hippocampus; ISH, in situ hybridization; CeA, central nucleus of the amygdala; PVN, paraventricular nucleus of the hypothalamus; HPA, hypothalamic-pituitary-adrenal; LC, locus coeruleus; DR, dorsal raphe; MnR, median raphe; VTA, ventral tegmental area.
References
- 1.Akiskal, H. S., Maser, J. D., Zeller, P. J., Endicott, J., Coryell, W., Keller, M., Warshaw, M., Clayton, P. & Goodwin, F. (1995) Arch. Gen. Psychiatry 52, 114-123. [DOI] [PubMed] [Google Scholar]
- 2.El-Mallakh, R. S. & Karippot, A. (2002) Psychiatr. Serv. 53, 580-584. [DOI] [PubMed] [Google Scholar]
- 3.Sapolsky, R. M., Krey, L. C. & McEwen, B. S. (1984) Proc. Natl. Acad. Sci. USA 81, 6174-6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akil, H. & Morano, M. (1995) in the Fourth Generation of Progress, eds. Bloom, F. & Kupfer, D. (Raven, New York), pp. 773-785.
- 5.McEwen, B. S. & Sapolsky, R. M. (1995) Curr. Opin. Neurobiol. 5, 205-216. [DOI] [PubMed] [Google Scholar]
- 6.Meaney, M. J., Diorio, J., Francis, D., Widdowson, J., LaPlante, P., Caldji, C., Sharma, S., Seckl, J. R. & Plotsky, P. M. (1996) Dev. Neurosci. 18, 49-72. [DOI] [PubMed] [Google Scholar]
- 7.De Kloet, E. R., Vreugdenhil, E., Oitzl, M. S. & Joels, M. (1998) Endocr. Rev. 19, 269-301. [DOI] [PubMed] [Google Scholar]
- 8.Roozendaal, B., Griffith, Q. K., Buranday, J., De Quervain D. J. & McGaugh, J. L. (2003) Proc. Natl. Acad. Sci. USA 100, 1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holsboer, F. (2000) Neuropsychopharmacology 23, 477-501. [DOI] [PubMed] [Google Scholar]
- 10.Korte, S. M. (2001) Neurosci. Biobehav. Rev. 25, 117-142. [DOI] [PubMed] [Google Scholar]
- 11.Kabbaj, M., Devine, D. P., Savage, V. R. & Akil, H. (2000) J. Neurosci. 20, 6983-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reichardt, H. M., Kaestner, K. H., Tuckermann, J., Kretz, O., Wessely, O., Bock, R., Gass, P., Schmid, W., Herrlich, P., Angel, P., et al. (1998) Cell 93, 531-541. [DOI] [PubMed] [Google Scholar]
- 13.Tronche, F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P. C., Bock, R., Klein, R. & Schutz, G. (1999) Nat. Genet. 23, 99-103. [DOI] [PubMed] [Google Scholar]
- 14.Pepin, M. C., Pothier, F. & Barden, N. (1992) Nature 355, 725-728. [DOI] [PubMed] [Google Scholar]
- 15.Barden, N., Stec, I. S., Montkowski, A., Holsboer, F. & Reul, J. M. (1997) Neuroendocrinology 66, 212-220. [DOI] [PubMed] [Google Scholar]
- 16.Rochford, J., Beaulieu, S., Rousse, I., Glowa, J. R. & Barden, N. (1997) Psychopharmacology 132, 145-152. [DOI] [PubMed] [Google Scholar]
- 17.Dijkstra, I., Tilders, F. J., Aguilera, G., Kiss, A., Rabadan-Diehl, C., Barden, N., Karanth, S., Holsboer, F. & Reul, J. M. (1998) J. Neurosci. 18, 3909-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danielsen, M., Northrop, J. P. & Ringold, G. M. (1986) EMBO J. 5, 2513-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayford, M., Bach, M. E., Huang, Y. Y., Wang, L., Hawkins, R. D. & Kandel, E. R. (1996) Science 274, 1678-1683. [DOI] [PubMed] [Google Scholar]
- 20.Schramm, N. L., McDonald, M. P. & Limbird, L. E. (2001) J. Neurosci. 21, 4875-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucki, I. (1997) Behav. Pharmacol. 8, 523-532. [DOI] [PubMed] [Google Scholar]
- 22.West, A. P. (1990) Prog. Neuropsychopharmacol. Biol. Psychiatry 14, 863-877. [DOI] [PubMed] [Google Scholar]
- 23.Piazza, P. V. & Le Moal, M. (1998) Trends Pharmacol. Sci. 19, 67-74. [DOI] [PubMed] [Google Scholar]
- 24.Kalivas, P. W. & Stewart, J. (1991) Brain Res. Brain Res. Rev. 16, 223-244. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, T. E. & Berridge, K. C. (2003) Annu. Rev. Psychol. 54, 25-53. [DOI] [PubMed] [Google Scholar]
- 26.Koob, G. F. & Heinrichs, S. C. (1999) Brain Res. 848, 141-152. [DOI] [PubMed] [Google Scholar]
- 27.Torres, G. E., Gainetdinov, R. R. & Caron, M. G. (2003) Nat. Rev. Neurosci. 4, 13-25. [DOI] [PubMed] [Google Scholar]
- 28.Leonard, B. E. (1997) J. Psychopharmacol. 11, Suppl. 4, 39-47. [Google Scholar]
- 29.Pierce, R. C. & Kalivas, P. W. (1997) Brain Res. Brain Res. Rev. 25, 192-216. [DOI] [PubMed] [Google Scholar]
- 30.Koob, G. F., Sanna, P. P. & Bloom, F. E. (1998) Neuron 21, 467-476. [DOI] [PubMed] [Google Scholar]
- 31.Meijer, O. C. & De Kloet, E. R. (1995) J. Neuroendocrinol. 7, 653-657. [DOI] [PubMed] [Google Scholar]
- 32.Lopez, J. F., Chalmers, D. T., Little, K. Y. & Watson, S. J. (1998) Biol. Psychiatry 43, 547-573. [DOI] [PubMed] [Google Scholar]
- 33.Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., Weisstaub, N., Lee, J., Duman, R., Arancio, O., et al. (2003) Science 301, 805-809. [DOI] [PubMed] [Google Scholar]
- 34.Blier, P. & de Montigny, C. (1994) Trends Pharmacol. Sci. 15, 220-226. [DOI] [PubMed] [Google Scholar]
- 35.Belanoff, J. K., Rothschild, A. J., Cassidy, F., DeBattista, C., Baulieu, E. E., Schold, C. & Schatzberg, A. F. (2002) Biol. Psychiatry 52, 386-392. [DOI] [PubMed] [Google Scholar]
- 36.Young, A. H., Gallagher, P., Watson, S., Del-Estal, D., Owen, B. M. & Ferrier, I. N. (May 5, 2004) Neuropsychopharmacology, doi:10.1038/sj.npp.1300471. [DOI] [PubMed]