The optic tectum is required for multisensory integration underlying sensorimotor behaviors. Communication between tectal lobes is thought to play important roles in tectal function. We investigated the development and plasticity of intertectal connections and found that excitatory and inhibitory intertectal connections converge with retinotectal input and are plastic in response to visual experience. This suggests that intertectal inputs are key players in tectal development and may preserve excitatory-inhibitory balance in response to changing sensory input.
Keywords: convergent, excitatory-inhibitory balance, experience-dependent plasticity, intertectal, retinotectal
Abstract
Communication between optic tecta/superior colliculi is thought to be required for sensorimotor behaviors by comparing inputs across the midline; however, the development of and the role of visual experience in the function and plasticity of intertectal connections are unclear. We combined neuronal labeling, in vivo time-lapse imaging, and electrophysiology to characterize the structural and functional development of intertectal axons and synapses in Xenopus tadpole optic tectum. We find that intertectal connections are established early during optic tectal circuit development. We determined the neurotransmitter identity of intertectal neurons using both rabies virus-mediated tracing combined with post hoc immunohistochemistry and electrophysiology. Excitatory and inhibitory intertectal neuronal somata are similarly distributed throughout the tectum. Excitatory and inhibitory intertectal axons are structurally similar and elaborate broadly in the contralateral tectum. We demonstrate that intertectal and retinotectal axons converge onto tectal neurons by recording postsynaptic currents after stimulating intertectal and retinotectal inputs. Cutting the intertectal commissure removes synaptic responses to contralateral tectal stimulation. In vivo time-lapse imaging demonstrated that visual experience drives plasticity in intertectal bouton size and dynamics. Finally, visual experience drives the maturation of excitatory intertectal inputs by increasing AMPA-to-N-methyl-d-aspartate (NMDA) ratios, comparable to experience-dependent maturation of retinotectal inputs, and coordinately increases intertectal GABA receptor-mediated currents. These data indicate that visual experience regulates plasticity of excitatory and inhibitory intertectal inputs, maintaining the balance of excitatory to inhibitory intertectal input. These studies place intertectal inputs as key players in tectal circuit development and suggest that they may play a role in sensory information processing critical to sensorimotor behaviors.
NEW & NOTEWORTHY
The optic tectum is required for multisensory integration underlying sensorimotor behaviors. Communication between tectal lobes is thought to play important roles in tectal function. We investigated the development and plasticity of intertectal connections and found that excitatory and inhibitory intertectal connections converge with retinotectal input and are plastic in response to visual experience. This suggests that intertectal inputs are key players in tectal development and may preserve excitatory-inhibitory balance in response to changing sensory input.
the optic tectum, or the superior colliculus, serves as an interface for adaptive multisensory motor processing across vertebrate species. This integration is essential for survival behaviors such as predator avoidance, prey capture, and orienting to a stimulus (King 2004; Murray et al. 2016; Mysore and Knudsen 2011; Wurtz and Albano 1980). Tectal neurons project axons across the midline to the contralateral tectum in many vertebrates (Herrero et al. 1999; Vanegas 1984). Communication between tecta is required for sensory processing, sensorimotor integration, and sensorimotor behaviors including sound localization and gaze tracking (Gaze et al. 1970; King 2004; Malmierca et al. 2005; Moschovakis et al. 1988; Munoz and Guitton 1991; Munoz and Istvan 1998; Munoz and Wurtz 1993; Olivier et al. 2000; Takahashi et al. 2007; Wurtz and Albano 1980).
In the adult frog, communication between the tectal lobes is accomplished by an indirect pathway in which retinorecipient tectal neurons project to neurons in the ipsilateral nucleus isthmi, which in turn project to the contralateral optic tectum, resulting in tectal neurons with binocular response properties (Grant and Keating 1989; Udin 2012; Udin and Grant 1999). The interhemispheric or crossed tecto-isthmo-tectal projections are first detectable anatomically at stage 52 of development (Udin and Fisher 1985) and electrophysiologically by stage 60 (Grant and Keating 1986). Lesioning the nucleus isthmi ablates prey-catching behavior in frogs, demonstrating that the crossed tecto-isthmo-tectal projection is necessary for such high-resolution visuomotor behaviors (Caine and Gruberg 1985). During developmental stages that precede the emergence of the tecto-isthmo-tectal projection, a direct commissural projection between the two tectal lobes is present (Herrero et al. 1999; Miraucourt et al. 2012; Vanegas 1984), which we refer to as the intertectal projection.
Studies in mammals have shown that postnatal sensory experience is required for the development of neuronal response properties underlying tectal multisensory integration (Murray et al. 2016; Stein 1984; Stein and Rowland 2011; Stein et al. 2014); however, little is known about the initial development of intertectal connections or the potential role of early sensory experience in developmental synaptic plasticity of these inputs. In particular, it is not clear whether direct sensory inputs innervate the tectum before intertectal inputs, when convergent intertectal and sensory inputs are established, or whether early sensory experience regulates the development and synaptic connectivity of intertectal inputs. In nonmammalian vertebrates that develop externally, such as frogs and fish, synaptic activity downstream of visual input regulates many aspects of visual circuit development that normally occur in utero or before the onset of natural vision in mammals (Cline 2001; Pratt et al. 2016). Therefore, the externally developing tadpole provides an ideal experimental system to investigate questions concerning development of intertectal connections because the brain is accessible for anatomic and electrophysiological analysis of the initial establishment and maturation of neuronal connections during early developmental stages that are intractable in mammalian systems.
In tadpoles, each retina projects axons via the optic nerve to excite neurons predominantly in the contralateral optic tectum (Munz et al. 2014). In addition to excitatory retinotectal inputs, excitatory and inhibitory intertectal axons extend across the intertectal commissure between the tecta (Miraucourt et al. 2012) and could mediate direct information transfer across the midline. Patterned visual stimulation drives structural elaboration of retinotectal axons, tectal neuron dendrites, and retinotectal synaptogenesis required for visual information processing (Dunn et al. 2016; Khakhalin et al. 2014; Munz et al. 2014; Pratt et al. 2008; Randlett et al. 2015; Ruthazer and Aizenman 2010). Visual stimulation also refines the temporal properties of excitatory and inhibitory recurrent activity in the tectum to optimize visual response properties (Pratt and Aizenman 2007; Pratt et al. 2008). Coordinated development of excitatory and inhibitory inputs to tectal neurons is required for the development of visual receptive fields (Tao and Poo 2005). Studies selectively interfering with synaptic GABAA receptor trafficking that decreased inhibitory input to tectal neurons showed that inhibitory inputs are required for normal development of tectal dendritic arbors, receptive field properties, and visual information processing required for visuomotor behavior (Shen et al. 2011). Together, these studies suggest that experience-dependent development of excitatory and inhibitory input is important for maintaining a balance of excitation to inhibition in tectal neurons that is required for optic tectal function. Here, we are interested in determining whether the development, connectivity, and plasticity of intertectal neurons might be regulated by visual experience and whether experience-dependent plasticity of excitatory and inhibitory intertectal connections might play a role in stabilizing the balance of excitation to inhibition in the optic tectum.
To address these questions, we examined the developmental time course of intertectal axon innervation and the relative distribution of excitatory and inhibitory intertectal input and of retinotectal and intertectal input using in vivo time-lapse imaging and neuronal tracing. We used electrophysiological recordings of tadpole tectal neurons to characterize the development of glutamatergic and GABAergic intertectal inputs and demonstrate the convergence of intertectal and retinotectal inputs onto the same optic tectal neurons. Finally, we exposed tadpoles to visual stimulation and evaluated experience-dependent structural and functional plasticity in excitatory and inhibitory intertectal boutons and synapses. Results from these studies indicate that enhanced visual experience regulates the plasticity of both excitatory and inhibitory intertectal inputs, thereby maintaining the excitatory-to-inhibitory (E:I) balance of intertectal input. Together, these studies place intertectal inputs as key players in early tectal circuit development and suggest that they may play a critical role in visual information processing critical to sensorimotor behaviors.
MATERIALS AND METHODS
Animals.
Albino Xenopus laevis tadpoles of either sex were obtained by in-house breeding or purchased from Xenopus Express (Brooksville, FL). Tadpoles were reared in 0.1× Steinberg solution in a 12:12-h light-dark cycle at 22–23°C and used for time-lapse imaging and electrophysiology experiments beginning at stage 46 (Nieuwkoop and Faber 1956). Animals were fed beginning at stage 46. Animals were anesthetized in 0.02% tricaine methanesulphonate (MS222) before all procedures. All animal protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Whole mount imaging of intertectal and retinotectal axons.
To examine the distribution of intertectal axons and assess the relationship between intertectal and retinotectal axons, we labeled intertectal axons alone or in combination with retinotectal axons. Retinotectal axons were traced using 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD; D-7757; Thermo Fisher Scientific), and intertectal axons were either electroporated with plasmid driving green fluorescent protein (GFP) expression or traced with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; D-3911; Thermo Fisher Scientific). DiI and DiD were injected at 0.2% in dimethylformamide. For labeling combined with electroporation, the left tectal lobe of stage 46 animals was electroporated with plasmid to drive GFP expression enhanced with the gal4-UAS system (Haas et al. 2002). For electroporation, plasmids were injected into the brain ventricle, and then platinum electrodes were placed on each side of the midbrain and voltage pulses were applied across the midbrain. Cells were electroporated with a plasmid using the vesicular GABA transporter (VGAT) promoter driving gal4 (pVGAT::gal4, 1 μg/μl) and 0.7 μg/μl pUAS::GFP and fixed with 4% paraformaldehyde 7 days following electroporation (dfe) at stage 48. Following fixation, 0.2% DiD was injected into the left eye and allowed to diffuse for 3 wk. For dual dye-labeling experiments, stage 46 tadpoles were fixed with 4% paraformaldehyde and then injected with 0.2% DiI in the left tectal lobe and 0.2% DiD in the left eye. Dyes were allowed to diffuse for 3 wk. Brains were dissected, mounted in 6 M urea in 50% glycerol to clear the tissue partially, and imaged whole mount with an Olympus FluoView 500 confocal microscope with a ×20 [0.8 numerical aperture (NA)] or ×40 (1.0 NA) oil-immersion lens. For dual dye-labeling experiments, brains were incubated in 1:1,000 SYTOX Orange Nucleic Acid Stain (S11368; Life Technologies) in PBS for 15 min before mounting to label the nuclei of tectal neurons.
Rabies virus-mediated retrograde labeling and GABA immunohistochemistry of intertectal neurons.
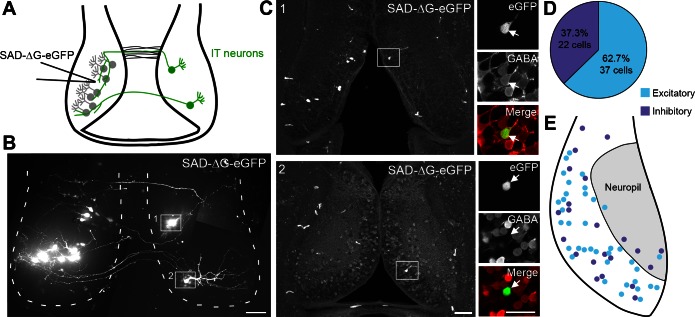
To label intertectally projecting optic tectal neurons retrogradely, we injected glycoprotein (G)-deleted rabies virus expressing enhanced GFP [eGFP; SAD-ΔG-eGFP, 1.84 × 109 transducing units (TU)/ml, provided by the Salk GT3 viral vector core] into the left tectum. Seven days later, we collected in vivo images of eGFP+ cells in the tectum using a PerkinElmer UltraVIEW VoX spinning-disk confocal microscope with a ×25 Nikon water-immersion objective lens (1.1 NA). Immediately following in vivo imaging, tadpoles were anesthetized with 0.02% MS222, immersed in 4% paraformaldehyde and 1–2% glutaraldehyde, and fixed using two bouts of microwave fixation at 150 W for 1 min followed by overnight fixation at 4°C. Brains were dissected, embedded in a gelatin-albumin mixture, and sectioned at 40 μm on a vibratome. Sections were blocked and permeabilized in 5% normal donkey serum and 2% Triton X-100 for 1 h at room temperature. Then, sections were incubated in 1:2,000 rabbit anti-GABA (A2052; Sigma) for 2–3 days at 4°C followed by 2 h in 1:200 anti-rabbit Alexa Fluor 647 (Life Technologies) at room temperature. Sections were mounted in Gel mount (Accurate) and imaged with an Olympus FluoView 500 confocal microscope. Confocal z-stacks of the entire tectum were first acquired with a ×20 (0.8 NA) oil-immersion lens to identify eGFP+ cells in the tectal lobe contralateral to G-deleted rabies virus injection. Then, two-channel GFP and GABA/Alexa 647 z-stacks of retrogradely traced intertectal neurons were acquired with a ×40 (1.0 NA) or ×60 (1.4 NA) oil-immersion lens. The percentage of eGFP+ neurons that were immunopositive for GABA was quantified. A map of intertectal neuron locations was created by overlaying eGFP+ neurons from the ×20 images after sectioning onto a diagram of the optic tectum.
In vivo time-lapse imaging of intertectal axon development.
To visualize intertectal axons, one tectal lobe of stage 46 animals was electroporated with either 1 μg/μl pα-actin::gal4-UAS::eGFP or 1 μg/μl pVGAT::gal4 + 1 μg/μl pUAS::eGFP. Tadpoles were anesthetized with 0.02% MS222 and were imaged on a custom-built two-photon microscope with a ×20 (0.95 NA) water-immersion lens at a 2× digital scan zoom beginning at 4 dfe. Animals were imaged daily from 4 to 10 dfe and then again at 12 dfe and 14 dfe. Beginning on the 1st day of imaging, animals were housed individually in the wells of a six-well tissue culture plate containing 0.1× Steinberg. Intertectal axons were traced and reconstructed using Imaris software (Bitplane, Zurich, Switzerland). Total axon length and total axonal branch tip number were quantified each day. For presentation purposes, autofluorescing skin cells were removed from the images in Fig. 1E.
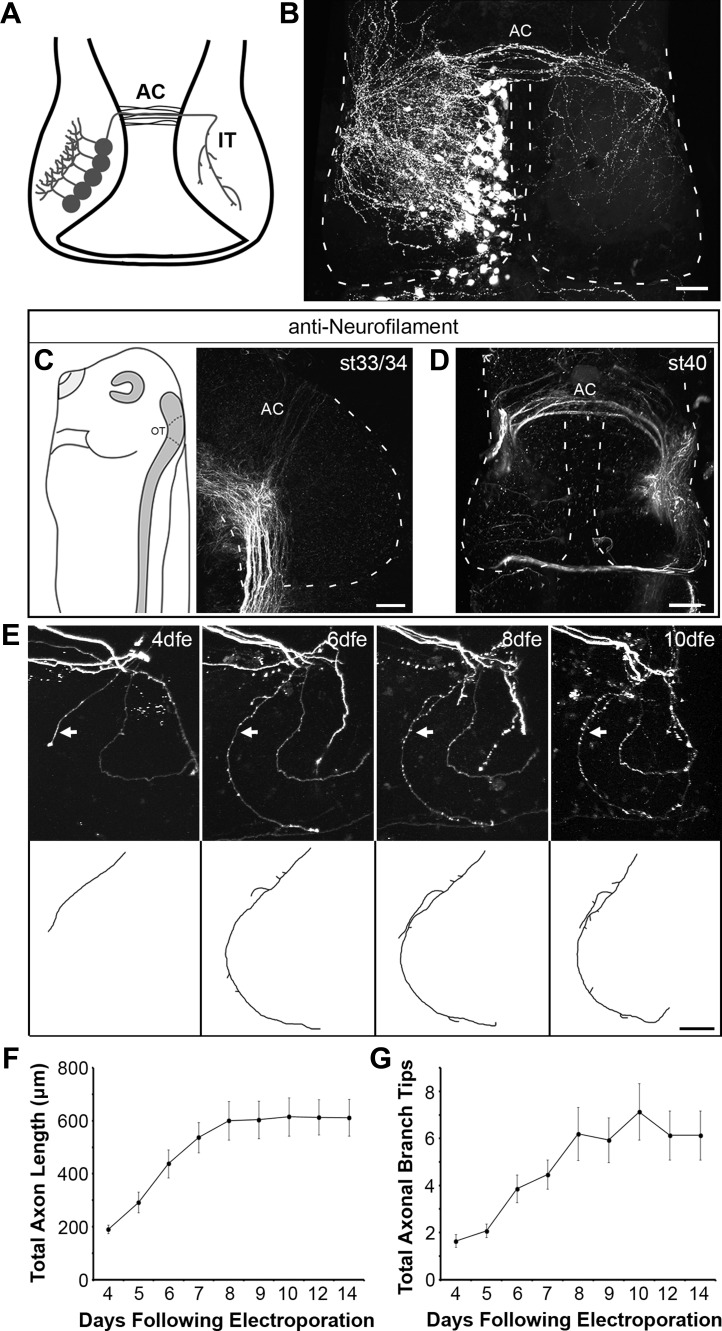
Fig. 1.
Anatomic development of intertectal axons. A: schematic showing tectal neurons projecting intertectal (IT) axons across the anterior commissure (AC), the anterior boundary of the optic tectum, which elaborate in the contralateral tectal lobe. B: confocal z-projection of GFP-expressing intertectal axons imaged in stage 48 whole mount brain. Intertectal axons cross the dorsal midline in a band across the anterior commissure and more sparsely along the anterior-posterior axis. Intertectal axons broadly innervate the contralateral tectal lobe. Dashed lines mark the boundary of the optic tectum. C and D: confocal z-projections of whole mount neurofilament immunostaining show that axons are 1st detected in the AC at stage (st) 33/34 (C), and a band of axons is present in the AC at stage 40 (D). At stage 33/34, the brain is lying on its right side with the midline facing the right edge (C, left). The optic tectum (OT) in the sketch is demarcated by dashed lines. Neurofilament-immunolabeled axons in the AC extend upward, away from the intensely labeled axon tract at the floor of the midbrain (C, right). D: image of a stage 40 midbrain in which the orientation of the brain matches the schematic in A. Dashed lines mark the boundary of the optic tectum. E: in vivo time-lapse confocal imaging of GFP-expressing intertectal axons shows the time course of elaboration within the contralateral tectum. Confocal z-projections (top) highlight 1 intertectal axon (arrow), which is reconstructed (bottom). dfe, Days following electroporation. This image has the same orientation as shown in A and B but includes only the right tectal lobe. F and G: quantification of total axon length (F) and branch tip number (G) of GFP-expressing axons (n = 15) imaged with in vivo time-lapse confocal microscopy. Intertectal axons become increasingly elaborate over 4 days following innervation of the contralateral tectum, and then their growth plateaus. F: total axon length (in μm): 4 dfe, 190.27 ± 15.81; 5 dfe, 291.84 ± 38.73; 6 dfe, 438.12 ± 52.95; 7 dfe, 537.15 ± 57.04; 8 dfe, 600.80 ± 72.94; 9 dfe, 604.07 ± 71.26; 10 dfe, 615.20 ± 72.33; 12 dfe, 613.45 ± 66.41; 14 dfe, 611.77 ± 69.52. G: branch tip number: 4 dfe, 1.64 ± 0.27; 5 dfe, 2.08 ± 0.29; 6 dfe, 3.87 ± 0.58; 7 dfe, 4.47 ± 0.62; 8 dfe, 6.20 ± 1.13; 9 dfe, 5.93 ± 0.95; 10 dfe, 7.13 ± 1.20; 12 dfe, 6.13 ± 1.04; 14 dfe, 6.13 ± 1.04. Data are presented as means ± SE. Scale bars = 50 μm.
Post hoc VGAT immunohistochemistry.
Immediately following in vivo imaging, tadpoles were anesthetized with 0.02% MS222, immersed in 4% paraformaldehyde, and fixed using two bouts of microwave fixation at 150 W for 1 min followed by overnight fixation at 4°C. Brains were dissected, embedded in a gelatin-albumin mixture, and sectioned at 40 μm on a vibratome. Sections were blocked and permeabilized in 5% normal donkey serum and 2% Triton X-100 for 1 h at room temperature. Then, sections were incubated in 1:400 rabbit anti-VGAT (cat. no. 131 003; Synaptic Systems) for 3 days at 4°C followed by 2 h in 1:200 anti-rabbit Alexa Fluor 647 (Life Technologies) at room temperature. Sections were mounted in Gel mount (Accurate) and imaged with an Olympus FluoView 500 confocal microscope. Confocal z-stacks were first acquired with a ×20 (0.8 NA) or ×40 (1.0 NA) oil-immersion lens to determine the location of the GFP+ axon from in vivo imaging. Then, two-channel GFP and VGAT/Alexa 647 z-stacks were acquired of axons with a ×60 (1.4 NA) oil-immersion lens at 2× digital scan zoom to yield a pixel size of 0.10 μm. To determine colocalization between GFP and VGAT, we used the Colocalization plugin for ImageJ. Fluorescence thresholds were set to 2× background levels for each channel. Then, the Colocalization plugin identified pixels in the z-stack that were above threshold for both channels, and those pixels were displayed in white. GFP+ boutons that were colocalized with VGAT/Alexa 647 (white) throughout their centers were deemed VGAT+. Finally, we quantified the percentage of GFP+ boutons that were VGAT+ for each axon. To determine a cutoff point at which to call an axon excitatory (VGAT−) or inhibitory (VGAT+), we took six sets of two-channel GFP and VGAT/Alexa 647 images and offset the two channels by 10 μm to assess the amount of colocalization that occurs at random. We found that the mean percentage of VGAT+ boutons for the offset images was 20.85%. Using this mean + 3SD [20.85% + (3 × 5.47%)], we set a cutoff for VGAT positivity at 37.25%. Therefore, any axon with a percentage of VGAT+ boutons >37.25% was deemed inhibitory (VGAT+), and any below that cutoff was deemed excitatory (VGAT−). For presentation purposes, autofluorescing skin cells were removed from the image in Fig. 4A.
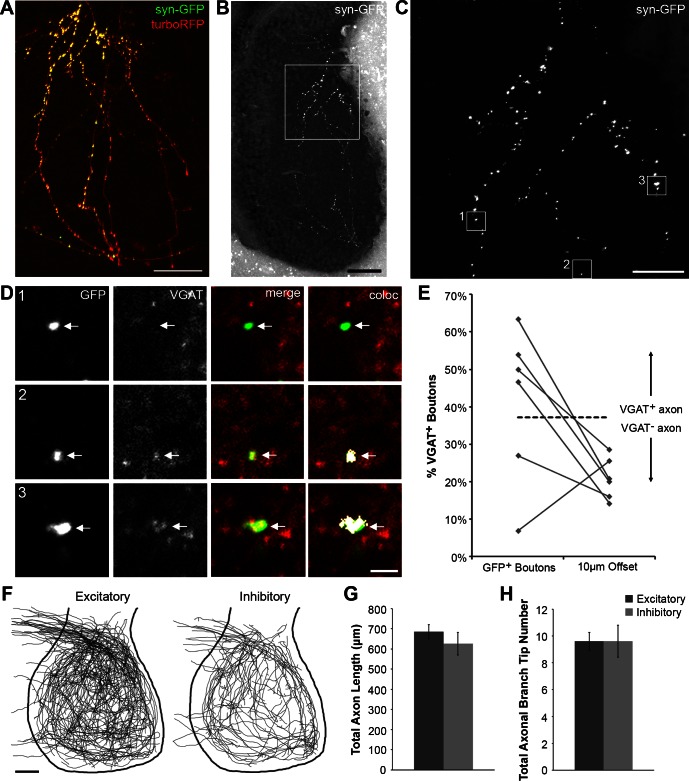
Fig. 4.
Immunohistochemistry and morphometrics of excitatory and inhibitory intertectal axons. A: z-projection of synaptophysin-GFP (syn-GFP) and turboRFP-expressing intertectal axons collected using in vivo 2-photon imaging. Scale bar = 25 μm. B: low-magnification confocal z-projection of imaged syn-GFP-expressing axons following fixation, sectioning, and VGAT immunohistochemistry was used to identify the axons imaged in A. Scale bar = 50 μm. C: high-magnification confocal z-projection of syn-GFP-expressing axons. Corresponds to boxed region in B. Scale bar = 20 μm. D: enlarged, single-optical sections of syn-GFP (green) and VGAT immunostaining (red) corresponding to boxed regions in C. Colocalization between GFP and VGAT was determined using the Colocalization plugin for ImageJ. In the “coloc” images (right), regions of colocalization between GFP and VGAT are shown as white pixels. The bouton in box 1 is VGAT− (excitatory), whereas boutons in boxes 2 and 3 are VGAT+ (inhibitory). Scale bar = 2 μm. Arrows: syn-GFP+ boutons. E: the percentage of boutons that are VGAT+ is quantified for each axon. The percentage of VGAT+ boutons for 6 imaged axons is shown to the left. To determine a cutoff point at which to call an axon excitatory or inhibitory, we offset GFP and VGAT channels by 10 μm for these 6 imaged axons and determined the background level of GFP and VGAT colocalization (right). Using the mean + 3SD from this offset data, a cutoff value of 37.25% VGAT+ boutons was set for VGAT positivity (dashed line). F: overlays of z-projected reconstructions of excitatory (n = 73) and inhibitory (n = 31) GFP-expressing intertectal axons collected in vivo with a 2-photon microscope. Excitatory and inhibitory axons have a similar distribution within the tectum. Scale bar = 50 μm. G: total axon length is similar for excitatory (685.64 ± 34.1 μm) and inhibitory (625.98 ± 56.87 μm) axons (P = 0.35). H: total axonal branch tip number is similar for excitatory (9.60 ± 0.66) and inhibitory (9.61 ± 1.20) axons (P = 0.99). Data are presented as means ± SE.
Morphometrics of excitatory and inhibitory axons.
To examine the morphologies of excitatory and inhibitory intertectal axons, one tectal lobe of stage 46 animals was electroporated with 1 μg/μl pα-actin::gal4-UAS::eGFP, 1 μg/μl pCMV::gal4 + 1 μg/μl pUAS::eGFP, or 1 μg/μl pVGAT::gal4 + 1 μg/μl pUAS::eGFP. Seven to eleven dfe animals with sparse labeling of intertectal axons were imaged on a custom-built two-photon microscope with a ×20 (0.95 NA) water-immersion lens at 2× or 2.5× digital scan zoom. Immediately following imaging, animals were fixed and processed for post hoc VGAT immunostaining. Intertectal axons were traced and reconstructed using Imaris software (Bitplane). Total axon length and total axonal branch tip number were quantified blind to neurotransmitter phenotype.
Neurofilament immunohistochemistry.
Tadpoles between developmental stages 32 and 41 were anesthetized with 0.02% MS222, immersed in 4% paraformaldehyde, and fixed using two bouts of microwave fixation at 150 W for 1 min followed by overnight fixation at 4°C. Brains were dissected, incubated in 100% methanol for 1 h at −20°C, and then rehydrated in a methanol gradient. Brains were blocked and permeabilized in 5% normal donkey serum and 2% Triton X-100 for 1 h at room temperature. Then, brains were incubated in 1:500 mouse anti-neurofilament-M (cat. no. 13-0700; Invitrogen) for 3 days at 4°C followed by 2 h in 1:200 anti-mouse Alexa Fluor 488 (Life Technologies) at room temperature. Brain were mounted in 6 M urea in 50% glycerol to clear the tissue partially and imaged whole mount with an Olympus FluoView 500 confocal microscope. Confocal z-stacks were acquired with a ×20 (0.8 NA) oil-immersion lens.
In vivo time-lapse imaging of intertectal axon bouton experience-dependent plasticity.
To visualize the synaptic boutons of intertectal axons, one tectal lobe of stage 46 animals was electroporated with 1 μg/μl pVGAT::gal4 + 2 μg/μl pUAS::synaptophysin-GFP + 1 μg/μl pUAS::turboRFP. Synaptophysin-GFP labeled presynaptic boutons (Ruthazer et al. 2006), and cytosolic turboRFP labeled the entire axonal arbor. Seven days following electroporation, animals with sparse labeling of intertectal axons were subjected to a visual stimulus protocol (Sin et al. 2002) and imaged on a custom-built two-photon microscope with a ×20 (0.95 NA) or ×25 (1.05 NA) water-immersion lens. First, a 512- × 512-pixel image of the entire axon was acquired, and then a region of the axonal arbor was selected and imaged at 1,024 × 1,024 pixels with 2–3× digital scan zoom (T1). Then, tadpoles were placed in the dark for a period of 4 h, and the selected axonal subregion was imaged again immediately thereafter (T2). Finally, animals were exposed to a simulated motion stimulus for 4 h. We refer to this stimulus period as short-term visual enhancement (STVE; Sin et al. 2002). Intertectal axons were imaged again immediately following STVE (T3). To compare boutons from single axons over time, the same imaging parameters were used for all three images. Immediately following imaging, animals were fixed for post hoc VGAT immunostaining. For presentation purposes, autofluorescing skin cells were removed from the images in Fig. 7B.
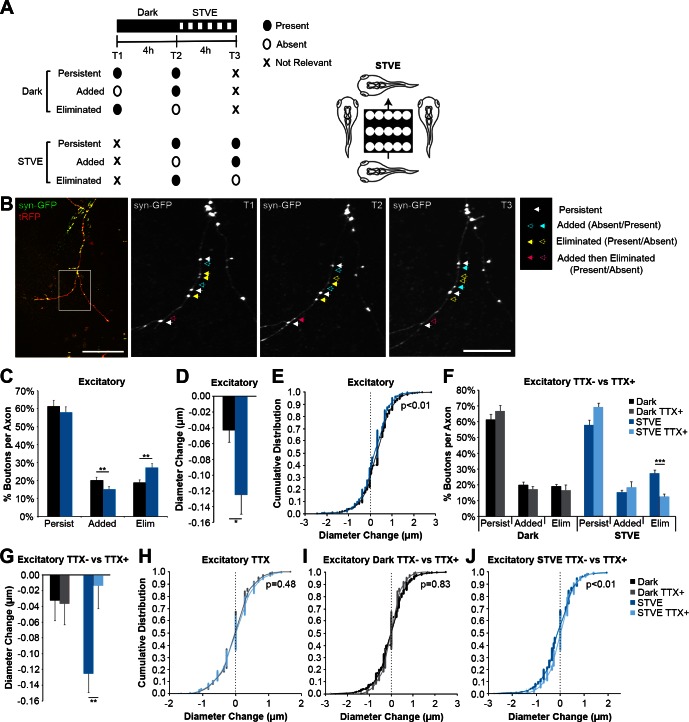
Fig. 7.
Visual experience induces structural plasticity of excitatory intertectal axon boutons in vivo. A: experimental protocol: 3 in vivo time-lapse 2-photon images were collected before (T1) and after (T2) free-swimming tadpoles were exposed to 4 h in the dark and again after exposure to 4 h of short-term visual enhancement (STVE; T3; left). Bouton dynamics were compared across the 3 time points. Some experiments were performed in the presence of tetrodotoxin (TTX) to uncouple bouton dynamics from visual stimulation. B: 2-photon z-projection of an intertectal axon expressing synaptophysin tagged with GFP (syn-GFP) and cytoplasmic turboRFP (tRFP). Scale bar = 100 μm. The boxed region was imaged at higher magnification (right). Scale bar = 25 μm. Bouton dynamics are highlighted with arrows. Closed and open arrows indicate the presence or absence of a bouton, respectively. White arrows: persistent through all time points; blue arrows: added; yellow arrows: eliminated; pink arrows: added and then eliminated. C: quantification of the percentage of excitatory boutons that persist, are added, or are eliminated during dark or STVE. STVE decreases addition (20.0 ± 1.9 vs. 15.1 ± 1.6%) and increases loss (18.8 ± 1.6 vs. 27.2 ± 2.3%) of excitatory boutons compared with dark (n = 21 axons; **P < 0.01). D and E: quantification of the average change in diameter (D) and the cumulative distribution of diameter changes (E) of persistent excitatory boutons. Persistent excitatory boutons shrink in STVE compared with dark (D; −0.125 ± 0.024 vs. −0.032 ± 0.026 μm; *P < 0.05), and the shift in distribution demonstrates decreases in the size of growing boutons and shrinking boutons (E; n = 639 boutons). F: TTX eliminates the difference in additions (17.3 ± 1.9 vs. 18.4 ± 3.7%, dark vs. STVE; P = 0.82) and subtractions (16.6 ± 3.4 vs. 12.6 ± 1.6%, dark vs. STVE; P = 0.42) of excitatory boutons with STVE (gray vs. light blue, n = 8 axons). TTX blocks the STVE-induced increase in excitatory bouton eliminations (12.6 ± 1.6 vs. 27.2 ± 2.3%, TTX+ vs. TTX−, light blue vs. dark blue; ***P < 0.0001). TTX− data are the same as in C. G: in TTX, changes in excitatory bouton diameter are comparable in dark vs. STVE (−0.037 ± 0.027 vs. −0.014 ± 0.029 μm, gray vs. light blue; P = 0.62; n = 314 boutons). TTX blocks STVE-induced shrinkage of excitatory boutons (−0.014 ± 0.029 vs. −0.125 ± 0.024 μm, TTX+ vs. TTX−, light blue vs. dark blue; **P < 0.01). TTX− data are the same as in D. H: TTX eliminates the difference in the distribution of excitatory bouton diameter changes between dark and STVE (n = 314 boutons). I and J: cumulative distribution plots of the diameter changes for all persistent excitatory boutons in the dark (I) or STVE (J) in the presence or absence of TTX. There is no difference in the distribution of diameter changes in the dark in the presence or absence of TTX (I). TTX blocks the leftward shift in diameter of shrinking excitatory boutons in STVE (J). TTX− data are the same as in E, and TTX+ data are the same as in H. Data in bar graphs are means + SE. Individual data points are presented in cumulative distribution plots. A Bonferroni correction was used for multiple comparisons.
To identify and measure boutons at each time point, we used the 3D Objects Counter plugin for ImageJ. The background fluorescence intensity for each axon was measured along the axonal backbone, and the threshold was set as 2× background. Each object (bouton) with at least two neighboring pixels above threshold was identified (pixel sizes ranged from 0.23 to 0.35 μm depending on the combination of objective and digital scan zoom). Then, images were compared across time points to identify boutons that were present in all time points or were newly added or eliminated. We examined the dark period (T1 to T2) and the STVE period (T2 to T3) and quantified the fraction of boutons during each time period that were added, eliminated, or persisted (Fig. 7A). These data are graphed as the average percentage of boutons per axon. The diameters of boutons present at each time point were measured at the widest point of each bouton. For persistent boutons, we quantified the change in bouton diameter during the dark (T2 − T1) or STVE (T3 − T2). These data are graphed as the average diameter change for all persistent boutons. To determine the amount of bouton change that occurs in the absence of activity, we repeated the experiment in the presence of 1 μM tetrodotoxin (TTX). For these experiments, TTX was introduced immediately after the baseline image and was present during the dark and STVE periods and during imaging time points 2 and 3.
Electrophysiology.
Following anesthesia in 0.2% MS222, brains were removed and cut along the ventral midline to expose the tectal cell layers while preserving the intertectal axons. For experiments that required severed intertectal connections, the preparation was the same with the exception that brains were cut along the dorsal midline.
The dissected preparation was placed in room temperature extracellular saline (115 mM NaCl, 4 mM KCl, 3 mM CaCl2, 0.5 mM MgCl2, 5 mM HEPES, 10 mM glucose, pH 7.2, osmolality of 255 mosmol/kgH2O). When appropriate, toxins were added to the bath [100 μM picrotoxin (PTX), 20 μM NBQX, 9 μM ifenprodil; Tocris]. Electrical stimulation was done using bipolar electrodes (FHC, Bowdoin, ME) placed in either the opposite tectal hemisphere or the lateral optic tract. Responses to electrical stimulation were collected in voltage-clamp configuration at 0.05 Hz. Recordings containing backpropagated action potentials, as distinguished by time course and waveform, were discarded. Recordings were made using glass micropipettes (5–15 MΩ) containing a Cs-based internal solution (80 mM CsMethSul, 5 mM MgCl2·6H2O, 20 mM TEA-Cl, 10 mM EGTA, 20 mM HEPES, 2 mM ATP, 0.3 mM GTP, 7.2 pH, 255 mosmol/kgH2O). Input and series resistance were monitored for the duration of the recording.
Data were acquired and analyzed using MultiClamp 700A, Clampex 9.2, and Clampfit 10.2 (Molecular Devices).
Statistical analysis.
All data were normally distributed. All data are presented as means ± SE or individual data points. Statistical differences between mean values were determined using two-tailed Student's t-tests or ANOVA followed by post hoc Tukey. Where noted, a Bonferroni correction for multiple comparisons was used following t-test. Statistical differences between data distributions were determined using χ2 tests. Where noted, a Bonferroni correction for multiple comparisons was used following χ2.
RESULTS
The anatomic development of intertectal axons.
To assess the contribution of intertectal axons to the visual circuit, we characterized their anatomic and synaptic properties. To visualize intertectal axons, we electroporated neurons in one tectal lobe with GFP expression plasmids in stage 46 tadpoles and fixed tadpoles after 7 days. Confocal images of fixed whole mount brains indicated that intertectal axons cross between the tectal lobes on the dorsal surface of the brain and broadly innervate the contralateral tectum (Fig. 1, A and B). At the stages we study, a majority of intertectal axons cross in the anterior commissure. Neurofilament immunostaining of axons in whole mount tadpole brains indicated that axons can be detected in the anterior commissure as early as stage 33/34, and a band of commissural axons was present by stage 40 (Fig. 1, C and D). We next characterized the structural development of intertectal axons to determine when they might contribute to visual circuit function. We used in vivo two-photon time-lapse imaging of sparsely labeled intertectal axons to determine the timing of their elaboration in contralateral tectum. At 4 days following electroporation in stage 46 tadpoles, we detected intertectal axons in the contralateral tectum (Fig. 1E). Intertectal axon length (Fig. 1F) and branch tip number (Fig. 1G) increased over the next 4 days, when animals had reached stage 48 and single intertectal axons expanded over the neuropil. The presence of intertectal axon innervation at such an early stage suggests that intertectal axons are an important source of tectal input from the onset of visual circuit development.
Tectal innervation by intertectal and retinotectal inputs.
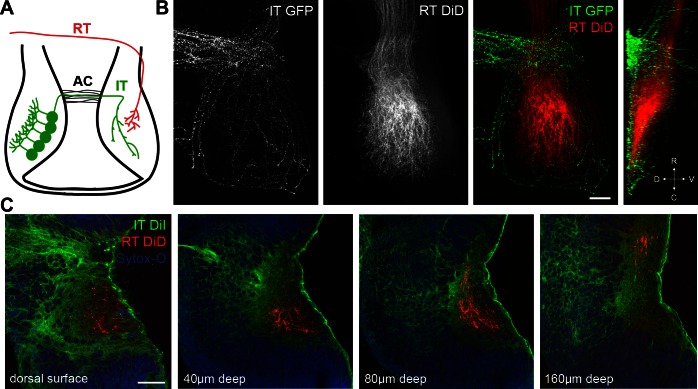
To assess convergence between intertectal and retinotectal axons, we performed dual-labeling experiments. We electroporated the left tectal lobe of stage 46 tadpoles with GFP expression constructs, fixed tadpoles after 7 days, and injected DiD into the left eye to label the retinotectal projection (Fig. 2A). Confocal images of the contralateral optic tectum show that intertectal axons terminate in a shell surrounding retinotectal axons (Fig. 2B). A y-projection shows that intertectal axons are dorsal and ventral to retinotectal axons (Fig. 2B, far right). To label a more complete population of intertectal axons, we injected DiI and DiD into the left tectum and left eye, respectively, of fixed stage 46 tadpoles and imaged the right tectum of whole mount brains. Intertectal axons completely surround retinotectal axons on the dorsal, medial, and ventral sides of the tectal neuropil (Fig. 2C and Supplemental Video S1, available in the data supplement online at the Journal of Neurophysiology Web site). Since most postsynaptic tectal neurons extend their dendrites from the medial cell body layer toward the neuropil in lateral tectum, this axon projection pattern suggests that tectal neurons receive convergent inputs from the retina and contralateral tectum. Furthermore, the contacts may be spatially segregated on the dendritic arbor with intertectal synapses on proximal dendrites and retinotectal synapses on more distal dendrites.
Fig. 2.
Tectal innervation by intertectal and retinotectal axons. A: schematic showing tectal neurons projecting intertectal (IT) axons across the anterior commissure (AC) and converging with retinotectal (RT) axons in the contralateral tectal lobe. B: confocal z- and y-projections (far right) of GFP-expressing intertectal axons and DiD-labeled retinotectal axons imaged in stage 48 whole mount brain. Intertectal axons create a shell surrounding retinotectal axons. D, dorsal; V, ventral; R, rostral; C, caudal. C: single-optical sections of DiI-labeled intertectal axons, DiD-labeled retinotectal axons, and SYTOX Orange Nucleic Acid Stain (Sytox-O) imaged at stated depths from the tectal surface in whole mount brain. Intertectal axons are dorsal, medial, and ventral to RT axons. Full z-stack is in Supplemental Video S1. Scale bars = 50 μm.
Distribution of excitatory and inhibitory intertectal neurons and axons.
Inhibitory and excitatory neurons in the midbrain are generated in a common proliferative zone and integrate into the optic tectal circuit at the same time (Akerman and Cline 2006; Muldal et al. 2014). About 30% of optic tectal neurons are GABAergic (Antal 1991; Miraucourt et al. 2012), as seen in other brain regions and other species (Caputi et al. 2013; Mize 1992). To determine the percentage of intertectal neurons that are inhibitory, we used retrograde tracing with glycoprotein (G)-deleted rabies virus in combination with GABA immunohistochemistry. Previous experiments indicated that GABA− tectal neurons are predominantly excitatory (Miraucourt et al. 2012). We injected SAD-ΔG-eGFP into the left tectal lobe of stage 46–47 tadpoles and identified eGFP+ retrogradely labeled intertectal neurons in the contralateral tectum 7 days later using in vivo confocal imaging (Fig. 3, A and B). Using post hoc GABA immunohistochemistry, we found that 37.3% of eGFP+ intertectal neurons are inhibitory (22/59 cells in 17 tadpoles; Fig. 3, C and D). The retrogradely labeled excitatory and inhibitory intertectal neurons were similarly distributed throughout the cell body layer of the optic tectum, with a few cells located in the tectal neuropil (Fig. 3E).
Fig. 3.
Intertectal neurons are excitatory and inhibitory. A: schematic showing injection of G-deleted rabies virus expressing eGFP (SAD-ΔG-eGFP) into the left tectal lobe and retrograde labeling of local (gray) and intertectal (IT; green) neurons. B: montage of z-projections of SAD-ΔG-eGFP-expressing retrogradely labeled neurons collected using in vivo confocal imaging. Seven days following injection of SAD-ΔG-eGFP in the left tectum, eGFP+ neurons were visible in the left (locally projecting) and right (intertectally projecting) tectal lobes. Intertectal neurons in boxed regions 1 and 2 are shown in C. Dashed lines mark the boundary of the optic tectum. Scale bar = 50 μm. C: confocal images of eGFP+ neurons following sectioning and post hoc GABA immunohistochemistry. Left: low-magnification confocal z-projections from 40-μm sections of SAD-ΔG-eGFP-injected optic tectum from animal in B. Boxed cells in images 1 and 2 correspond to cells identified using in vivo confocal imaging in B. Scale bar = 50 μm. Right: high-magnification, single-optical sections of boxed neurons (arrows) show eGFP+ intertectal neurons (green) and GABA immunostaining (red). Cell 1 is GABA− (excitatory), and cell 2 is GABA+ (inhibitory). Scale bar = 25 μm. D: quantification of the percentage of retrogradely labeled intertectal neurons that are excitatory and inhibitory. These percentages are similar to the proportions of excitatory and inhibitory cells present within the optic tectum as a whole. n = 59 Cells from 17 animals. E: retrogradely labeled excitatory and inhibitory intertectal neurons are distributed throughout the tectum in overlapping regions. Few neurons are located in the tectal neuropil (shaded region).
To determine whether there were gross morphological differences between excitatory and inhibitory intertectal axons, we combined in vivo two-photon imaging of GFP-expressing intertectal axons with post hoc immunostaining for vesicular GABA transporter (VGAT) to label inhibitory presynaptic boutons (Fig. 4, A–E). We electroporated neurons in one tectal lobe with cytoplasmic GFP expression plasmids or cytoplasmic turboRFP and synaptophysin tagged with GFP to label presynaptic boutons in stage 46 tadpoles and performed in vivo two-photon imaging of fluorescent protein-expressing intertectal axons 7–11 days later (Fig. 4A). Following post hoc VGAT immunohistochemistry, low-magnification images were acquired to determine the location of the GFP+ axon from in vivo imaging (Fig. 4B). Then, high-magnification images of GFP and VGAT channels were acquired, and the percentage of GFP+ boutons that colocalized with VGAT was quantified using the Colocalization plugin for ImageJ (Fig. 4D; see materials and methods). To determine a cutoff point at which to call an axon excitatory (VGAT−) or inhibitory (VGAT+), we took six sets of images and offset the GFP and VGAT channels by 10 μm to assess the amount of colocalization that occurs at random (Fig. 4E). We found that the mean percentage of VGAT+ boutons for the offset images was 20.85%. Using this mean + 3SD [20.85% + (3 × 5.47%)], we set a cutoff for VGAT positivity at 37.25%. Therefore, any axon with a %value of VGAT+ boutons >37.25% was deemed inhibitory (VGAT+), and any below that cutoff was deemed excitatory (VGAT−). Each axon that was imaged in vivo was reconstructed in three dimensions and categorized as excitatory or inhibitory based on its VGAT expression. Overlays of all imaged axons show that both excitatory and inhibitory axons cover the full extent of the tectal neuropil (Fig. 4F). The projection pattern, axon length, and axon branch tip number were similar for excitatory and inhibitory intertectal axons (Fig. 4, F–H).
Taken together, these experiments demonstrate that the proportion of excitatory and inhibitory intertectal neurons is comparable to the proportion of the overall population of excitatory and inhibitory neurons in the optic tectum (Miraucourt et al. 2012). Furthermore, excitatory and inhibitory intertectal neurons are located throughout the tectum in overlapping populations. Finally, the axonal arbors of excitatory and inhibitory intertectal neurons are similar in size and distribution and cover the full extent of the tectal neuropil.
The functional development of intertectal input.
The structural data in Figs. 3 and 4 indicate that intertectal axons carry both excitatory and inhibitory information. Next, we used electrophysiology to investigate the functional composition of the intertectal postsynaptic currents (PSCs) by testing the sensitivity to GABA receptor and AMPA receptor antagonists, using PTX and NBQX. Thus far, all electrophysiological recordings of tadpole tectal neurons have been done in a preparation in which the dorsal midline, including the intertectal commissure, is cut to expose tectal cell somata for recording, so there has been no electrophysiological study of intertectal connections (Aizenman et al. 2002, 2003; Aizenman and Cline 2007; Akerman and Cline 2006; Bestman and Cline 2008; Ciarleglio et al. 2015; Pratt and Aizenman 2007; Pratt et al. 2008; Tao and Poo 2005; Wu et al. 1996). We developed an electrophysiological preparation in which the ventral side of the midbrain is cut to expose tectal cell bodies for recording, thereby preserving intertectal fibers. PSCs recorded in tectal neurons held at 0 mV, in response to stimulation of the contralateral lobe of the tectum, decreased in PTX (Fig. 5A). Responses recorded at −60 mV decreased in NBQX (Fig. 5B), indicating both GABAergic and glutamatergic axons cross between the hemispheres and synapse onto tectal neurons.
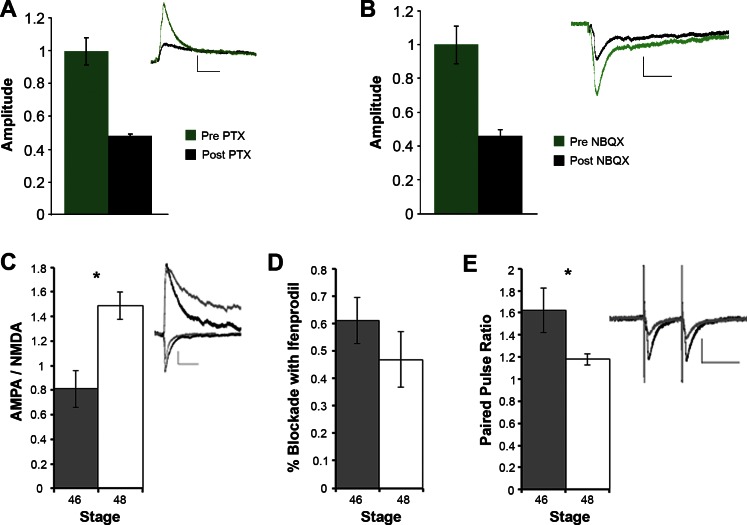
Fig. 5.
Development of intertectal axons and their synapses. A: intertectal synaptic current is sensitive to picrotoxin (PTX). Tectal neurons were recorded from in whole cell, voltage-clamp configuration. Membrane potential was held at 0 mV, and 20 μM NBQX was added to the bath to isolate inhibitory components of the postsynaptic current (PSC). A bipolar electrode in the opposite tectal hemisphere was used to generate PSCs. A baseline of 50 traces was recorded (inset shows averaged responses, green trace). Then, 100 μM PTX was added to the bath. The cell was allowed to incubate in PTX for 10 min before recording was resumed. Inset black trace shows averaged response after PTX addition. The amplitude of the PSC was measured and is shown normalized to baseline (normalized baseline: 1.00 ± 0.08; +PTX: 0.48 ± 0.06; n = 12 cells, scale bar = 50 ms, 10 pA). B: intertectal synaptic current is sensitive to NBQX. A 2nd set of tectal neurons was recorded at −60 mV in the presence of 100 μM PTX. Baseline traces were collected as in A, and then 20 μM NBQX was added to the bath and allowed to circulate for 10 min. Inset: averaged traces before (green) and after (black) NBQX. Amplitudes of the PSCs were averaged and normalized to baseline (normalized baseline: 1.00 ± 0.11; +NBQX: 0.46 ± 0.04; n = 25 cells, scale bar = 50 ms, 20 pA). C: developmental increase in AMPA/NMDA postsynaptic currents (PSCs) in response to stimulating intertectal axons. PSCs were measured at −60 mV (AMPA, peak taken 10–30 ms following stimulus artifact) and +40 mV (NMDA, peak taken at >50 ms following stimulus artifact). AMPA/NMDA (absolute value of measured AMPA current amplitude divided by NMDA current amplitude) increased significantly from stage 46 (n = 9) to stage 48 (n = 33); *P < 0.05. Inset: example traces of currents recorded at −60 and +40 mV in stage 46 (gray) and stage 48 (black) neurons. Scale bar = 20 ms/50 pA. D: no developmental change in the GluN2B component of intertectal NMDAR-mediated currents. Pharmacologically isolated NMDAR-mediated responses were recorded at +40 mV before and after exposure to ifenprodil. Current amplitudes were normalized to baseline and are presented as %blockade (1 − remaining amplitude). The proportion of ifenprodil-sensitive NMDAR-mediated current was not different between stage 46 (n = 6) and stage 48 (n = 6). E: developmental decrease in paired-pulse ratio. Cells from stage 48 tadpoles (n = 13) showed significantly less facilitation (*P < 0.05) than at stage 46 (n = 17) in response to paired stimuli (50-ms interstimulus interval) to intertectal axons. Inset: example traces showing paired-pulse ratio responses in stage 46 (gray) and stage 48 (black) tadpoles. Scale bar = 20 ms/50 pA. Data are presented as means ± SE.
We then assessed the functional development of intertectal synapses. The electrophysiological maturation of retinotectal synapses is characterized by an increase in the ratio of AMPA to N-methyl-d-aspartate (NMDA) currents, a switch in GluN2 subunit composition, and changes to the paired-pulse ratio (Aizenman and Cline 2007; Ruthazer and Aizenman 2010; Wu et al. 1996). To determine whether glutamatergic intertectal synapses follow a similar progression compared to retinotectal synapses, we recorded intertectal PSCs at stages 46 and 48 (Fig. 5C). AMPA-to-NMDA ratios increased significantly between stages 46 and 48, consistent with the incorporation of AMPA receptors into developing intertectal synapses. Ifenprodil, a GluN2B-specific antagonist, blocked 61 ± 8.5% of isolated NMDA receptor (NMDAR)-mediated currents at stage 46 and 47 ± 10.1% at stage 48, indicating that intertectal synapses maintain a significant population of GluN2B-containing NMDARs over this developmental window (Fig. 5D). Finally, paired-pulse facilitation in intertectal synapses decreased between stages 46 and 48 (Fig. 5E). These data indicate that glutamatergic intertectal inputs follow a similar maturation program as seen in retinotectal inputs.
Intertectal and retinotectal inputs converge onto optic tectal neurons.
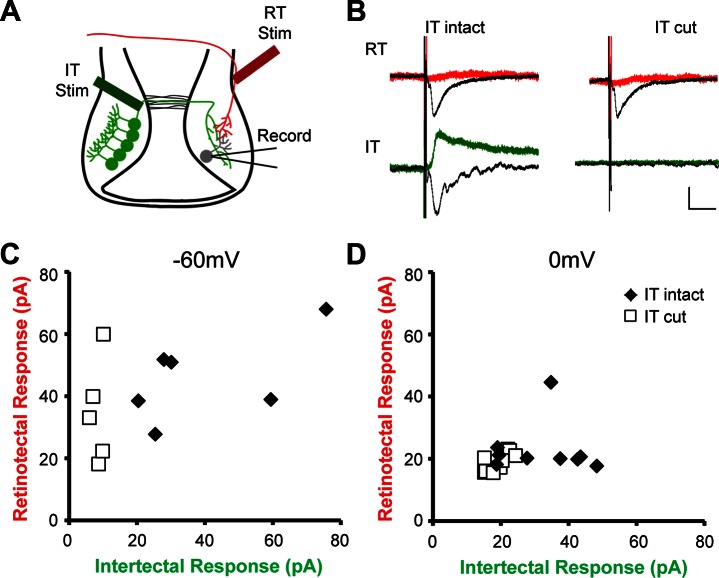
To test whether intertectal and retinotectal inputs converge onto common postsynaptic tectal neurons, we recorded PSCs in tectal neurons while stimulating the intertectal commissure and retinotectal axons in the optic tract (Fig. 6A). In preparations with the intertectal commissure intact, we recorded PSCs in response to stimulating both intertectal and retinotectal fiber populations in the same postsynaptic neurons. In addition, we occasionally observed retrograde action potentials, identified by their distinct waveform, in response to intertectal stimulation, indicating that tectal neurons that receive convergent retinotectal and intertectal inputs also project axons across the intertectal commissure. However, for analysis presented here and all further experiments, recordings containing retrograde action potentials were discarded because they can interfere with analysis of synaptic responses. When the intertectal commissure was cut, responses to intertectal stimulation were lost, whereas responses to retinotectal stimulation persisted (Fig. 6, B–D). PSCs generated in response to optic tract (retinotectal input) stimulation were excitatory (Fig. 6B, black trace examples), but PSCs recorded at 0 mV, in an attempt to isolate inhibitory responses, were not significantly different from noise (Fig. 6B, red lines), consistent with solely excitatory input from the retina.
Fig. 6.
Intertectal axons provide convergent input onto retinorecipient tectal neurons. A: tectal neurons receive direct intertectal (IT) and retinotectal (RT) input. Schematic of the tectal circuit showing retinotectal (red) and intertectal (green) axons, the placement of bipolar stimulating (Stim) electrodes, and the location of recorded tectal neurons (gray). B: averaged example traces showing postsynaptic response of a cell to intertectal and retinotectal stimulation, with intertectal commissure intact and cut. Black traces show PSCs recorded at −60 mV; red/green traces show PSCs recorded at 0 mV. Current following the initial peak response is due to recurrent activity in the tectum. Both components of the intertectal response are lost when the commissure is cut. Stimulation artifact shown, scale bar = 20 ms/20 pA. C: convergent retinotectal and intertectal excitatory response amplitudes. Each data point shows the amplitude (in picoamperes) of synaptic responses of a single cell (whole cell, voltage-clamp at −60 mV) to intertectal and retinotectal stimulation with the intertectal commissure intact (n = 6) or cut (n = 5). Intertectal response amplitudes in the cut preparation were not significantly different from noise. D: convergent retinotectal and intertectal inhibitory response amplitudes. Individual data points show amplitude (in picoamperes) of synaptic responses as above but recorded at 0 mV. Commissure intact n = 18; cut n = 24. RT response amplitude in both conditions and intertectal response in cut condition were not significantly different from noise.
In contrast, stimulation of intertectal fibers generated robust responses at both −60 mV (Fig. 6B, black line) and 0 mV (Fig. 6B, lower left, green line). Both responses were lost in preparations in which the intertectal commissure was cut (Fig. 6B, lower right). PSCs in response to both retinotectal and intertectal stimulation had a fast, low-variability time course, consistent with a monosynaptic connection. These results demonstrate that intertectal axons provide convergent excitatory and inhibitory input onto retinorecipient tectal neurons via the intertectal commissure.
Together, these data indicate that intertectal neurons extend axons in the contralateral tectum at the onset of visual circuit formation. Intertectal synapses mature during a crucial developmental window in which visually guided behavior emerges (Dong et al. 2009), by following a maturation program similar to glutamatergic synapses in other systems. The extensive elaboration of intertectal axons and the retention of GluN2B-containing receptors in their synapses make intertectal input a strong candidate for mediating visual circuit plasticity.
Visual experience drives distinct structural plasticity in excitatory and inhibitory intertectal axons.
Exposing free-swimming tadpoles to 4 h of dark followed by 4 h of short-term visual enhancement (STVE) using a simulated motion stimulus induces structural and functional experience-dependent plasticity of retinotectal connections (Aizenman and Cline 2007; Haas et al. 2006; Ruthazer et al. 2006; Sin et al. 2002). For retinotectal axons, which are highly branched, experience-dependent changes in synaptic connectivity occur via growth and retraction of terminal axon branches (Ruthazer et al. 2006). Given the relatively sparse branching pattern of intertectal axons and the fact that locally projecting tectal axons have developmental increases in synapse density preferentially on stable axon branches (Li et al. 2011), we hypothesized that experience-dependent changes in intertectal synaptic connectivity may occur en passant. Therefore, we tested whether intertectal synaptic boutons undergo dynamic changes in response to sensory input.
We labeled intertectal axons and presynaptic boutons by electroporating one tectal lobe of stage 46 tadpoles with plasmids expressing cytoplasmic turboRFP (tRFP) and synaptophysin tagged with GFP (syn-GFP) and collected in vivo time-lapse two-photon images of syn-GFP+ intertectal axon boutons 7 days later (Fig. 7, A and B). Previous electr on microscopy studies indicated that 95% of syn-GFP boutons are bona fide synapses (Ruthazer et al. 2006). We exposed free-swimming tadpoles to the 4-h dark-4-h STVE visual stimulus paradigm and analyzed intertectal bouton dynamics over time (Fig. 7A). We quantified the percentage of boutons that persisted, were added, or were eliminated during dark and STVE (Fig. 7, A and B). In addition, we measured the diameter of persistent boutons to ascertain whether they grow or shrink in response to visual stimulation. Next, we used post hoc VGAT immunohistochemistry to determine the neurotransmitter expression of each axon (Fig. 4) and assess whether visual activity affects excitatory and inhibitory intertectal axon boutons in similar or different ways. Finally, we performed the 4-h dark-4-h STVE visual stimulation paradigm in the presence of 1 μM tetrodotoxin (TTX) to block action potentials and assess whether intertectal axons respond to both dark and STVE as salient forms of visual input.
For excitatory intertectal axons, increased visual input during STVE decreased bouton additions and increased bouton eliminations compared with dark (Fig. 7C). Excitatory boutons that persisted through all three time points shrank more on average in STVE compared with dark (Fig. 7D). As expected, we found that persistent boutons are significantly larger than boutons that have just been added or will be eliminated in the subsequent time point (data not shown). We plotted diameter changes as a cumulative distribution to assess whether increased average bouton shrinkage was due to changes in shrinking or growing boutons. We found both an increase in bouton shrinkage and a decrease in bouton growth in STVE compared with dark (Fig. 7E).
TTX eliminated the differences in excitatory bouton dynamics between dark and STVE (Fig. 7F). Bouton dynamics were similar in the dark whether TTX was present or absent, but TTX prevented the STVE-induced increase in excitatory bouton eliminations (Fig. 7F). TTX also eliminated the differences in persistent excitatory bouton diameter change between dark and STVE (Fig. 7, G and H). Furthermore, comparing TTX− and TTX+ groups demonstrated that the size of persistent boutons was similar in the dark (Fig. 7, G and I), but TTX blocked STVE-induced shrinkage of excitatory boutons (Fig. 7, G and J). Together, these results demonstrate that excitatory intertectal axon boutons are lost and decrease in size in response to enhanced visual input. Furthermore, these results suggest that STVE, but not dark, is an active form of stimulation for excitatory intertectal axons.
For inhibitory intertectal axons, STVE decreased bouton eliminations compared with dark (Fig. 8A), the opposite of what we observed for excitatory boutons. STVE also increased inhibitory bouton additions on axonal branch tips (data not shown) but not arbor-wide. On average, persistent inhibitory boutons grew in STVE compared with dark (Fig. 8B). Plotting the cumulative distribution of diameter changes reveals a decrease in bouton shrinkage and an even larger increase in bouton growth in STVE (Fig. 8C).
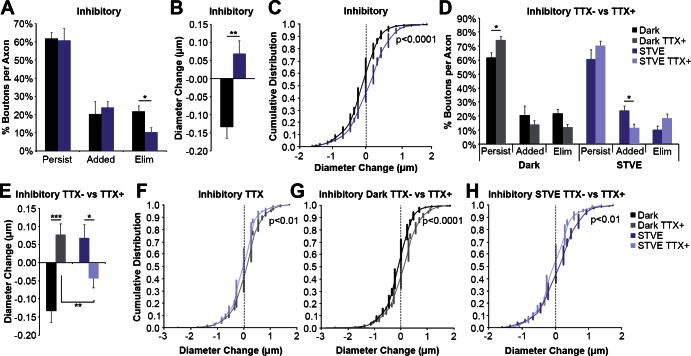
Fig. 8.
Visual deprivation and experience induce structural plasticity of inhibitory intertectal axon boutons in vivo. A: quantification of the percentage of inhibitory boutons that persist, are added, or are eliminated during dark or STVE. Inhibitory bouton loss is decreased in STVE (10.3 ± 2.6 vs. 21.5 ± 3.4%; *P < 0.05; n = 10 axons). B and C: quantification of the average change in diameter (B) and cumulative distribution of diameter changes (C) of persistent inhibitory boutons. Persistent inhibitory boutons grow in STVE compared with dark (B; 0.068 ± 0.037 vs. −0.133 ± 0.032 μm; **P < 0.01), and the shift in distribution is seen across bouton diameter changes but is greatest with growing boutons (C; n = 236 boutons). D: the difference in eliminations with visual experience in A is eliminated with TTX (12.0 ± 2.0 vs. 18.3 ± 2.5%, dark vs. STVE, gray vs. light purple; P = 0.14; n = 7 axons). Comparing TTX− and TTX+ demonstrates that bouton dynamics are different during both the dark and STVE. In the absence of TTX, fewer inhibitory boutons persist in dark (61.7 ± 3.4 vs. 74.3 ± 2.7%; *P < 0.05; black vs. gray) and more boutons are added in STVE (23.9 ± 3.4 vs. 11.5 ± 2.8%; *P < 0.05; dark purple vs. light purple). There are trends toward differences in eliminations between TTX− and TTX+ in both the dark (21.5 ± 3.4 vs. 12.0 ± 2.0%; P = 0.05; black vs. gray) and STVE (10.3 ± 2.6 vs. 18.3 ± 3.1%; P = 0.06; dark purple vs. light purple). TTX− data are the same presented in A. E: in TTX, there is a significant decrease in inhibitory bouton diameter change in STVE compared with dark (−0.043 ± 0.026 vs. 0.078 ± 0.030 μm; **P < 0.01; light purple vs. gray, n = 331 boutons). We interpret this as a homeostatic response to 8 h of TTX. Bouton diameter changes are different in both dark and STVE for TTX− vs. TTX+. TTX blocks dark-induced decrease in inhibitory bouton size (−0.132 ± 0.031 vs. 0.078 ± 0.030 μm; ***P < 0.0001; TTX− vs. TTX+, black vs. gray) and STVE-induced increase in inhibitory bouton size (0.068 ± 0.037 vs. −0.043 ± 0.026 μm; **P < 0.05; TTX− vs. TTX+, dark purple vs. light purple). TTX− data are the same as in B. F: in TTX, there is a leftward shift in the distribution of inhibitory bouton diameter changes in STVE, which we interpret as a homeostatic change in response to 8 h of TTX (n = 331 boutons). G and H: cumulative distribution plots of diameter changes for all persistent inhibitory boutons in dark (G) or STVE (H) in the presence or absence of TTX. There is a leftward shift in the distribution of diameter changes in dark in the absence of TTX, which is most apparent for growing boutons (G). There is a rightward shift of TTX− bouton diameter changes in STVE compared with TTX+ (H). This shift is also greatest for growing boutons. TTX− data are the same as in C, and TTX+ data are the same as in F. Data in bar graphs are means ± SE. Individual data points are presented in cumulative distribution plots. A Bonferroni correction was used for multiple comparisons.
TTX eliminated the differences in inhibitory bouton dynamics between dark and STVE (Fig. 8D). Comparing TTX− and TTX+ indicated that TTX increased the persistence of inhibitory boutons in the dark and decreased the addition of inhibitory boutons in STVE (Fig. 8D). Differences in bouton eliminations between TTX+ and TTX− groups nearly reached significance in both dark (P = 0.05) and STVE (P = 0.06). These data demonstrate that inhibitory intertectal axons respond to both dark and STVE as salient forms of visual input, and those responses are blocked by TTX. TTX also blocked the dark-induced shrinkage and STVE-induced growth of persistent inhibitory boutons (Fig. 8, E, G, and H). Interestingly, TTX did not eliminate the differences in bouton diameter change between dark and STVE. Instead, we observed growth of boutons during the 1st 4 h in TTX but a shrinkage in boutons during the 2nd 4 h of TTX (Fig. 8, E and F). This suggests that inhibitory axons were sensitive to the lack of activity over the course of 8 h and responded in a homeostatic manner with respect to bouton size. We did not see similar effects on excitatory boutons, suggesting that inhibitory axons may be more acutely sensitive to levels of network activity. Together, these results demonstrate that, in response to dark, inhibitory boutons shrink and are less persistent, with a trend toward increasing eliminations. In response to STVE, inhibitory bouton eliminations are decreased, bouton additions increase on axonal branch tips, and bouton growth increases.
These data show that synaptic connectivity of both excitatory and inhibitory intertectal axons can be modified by visual experience in vivo. Whereas excitatory axons only respond to increased visual stimulation, inhibitory axons respond actively to both decreased and increased visual stimulation. Interestingly, the valence of the response to STVE is opposite for excitatory and inhibitory boutons. Four hours of STVE increases loss of excitatory intertectal boutons and reduces loss of inhibitory intertectal boutons. Furthermore, STVE decreases addition of excitatory boutons arbor-wide and increases addition of inhibitory boutons specifically on axonal branch tips. Finally, STVE causes shrinkage of persistent excitatory boutons and growth of persistent inhibitory boutons. Together, these changes would be predicted to decrease excitatory intertectal output while increasing inhibitory intertectal output in response to enhanced visual stimulation. This outcome would drive changes in the E:I balance of intertectal input onto tectal neurons. Alternatively, the plasticity in bouton number described above might occur in concert with changes in efficacy of synaptic transmission that maintain the E:I balance of intertectal input. To elucidate the functional component of visual-experience-induced plasticity in intertectal connections and the effect of these changes on network function, we collected electrophysiological recordings from intertectal-recipient tectal neurons.
Visual experience potentiates excitatory and inhibitory intertectal synaptic input.
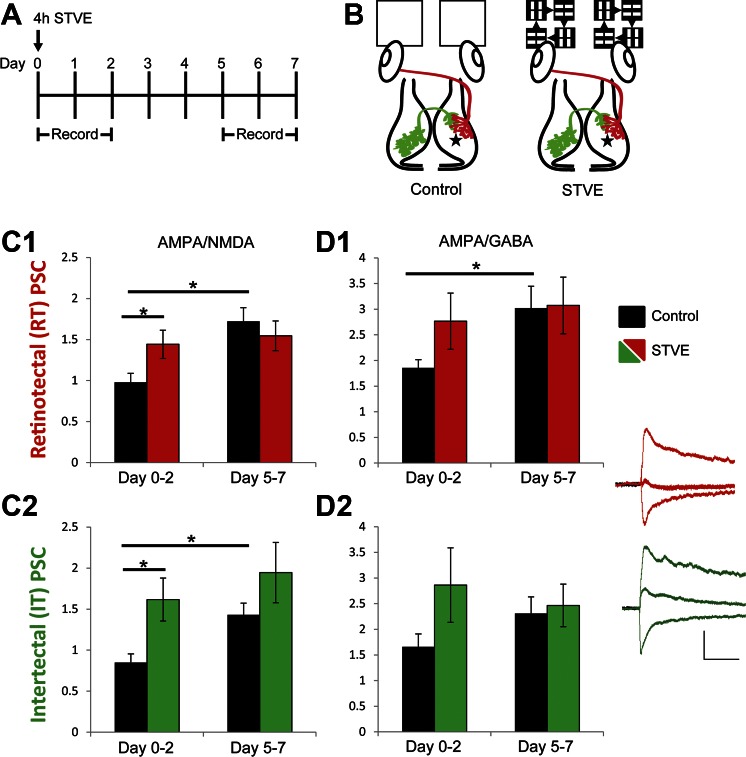
We recorded intertectally and retinotectally mediated PSCs in tadpoles that had been exposed to 4 h of STVE at stage 47. We recorded PSCs directly following STVE (∼1 h to 2 days afterward) to capture acute changes and at a later time point (5–7 days afterward) to determine the duration of functional change (Fig. 9A). We recorded PSCs at −60, 0, and +40 mV, which enabled us to examine shifts in both AMPA-to-NMDA and AMPA-to-GABA ratios. These ratios are indicative of the maturation of excitatory synapses (via addition of AMPA receptors) and the relative amounts of excitatory/inhibitory input. The use of ratios enabled comparison across multiple animals, and the ratios from STVE-treated tadpoles were compared with a control condition in which tadpoles were exposed only to the normal 12:12-h light-dark cycle (Fig. 9B). Like the STVE-treated tadpoles, tadpoles in the control condition were housed in groups and able to see other individuals swimming; their environment differed from the STVE-treated tadpoles only in that they did not receive the additional moving light stimulus.
Fig. 9.
Visual experience potentiates excitatory and inhibitory intertectal synaptic input. A: experimental timeline: STVE was provided to freely swimming animals on day 0. Animals were otherwise kept in a 12:12-h light-dark cycle. Recordings were made immediately after STVE on day 0 and on days 1–2 and 5–7. B: schematic of treatment groups. Control tadpoles received only ambient light. STVE tadpoles received STVE exposure for 4 h. Tectal cell recordings were made at −60, 0, and +40 mV in dissected brains with electrodes placed on retinotectal (red) or intertectal (green) axons to evoke PSCs. Recording location indicated with star. C: STVE increased retinotectal (C1) and intertectal (C2) AMPA-to-NMDA ratio compared with control at the days 0–2 time point, and no further increase in AMPA-to-NMDA ratio was seen at the later time point. Control conditions showed a developmental increase in AMPA-to-NMDA ratio from early to later time points. Summary data of PSC amplitudes at −60/+40 mV (+40-mV values were measured 50 ms after the peak). Data were pooled from days 0–2 and days 5–7 after STVE. Black bars = control [n = 20 (days 0–2), 12 (days 5–7)]. Colored bars = STVE [n = 12 (days 0–2), 11 (days 5–7)]. D: STVE does not affect AMPA-to-GABA ratio. Controls show a developmental increase in AMPA-to-GABA retinotectal ratios, where GABA currents are from intratectal recurrent inhibition (D1), whereas AMPA-to-GABA ratios in STVE were similarly increased at early and late time points. Intertectal AMPA-to-GABA ratios were not significantly different between early and late time points in either control or STVE conditions (D2), suggesting a concomitant increase in both AMPA receptor- and GABA receptor-mediated PSCs. Summary data of PSC amplitudes at −60/0 mV. Data pooled, n values, and color coding as in C. Inset traces show example retinotectal (red) and intertectal (green) PSCs from STVE-treated condition (days 0–2) at −60, 0, and +40 mV and demonstrate the robust excitatory component of both retinotectal and intertectal PSCs, the robust intertectal inhibitory response, and the small inhibitory component of the retinotectal response, most likely generated by contamination by recurrent inhibitory fibers. Scale bar = 50 ms/50 pA. Data are presented as means ± SE. *P < 0.05.
During the period between the initial and final data collection points, tadpoles undergo a developmental increase in the AMPA-to-NMDA ratio in both retinotectal and intertectal-recipient synapses. Control tadpoles increased retinotectal AMPA-to-NMDA ratio from 0.97 ± 0.11 at days 0–2 to 1.72 ± 0.17 at days 5–7 (Fig. 9C1, black bars), replicating data in Fig. 5. Over the same period, control tadpoles also increased the AMPA-to-NMDA ratio in intertectal synapses from 0.85 ± 0.10 to 1.43 ± 0.14 (Fig. 9C2, black bars). The AMPA-to-NMDA ratio of STVE-treated tadpoles was significantly larger than controls at the initial time point [retinotectal: 0.97 ± 0.11 (control) vs. 1.44 ± 0.17 (STVE), P = 0.03, Fig. 9C1; intertectal: 0.85 ± 0.11 (control) vs. 1.62 ± 0.26 (STVE), P = 0.01, Fig. 9C2], consistent with visual experience-dependent maturation of glutamatergic synapses, but the AMPA-to-NMDA ratios in STVE-treated tadpoles did not change between earlier and later time points for retinotectal or intertectal inputs.
Current ratios can also be used to examine shifts in the relative contributions of excitatory and inhibitory input. In control conditions, retinotectal responses, which are exclusively glutamatergic, show a developmental increase in the AMPA-to-GABA ratio (1.86 ± 0.16 to 3.02 ± 0.43; Fig. 9D1, black bars). The GABA receptor-mediated current is due to intratectal recurrent inhibitory connections (Pratt et al. 2008; Shen et al. 2011). The amplitude of the response at 0 mV did not change across the developmental window we studied, indicating the shift in ratio reflects an increase in AMPA receptor-mediated current. In the STVE condition, the AMPA-to-GABA ratio is comparable at days 0–2 and 5–7 (2.76 ± 0.55 to 3.07 ± 0.55; Fig. 9D1, red bars), consistent with the STVE-dependent increase in AMPA receptor-mediated current seen in Fig. 9C1. In control conditions, the AMPA-to-GABA ratio in intertectal inputs does not change over development (1.65 ± 0.25 to 2.31 ± 0.32; Fig. 9D2, black bars), which, together with the developmental increase in the AMPA-to-NMDA ratio, suggests a concomitant increase in both excitatory and inhibitory inputs. Although STVE-treated tadpoles show a trend of a larger AMPA-to-GABA ratio at days 0–2 compared with controls [1.65 ± 0.25 (control) vs. 2.86 ± 0.72 (STVE); Fig. 9D2], these values are not statistically significantly different. The trend toward an increased AMPA-to-GABA ratio and the increased variability of the ratio, as shown in the standard error, suggest that different postsynaptic cells receive differ amounts of inhibitory input.
These data indicate that STVE drives visual experience-dependent maturation of excitatory and inhibitory intertectal inputs, similar to that seen in excitatory retinotectal inputs (Aizenman and Cline 2007). This experience-dependent synaptic plasticity may function to maintain a balance of excitatory to inhibitory activity within the tectal network and allow the network to function in diverse sensory environments.
DISCUSSION
Development of intertectal communication.
In frogs, intertectal communication is accomplished by an indirect pathway through the nucleus isthmi that is only established after metamorphosis (Udin 2012). Here, we find that intertectal communication is established early in visual system development in tadpoles via a direct commissural projection. Retinal inputs begin to innervate the optic tectum at stage 39/40 of tadpole development, and retinotectal responses are apparent shortly thereafter (Gaze et al. 1974; Holt and Harris 1983). Similarly, auditory and mechanosensory inputs from the hindbrain first innervate the tectum at stage 40 (Deeg et al. 2009; Hiramoto and Cline 2009). We first detect intertectal inputs extending into the contralateral optic tectum at stage 33/34, ∼1 day earlier than retinotectal or hindbrain axons. This suggests that intertectal communication is present before the onset of direct visual and mechanosensory activity in the tectum and may play an important role in establishing early tectal circuitry. Excitatory and inhibitory intertectal neurons are distributed throughout the tectum and are present in proportions comparable to the overall population of tectal excitatory and inhibitory neurons (Miraucourt et al. 2012). Excitatory and inhibitory intertectal axons project widely in the contralateral tectum, suggesting that they are not topographically organized at the stages we studied. Intertectal projections may become topographically organized later in development, akin to tecto-isthmo-tectal projections (Udin 2012). In the frog, there is alignment of the direct retinotectal and convergent crossed tecto-isthmo-tectal projections that provides frogs with the substrate for binocular vision (Udin and Grant 1999). Visuomotor behavior exhibited by frogs, such as prey-catching, would require precise, aligned representations of the visual field in the optic tectum by converging retinotectal and tecto-isthmo-tectal projections. In contrast, the visual avoidance behavior exhibited by tadpoles (Dong et al. 2009) may only require detection of a looming stimulus, which could occur even if the intertectal projection is not topographically organized.
We also found that excitatory and inhibitory intertectal inputs converge onto postsynaptic tectal neurons with direct excitatory retinal input. Cutting the intertectal commissure selectively removes intertectal inputs without affecting convergent retinotectal inputs. In addition, we find that visual experience promotes maturation of both excitatory and inhibitory intertectal synapses, detected as increases in the strength of synaptic inputs, a developmental shift in NMDAR subunits and the paired-pulse ratio similar to retinotectal synapses (Aizenman and Cline 2007; Wu et al. 1996). Together, these findings suggest that intertectal inputs play an important role in sensory processing within the optic tectum from the onset of visual circuit development.
Increased visual input and homeostatic plasticity.
In the tadpole tectum, visual input drives experience-dependent plasticity concurrent with neurogenesis, circuit assembly, and maturation. During development and experience-dependent plasticity, homeostasis ensures that neurons respond to and integrate changing input while maintaining their output within a functional dynamic range (Turrigiano 2012). The dynamic changes in tectal circuit connectivity during development and experience-dependent plasticity suggest that homeostatic regulation of the tectal circuit might be particularly important to maintain visuomotor circuit function during development. Homeostasis is achieved by multiple mechanisms, including regulation of intrinsic excitability, synaptic scaling, and regulation of E:I balance (Feldman 2009; Turrigiano 2012). Our data show that intertectal input responds dynamically to changes in visual experience, and this plasticity may help to maintain the E:I balance within the tectum (Fig. 10).
Fig. 10.
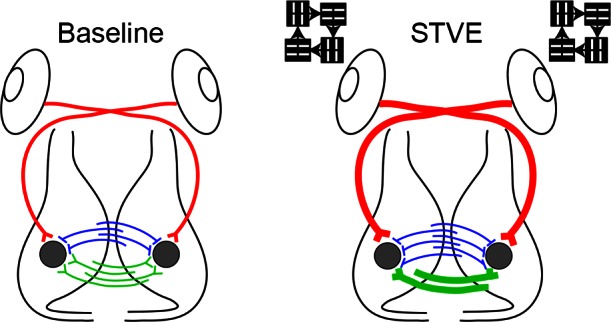
Summary diagram. Baseline: tectal neurons (gray circles) receive excitatory retinotectal (red), excitatory intertectal (green), and inhibitory intertectal (blue) inputs. STVE: with short-term visual enhancement (STVE), excitatory retinotectal input increases through addition of new synapses and strengthening of existing synapses. STVE increases both excitatory and inhibitory intertectal drive but via different mechanisms. STVE refines excitatory intertectal synapses by eliminating some boutons and by stabilizing and strengthening other synapses. In contrast, STVE increases inhibitory intertectal synapse number. The experience-dependent plasticity of intertectal inputs may help maintain the balance of excitation to inhibition in the optic tectum and keep tectal neurons functioning within a dynamic range in response to a changing visual environment.
STVE drives experience-dependent increases in retinotectal synapse number and strength (Aizenman and Cline 2007; Ruthazer et al. 2006). However, the overall E:I ratio of visually driven inputs to tectal neurons remains stable following STVE due to potentiation of both excitatory and inhibitory components of the visually evoked response (He et al. 2016). Experiments selectively interfering with inhibitory inputs indicate that the proper balance of excitation to inhibition in the tectum is critical to the performance of visually guided behavior in tadpoles (Shen et al. 2011). Through a combination of time-lapse imaging and electrophysiological recordings, we found that the net effect of increased visual input is to increase inhibitory intertectal drive and refine excitatory intertectal drive onto tectal neurons concomitant with increasing retinotectal synaptic communication. Inhibitory intertectal drive was increased following STVE, and the average AMPA-to-NMDA ratio of the excitatory input was also increased. The structural plasticity mechanisms that contribute to these changes were different. Increased visual experience decreased new excitatory bouton additions and caused shrinkage and elimination of existing excitatory synaptic boutons. In contrast, STVE enlarged and slowed the loss of existing inhibitory boutons. Therefore, STVE results in a reduction in the number of excitatory intertectal boutons and an increase in inhibitory intertectal boutons. The increase in the strength of the inhibitory component of the intertectal input we observed in response to STVE (larger and more numerous boutons as well as increased GABA receptor-mediated postsynaptic currents) in combination with recurrent tectal inhibition (Pratt et al. 2008) might function to balance STVE-driven increases in retinotectal drive.
Visual stimulation also tunes the AMPA receptor-mediated responses of retinotectal synapses to respond selectively to bursts of input or coactive convergent excitatory input, resulting in improved stimulus detection (Aizenman et al. 2002, 2003). We observed that STVE increased the AMPA-to-NMDA ratio of excitatory intertectal synapses. In systems in which excitatory synapses terminate on dendritic spines, increased AMPA receptor-mediated currents correlate with synapse stability and maintenance (Hill and Zito 2013; Holtmaat and Svoboda 2009; Matsuzaki et al. 2004). In the Xenopus tadpole retinotectal projection, STVE selectively stabilizes some retinotectal synapses by strengthening AMPA and NMDA receptor currents (Aizenman and Cline 2007) while destabilizing synapses in other regions of the axonal arbor in the process of experience-dependent topographic map refinement (Ruthazer et al. 2003, 2006). Our combined observations of STVE-induced increased intertectal AMPA-to-NMDA ratio and decreased excitatory bouton number suggest that visual activity is driving the refinement of excitatory intertectal input by eliminating synapses with low AMPA-to-NMDA ratios and selectively strengthening the remaining excitatory synapses. Together, these concomitant changes in excitatory and inhibitory intertectal inputs function to maintain E:I balance within the intertectal projection. In addition to the intertectal input we studied, the tectal network has other sources of input, including recurrent inhibitory connections (Pratt et al. 2008). Together, these inputs maintain E:I balance within the developing tectal network as a whole.
Response to brief visual deprivation.
We also investigated the response of excitatory and inhibitory intertectal boutons to decreased visual input (dark) by comparing the structural plasticity of boutons in the presence and absence of TTX during periods when the animal was kept in the dark. Acute visual deprivation in adult animals rapidly increases elimination of inhibitory synapses in the visual cortex (Chen et al. 2011, 2012; Keck et al. 2011; van Versendaal et al. 2012), whereas excitatory boutons are added (Yamahachi et al. 2009) or unchanged (Keck et al. 2011). These changes are correlated with increased responsiveness to the nondeprived eye (van Versendaal et al. 2012) and would be expected to maintain the E:I balance within the visual circuit in response to decreased excitatory drive. Here, we show that inhibitory intertectal boutons are plastic in response to decreased visual input from a 4-h period in the dark. We found that inhibitory intertectal output decreased after 4 h in the dark by shrinking presynaptic boutons, but a comparable dark period did not affect excitatory intertectal output. Similarly, inhibitory tectal neurons decrease their visually driven somatic calcium responses, and presumably synaptic output, in response to 4 h in dark, whereas excitatory neurons do not (He et al. 2016). Interestingly, He et al. (2016) identified two types of inhibitory tectal neurons, each of which exhibits bimodal dendritic structural plasticity in response to visual experience. In one group of inhibitory neurons, dark increased dendritic arbor growth and visually induced calcium responses, whereas STVE induced arbor retraction and decreased visually induced calcium responses. The other group of inhibitory neurons retracted branches and reduced visual responses in the dark and increased neuronal input and output with STVE. Although these data suggest two functionally distinct sets of inhibitory neurons are present in the optic tectum, the projection patterns of these two groups of inhibitory neurons were not explored. Here, we found that inhibitory intertectal neurons respond homogenously with respect to experience-dependent structural plasticity of their axons, suggesting that they might represent one of the functional groups of inhibitory neurons described by He et al. (2016). Together, our data suggest a homeostatic role for intertectal input in which input from the contralateral tectum as well as other sources preserves the E:I balance of inputs onto tectal neurons in the face of retinotectal experience-dependent plasticity and maintains visual responsiveness within functional limits.
Conclusions.
We took advantage of the highly plastic developing Xenopus visual circuit to study the development and experience-dependent plasticity of convergent retinotectal and intertectal inputs. The activity-dependent plasticity of early-stage developing axons and synapses are readily accessible in the Xenopus tadpole. We show that physiologically relevant increases and decreases in visual input trigger different structural and functional plasticity responses in retinotectal input and in both the excitatory and inhibitory components of intertectal inputs. Together, these data suggest that intertectal inputs maintain the function of the tectal circuit in changing sensory environments. Recent studies have highlighted the roles of sensory inputs in organizing multisensory circuits required for integrating multisensory inputs and generating coherent motor outputs in the developing and mature brain (Murray et al. 2016). We suggest that the early activity-dependent events identified in this study contribute to the development of the sensorimotor circuit in Xenopus.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants NS-084749 to A. C. Gambrill, NS-071807 to R. L. Faulkner, and EY-011261 to H. T. Cline, an endowment from the Hahn Family Foundation to H. T. Cline, and funding to research cores through NIH Grant P30-EY-019005.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.C.G., R.L.F., and H.T.C. conception and design of research; A.C.G. and R.L.F. performed experiments; A.C.G. and R.L.F. analyzed data; A.C.G., R.L.F., and H.T.C. interpreted results of experiments; A.C.G. and R.L.F. prepared figures; A.C.G., R.L.F., and H.T.C. drafted manuscript; A.C.G., R.L.F., and H.T.C. edited and revised manuscript; A.C.G., R.L.F., and H.T.C. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Cline laboratory for helpful discussions and Ed Ruthazer for comments on the manuscript.
REFERENCES
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39: 831–842, 2003. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Cline HT. Enhanced visual activity in vivo forms nascent synapses in the developing retinotectal projection. J Neurophysiol 97: 2949–2957, 2007. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Munoz-Elias G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron 34: 623–634, 2002. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci 26: 5117–5130, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal M. Distribution of GABA immunoreactivity in the optic tectum of the frog: a light and electron microscopic study. Neuroscience 42: 879–891, 1991. [DOI] [PubMed] [Google Scholar]
- Bestman JE, Cline HT. The RNA binding protein CPEB regulates dendrite morphogenesis and neuronal circuit assembly in vivo. Proc Natl Acad Sci USA 105: 20494–20499, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine HS, Gruberg ER. Ablation of nucleus isthmi leads to loss of specific visually elicited behaviors in the frog Rana pipiens. Neurosci Lett 54: 307–312, 1985. [DOI] [PubMed] [Google Scholar]
- Caputi A, Melzer S, Michael M, Monyer H. The long and short of GABAergic neurons. Curr Opin Neurobiol 23: 179–186, 2013. [DOI] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci 14: 587–594, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74: 361–373, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Khakhalin AS, Wang AF, Constantino AC, Yip SP, Aizenman CD. Multivariate analysis of electrophysiological diversity of Xenopus visual neurons during development and plasticity. Elife 4: pii: e11351, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol 11: 118–126, 2001. [DOI] [PubMed] [Google Scholar]
- Deeg KE, Sears IB, Aizenman CD. Development of multisensory convergence in the Xenopus optic tectum. J Neurophysiol 102: 3392–3404, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Lee RH, Xu H, Yang S, Pratt KG, Cao V, Song YK, Nurmikko A, Aizenman CD. Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J Neurophysiol 101: 803–815, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TW, Gebhardt C, Naumann EA, Riegler C, Ahrens MB, Engert F, Del Bene F. Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron 89: 613–628, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci 32: 33–55, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze RM, Keating MJ, Chung SH. The evolution of the retinotectal map during development in Xenopus. Proc R Soc Lond B Biol Sci 185: 301–330, 1974. [DOI] [PubMed] [Google Scholar]
- Gaze RM, Keating MJ, Szekely G, Beazley L. Binocular interaction in the formation of specific intertectal neuronal connexions. Proc R Soc Lond B Biol Sci 175: 107–147, 1970. [DOI] [PubMed] [Google Scholar]
- Grant S, Keating MJ. Changing patterns of binocular visual connections in the intertectal system during development of the frog, Xenopus laevis. I. Normal maturational changes in response to changing binocular geometry. Exp Brain Res 75: 99–116, 1989. [DOI] [PubMed] [Google Scholar]
- Grant S, Keating MJ. Normal maturation involves systematic changes in binocular visual connections in Xenopus laevis. Nature 322: 258–261, 1986. [DOI] [PubMed] [Google Scholar]
- Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo–from single cells to the entire brain. Differentiation 70: 148–154, 2002. [DOI] [PubMed] [Google Scholar]
- Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci USA 103: 12127–12131, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Shen W, Hiramoto M, Cline HT. Experience-dependent bimodal plasticity of inhibitory neurons in early development. Neuron 90: 1203–1214, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero L, Perez P, Nunez Abades P, Hardy O, Torres B. Tectotectal connectivity in goldfish. J Comp Neurol 411: 455–471, 1999. [DOI] [PubMed] [Google Scholar]
- Hill TC, Zito K. LTP-induced long-term stabilization of individual nascent dendritic spines. J Neurosci 33: 678–686, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto M, Cline HT. Convergence of multisensory inputs in Xenopus tadpole tectum. Dev Neurobiol 69: 959–971, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Harris WA. Order in the initial retinotectal map in Xenopus: a new technique for labelling growing nerve fibres. Nature 301: 150–152, 1983. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10: 647–658, 2009. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science 301: 521–525, 2003. [DOI] [PubMed] [Google Scholar]
- Keck T, Scheuss V, Jacobsen RI, Wierenga CJ, Eysel UT, Bonhoeffer T, Hubener M. Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron 71: 869–882, 2011. [DOI] [PubMed] [Google Scholar]
- Khakhalin AS, Koren D, Gu J, Xu H, Aizenman CD. Excitation and inhibition in recurrent networks mediate collision avoidance in Xenopus tadpoles. Eur J Neurosci 40: 2948–2962, 2014. [DOI] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol 14: R335–R338, 2004. [DOI] [PubMed] [Google Scholar]
- Li J, Erisir A, Cline H. In vivo time-lapse imaging and serial section electron microscopy reveal developmental synaptic rearrangements. Neuron 69: 273–286, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Hernandez O, Rees A. Intercollicular commissural projections modulate neuronal responses in the inferior colliculus. Eur J Neurosci 21: 2701–2710, 2005. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature 429: 761–766, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraucourt LS, Silva JS, Burgos K, Li J, Abe H, Ruthazer ES, Cline HT. GABA expression and regulation by sensory experience in the developing visual system. PLoS One 7: e29086, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res 90: 219–248, 1992. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. I. Morphological classification of efferent neurons. J Neurophysiol 60: 232–262, 1988. [DOI] [PubMed] [Google Scholar]
- Muldal AM, Lillicrap TP, Richards BA, Akerman CJ. Clonal relationships impact neuronal tuning within a phylogenetically ancient vertebrate brain structure. Curr Biol 24: 1929–1933, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. II. Sustained discharges during motor preparation and fixation. J Neurophysiol 66: 1624–1641, 1991. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol 79: 1193–1209, 1998. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol 70: 576–589, 1993. [DOI] [PubMed] [Google Scholar]
- Munz M, Gobert D, Schohl A, Poquerusse J, Podgorski K, Spratt P, Ruthazer ES. Rapid Hebbian axonal remodeling mediated by visual stimulation. Science 344: 904–909, 2014. [DOI] [PubMed] [Google Scholar]
- Murray MM, Lewkowicz DJ, Amedi A, Wallace MT. Multisensory processes: a balancing act across the lifespan. Trends Neurosci 39: 567–579, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore SP, Knudsen EI. The role of a midbrain network in competitive stimulus selection. Curr Opin Neurobiol 21: 653–660, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). Amsterdam: North-Holland Publishing, 1956. [Google Scholar]
- Olivier E, Corvisier J, Pauluis Q, Hardy O. Evidence for glutamatergic tectotectal neurons in the cat superior colliculus: a comparison with GABAergic tectotectal neurons. Eur J Neurosci 12: 2354–2366, 2000. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci 27: 8268–8277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Dong W, Aizenman CD. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci 11: 467–475, 2008. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Hiramoto M, Cline HT. An evolutionarily conserved mechanism for activity-dependent visual circuit development. Front Neural Circuits 10: 79–86, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randlett O, Wee CL, Naumann EA, Nnaemeka O, Schoppik D, Fitzgerald JE, Portugues R, Lacoste AM, Riegler C, Engert F, Schier AF. Whole-brain activity mapping onto a zebrafish brain atlas. Nat Methods 12: 1039–1046, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Aizenman CD. Learning to see: patterned visual activity and the development of visual function. Trends Neurosci 33: 183–192, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science 301: 66–70, 2003. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci 26: 3594–3603, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, McKeown CR, Demas JA, Cline HT. Inhibition to excitation ratio regulates visual system responses and behavior in vivo. J Neurophysiol 106: 2285–2302, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419: 475–480, 2002. [DOI] [PubMed] [Google Scholar]
- Stein BE. Development of the superior colliculus. Annu Rev Neurosci 7: 95–125, 1984. [DOI] [PubMed] [Google Scholar]
- Stein BE, Rowland BA. Organization and plasticity in multisensory integration: early and late experience affects its governing principles. Prog Brain Res 191: 145–163, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Rowland BA. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci 15: 520–535, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Sugiuchi Y, Shinoda Y. Commissural mirror-symmetric excitation and reciprocal inhibition between the two superior colliculi and their roles in vertical and horizontal eye movements. J Neurophysiol 98: 2664–2682, 2007. [DOI] [PubMed] [Google Scholar]
- Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron 45: 829–836, 2005. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol 4: a005736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udin SB. Binocular maps in Xenopus tectum: visual experience and the development of isthmotectal topography. Dev Neurobiol 72: 564–574, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udin SB, Fisher MD. The development of the nucleus isthmi in Xenopus laevis. I. Cell genesis and the formation of connections with the tectum. J Comp Neurol 232: 25–35, 1985. [DOI] [PubMed] [Google Scholar]
- Udin SB, Grant S. Plasticity in the tectum of Xenopus laevis: binocular maps. Prog Neurobiol 59: 81–106, 1999. [DOI] [PubMed] [Google Scholar]
- van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, Sommeijer JP, De Zeeuw CI, Hofer SB, Heimel JA, Levelt CN. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74: 374–383, 2012. [DOI] [PubMed] [Google Scholar]
- Vanegas H. Comparative Neurology of the Optic Tectum. New York: Plenum Press, 1984. [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science 274: 972–976, 1996. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci 3: 189–226, 1980. [DOI] [PubMed] [Google Scholar]
- Yamahachi H, Marik SA, McManus JN, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron 64: 719–729, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.