Progressive resistance exercise training over 24 mo in patients with mild-to-moderate Parkinson's disease partially restores the triphasic electromyographic pattern and improves movement velocity. This finding is similar to the effect of medication and deep brain stimulation. Additionally, the improvement in the triphasic electromyographic pattern and muscle strength is significantly associated with improvement in peak velocity. Our findings indicate that resistance exercise can drive neurophysiological changes that underlie the improvement in movement velocity in Parkinson's disease.
Keywords: progressive resistance exercise, Parkinson's disease, PRET-PD randomized clinical trial, bradykinesia, EMG triphasic muscle activation pattern
Abstract
In Parkinson's disease (PD), the characteristic triphasic agonist and antagonist muscle activation pattern during ballistic movement is impaired: the number of agonist muscle bursts is increased, and the amplitudes of the agonist and antagonist bursts are reduced. The breakdown of the triphasic electromyographic (EMG) pattern has been hypothesized to underlie bradykinesia in PD. Progressive resistance exercise has been shown to improve clinical measures of bradykinesia, but it is not clear whether the benefits for bradykinesia are accompanied by changes in agonist and antagonist muscle activity. This study examined the spatiotemporal changes in agonist and antagonist muscle activity following 24 mo of progressive resistance exercise and the combined relationship between spatiotemporal muscle activity and strength measures and upper limb bradykinesia. We compared the effects of progressive resistance exercise training (PRET) with a nonprogressive exercise intervention, modified Fitness Counts (mFC), in patients with PD. We randomized 48 participants with mild-to-moderate PD to mFC or PRET. At the study endpoint of 24 mo, participants randomized to PRET compared with mFC had significantly faster movement velocity, accompanied by significant increases in the duration, magnitude, and magnitude normalized to duration of the 1st agonist burst and fewer number of agonist bursts before peak velocity. The antagonist muscle activity was increased relative to baseline but did not differ between groups. Spatiotemporal EMG muscle activity and muscle strength were significantly associated with upper limb bradykinesia. These findings demonstrate that progressive resistance exercise improves upper limb movement velocity and restores some aspects of the triphasic EMG pattern.
NEW & NOTEWORTHY
Progressive resistance exercise training over 24 mo in patients with mild-to-moderate Parkinson's disease partially restores the triphasic electromyographic pattern and improves movement velocity. This finding is similar to the effect of medication and deep brain stimulation. Additionally, the improvement in the triphasic electromyographic pattern and muscle strength is significantly associated with improvement in peak velocity. Our findings indicate that resistance exercise can drive neurophysiological changes that underlie the improvement in movement velocity in Parkinson's disease.
bradykinesia is slowness of movement (Berardelli et al. 2001) and is the only symptom of Parkinson's disease (PD) that is always required to receive a PD diagnosis (Hughes et al. 1992). Bradykinesia is also considered the most functionally debilitating symptom in PD (Hallett and Khoshbin 1980). The pathophysiology of bradykinesia is related to dopaminergic deficits in the substantia nigra pars compacta, and this results in a reduction in the excitatory drive to the motor cortex that may disrupt cortical activation of the muscle (DeLong and Wichmann 2010; DeLong 1990; Wichmann and DeLong 2003, 2007). Using functional magnetic resonance imaging in humans, it is established that the hypoactivity within the basal ganglia and motor cortex is related to bradykinesia in PD (Prodoehl et al. 2010). In addition, during upper and lower limb movements, the pattern of muscle activity for the agonist and antagonist muscles breaks down in PD, which directly affects bradykinesia (Berardelli et al. 1996; Hallett and Khoshbin 1980; Pfann et al. 2001; Vaillancourt et al. 2006).
The seminal work of Hallett and colleagues (1975) showed that ballistic movements typically exhibit a characteristic triphasic pattern measured using electromyography (EMG) of the agonist and antagonist muscle. The first agonist burst accelerates the limb. The slope and magnitude of the first agonist burst scales with movement velocity for movements made over a fixed distance; steeper slopes and larger magnitudes correspond to faster movement velocities (Corcos et al. 1989). The second phase is the antagonist burst that decelerates the limb. The third phase is the second agonist burst that clamps the limb at the end of the movement (Hannaford and Stark 1985). Participants with PD do not exhibit this triphasic pattern; instead, they exhibit a fractionated bursting pattern (Hallett and Khoshbin 1980). Specifically, the magnitude and duration of the first agonist burst are reduced, the number of agonist bursts during the acceleration phase of the movement is increased, and the magnitude of the antagonist burst is reduced (Pfann et al. 2001; Teasdale et al. 1990). Treatments that alter the fundamental pathophysiology of PD should improve bradykinesia and restore some properties of the triphasic pattern during limb movement.
In prior work, treatments aimed at modifying basal ganglia physiology such as dopaminergic medications and deep brain stimulation of the subthalamic nucleus increase the first agonist burst magnitude and duration, reduce the number of agonist bursts during the acceleration phase of movement, and increase antagonist magnitude (Vaillancourt et al. 2004). All of these features facilitate the improvement in bradykinesia. However, neither medication nor deep brain stimulation of the subthalamic nucleus normalizes the abnormal agonist and antagonist muscle activation pattern observed in PD. Furthermore, pharmacological treatment can be accompanied with side effects, such as dyskinesias, cognitive decline, confusion, psychosis, hallucinations, and sedative effects (Lang and Lozano 1998; Olanow et al. 2001), which reduce tolerance and efficacy of such treatment. Similarly, brain surgery entails side effects that can include adverse events related to the surgical procedure itself, such as intracerebral hemorrhage with persistent neurological deficit (Olanow et al. 2001). Additionally, there can be device-related side effects such as infections and mechanical complications (Olanow et al. 2001). The above-mentioned side effects related to surgery are rare and are offset by substantial benefits of surgery that significantly impact quality of life. More commonly, there can also be stimulation-related side effects, such as contralateral transient facial muscle twitch, speech impairments, and paresthesias (Olanow et al. 2001). Resistance exercise is a promising adjunct treatment that has been shown to improve bradykinesia in patients with PD without the debilitating side effects observed as a result of medication and/or surgery.
Recently, the progressive resistance exercise training (PRET)-PD randomized controlled trial (Corcos et al. 2013) demonstrated that over 24 mo, PRET was significantly more efficacious at improving movement velocity and isometric muscle strength than an exercise regimen that used stretching, balance, breathing, and nonprogressive strengthening exercises. It remains unclear whether the improvements observed in upper limb movement velocity following PRET are accompanied with changes in the muscle activation pattern. Increases in velocity and changes in muscle activation patterns have previously been observed following dopaminergic medication and deep brain stimulation (Vaillancourt et al. 2004). Our first question is which aspects of the EMG muscle activation PRET modifies. We tested the hypothesis that PRET restores some properties of the triphasic pattern that is known to underlie the pathophysiology of bradykinesia in PD (Berardelli et al. 2001; Hallett and Khoshbin 1980). Our second question examines the relationships of muscle activation patterns, muscle strength, and movement velocity to each other. Specifically, we tested the hypothesis that improving muscle activation and muscle strength will be significantly associated with improvements in upper limb movement velocity.
METHODS
Study design and participants.
This study used data gathered for the PRET-PD trial, a prospective, parallel-group, single-center, randomized, controlled trial conducted between September 2007 and July 2011 (Corcos et al. 2013). The PRET-PD trial is registered at https://clinicaltrials.gov/ (NCT00591344). Participants with idiopathic PD confirmed by a movement disorders specialist (Hughes et al. 1992) were self-referred or recruited from Rush University Medical Center. Participants were evaluated at the University of Illinois at Chicago. Participants were eligible if they were 50–67 yr [the Physical Activity Readiness Questionnaire (Canadian Society for Exercise Physiology 2002), a screening tool used for exclusion, was validated only for use with persons younger than 69 yr, which determined the maximum age for eligibility], on stable dopaminergic therapy, and able to walk for 6 min. Participants were ineligible if they had a neurological history other than PD, had significant arthritis, failed the Physical Activity Readiness Questionnaire (Canadian Society for Exercise Physiology 2002), had a Mini-Mental State examination score <23 (Folstein et al. 1975), were already exercising, or had deep brain stimulation surgery for PD. Participants were followed every 6 mo for 24 mo or until they withdrew from the study. The Institutional Review Boards at Rush University Medical Center and University of Illinois at Chicago approved the study. Participants provided written, informed consent.
Interventions.
Details of the exercise intervention used in the PRET-PD trial have been published previously (Corcos et al. 2013). In brief, modified Fitness Counts (mFC) is an exercise program recommended by the National Parkinson Foundation and focused on stretches, balance exercises, breathing, and nonprogressive strengthening (chapts. 2 and 3 in the manual; Cianci 2006). The PRET was a weightlifting program in which the load against which the muscle worked was systematically and progressively increased. The PRET program consisted of strengthening exercises that were directed at all of the major muscle groups (Feigenbaum and Pollock 1999). The two exercise programs were identical in all aspects (duration of exercise, number of exercise sessions, and time with the personal trainer) except for the specific progressive resistance exercises. Participants participated in their respective interventions twice a week (Feigenbaum and Pollock 1999) for 24 mo. One-on-one exercise with a certified personal trainer was provided for both weekly exercise sessions during the 1st 6 mo; the trainer-assisted sessions reduced to once per week after 6 mo. Participants in the mFC and PRET groups were instructed not to engage in additional exercise.
Study testing procedures.
The movement velocity task was performed after 12-h overnight withdrawal of dopaminergic medication (Langston et al. 1992) at the Clinical Motor Control Laboratory at University of Illinois at Chicago. Off-medication assessment was completed in the morning by investigators blinded to group assignment. Participants then took their medication, had lunch, and 60 min later repeated the assessments (on-medication). It should be noted that EMG data to assess muscle activation patterns were collected during the movement velocity task, and the movement velocity task was one of multiple outcome domains that were tested in the PRET-PD trial. These domains included clinical status that was the primary outcome (Corcos et al. 2013). Secondary domains were bradykinesia (movement velocity), strength, tremor, physical function (Prodoehl et al. 2015) and gait, quality of life (Corcos et al. 2013), and cognition (David et al. 2015). Here, we report findings related to only the muscle activation data collected during the movement velocity task. The order of testing was pseudorandomized between outcome domains. The most impaired limb as reported by the patient was tested.
Apparatus.
The apparatus used to test the movement velocity and isometric strength was a single-degree-of-freedom manipulandum that consisted of a metal bar with a handle (combined moment of inertia 0.14 kg·m2) freely rotating in a horizontal plane around a pivot centered at the elbow joint. The manipulandum could be locked in and could engage a torque transducer, for which the axis of rotation was aligned with axis of rotation of the subject's elbow joint. For the right (left) elbow, full extension was defined as 90° (−90°), elbow flexion was in the negative (positive) direction, and the initial position for the experiments was 35° (−35°). The movement amplitude was 72° (−72°), thus the target location was set at 37° (−37°). The width of the target corresponded to 6° of angular elbow movement. Joint angle was measured by a capacitive transducer mounted on a shaft at the axis of rotation. Joint angle was digitally differentiated to generate joint velocity. Joint acceleration was measured by a piezoresistive accelerometer mounted 47.6 cm from the center of rotation. Surface EMGs were recorded from the biceps brachii, brachioradialis, and the lateral and long heads of the triceps brachii. The EMG signals were band-pass filtered between 20 and 450 Hz using built-in Bagnoli filters and then amplified (gain 1,000; Delsys). All signals were digitized at 1,000 Hz using a 16-bit analog-to-digital converter.
Experimental tasks.
The movement velocity task was used to measure peak elbow velocity. Participants were seated with their arm abducted between 75 and 90° and their forearm supported by the manipulandum. The participants viewed a computer monitor that displayed the initial position, the target, and a vertical cursor that corresponded to the angular displacement of the elbow. The threshold for visual feedback of the vertical cursor was set at 100°/s, i.e., when the movement velocity exceeded 100°/s, the vertical cursor was extinguished to facilitate feedforward, ballistic elbow flexion movements. Thus the vertical cursor was only visible at the beginning and at the end of the movement. At the start of each trial, the subject was asked to line up the vertical cursor with the initial position. When the subject heard a “GO” beep, he/she was asked to move the vertical cursor to the target as fast as possible in one smooth movement. Both velocity and accuracy were stressed. At the end of the trial, when the subject heard an “END” beep, he/she was asked to return to the initial position. Data were collected only when the subject moved from the initial position to the target. Each subject was given 10–20 practice trials and 30 test trials. Practice trials were not analyzed. The same manipulandum and a similar setup were used for the strength task. For the muscle strength task, the subject held an adjustable handle and pulled/pushed the manipulandum as hard as possible that was locked in at 90° of elbow flexion. For each muscle strength task, i.e., flexion and extension, we conducted three trials, each of which was 8 s in duration.
Follow-up.
Participants in the mFC and PRET groups returned to the laboratory at 6, 12, 18, and 24 mo for evaluations that consisted of the entire baseline assessment procedure. Procedural fidelity was maintained for each subject at follow-up testing by keeping the elbow and shoulder angle and seat height the same at each testing session. Previous studies have shown that the recorded surface EMG without normalizing is sensitive to change brought about by medical (Robichaud et al. 2002, 2004) and/or surgical intervention (Vaillancourt et al. 2004, 2006) as well as disease progression (Robichaud et al. 2009) when careful repeated measurements are taken over time. In addition, we have found that normalizing EMG to a maximum voluntary contraction or fixed level of force can also include considerable variability. This is especially true in the present study as PRET is designed to increase maximum voluntary contraction and force production. As such, we have chosen not to normalize the EMGs across the five testing time points. Instead, we assume that randomization creates initial group equivalence in the surface EMGs.
Randomization and blinding.
Details of randomization and blinding in the PRET-PD trial have been published previously (Corcos et al. 2013). In brief, the statistician matched the enrolled participants in pairs by sex and off-medication Unified Parkinson's Disease Rating Scale, motor subscale (UPDRS-III) scores (Fahn et al. 1987) and randomly assigned one member of each pair to PRET and the other member to mFC using a random-length permuted block design (Friedman et al. 1998). Randomization resulted in a parallel group design with a 1:1 allocation ratio. Participants started exercising within a month of randomization. Research personnel involved in data collection were blinded to group assignment. The participants knew their treatment assignment but were unaware of the study hypothesis and were explicitly instructed not to discuss their exercise program with the raters.
Data analysis.
Data processing was performed offline with the investigator blinded to group allocation. The kinetic and kinematic signals were low-pass filtered at 20 Hz (2nd-order Butterworth dual-pass filter). The EMG signals were full-wave rectified and then low-pass filtered at 50 Hz.
Peak velocity and EMG muscle activation outcomes.
The EMG muscle activation outcome variables were determined from the kinematic and EMG signals obtained during the movement velocity task. The onset and offset of the agonist EMG were determined by a well-trained rater blinded to group assignment (J. A. Robichaud) using an automated algorithm with the following steps. 1) The algorithm identified movement onset by finding peak acceleration and then searching backward to locate the first acceleration data sample that fell below 5% of peak acceleration. 2) Because of the electromechanical delay (∼30 ms) between EMG and kinematic signals (Corcos et al. 1992), the algorithm searched for the onset of the EMG signal before the onset of acceleration. Because rest tremor (4–7 Hz) is common in participants with PD, a notch filter (4–7 Hz) was used to filter out rest tremor from the agonist EMG signal. Each data sample from the agonist EMG during that period was compared with the baseline agonist EMG activity (this was defined as mean agonist EMG activity from −200 to −100 ms before acceleration onset). When the agonist EMG data sample was greater than an onset threshold (sum of 5 times the standard deviation of mean baseline agonist EMG and the mean baseline agonist EMG activity) for 30 consecutive samples, this was marked as the onset of the EMG burst. 3) The algorithm searched forward to find when the agonist EMG signal fell below an offset threshold (sum of 5 times the standard deviation of mean baseline EMG and the mean baseline agonist EMG activity) for 3 consecutive samples; this time point was set as the offset of the agonist EMG burst. 4) Steps 2 and 3 were repeated to mark any additional agonist EMG bursts that occurred before peak velocity. After automated algorithm marking, a well-trained rater (J. A. Robichaud), blinded to intervention group membership, visually inspected each trial to ensure agonist EMG burst marking accuracy. If necessary, visual adjustments were made. Approximately 20% of the trials were visually adjusted. Below are the operational definitions of each of the outcome variables.
1) Peak velocity (in degrees per second): the maximum of the absolute value of the velocity signal.
2) Time to peak velocity (in milliseconds): the duration from the onset of movement to the maximum of the absolute value of the velocity signal.
3) Duration of the first agonist burst (in milliseconds): the time period between the onset and the offset of the first agonist burst.
4) Qag1, magnitude of the first agonist burst: the integral of the agonist EMG during the first agonist burst.
5) Qag1/t1, magnitude of the first agonist burst normalized to burst duration: the integral of EMG activity during the first agonist burst divided by the duration of the first agonist burst. This is a normalized metric that represents the magnitude of the first agonist burst per unit time.
6) Number of agonist bursts: the number of agonist bursts that began before peak velocity.
7) Q30, magnitude of the 1st 30 ms of the agonist burst: the integral of EMG activity during the 1st 30 ms of the agonist burst.
8) Qag, magnitude of the agonist burst: the integral of EMG activity from the onset of the first agonist burst to the time of peak velocity.
9) Qant, magnitude of the antagonist burst: the integral of the antagonist EMG activity from the onset of the first agonist burst to the end of the movement (the time when the absolute value of the deceleration signal dropped below 5% of maximum).
10) Cocontraction during limb acceleration and deceleration: the degree of cocontraction from movement onset to peak acceleration and from peak acceleration to movement offset was calculated according to the algorithm by Winter (1990):
where min is the minimum of values at time t.
Elbow flexion/extension strength (in newton meters).
Each subject performed three flexion and three extension trials. For each trial, the average of the torque signal ± 200 ms from peak torque was calculated. Of the three trials, the maximum observed torque value was used as the flexion/extension maximal voluntary torque.
Statistical analysis.
The focus of the statistical analyses was on the off-medication outcomes measured following a 12-h withdrawal of anti-Parkinsonian medications. This was because anti-Parkinsonian medication could possibly mask the effects of exercise. We performed three sets of statistical analyses on the off-medication outcomes. The first sets of statistical analyses were performed to test for differences at baseline between mFC and PRET on the outcomes listed above. This was done because, before randomization, participants were matched only on off-medication UPDRS-III scores and sex. Appropriate parametric (t-tests) and nonparametric (Fisher exact test for binary variables and Wilcoxon rank-sum test for nonnormal distributions) methods were performed to test for differences at baseline. In addition, we calculated confidence intervals (CI) on the difference between mFC and PRET on demographic, clinical, movement velocity, muscle activation, and strength outcomes.
The second sets of analyses were performed to test our hypothesis that compared with mFC, PRET modifies the triphasic pattern. For this purpose, mixed-effects regression models were used on each of the outcomes listed above. Mixed-effects regression models were preferred over repeated-measures analysis of variance because of missing data. Mixed-effects regression models allow retaining cases with missing data, whereas repeated-measures analysis of variance does not (Hedeker and Gibbons 2006). Fixed effects were group (mFC and PRET), time (0, 6, 12, 18, and 24 mo), and the group-by-time interaction. The random effect was subject. If the omnibus F test was significant for the group-by-time interaction, then post hoc pairwise comparisons were performed using t-tests. Because our research question dealt with the effect of PRET on muscle activation patterns, these pairwise comparisons primarily examined differences between groups for a given time point. Main effects are reported when the group-by-time interaction was not significant.
The third sets of analyses were performed to examine the relationship between change in muscle activation patterns, muscle strength, and improvement in movement velocity. We used a principal component regression analysis for this purpose because of the redundancy in muscle activation outcomes and strength outcomes (Jeffers 1967). First, a principal component analysis (PCA) was performed. We did not use peak velocity and time to peak velocity because our goal was to explain the variation in peak velocity using muscle activation and strength variables. Only the 24-mo changes from baseline were used for the principal component regression analysis. We used 11 variables in the PCA. They were the duration of the 1st agonist burst, magnitude of the 1st agonist burst, magnitude of the 1st agonist burst normalized to burst duration, number of agonist bursts, magnitude of the 1st 30 ms of the agonist burst, magnitude of the agonist burst, magnitude of the antagonist burst, cocontraction during limb acceleration, cocontraction during limb deceleration, maximum elbow flexion strength, and maximum elbow extension strength. The correlation matrix was used for PCA because the variables used for the PCA were on different scales. The plan was to use principal component scores from those principal components with an eigenvalue >1 as independent variables in a standard linear regression analysis. For the standard linear regression analysis, the dependent variable was the 24-mo change in peak velocity. The independent variables were principal component scores from the PCA. The principal component scores were entered into the regression at once.
All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). All statistical tests were two-sided, and we used a P value <0.05. Missing data were not imputed; data were assumed to be missing at random.
RESULTS
Fifty-one subjects were enrolled in the study. Forty-eight of these were randomized, and three were replacements. Treatment groups did not differ significantly at baseline (Table 1). Of the fifty-one subjects enrolled in the study, thirty-eight subjects (mFC, n = eighteen; PRET, n = twenty) completed the study. First, we present kinematic and EMG data from single trials from representative subjects in the mFC and PRET groups. Then, we present statistical results for movement velocity, key muscle activation variables, and strength variables. The figures and the tables record the complete list of variables used in the PCA.
Table 1.
Characteristics of patients at baseline by treatment group*
| Characteristic | Treatment Group |
Difference Between Groups (95% Confidence Interval) | P Value† | |
|---|---|---|---|---|
| Modified Fitness Counts (n = 24) | Progressive Resistance Training (n = 24) | |||
| Demographic | ||||
| Age, yr | 58.6 ± 5.6 | 59.0 ± 4.6 | −0.4 (−2.6 to 3.4) | 0.78 |
| Sex, no. (%) | ||||
| Male | 14 (58.3) | 14 (58.3) | ||
| Female | 10 (41.7) | 10 (41.3) | ||
| Ethnicity, no. (%)a | 0.19 | |||
| Hispanic or Latino | 5 (20.8) | 1 (4.2) | ||
| Not Hispanic or Latino | 19 (79.2) | 23 (95.8) | ||
| Race, no. (%)a | 0.49 | |||
| African-American | 0 (0) | 2 (8.3) | ||
| White | 24 (100) | 22 (91.7) | ||
| Handedness, no. (%)a | 1.00 | |||
| Right | 22 (91.7) | 23 (95.8) | ||
| Left | 2 (8.3) | 1 (4.2) | ||
| Clinical | ||||
| Years since diagnosis | 6.5 ± 4.7 | 6.5 ± 4.1 | 0.0 (−2.5 to 2.6) | 0.97 |
| Mini-Mental State examination | 29.1 ± 1.4 | 29.3 ± 1.1 | 0.2 (−0.5 to 0.9) | 0.56 |
| Most affected side, no. (%) | 0.5 (0.2–1.6)b | 0.37 | ||
| Right | 17 (70.8) | 13 (54.2) | ||
| Left | 7 (29.2) | 11 (45.8) | ||
| Motor status | ||||
| Unified Parkinson's Disease Rating Scale, part III, motor subscale score (range, 0–108) (primary outcome; off-medication) | 34.7 ± 11.5 | 34.5 ± 11.9 | −0.2 (−7.0 to 6.6) | 0.95 |
| Hoehn and Yahr staging scale (disability; range, 0–5; off-medication) | 2.3 ± 0.53 | 2.2 ± 0.41 | −0.1 (−0.4 to 0.2) | 0.55 |
| Medication§‡ | ||||
| Levodopa equivalent dose | 705 ± 405 | 598 ± 355 | −100 (−125 to 350)c | 0.37 |
| Movement velocity | ||||
| Elbow flexion velocity, °/s; off-medication | 330.3 ± 86.3 | 327.2 ± 79.7 | −3.1 (−51.4 to 45.1) | 0.90 |
| Time to peak velocity, ms; off-medication | 223 ± 55 | 221 ± 67 | −2 (−38 to 34) | 0.93 |
| Muscle activation outcomes | ||||
| Duration of 1st agonist burst, ms | 129.5 ± 48.8 | 123.7 ± 57.4 | −5.8 (−36.8 to 25.1) | 0.71 |
| Magnitude of 1st agonist burst, au; off-medication | 8.8 ± 6.1 | 8.6 ± 6.5 | −0.2 (−3.8 to 3.5) | 0.92 |
| Magnitude of 1st agonist burst normalized to duration of 1st agonist burst, au/ms; off-medication | 0.07 ± 0.04 | 0.08 ± 0.07 | 0.01 (−0.03 to 0.04) | 0.68 |
| Number of agonist bursts during acceleration phase; off-medication | 2.1 ± 0.6 | 2.2 ± 0.7 | 0.1 (−0.3 to 0.5) | 0.47 |
| Magnitude of 1st 30 ms of 1st agonist burst, au; off-medication | 1.5 ± 0.9 | 2.1 ± 2.2 | 0.6 (−0.4 to 1.6) | 0.23 |
| Magnitude of agonist burst, au; off-medication | 15.6 ± 11 | 19.4 ± 14.1 | 3.8 (−3.6 to 11.1) | 0.31 |
| Magnitude of the antagonist burst, au; off-medication | 9.5 ± 3.9 | 9.3 ± 5.5 | −0.2 (−3 to 2.6) | 0.89 |
| Cocontraction during acceleration; off-medication | 44.1 ± 18.4 | 39.4 ± 19 | −4.7 (−15.6 to 6.2) | 0.39 |
| Cocontraction during deceleration; off-medication | 48.9 ± 12.9 | 49.9 ± 13.8 | −1 (−6.7 to 8.8) | 0.79 |
| Strength | ||||
| Elbow flexion torque, Nm; off-medication | 50.2 ± 17.8 | 47.6 ± 15.7 | −2.6 (12.4–7.2) | 0.60 |
| Elbow extension torque, Nm; off-medication | 30.9 ± 11.8 | 27.1 ± 7.2 | 3.8 (−1.9 to 9.5) | 0.19 |
Plus-minus values are means ± 1 SD.
P values calculated with the use of t-tests for continuous variables and Fisher exact test for binary variables unless mentioned otherwise.
P values calculated with the use of Wilcoxon rank-sum test.
Medication is measured in levodopa equivalent units in milligrams per day.
Confidence intervals not estimated for binary variables if total frequency (combined groups) is <10.
Mantel-Haenszel estimate of the common odds ratio for binary variables. If 1 is contained within the confidence interval, then there is no difference between groups.
Hodges-Lehmann estimate of location shift. All P values are 2-sided.
Peak velocity and EMG patterns of individual representative subjects.
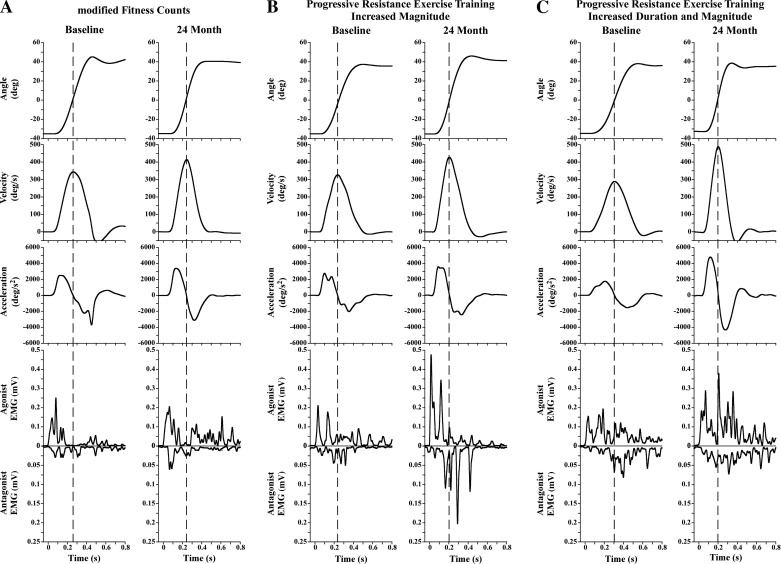
Figure 1 shows kinematic and EMG muscle activation patterns of subjects from both groups during a single trial of a 72° flexion movement at baseline and at 24 mo. Two subjects are shown for the PRET group to represent the two different agonist EMG patterns observed. The subject in the mFC group was a 63-yr-old man with an off-medication UPDRS-III of 39 at baseline (Fig. 1A). The subjects in the PRET group were a 59-yr-old man with an off-medication UPDRS-III of 42 (Fig. 1B) and a 61-yr-old woman with an off-medication UPDRS-III of 24 at baseline (Fig. 1C). These data are presented for descriptive purposes only. The first columns in Fig. 1, A–C, represent data at baseline. At baseline, all participants presented with comparable peak velocities (∼300–350°/s), first agonist burst durations (∼100 ms), and number of agonist bursts before peak velocity (∼2). The second columns in Fig. 1, A–C, represent data 24 mo after the intervention. At the 24-mo time point, relative to baseline, peak velocity improved substantially more in the subjects in the PRET group than in the subject in the mFC group. The magnitude and/or duration of the first agonist burst substantially increased in the subjects in the PRET group compared with the subject in the mFC group. One representative subject shows increased magnitude of agonist EMG bursts that does not fully return to baseline (Fig. 1B), whereas another representative subject shows increased agonist EMG magnitude with prolonged duration (Fig. 1C). Consequently, the number of agonist bursts before peak velocity also differed substantially between the two groups. The subject in the PRET group (Fig. 1C) presented with one agonist burst, whereas the subject in the mFC group presented with two bursts.
Fig. 1.
Shown are the kinematic and EMG muscle activation patterns of 1 representative subject from the mFC group (A) and 2 representative subjects from the PRET group (B and C) during a single trial of a 72° flexion movement at baseline and at 24 mo. The vertical dashed line corresponds to peak velocity. The data shown are commonly observed patterns from both groups. A: subject from the modified Fitness Counts (mFC) group (age, 63 yr; sex, male; baseline off-medication UPDRS-III, 39). Fractionation of agonist EMG before peak velocity is observed at baseline and at 24 mo. B: subject from the progressive resistance exercise training (PRET) group (age, 59 yr; sex, male; baseline off-medication UPDRS-III, 42). This subject shows a pattern of increased magnitude of agonist EMG before peak velocity at 24 mo relative to baseline. C: subject from the PRET group (age, 61 yr; sex, female; baseline off-medication UPDRS-III, 24). This subject shows a pattern of increased magnitude and duration of agonist EMG before peak velocity at 24 mo relative to baseline.
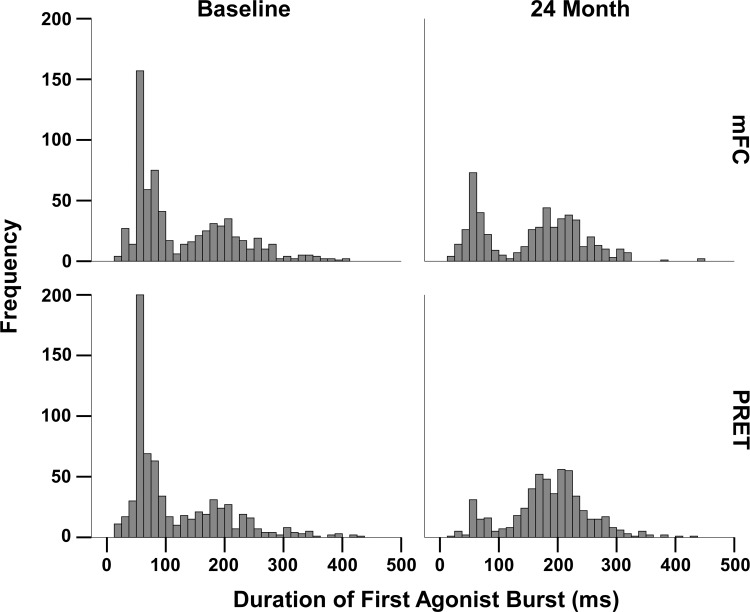
The frequency distribution of the individual durations of the first agonist burst at baseline and 24 mo is shown in Fig. 2. The similarity between the mFC and PRET at baseline as well as the differences at 24 mo are evident. In the PRET group, relative to baseline, at 24 mo, there are fewer short bursts <100 ms, and most bursts occur around 200 ms. This is important as this drives the increase in the magnitude of the first agonist burst as well as the reduction in the number of agonist burst before peak velocity possibly contributing to increase in movement velocity.
Fig. 2.
Frequency distribution of durations of the 1st agonist burst at baseline and the study endpoint of 24 mo for the modified fitness counts and progressive exercise training group are shown.
In summary, by the study endpoint of 24 mo, both of the individuals from the PRET group presented with greater change relative to baseline compared with the individual from mFC group. These are particularly evident in the peak velocity, duration, and magnitude of the first agonist burst and the number of agonist bursts before peak velocity. Next, the results at the group level along with accompanying statistics are presented for the most important variables. Henceforth, in the presence of a significant interaction, main effects are not discussed.
Peak velocity (in degrees per second).
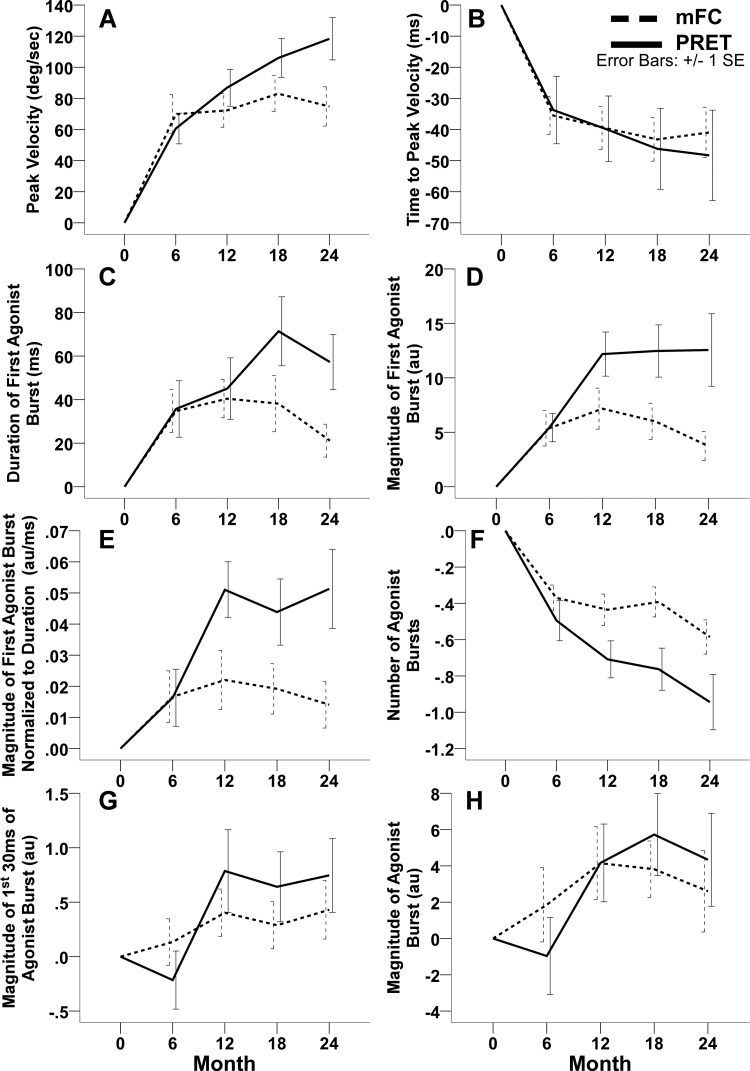
A significant group-by-time interaction (F4, 167 = 3.29; P = 0.01) was observed. As can be seen in Fig. 3A and Table 2, the mFC and the PRET groups presented with increases in peak velocity at the 6-mo time point. However, by the 24-mo study endpoint, the mFC group exhibited only an additional 5°/s increase in peak velocity relative to the 6-mo time point (Table 2). In contrast, the PRET group almost doubled their peak velocity relative to baseline from 61°/s at 6 mo to 118°/s at the study endpoint. Pairwise comparisons revealed that the gain in peak velocity relative to baseline was significantly greater in the PRET group compared with the mFC group at the study endpoint of 24 mo (mean difference, 37°/s; 95% CI, 9–65; P = 0.009; Table 2).
Fig. 3.
These plots illustrate the change from baseline scores in the 2 exercise groups, including the mean (± standard error) change from baseline in the off-medication peak elbow flexion velocity (A), time to peak elbow flexion velocity (B), duration of the 1st agonist burst (C), magnitude of the 1st agonist burst (D), magnitude of 1st agonist burst normalized to the duration of the 1st agonist burst (E), number of agonist bursts (F), magnitude of the 1st 30 ms of the agonist burst (G), and magnitude of the agonist burst (H) at 6, 12, 18, and 24 mo. The dashed lines indicate the modified fitness counts group (mFC), and the solid lines indicate the progressive resistance exercise training group (PRET). Positive change scores indicate improvement in peak velocity, duration of the 1st agonist burst, magnitude of the 1st agonist burst, and magnitude of 1st agonist burst normalized to the duration of the 1st agonist burst. Negative change scores indicate improvement in time to peak velocity and number of agonist bursts.
Table 2.
Muscle activation outcomes at each visit by treatment group while off-medication
| Score at Visit: Mean ± SD | Change from Baseline: Mean ± SD | Estimated Difference in Change from Baseline: PRET vs. mFC (95% CI) | P Valuea | |||
|---|---|---|---|---|---|---|
| mFC | PRET | mFC | PRET | |||
| Peak velocity, °/sb | ||||||
| Baseline | 330 ± 86 | 327 ± 80 | ||||
| 6 mo | 400 ± 100 | 388 ± 78 | 70 ± 61 | 61 ± 48 | −9 (−35 to 16) | 0.48 |
| 12 mo | 407 ± 96 | 413 ± 99 | 72 ± 52 | 87 ± 57 | 15 (−11 to 41) | 0.26 |
| 18 mo | 422 ± 91 | 424 ± 81 | 83 ± 54 | 106 ± 57 | 21 (−6 to 47) | 0.13 |
| 24 mo | 404 ± 74 | 438 ± 79 | 75 ± 54 | 118 ± 61 | 37 (9–65) | 0.009 |
| Time to peak velocity, msc | ||||||
| Baseline | 223 ± 55 | 221 ± 67 | ||||
| 6 mo | 188 ± 55 | 188 ± 41 | −36 ± 30 | −34 ± 53 | 2 (−17 to 21) | 0.86 |
| 12 mo | 181 ± 51 | 182 ± 39 | −40 ± 33 | −40 ± 51 | 1 (−18 to 20) | 0.93 |
| 18 mo | 174 ± 44 | 177 ± 40 | −43 ± 33 | −46 ± 60 | 0 (−20 to 20) | 0.99 |
| 24 mo | 185 ± 37 | 174 ± 43 | −41 ± 34 | −48 ± 65 | −5 (−26 to 15) | 0.62 |
| Duration of 1st agonist burst, msd | ||||||
| Baseline | 130 ± 49 | 124 ± 57 | ||||
| 6 mo | 164 ± 80 | 159 ± 72 | 35 ± 48 | 36 ± 64 | 1 (−32 to 34) | 0.95 |
| 12 mo | 172 ± 68 | 170 ± 75 | 40 ± 42 | 45 ± 68 | 5 (−29 to 38) | 0.78 |
| 18 mo | 173 ± 80 | 202 ± 80 | 38 ± 61 | 71 ± 73 | 35 (1–69) | 0.04 |
| 24 mo | 154 ± 48 | 186 ± 29 | 21 ± 32 | 57 ± 57 | 43 (7–78) | 0.02 |
| Magnitude of 1st agonist burst, aue | ||||||
| Baseline | 8.8 ± 6.1 | 8.6 ± 6.5 | ||||
| 6 mo | 14.2 ± 9.5 | 14.1 ± 9.2 | 5.4 ± 8 | 5.4 ± 6.3 | 0.1 (−5.3 to 5.4) | 0.99 |
| 12 mo | 16.2 ± 11.8 | 21 ± 12.9 | 7.2 ± 9 | 12.2 ± 9.7 | 5 (−0.5 to 10.5) | 0.07 |
| 18 mo | 15.4 ± 10.5 | 20.5 ± 13.8 | 6 ± 7.8 | 12.5 ± 11 | 6.5 (1–12.1) | 0.02 |
| 24 mo | 12.9 ± 8.8 | 20.5 ± 15.9 | 3.7 ± 5.7 | 12.6 ± 14.9 | 9.3 (3.5–15.1) | 0.002 |
| Magnitude of 1st agonist burst normalized to duration of 1st agonist burst, au/msf | ||||||
| Baseline | 0.07 ± 0.04 | 0.08 ± 0.07 | ||||
| 6 mo | 0.09 ± 0.05 | 0.09 ± 0.06 | 0.02 ± 0.04 | 0.02 ± 0.04 | 0 (−0.02 to 0.02) | 0.98 |
| 12 mo | 0.09 ± 0.05 | 0.13 ± 0.08 | 0.02 ± 0.05 | 0.05 ± 0.04 | 0.03 (0–0.05) | 0.02 |
| 18 mo | 0.09 ± 0.05 | 0.1 ± 0.06 | 0.02 ± 0.04 | 0.04 ± 0.05 | 0.02 (0–0.05) | 0.11 |
| 24 mo | 0.08 ± 0.04 | 0.11 ± 0.08 | 0.01 ± 0.03 | 0.05 ± 0.06 | 0.03 (0.01–0.06) | 0.01 |
| Number of agonist bursts during acceleration phaseg | ||||||
| Baseline | 2.1 ± 0.6 | 2.2 ± 0.7 | ||||
| 6 mo | 1.7 ± 0.6 | 1.7 ± 0.6 | −0.4 ± 0.3 | −0.5 ± 0.5 | −0.1 (−0.4 to 0.1) | 0.28 |
| 12 mo | 1.6 ± 0.6 | 1.5 ± 0.5 | −0.4 ± 0.4 | −0.7 ± 0.5 | −0.3 (−0.5 to 0) | 0.03 |
| 18 mo | 1.6 ± 0.6 | 1.4 ± 0.4 | −0.4 ± 0.4 | −0.8 ± 0.5 | −0.4 (−0.6 to −0.2) | 0.002 |
| 24 mo | 1.5 ± 0.5 | 1.2 ± 0.3 | −0.6 ± 0.4 | −0.9 ± 0.7 | −0.4 (−0.6 to −0.1) | 0.002 |
| Magnitude of 1st 30 ms of 1st agonist burst, auh | ||||||
| Baseline | 1.5 ± 0.9 | 2.1 ± 2.2 | ||||
| 6 mo | 1.6 ± 1 | 1.9 ± 1.8 | 0.1 ± 1 | −0.2 ± 1.3 | −0.3 (−1.1 to 0.4) | 0.34 |
| 12 mo | 1.9 ± 1.2 | 2.9 ± 2.9 | 0.4 ± 1 | 0.8 ± 1.8 | 0.4 (−0.4 to 1.1) | 0.3 |
| 18 mo | 1.8 ± 1.1 | 2.2 ± 1.8 | 0.3 ± 1 | 0.6 ± 1.5 | 0.2 (−0.5 to 1) | 0.53 |
| 24 mo | 1.8 ± 1.2 | 2.4 ± 2 | 0.4 ± 1.1 | 0.7 ± 1.5 | 0.3 (−0.5 to 1.1) | 0.46 |
| Magnitude of the agonist burst, aui | ||||||
| Baseline | 15.6 ± 11 | 19.4 ± 14.1 | ||||
| 6 mo | 17.5 ± 7.7 | 18.4 ± 10.4 | 1.9 ± 10 | −1 ± 10.4 | −2.8 (−7.7 to 2) | 0.25 |
| 12 mo | 20.2 ± 8.8 | 23.8 ± 13.1 | 4.1 ± 9.6 | 4.2 ± 10.3 | 0.2 (−4.7 to 5.2) | 0.92 |
| 18 mo | 19.9 ± 9.6 | 23 ± 12.6 | 3.8 ± 7.3 | 5.7 ± 10.4 | 1.8 (−3.3 to 6.8) | 0.49 |
| 24 mo | 19 ± 9.2 | 21.4 ± 12.6 | 2.6 ± 9.5 | 4.3 ± 11.5 | 1.7 (−3.5 to 7) | 0.52 |
| Magnitude of the antagonist burst, auj | ||||||
| Baseline | 9.5 ± 3.9 | 9.3 ± 5.5 | ||||
| 6 mo | 12.4 ± 5.5 | 10.3 ± 4.8 | 2.9 ± 3.9 | 1 ± 3.8 | −1.9 (−4.5 to 0.7) | 0.16 |
| 12 mo | 13.3 ± 6.7 | 10.5 ± 5.3 | 3.6 ± 5.6 | 1 ± 5.1 | −2.7 (−5.3 to 0) | 0.05 |
| 18 mo | 13.5 ± 5.6 | 11.6 ± 6 | 3.8 ± 5.3 | 2.6 ± 4.8 | −1.1 (−3.9 to 1.6) | 0.41 |
| 24 mo | 13.7 ± 5.7 | 13.1 ± 7.3 | 3.9 ± 4.9 | 3.9 ± 5.1 | 0 (−2.9 to 2.8) | 0.99 |
| Cocontraction during accelerationk | ||||||
| Baseline | 44.1 ± 18.4 | 39.4 ± 19 | ||||
| 6 mo | 43.3 ± 15.1 | 38 ± 18.5 | −0.9 ± 15.3 | −1.4 ± 19.1 | −0.5 (−9.5 to 8.5) | 0.91 |
| 12 mo | 42.2 ± 16.5 | 34.2 ± 14.4 | −1.2 ± 18.5 | −6 ± 14.7 | −4.3 (−13.4 to 4.9) | 0.36 |
| 18 mo | 42.1 ± 12.9 | 37.7 ± 12.7 | −1.8 ± 14.9 | −3.9 ± 18.3 | −1.6 (−11 to 7.7) | 0.73 |
| 24 mo | 45.6 ± 16.8 | 39.7 ± 15.5 | 0 ± 15.2 | −2.6 ± 19.5 | −3.4 (−13.2 to 6.3) | 0.49 |
| Cocontraction during decelerationl | ||||||
| Baseline | 48.9 ± 12.9 | 49.9 ± 13.8 | ||||
| 6 mo | 50.3 ± 12.2 | 46.7 ± 13.3 | 1.4 ± 11.7 | −3.2 ± 15.3 | −4.6 (−11.4 to 2.1) | 0.18 |
| 12 mo | 49 ± 11.8 | 47.2 ± 13.7 | 0.8 ± 14.1 | −3 ± 11.7 | −3.9 (−10.8 to 3) | 0.26 |
| 18 mo | 51.6 ± 9.9 | 50.2 ± 14.7 | 2.7 ± 12.4 | −1.4 ± 14.5 | −4.3 (−11.3 to 2.7) | 0.23 |
| 24 mo | 53.2 ± 11.3 | 49.5 ± 12.1 | 4.8 ± 9.8 | −2.7 ± 13.5 | −6 (−13.3 to 1.3) | 0.11 |
| Elbow flexion strength, Nmm | ||||||
| Baseline | 50.2 ± 17.8 | 47.6 ± 15.7 | ||||
| 6 mo | 54.1 ± 21.9 | 55.3 ± 17.2 | 4 ± 8.7 | 7.7 ± 7.3 | 3.7 (−0.8 to 8.3) | 0.11 |
| 12 mo | 51.1 ± 20.4 | 54.8 ± 17.5 | 0.1 ± 9.3 | 7.7 ± 6.9 | 7.9 (3.2–12.5) | 0.001 |
| 18 mo | 49.9 ± 21.2 | 53.9 ± 13.6 | −1.1 ± 10 | 6.8 ± 6.2 | 8.5 (3.7–13.2) | <0.001 |
| 24 mo | 43.2 ± 16.2 | 56.3 ± 15.2 | −5.3 ± 9.5 | 9 ± 6.9 | 14.3 (9.4–19.3) | <0.001 |
| Elbow extension strength, Nmn | ||||||
| Baseline | 30.9 ± 11.8 | 27.1 ± 7.2 | ||||
| 6 mo | 34.1 ± 16 | 34.9 ± 9.7 | 3.2 ± 7 | 7.7 ± 7.9 | 4.6 (1–8.1) | 0.01 |
| 12 mo | 30.6 ± 12.2 | 32.5 ± 7.8 | 0.2 ± 7 | 5.9 ± 6.6 | 5.9 (2.3–9.6) | 0.002 |
| 18 mo | 31.7 ± 13.1 | 33 ± 7.7 | 0.2 ± 8 | 6.1 ± 5 | 6.7 (3–10.4) | <0.001 |
| 24 mo | 28.7 ± 12.5 | 32.3 ± 7.9 | −1.3 ± 8.1 | 5.3 ± 4.7 | 7.3 (3.4–11.1) | <0.001 |
SD, standard deviation; mFC, modified Fitness Counts; PRET, progressive resistance exercise training; CI, confidence interval.
P values are based on pairwise between-group contrasts using a mixed-effects regression model unless mentioned otherwise.
Positive change scores indicate faster elbow flexion velocity in degrees per second.
Negative change scores indicate faster time to peak velocity in milliseconds.
Positive change scores indicate longer duration of 1st agonist burst in milliseconds.
Positive change scores indicate greater magnitude of 1st agonist burst.
Positive change scores indicate greater magnitude of 1st agonist burst normalized to duration of 1st agonist burst.
Positive change scores indicate fewer number of agonist bursts during the acceleration phase.
Positive change scores indicate greater magnitude of 1st 30 ms of 1st agonist burst.
Positive change scores indicate greater magnitude of agonist burst.
Positive change scores indicate greater magnitude of antagonist burst.
Negative change scores indicate lesser cocontraction.
Duration of the first agonist burst (in milliseconds).
A significant group-by-time interaction (F4, 167 = 2.58; P = 0.04) was observed. As can be seen in Fig. 3C and Table 2, the mFC and the PRET groups presented with a similar increase in the duration of the first agonist burst at the 6- and 12-mo time point. However, at the 18- and 24-mo time points, the mFC group started to return to baseline levels, whereas the PRET group increased their first agonist burst duration at the 18-mo time point and presented with a slight decrease at the 24-mo time point. At the study endpoint, the mFC group presented with a 21-ms increase, whereas the PRET presented with a 57-ms increase in the duration of the first agonist burst relative to baseline. Pairwise comparisons revealed that the increase in first agonist burst duration relative to baseline, as shown in Fig. 3C and Table 2, was significantly greater in the PRET group compared with the mFC group at 18 mo (35 ms; 1–69; P = 0.04) and at the study endpoint of 24 mo (43 ms; 7–78; P = 0.02). Additionally, at the study endpoint of 24 mo, the first agonist burst duration in the mFC group returned toward baseline levels and was not significantly different from baseline (P = 0.15).
Qag1, magnitude of the first agonist burst.
A significant group-by-time interaction (F4, 167 = 3.89; P = 0.005) was observed. As can be seen in Fig. 3D and Table 2, the mFC and the PRET groups presented with increases in Qag1 at the 6- and 12-mo time points. However, at the 18- and 24-mo time points, the mFC group started to return toward baseline levels. At the study endpoint, the mFC group was only 3.7 arbitrary units (au) greater than baseline. On the other hand, the PRET group maintained their initial increase in Qag1 and was 12.6 au greater than baseline at the study endpoint. Pairwise comparisons revealed that the increase in Qag1 relative to baseline, as shown in Fig. 3D, was significantly greater for the PRET group compared with the mFC group at 18 mo (6.5 au; 1–12.1; P = 0.02) and at the study endpoint of 24 mo (9.3 au; 3.5–15.1; P = 0.002). Additionally, at the study endpoint of 24 mo, Qag1 in the mFC group trended toward baseline levels and was not significantly different from baseline (P = 0.13).
Qag1/t1, magnitude of the first agonist burst normalized to burst duration.
A significant group-by-time interaction (F4, 167 = 2.95; P = 0.02) was observed. As can be seen in Fig. 3E and Table 2, the mFC and the PRET groups increased Qag1/t1 relative to baseline at the 6- and 12-mo time points. However, at the 18- and 24-mo time points, the mFC group started to return to baseline levels, whereas the PRET group maintained the increase in Qag1/t1. At the study endpoint, the mFC group presented with an increase of 0.01 au/ms, whereas the PRET group exhibited a 0.05 au/ms increase in Qag1/t1 relative to baseline. Pairwise comparisons revealed that the increase in Qag1/t1 relative to baseline, as shown in Fig. 3E, was significantly greater for the PRET group compared with the mFC group at 12 mo (0.03 au/ms; 0–0.05; P = 0.02) and at the study endpoint of 24 mo (0.03 au/ms; 0.01–0.06; P = 0.01). Additionally, at the study endpoint of 24 mo, the Qag1/t1 in the mFC group returned to baseline levels and was not significantly different from baseline (P = 0.16).
Number of agonist bursts.
A significant group-by-time interaction (F4, 167 = 3.82; P = 0.005) was observed for the number of agonist bursts before peak velocity. Both the mFC and the PRET groups exhibited a reduction in number of agonist bursts before peak velocity relative to baseline from 6 to 24 mo (Fig. 3F and Table 2). As can be seen in Fig. 3F and Table 2, the PRET group exhibited a reduction at all study time points, whereas the mFC group plateaued at 6, 12, and 18 mo and was slightly reduced at the study endpoint of 24 mo. At the study endpoint, the mean number of agonist bursts relative to baseline reduced by 0.6 in the mFC group and by 0.9 in the PRET group. PRET was able to reduce the mean number of agonist bursts by almost one entire burst. Pairwise comparisons revealed that the reduction in the number of agonist bursts relative to baseline, as shown in Fig. 3F and Table 2, was significantly greater for the PRET group compared with the mFC group at 12 mo (−0.3; −0.5 to 0; P = 0.03), at 18 mo (−0.4; −0.6 to −0.2; P = 0.002), and at the study endpoint of 24 mo (−0.4; −0.6 to −0.1; P = 0.002).
Qant, magnitude of the antagonist burst.
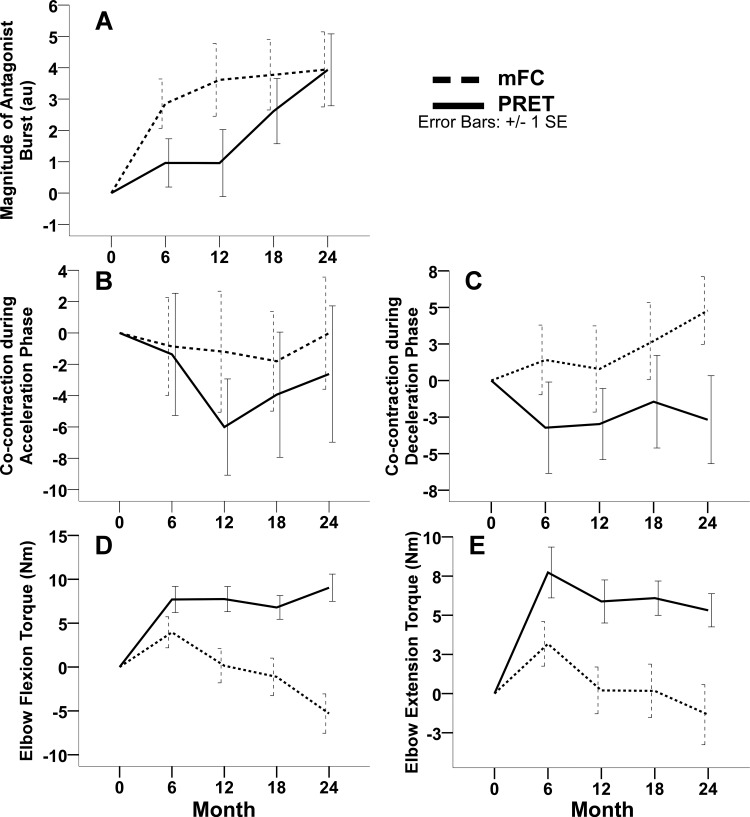
As can be seen in Fig. 4A and Table 2, there was a significant increase in Qant for both groups across time (F4, 166 = 5.52; P < 0.001).
Fig. 4.
These plots illustrate the change from baseline scores in the 2 exercise groups, including the mean (± standard error) change from baseline in the off-medication magnitude of the antagonist burst (A), percentage cocontraction during the acceleration phase (B), percentage cocontraction during the deceleration phase (C), elbow flexion torque (D), and elbow extension torque (E) at 6, 12, 18, and 24 mo. The dashed lines indicate the modified fitness counts group (mFC), and the solid lines indicate the progressive resistance exercise training group (PRET). Positive change scores indicate improvement in magnitude of the agonist burst, magnitude of the antagonist burst, and elbow flexion and extension torque. Negative change scores indicate improvement in percentage cocontraction.
Elbow flexion and extension torque.
A significant group-by-time interaction was observed for elbow flexion (F4, 167 = 9.29; P < 0.001) and elbow extension (F4, 166 = 4.79; P = 0.001) torque. At 6 mo, both groups presented with an increase in elbow flexion torque (mFC, 4 Nm; PRET, 7.7 Nm). The groups did not differ at the 6-mo time point (Fig. 4D and Table 2). At 12, 18, and 24 mo, the mFC presented with reductions in flexion torque production and went down below baseline levels (Fig. 4D). The PRET group maintained the gains in flexion torque production at 12 and 18 mo and presented with a slight increase in torque production at 24 mo. Pairwise comparisons revealed that the increase in flexion torque relative to baseline, as shown in Fig. 4D and Table 2, was significantly greater for the PRET group compared with the mFC group at 12 mo (7.9; 3.2–12.5; P = 0.001), at 18 mo (8.5; 3.7–13.2; P < 0.001), and at the study endpoint of 24 mo (14.3; 9.4–19.3; P < 0.001). Elbow extension torque presentation patterns paralleled those of elbow flexion torque. Both groups presented with elevated extension torque levels at 6 mo, which progressively reduced in the mFC group and were maintained in the PRET group out to 24 mo. Pairwise comparisons revealed that the increase in extension torque relative to baseline, as shown in Fig. 4E and Table 2, was significantly greater for the PRET group compared with the mFC group at 6 mo (4.6; 1–8.1; P = 0.01), at 12 mo (5.9; 2.3–9.6; P = 0.002), at 18 mo (6.7; 3–10.4; P < 0.001), and at the study endpoint of 24 mo (7.3; 3.4–11.1; P < 0.001).
We also conducted analyses on the above outcomes with the last available observation carried forward, and the results were similar except for cocontraction during deceleration, which was significantly lower in the PRET group at the study endpoint (−7.1; −13.6 to −0.5; P = 0.03). In addition, within each group, we compared baseline demographic, clinical, motor status (Corcos et al. 2013) and muscle activation outcomes between those who did and those who did not complete the study. No differences between completers and noncompleters were observed.
Relationship of movement velocity, muscle activation patterns, and muscle strength.
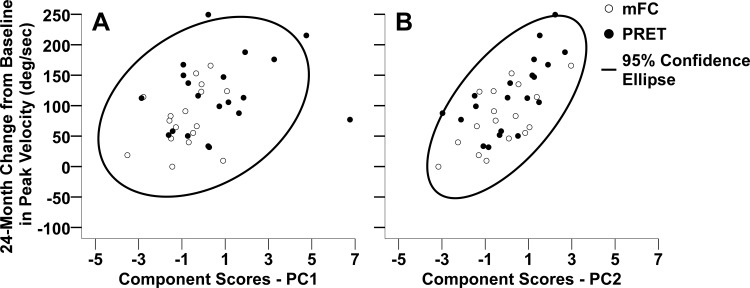
To determine the relationship of movement velocity, muscle activation, and muscle strength, we employed a principal component regression analysis. The principal component (PC) analysis resulted in three PCs with eigenvalues >1 (PC1, 3.94; PC2, 2.25; and PC3, 1.58; Table 3). Examination of the eigenvectors (Table 4) revealed that PC1 was positively correlated with the duration (r = 0.32) and magnitude (r = 0.45) of the first agonist burst, duration of the first agonist burst normalized to its duration (r = 0.45), magnitude of the 1st 30 ms of agonist EMG (r = 0.39), and the magnitude of the agonist EMG (r = 0.39). PC2 was positively correlated with the magnitude of the antagonist burst (r = 0.47), cocontraction during the acceleration (r = 0.5) and deceleration (r = 0.43) phases of movement, and elbow flexor (r = 0.43) and extensor (r = 0.38) maximal isometric torque. Number of agonist bursts correlated negatively (r = −0.26) with PC1 and correlated positively (r = 0.38) with PC3. In the context of muscle activation outcomes being associated with changes in peak velocity, the negative correlation was more meaningful, i.e., the more agonist bursts, the slower the peak velocity. In addition, only PC1 and PC2 were significantly correlated with change in peak velocity (r = 0.35 and 0.68, respectively). Consequently, PC3 was not included in any further analysis.
Table 3.
Principal component analysis eigenvalues
| PC | Eigenvalue | Difference | Proportion | Cumulative |
|---|---|---|---|---|
| 1 | 3.94 | 1.69 | 0.36 | 0.358 |
| 2 | 2.25 | 0.67 | 0.2 | 0.563 |
| 3 | 1.58 | 0.59 | 0.14 | 0.706 |
| 4 | 0.99 | 0.36 | 0.09 | 0.797 |
| 5 | 0.64 | 0.15 | 0.06 | 0.855 |
| 6 | 0.49 | 0.05 | 0.04 | 0.899 |
| 7 | 0.44 | 0.11 | 0.04 | 0.939 |
| 8 | 0.33 | 0.15 | 0.03 | 0.969 |
| 9 | 0.18 | 0.05 | 0.02 | 0.985 |
| 10 | 0.13 | 0.11 | 0.01 | 0.998 |
| 11 | 0.03 | 0.003 | 1 |
PC, principal component.
Table 4.
Principal component analysis eigenvectors
| PC1 | PC2 | |
|---|---|---|
| Duration of the 1st agonist burst | 0.32 | −0.11 |
| Magnitude of the 1st agonist burst | 0.45 | −0.04 |
| Magnitude of the 1st agonist burst normalized to burst duration | 0.45 | 0.04 |
| Number of agonist bursts | −0.26 | −0.02 |
| Magnitude of the 1st 30 ms of the agonist burst | 0.39 | 0.08 |
| Magnitude of the agonist burst | 0.39 | −0.05 |
| Magnitude of the antagonist burst | 0.13 | 0.47 |
| Cocontraction during limb acceleration | −0.17 | 0.5 |
| Cocontraction during limb deceleration | −0.16 | 0.43 |
| Maximum elbow flexion strength | 0.15 | 0.43 |
| Maximum elbow extension strength | 0.14 | 0.38 |
PC, principal component.
Principal component scores from PC1 and PC2 from each subject were used as continuous independent variables in a linear regression with the 24-mo change in peak velocity as the dependent outcome. Both PC1 and PC2 (Fig. 5, A and B, respectively) were significantly associated with change in peak velocity, with PC2 being the stronger of the two. A unit increase in PC1 was associated with a 10°/s increase in peak velocity (95% confidence level, 3.7–17; P = 0.003). A unit increase in PC2 was associated with a 26°/s increase in peak velocity (17.5–35.1; <0.0001). Together, PC1 and PC2 explained ∼55% of the variance in change in peak velocity. Treatment group and the treatment group-by-PC interaction were not included in the final model as they were not significant.
Fig. 5.
These scatter plots illustrate the relationship between 24-mo change from baseline in peak velocity and component scores on principal component 1 (A) and principal component 2 (B) for each subject. The open circles represent subjects in the modified fitness counts group (mFC), and the closed circles represent subjects in the progressive resistance training group (PRET). The ellipse in each plot represents the 95% confidence ellipse of the joint mean.
DISCUSSION
Bradykinesia is a cardinal feature of PD that is accompanied by impaired muscle activation patterns. These impaired muscle activation patterns include reduction in the magnitude and duration of the first agonist burst, increase in the number of agonist bursts during the acceleration phase of the movement, and the reduction of the magnitude of the antagonist burst (Pfann et al. 2001; Teasdale et al. 1990). These impaired muscle activation patterns offer a physiological basis for bradykinesia in PD. Dopaminergic medication and deep brain stimulation of the subthalamic nucleus increase movement velocity and partially restore the triphasic muscle activation pattern in PD but do not normalize it. Recently, bradykinesia quantified after 12-h withdrawal of dopaminergic medication has been shown to improve significantly following 24 mo of PRET despite the progressive nature of PD (Corcos et al. 2013). In the current study, our findings demonstrate that PRET restores some properties of the triphasic EMG muscle activation pattern and improves elbow flexor and extensor muscle strength. Together, the changes in muscle activation and muscle strength are significantly associated with improvement in bradykinesia. These findings are consistent with changes that have been shown with dopaminergic medication and deep brain stimulation of the subthalamic nucleus (Vaillancourt et al. 2004).
Bradykinesia, EMG muscle activation, and muscle strength.
The results of the principal component regression support the idea that partial restoration of the triphasic EMG pattern and the increase in muscle strength are likely to underlie the improvement in bradykinesia (Fig. 5). An adjusted r2 of 0.55 suggests that 55% of the variance in bradykinesia is explained by muscle activation and muscle strength outcomes. During ballistic movements, peak torque is directly related to peak velocity, i.e., greater the ability to generate torque, greater will be the movement velocity. It is also known that the magnitude of the first agonist burst is related to the magnitude of torque produced (Corcos et al. 1989). Therefore, the increase in peak velocity observed in the PRET group could be explained in part by the increase in torque generation brought about by the increase in the magnitude of the first agonist burst. In the PRET group, two mutually exclusive patterns of increase in the first agonist magnitude were observed. The first pattern was that the magnitude was increased by increasing the peak magnitude with minimal changes in the duration of the first agonist burst. The second pattern was that the peak magnitude and the duration were increased simultaneously. Additionally, because the first agonist burst is of larger magnitude or larger magnitude and longer duration, overall, the number of additional bursts required to accelerate the limb are reduced. Finally, given that the magnitude of the first agonist burst increased and the peak movement velocity also increased, there was a corresponding increase in antagonist muscle activation to slow the limb as it approached the target. Our results support the idea that by increasing the magnitude or magnitude and duration of the first agonist burst, reducing the number of agonist bursts, increasing the magnitude of the antagonist burst, and increasing elbow flexor and extensor torque, PRET partially restored the triphasic EMG pattern and mitigated bradykinesia.
At the study endpoint of 24 mo, change from baseline scores in the duration and magnitude of the first agonist burst, number of agonist bursts before peak velocity, elbow flexion torque, and elbow extension torque were significantly improved in PRET relative to mFC. Despite these statistically significant differences between groups, with respect to the principal component regression, there were no differences between groups, nor was there any interaction between groups and PC1 and PC2. This suggests that the relationship between PC1 and PC2 with respect to peak velocity is similar between groups (Fig. 5). To elaborate, given that peak velocity improved relative to baseline in the mFC group, it is likely that in the mFC group the significant improvements in number of agonist bursts before peak velocity and antagonist magnitude combined with the nonsignificant improvements in the first agonist duration and magnitude are associated with increase in peak velocity. It is known that agonist activity dominates during the acceleration phase of movement (Hallett et al. 1975), whereas antagonist/cocontraction dominates during the deceleration phase of movement (Hallett et al. 1975; Hannaford and Stark 1985). This parallels our findings wherein agonist measures correlated with PC1 and antagonist, cocontraction, and strength measures correlated with PC2 (Table 4). This pattern is fundamental to ballistic movement generation, and it appears that it is not altered by exercise. Another finding from the principal component regression is that unit change in PC2 brings about a greater change in peak velocity compared with PC1. This indicates that altering magnitude of antagonist burst, cocontraction, and strength is likely to bring about greater increases in peak velocity.
Another important variable that correlated with PC2 was the agonist and antagonist muscle cocontraction during movement (Table 4). Percentage cocontraction has been reported to be increased in participants with PD, and reduction in cocontraction has been shown to accompany increases in movement velocity (Vaillancourt et al. 2004). Cocontraction was also shown to be reduced with medication as well as deep brain stimulation of the subthalamic nucleus. Even though PRET brought about reduction in cocontraction relative to mFC during the acceleration and deceleration phases of movement at all time points, none of these was significant (Fig. 4, B and C). Of note, the last available observation carried forward analysis showed that cocontraction during deceleration was in fact significantly lower in the PRET group at 24 mo. Therefore, it is likely that the inability to find statistical significant differences between groups with respect to cocontraction is likely due to a lack of statistical power. PRET led to mostly consistent effects on the muscle activation pattern as deep brain stimulation (Vaillancourt et al. 2004), and it appears that PRET partially restores some aspects of the triphasic EMG pattern.
PRET and central and peripheral changes.
One likely explanation for the positive changes observed in the PRET group is that PRET potentially changes basal ganglia neurophysiology (David et al. 2012). It has previously been proposed that the basal ganglia are involved in modulating both the magnitude and duration of the signal arriving at the motoneuron pool (Vaillancourt et al. 2004). Dopamine replacement therapy and deep brain stimulation of the subthalamic nucleus, both therapeutic interventions aimed at modifying basal ganglia output, have been shown to increase first agonist magnitude and duration (Vaillancourt et al. 2004). Here, we show that PRET can also increase burst magnitude and duration. Consequently, it is possible that some of the changes observed in muscle activation patterns in the current study are mediated by changes in basal ganglia neurophysiology. This may also partially account for the percentage of the variance in bradykinesia that is not accounted for by improving muscle activation and muscle strength. Future studies are needed to determine whether this is the case.
One possible underlying mechanism that drives changes in basal ganglia neurophysiology is use-dependent synaptic plasticity following intensive physical activity. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mice, Petzinger and colleagues (2007) have shown an increase in the stimulus-evoked dopamine release within the dorsolateral striatum following intensive treadmill training. Because the dorsolateral striatum is engaged to a high degree during fore- and hindlimb movements during treadmill exercise, they attributed the observed striatal plasticity to use-dependent synaptic plasticity. Similarly, there may also be use-dependent synaptic plasticity in the putamen, the globus pallidus internus, and the subthalamic nucleus following PRET. Evidence to support this statement comes from a series of studies where we have shown that the nuclei within the basal ganglia scale with force in both healthy individuals and those with PD. Specifically, both the globus pallidus and the subthalamic nucleus increase the percent signal change when generating progressively larger forces in healthy participants (Spraker et al. 2007). Individuals with PD exhibit a reduced percent signal change in all nuclei of the basal ganglia during force production tasks, even early in the disease process and in not yet medicated individuals with PD (Spraker et al. 2010). Because of the progressive increase in torque generation and the high repetition, it is quite possible that PRET activates the basal ganglia regions that are deficient and brings about use-dependent synaptic plasticity that may be mediated by increased release of striatal dopamine.
It should be noted that physical activity has also been linked with plastic changes in several cortical and subcortical regions (Carroll et al. 2002; Falvo et al. 2010; Liu-Ambrose et al. 2012; Griesbach et al. 2004; Matsuda et al. 2011). The findings of the current study clearly show that PRET could bring about changes in the control signal that arrives at the muscle in PD. It does not identify where in the neural axes these plastic changes occur. Thus it is likely that plastic changes following PRET could occur in brain regions other than the basal ganglia. Further research is required to identify specific loci of neuroplasticity along the neural axes.
In addition to being altered by central factors, peripheral factors can also be altered by PRET (Kelly et al. 2014). Peripheral factors like muscle fiber diameter, fiber type composition, and amount of subcutaneous fat can all affect EMG magnitude (De Luca 1997). This is certainly quite possible in the current study following 24 mo of PRET. We do not discount the effect these peripheral changes have on the magnitude of the EMG. However, Corcos et al. (1996) showed that muscle strength and muscle activation were significantly reduced after withdrawal of dopaminergic medication. Peripheral factors are also unlikely to affect duration modulation of the first agonist burst or the number of agonist bursts. These are amenable to modulation primarily through central mechanisms. In addition, during ballistic movements, the triphasic EMG pattern is a consequence of central programming (Hallett et al. 1991); as such, any change in the triphasic EMG pattern following PRET is most likely to be central in origin as well. Moreover, change in the triphasic EMG pattern following medication or deep brain stimulation is immediate with no change in muscle fiber diameter, amount of subcutaneous fat, and fiber type composition. Therefore, the current findings suggest that a major contributor to improvement in bradykinesia of the upper limb following 24 mo of PRET is central.
In conclusion, at the study endpoint of 24 mo, PRET was associated with significant gains in upper limb movement velocity compared with mFC. The increase in movement velocity was associated with an alteration in the myoelectric pattern thus partially restoring the triphasic EMG pattern. Additionally, by positively modifying the control signal that arrives at the muscle, PRET also shows promise in affecting change in the striatothalamocorticospinal circuit.
GRANTS
This study was supported by the National Institute of Neurological Disorders and Stroke (R01-NS-28127-12 to -16).
DISCLAIMERS
The sponsors were not involved in the design, conduct, collection, management, analysis, and/or interpretation of the study results and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institutes of Health (NIH).
DISCLOSURES
F. J. David, J. A. Robichaud, and C. Poon received grant support from the NIH. D. E. Vaillancourt received grant support from the NIH, State of Florida, Bachmann-Strauss Dystonia & Parkinson Foundation, and Tyler's Hope for a Dystonia Cure and is cofounder of Neuroimaging Solutions. W. M. Kohrt received grant support from the NIH and Department of Defense and consulting fees from the NIH. C. L. Comella is receiving or has received research support from Allergan, Inc., Merz Pharma Group, Ipsen Limited, the NIH, and Parkinson's Disease Foundation and consulting fees from NuPathe, Allergan, Inc., Merz Pharma Group, Ipsen Limited, and Medtronic. D. M. Corcos received grant support from the NIH and the Michael J. Fox Foundation and receives lecture and reviewer fees from the NIH.
AUTHOR CONTRIBUTIONS
F.J.D. was responsible for acquisition of data, analysis and interpretation of the data, statistical analysis, drafting the manuscript, and administrative, technical, or material support. J.A.R. was responsible for study concept and design, obtaining funding, acquisition of data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support. D.E.V. was responsible for study concept and design, obtaining funding, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support. C.P. was responsible for acquisition of data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support. W.M.K. was responsible for study concept and design, obtaining funding, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support. C.L.C. was responsible for study concept and design, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support. D.M.C. was responsible for study concept and design, obtaining funding, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support.
REFERENCES
- Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD, Marsden CD. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain 119: 661–674, 1996. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallet M. Pathophysiology of bradykinesia in Parkinson's disease. Brain 124: 2131–2146, 2001. [DOI] [PubMed] [Google Scholar]
- Canadian Society for Exercise Physiology. Physical Activity Readiness Questionnaire - PAR-Q (Revised 2002) (Online). http://uwfitness.uwaterloo.ca/PDF/par-q.pdf [4 May 2013]. [Google Scholar]
- Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol 544: 641–652, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianci H. Parkinson Disease: Fitness Counts. Miami, FL: National Parkinson Foundation, 2006. [Google Scholar]
- Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson's disease: relationship to rate of force generation and clinical status. Ann Neurol 39: 79–88, 1996. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Agarwal GC. Organizing principles for single-joint movements. II. A speed-sensitive strategy. J Neurophysiol 62: 358–368, 1989. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Latash ML, Almeida GL, Agarwal GC. Electromechanical delay: an experimental artifact. J Electromyogr Kinesiol 2: 59–68, 1992. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, Rafferty MR, Kohrt WM, Comella CL. A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Mov Disord 28: 1230–1240, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David FJ, Rafferty MR, Robichaud JA, Prodoehl J, Kohrt WM, Vaillancourt DE, Corcos DM. Progressive resistance exercise and Parkinson's disease: a review of potential mechanisms. Parkinsons Dis 2012: 124527, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David FJ, Robichaud JA, Leurgans SE, Poon C, Kohrt WM, Goldman JG, Comella CL, Vaillancourt DE, Corcos DM. Exercise improves cognition in Parkinson's disease: the PRET-PD randomized, clinical trial. Mov Disord 30: 1657–1663, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C. The use of surface electromyography in biomechanics. J Appl Biomech 13: 135–163, 1997. [Google Scholar]
- DeLong M, Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin EEG Neurosci 41: 61–67, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, UPDRS Program Members. Unified Parkinson's Disease Rating Scale. In: Recent Developments in Parkinson's Disease, edited by Fahn S, Marsden CD, Goldstein M, andCalne DB. Florham Park, NJ: Macmillan Health Care Information, 1987, p. 153–163. [Google Scholar]
- Falvo MJ, Sirevaag EJ, Rohrbaugh JW, Earhart GM. Resistance training induces supraspinal adaptations: evidence from movement-related cortical potentials. Eur J Appl Physiol 109: 923–933, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbaum MS, Pollock ML. Prescription of resistance training for health and disease. Med Sci Sports Exerc 31: 38–45, 1999. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975. [DOI] [PubMed] [Google Scholar]
- Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. New York: Springer-Verlag, 1998. [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125: 129–139, 2004. [DOI] [PubMed] [Google Scholar]
- Hallett M, Berardelli A, Matheson J, Rothwell J, Marsden CD. Physiological analysis of simple rapid movements in patients with cerebellar deficits. J Neurol Neurosurg Psychiatry 54: 124–133, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain 103: 301–314, 1980. [DOI] [PubMed] [Google Scholar]
- Hallett M, Shahani BT, Young RR. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry 38: 1154–1162, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannaford B, Stark L. Roles of the elements of the triphasic control signal. Exp Neurol 90: 619–634, 1985. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons DE. Longitudinal Data Analysis. New York: John Wiley & Sons, 2006. [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181–184, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers JN. Two case studies in the application of principal component analysis. J R Stat Soc Ser C Appl Stat 16: 225–236, 1967. [Google Scholar]
- Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR, Tuggle SC, Williams JY, Lieb L, Windham ST, Bamman MM. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson's disease. J Appl Physiol (1985) 116: 582–592, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med 339: 1130–1143, 1998. [DOI] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 7: 2–13, 1992. [DOI] [PubMed] [Google Scholar]
- Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging 33: 1690–1698, 2012. [DOI] [PubMed] [Google Scholar]
- Matsuda F, Sakakima H, Yoshida Y. The effects of early exercise on brain damage and recovery after focal cerebral infarction in rats. Acta Physiol (Oxf) 201: 275–287, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson's disease (2001 ): treatment guidelines. Neurology 56: S1–S88, 2001. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci 27: 5291–5300, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson's disease. Mov Disord 16: 1048–1065, 2001. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Rafferty MR, David FJ, Poon C, Vaillancourt DE, Comella CL, Leurgans SE, Kohrt WM, Corcos DM, Robichaud JA. Two-year exercise program improves physical function in Parkinson's disease: the PRET-PD randomized clinical trial. Neurorehabil Neural Repair 29: 112–122, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Spraker M, Corcos D, Comella C, Vaillancourt D. Blood oxygenation level-dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson's disease. Mov Disord 25: 2035–2043, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud JA, Pfann KD, Comella CL, Brandabur M, Corcos DM. Greater impairment of extension movements as compared to flexion movements in Parkinson's disease. Exp Brain Res 156: 240–254, 2004. [DOI] [PubMed] [Google Scholar]
- Robichaud JA, Pfann KD, Comella CL, Corcos DM. Effect of medication on EMG patterns in individuals with Parkinson's disease. Mov Disord 17: 950–960, 2002. [DOI] [PubMed] [Google Scholar]
- Robichaud JA, Pfann KD, Leurgans S, Vaillancourt DE, Comella CL, Corcos DM. Variability of EMG patterns: a potential neurophysiological marker of Parkinson's disease? Clin Neurophysiol 120: 390–397, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naive Parkinson's disease. Hum Brain Mapp 31: 1928–1941, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol 98: 821–834, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale N, Phillips J, Stelmach GE. Temporal movement control in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 53: 862–868, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Sturman MM, Bakay RA, Metman LV, Corcos DM. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson's disease. Mov Disord 21: 50–58, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson's disease. Brain 127: 491–504, 2004. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Anatomy and physiology of the basal ganglia: relevance to Parkinson's disease and related disorders. Handb Clin Neurol 83: 1–18, 2007. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional neuroanatomy of the basal ganglia in Parkinson's disease. Adv Neurol 91: 9–18, 2003. [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. New York: John Wiley & Sons, 1990. [Google Scholar]