Abstract
Clear evidence of sleep in invertebrates is still meager. Defined as a distinct state of reduced activity, arousability, attention, and initiative, it is well established in mammals, birds, reptiles, and teleosts. It is commonly defined by additional electroencephalographic criteria that are only well established in mammals and to some extent in birds. Sleep states similar to those in mammals, except for electrical criteria, seem to occur in some invertebrates, based on behavior and some physiological observations. Currently the most compelling evidence for sleep in invertebrates (evidence that meets most standard criteria for sleep) has been obtained in the fruit fly Drosophila melanogaster. However, in mammals, sleep is also characterized by a brain state different from that at rest but awake. The electrophysiological slow wave criterion for this state is not seen in Drosophila or in honey bees. Here, we show that, in crayfish, a behavioral state with elevated threshold for vibratory stimulation is accompanied by a distinctive form of slow wave electrical activity of the brain, quite different from that during waking rest. Therefore, crayfish can attain a sleep state comparable to that of mammals.
Sleep is clearly recognized as a state during which the body, not the brain, is passive. It has been studied mostly in mammals and to some extent in vertebrates (1) whereas studies in invertebrates are few and mostly about behavioral and some electrophysiological aspects (2, 3). However, recent genetic studies in flies (4–6) seem to show that these animals have a clearly distinct sleep state; thus, its demonstration in other invertebrates would show that sleep arose very early in evolution.
A common assessment of brain states in mammals is based on the proportions of different frequency segments of the power spectrum of the electroencephalogram (EEG) (labeled alpha, beta, gamma, delta, and theta). To test in an invertebrate for the presence of a brain state that could be equivalent to sleep in vertebrates, we chose crayfish for three main reasons: (i) their relatively large size allows us to chronically implant electrodes on the brain (7); (ii) they are easy to maintain and record under laboratory conditions for extended periods of time, and (iii) we learned to recognize a resting position clearly distinguishable from other positions and during which they seem to be unaware of their environment (8) and had elevated thresholds for arousal.
We first videotaped animals during periods of 24 h and noted specific stereotypic body positions, the hours of the day at which these occur, and the length of time spent in each position. At these times, we simultaneously recorded their spontaneous brain electrical activity. We also measured arousal thresholds to mechanical stimulation and “event-related potentials” with trains of light flashes that tested expectation, a mildly cognitive function. Because crayfish show several resting states during which they remain motionless, we define “sleep” as that condition during which the animal is resting and apparently unaware of its environment, arousable only with strong stimuli. Because this condition occurs during a specific brain state and posture, we sought also to identify specific characteristics of its brain electrical activity.
Materials and Methods
Experiments were performed on crayfish Procambarus clarkii of either sex and ≈10 cm from rostrum to uropods in laboratories at Mexico City and La Jolla, CA. At Mexico City, animals were obtained from a local supplier whereas those at La Jolla were provided by Niles Biological (Sacramento, CA). As soon as received, crayfish were placed in well aerated “old” tap water and fed twice per week with lettuce, carrot, powdered dry fish, and hard boiled eggs.
For behavioral observations, we used intact crayfish of similar size and either sex freely moving about their aquarium. For electrophysiological recordings from the brain, animals were chronically implanted with Teflon-coated fine-wire electrodes. For studying the crayfish locomotor behavior, over a period of a year we placed ≈200 groups of three animals in aquaria (30 × 30 × 15 cm with 10 cm of well oxygenated tap water) without shelters in a room with controlled light (cycles of 12:12 h dark:light, switched on at 7:00 a.m. and off at 7:00 p.m.). Animals were in their aquaria several days before the recording.
Groups of three crayfish were videotaped continuously throughout 24-h cycles with an infrared Video Camera (CCD-TR416, Sony). Cartridges were changed every 4 h. For measuring the amount of crayfish displacement, videotapes were played at less than half-speed on a TV monitor with the screen divided in 5 × 5 cm2. We considered that an animal entered a new square when the head was in the new square up to the level of the eyes. Displacement for each animal was measured during periods of 5 min every hour, along with its body position. We also recorded the time spent by each animal in each position.
To test the crayfish threshold to mechanical stimuli, we used a brushless dc motor (Model 1E120, Yokyo Parts, Tokyo) attached to the dorsal cephalothorax and connected with a thin cable to a motor powered with rectangular pulses of various intensities and durations from a Stimulator (S-88, Grass Instruments, Quincy, MA). Pulses changed the speed of motor rotation from zero to a maximum of ≈3,000 rpm, as measured on the oscilloscope screen by the interruption of a laser beam.
Strength-duration curves were obtained increasing the intensity and duration of the square pulse powering the motor. Animals were stimulated every 15 s after >2 min in each of four positions. As soon as the threshold for a particular set of parameters was reached, we changed to new values. Maximal values were 10 V and 1 s. A complete run of pulses to obtain threshold values lasted ≈5 min.
The crayfish appendages most frequently moved during stimulation by the motor were antennae, antennulae and the third pereiopods. Recording electromyograms from leg muscles was not useful to detect a mechanical threshold because there is not an abrupt change in the number of spikes elicited at any intensity value. Therefore and because it is easy to observe, we defined threshold as any movement of the third pereiopod associated with the pulse, as seen in vivo by an experienced observer. We fitted the data points from mechanical threshold with the sum of two exponentials.
For recording the brain spontaneous electrical activity, we implanted electrodes following a technique previously described (7). Briefly, we inserted a segment of a stainless steel needle (12 gauge) carrying inside a fine platinum-iridium Teflon-coated wire (25 μm) through a hole in the dorsal cephalothorax. The needle, observed with a stereomicroscope, was placed on the median protocerebrum, and the ensemble was attached to the cephalothorax with dental cement.
Brain extracellular potentials were recorded by AC preamplifiers (P511, Grass Instrument, Quincy, MA), with filters passing from 3 Hz to 3 kHz. In most cases, an additional filter band-rejecting at 60 Hz was used. All recordings from the brain were analyzed with datawave (Longmont, CO) and/or McClune Tools.
Gross electrode localization was assessed during postmortem studies. Data shown here were recorded when electrodes rested on the upper third of the right lower protocerebrum and the upper deutocerebrum. When electrodes were found on other regions, spikes were absent or very small and data from these crayfish were not used.
We tested crayfish stimulating with light flashes and analyzing the omitted stimulus potentials (OSPs) in the records, as described (9). Briefly, we used trains of 10 light flashes of 10 μs duration at frequencies around 10 Hz, delivered by a reflector-diffuser lamp 13 cm in diameter, connected to a photic stimulator (PS22, Grass Instruments) placed 20–30 cm in front of the crayfish. Typical experiments involved recording segments of 3 s long: 1-s control prestimulus, 1-s during the stimuli, and 1-s after the train, delivered every 10 s. Groups of five trains were averaged to enhance the resultant spike burst or slow wave. Analyses were performed with datawave. Air temperature was 20–25°C.
Crayfish were deprived from sleep by suspending them in water in the aquarium for periods of 24 h while strongly bubbling air underneath. The bubbles move the animals continuously, and they are unable to rest.
To record the crayfish position for prolonged periods of time, we placed on the dorsal carapace a mercury switch that opened and closed a circuit including a 1.5-V battery. This device provided a voltage value recorded with a low-level dc amplifier (7P122) feeding an ink-writing oscillograph (Grass Instruments).
Results and Discussion
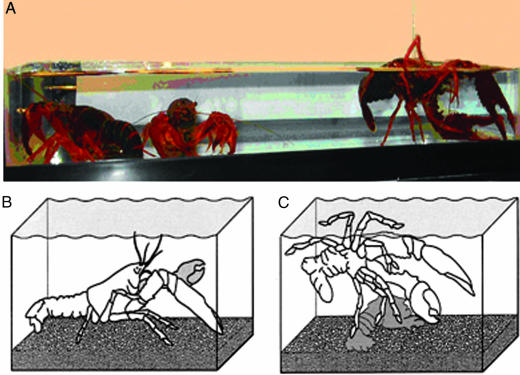
When crayfish have settled down in an aquarium without shelters, four general body positions are observed: (i) walking on all legs (pereiopods) at the bottom of the aquarium, with chelae extended at the level of the rostrum; (ii) standing up motionless on extended pereiopods (Fig. 1B); (iii) standing up motionless on flexed pereiopods, with chelae flexed, tail curled and antennae and antennulae low (Fig. 1 A, middle crayfish), and; (iv) lying on one side just under the surface of the water, with chelae extended, commonly against a wall of the aquarium (Fig. 1 A and C).
Fig. 1.
Crayfish in several positions. (A) Side view of an aquarium with three crayfish, one of them lying on one side (right) and two standing up (left). (B) Drawing of crayfish standing up. (C) Drawing of crayfish lying on one side.
In the aquarium, the walking behavior seen during most of the day (Fig. 2A) is interrupted at times, when animals remain standing up motionless for variable periods of time. Then, some crayfish are seen pushing themselves against the surface of the water and lying motionless on one side. Because at these times we also recorded slow waves from the brain and the crayfish has the highest threshold to mechanical stimulation, we consider such lying on one side to be a postural sign of sleep. It remains to be shown whether it is an invariable sign.
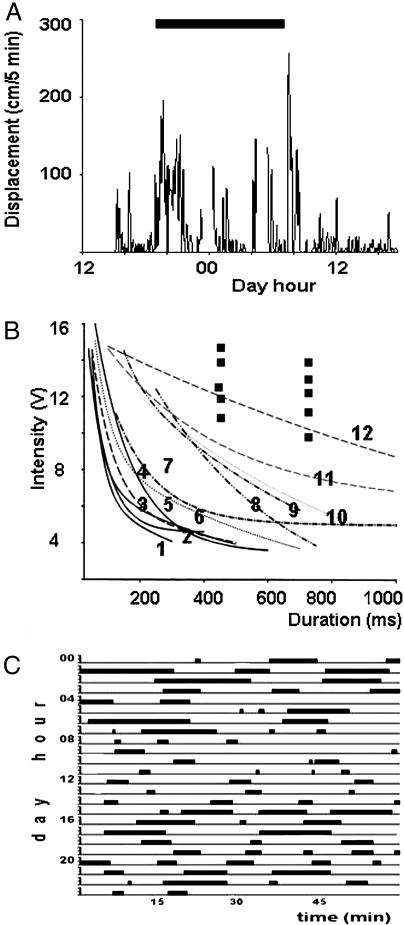
Fig. 2.
Parameters of crayfish behaviors. (A) Time-hours at which a crayfish is walking around its aquarium. The black bar indicates night hours. (B) Vibratory intensity-duration threshold curves while crayfish behavior is active (curves 1–7), standing up motionless (curves 8–11), and lying on one side against the surface of the water (curve 12; isolated points also correspond to this condition). (C) Time-hours at which a crayfish lies on a side against the surface of the water. A and C show an example of 12 recorded cases.
Crayfish have positive buoyancy and to adopt a lying-on-one-side position, they generally brace themselves at the surface of the water in the lateral recumbent position. The crayfish shown at the right in Fig. 1 A is supporting itself with three left pereiopods and a chela at the bottom of the aquarium.
Crayfish spend variable amounts of time lying on one side anytime day or night, but longer stays in such a position occur during night hours. This fact is illustrated with black bars in Fig. 2C, where we present one 24-h example out of nine similar cases recorded.
We defined mechanical threshold as the stimulus value at which we observed a just noticeable movement of either left or right third pereiopod after each mechanical pulse. With these values, we obtained strength-duration curves (Fig. 2B). Mechanical threshold was lowest (curves 1–7) when crayfish were walking about their aquaria or were motionless and slowly moving antennae or antennulae (position 2). Occasionally, crayfish could be seen in position 3 above, and under these conditions the threshold was somewhat higher (curves 8–11).
The highest thresholds were obtained when crayfish held themselves against the surface of the water (position 4). At these times, strong activation of the stepping motor did not elicit a response, and it was only when the stimulation was near the maximum that animals were seen reacting. It was difficult to obtain whole curves under these conditions because, as soon as threshold for a particular stimulus intensity was reached, the crayfish abruptly moved to a standing up position. It was in only one case that we could complete a curve (Fig. 2B, curve 12); however, almost all individual points obtained are shown above the curves for the other positions (Fig. 2B, isolated squares). All 9 animals tested while in this posture showed high thresholds whereas none of 10 animals in other postures had high thresholds. Thus, it is clear that mechanical thresholds are higher when crayfish are lying on one side.
We also recorded the spontaneous brain electrical activity of crayfish in all positions described above. Spontaneous brain electrical activity in the first posture is comprised of numerous fast spikes on a flat baseline. When occasionally we also recorded muscle spikes, these have characteristics that clearly distinguish them from neuronal spikes, as described (7, 10). The monophasic or biphasic neuronal spikes of different amplitude, as well as characteristics of the power spectrum of the record, are similar whether the crayfish are walking around the aquarium or are motionless in an upright position (Fig. 3 A and D). We do not show records when crayfish are walking around their aquaria because the baseline is very unstable due to movement of cables connecting the electrodes.
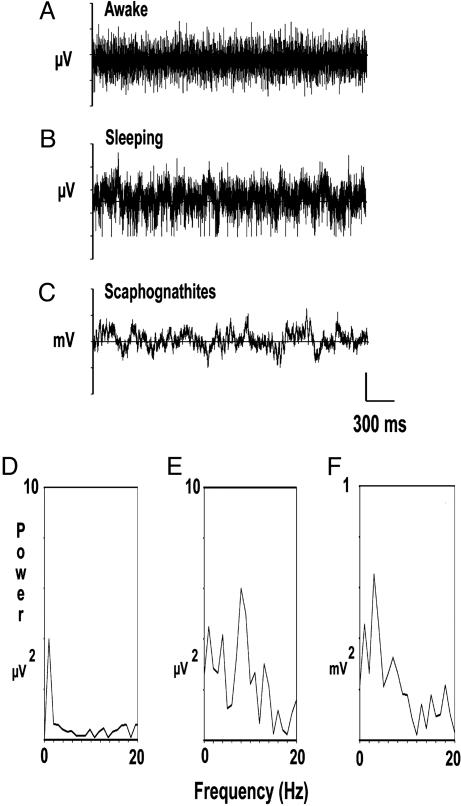
Fig. 3.
Spontaneous electrical activity of a crayfish. (A) Records obtained from the brain of a crayfish walking around its aquarium. (B and C) Records from the brain while lying asleep on one side against the surface of the water and from scaphognathites. The corresponding power spectra (0–20 Hz) of the records shown in A–C are in frames D–F. Note the different power scales.
During the spontaneous brain electrical activity from crayfish lying on one side, spikes are replaced by continuous slow waves with an amplitude of 50–100 μV (Fig. 3B). When a crayfish in such position is perturbed by other crayfish or the investigator, it immediately recovers a standing up position and the slow waves are replaced by spikes. However, non-brain activity can be also seen in the records as slow waves, and, to eliminate such possibility, we simultaneously recorded their most common source, the rhythmic beating of breathing parts (scaphognathites). Records show that, when crayfish are lying on one side, their scaphognathites activity have frequencies at or below 5 Hz (Fig. 3 C and F).
Most animals (30 of 33) with obvious slow waves were in the sleeping posture, where we know that every animal tested (n = 9) had high threshold to tactile awakenings. On the contrary, most animals recorded in a nonsleeping posture (48 of 50) lacked any such low-frequency waves.
The corresponding power spectra of records in Fig. 3 A–C are in Fig. 3 D–F. Power from record 3A is very small whereas that from record 3B shows that slow waves generated when crayfish are lying on one side have a frequency around 8 Hz. The peaks of power below 5 Hz are probably due to scaphognathite activity (Fig. 3F).
To compare records from crayfish under positions 2, 3, and 4 above, we divided the low range (0–100 Hz) of the power spectra into four bands (A, 0–5 Hz; B, 5–15 Hz; C, 15–50 Hz; and D, 50–100 Hz). Then, we obtained the frequency and power of the largest peak at each of these bands from 8 crayfish and 16 events and averaged them, as shown in Table 1.
Table 1. Frequency and power of largest peak at each of four bands of the power spectra.
| Frequency, Hz
|
Power, μV2
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Crayfish position | Bond A | Bond B | Bond C | Bond D | Bond A | Bond B | Bond C | Bond D |
| Lying on a side | 2.77 ± 1.77 | 8.48 ± 2.99 | 20.51 ± 3.66 | 85.56 ± 11.96 | 0.096 ± 0.059 | 0.052 ± 0.053 | 0.017 ± 0.013 | 0.005 ± 0.004 |
| Standing up ext. | 1.77 ± 1.45 | 7.44 ± 2.39 | 21.80 ± 6.41 | 61.30 ± 8.46 | 0.064 ± 0.046 | 0.017 ± 0.021 | 0.008 ± 0.004 | 0.004 ± 0.002 |
| Standing up flex. | 2.90 ± 1.41 | 9.77 ± 3.46 | 22.22 ± 2.93 | 86.70 ± 13.74 | 0.044 ± 0.038 | 0.019 ± 0.014 | 0.017 ± 0.007 | 0.004 ± 0.002 |
Frequency bands are 0-5 Hz (A), 5-15 Hz (B), 15-50 Hz (C), 50-100 Hz (D), while crayfish is in one of three positions: lying on a side, standing up with pereiopods extended (ext.), and standing up with pereiopods flexed (flex.). Values are mean ± SD. N = eight different crayfish and 16 events.
Table 1 shows power data from records in Fig. 3 A–C. We disregard data under band A (0–5 Hz) because filters for data acquisition were set at 3 Hz and because, in this range, we found the scaphognathite activity. The largest peak at bands B (5–15 Hz) and C (15–50 Hz) have similar frequencies (band B, 8.48, 7.44, and 9.77; band C, 20.5, 21.8, and 22.2 Hz) in all three body positions. However, when crayfish are lying on one side, the peak has larger power (0.05 mV2) than when standing up (0.017 and 0.019 μV2). Thus, although records from crayfish lying on one side or standing up motionless have similar frequency components, the larger power in the first case suggests more coherent slow waves. The t tests of power values show statistically significant differences (P < 0.5; n = 16 events) between values from animals lying on one side vs. standing up with pereiopods extended.
We also tested animal awareness of its environment with a stimulation paradigm known to test simple cognitive states in vertebrates and some invertebrates, a train of light pulses at frequencies around 10 Hz (9, 11). Omission of a stimulus within the train or its termination, elicits OSPs whose defining characteristic is their occurrence at a fixed time after the due time of the first missing stimulus (9).
OSPs generally consist of a burst of spikes following the train with a delay that for a given animal is fixed with respect to the due-time of the next stimulus after the train. They are elicited from the protocerebrum of crayfish, and scores of animals stimulated with this paradigm have always shown OSPs (9). However, when a crayfish is lying on one side in the aquarium, a train of light flashes fails to elicit OSPs (not illustrated).
A final test of sleep in crayfish was the homeostatic mechanism, which is indicated if after a period of sleep deprivation animals compensate by increasing their sleeping time. As control, we first recorded the animals position for a week and then deprived them of sleep as described in Materials and Methods, recording their position for 24 more hours.
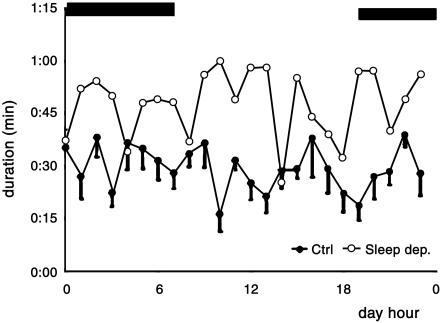
Data points (filled circles) in Fig. 4 show the time per hour that control crayfish spend lying on one side during a 24-h cycle. Points are mean (± SE) from 7 days continuous recording, and they have a rather constant value around 20 min/h with no apparent rhythm. Adding up all periods lying down, we obtained a total value of ≈11 h during a 24-h period (mean ± SD, 11 h, 6 min, 53 s ± 3 h, 0 min, 17 s, n = 6). In contrast, after animals are sleep-deprived, the periods lying on one side are prolonged (open circles in Fig. 4), greatly increasing the total time in this position (mean ± SD, 18 h, 31 min, 10 s ± 2 h, 45 min, 34 s. Fig. 4 shows only one example of 6 recorded). These data show that, similar to other animals, the lying-on-one-side position in crayfish also follows a homeostatic mechanism and further supports our assumption that this posture is a reliable behavioral sign of sleep in crayfish.
Fig. 4.
Time spent by crayfish lying on one side (sleeping) during a 24-h period (12 light/12 dark). Black bands above show the dark period. Filled circles are mean (± SE) values of sleep duration per hour during 7 control days. Open circles show first 24 h of recovery after sleep deprivation, starting at 1200 h. Data are from the same crayfish.
Our findings in crayfish (of a resting state during which the animal has high mechanical arousal threshold and the brain emits slow waves, stops processing external sensory signals, and is directed by a homeostatic mechanism) indicate the presence of the characteristic brain state named sleep. It also suggests that the presence of slow waves and their underlying slow neuronal ionic currents is a requirement or at least is associated with such a brain state. However, synchronization seems to occur also under other conditions in invertebrates and vertebrates (12), and we do not propose a homology with any particular wave in mammals, where no stage of sleep is characterized by a continuous regular wave near 8 Hz. But, because in humans and other endotherms some stages are commonly accompanied by somewhat irregular delta (2–4 Hz) slow waves, we can think of slow waves as having been conserved through evolution from invertebrates to mammals. This interpretation would strengthen the view that sleep is rather fundamental, possibly widespread among the taxa and worthy of new study, especially electrophysiologically. The use of crayfish, where slow wave sleep occurs without a REM-sleep state, provides advantages to unravel the underlying need fulfilled by such a state and which has been difficult to track in other preparations.
Acknowledgments
We appreciate the help of Karina Mendoza-Angeles in the analysis of some of the data. This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke, the Guggenheim Foundation, the University of California at San Diego, and the Facultad de Medicina and Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México.
Abbreviation: OSP, omitted stimulus potential.
References
- 1.Rattenborg, N. C. & Amlaner, C. J., Jr. (2002) in Phylogeny of Sleep, Sleep Medicine, eds. Lee-Chiong, T. L., Sayeia, T. L. & Carskadon, M. A. (Hanley and Belfus, Philadelphia), pp. 7-22.
- 2.Campbell, S. S. & Tobler, I. (1984) Neurosci. Biol. Behav. Rev. 8, 269-300. [DOI] [PubMed] [Google Scholar]
- 3.Tobler, I. & Stadler, J. J. (1988) Comp. Physiol. 163, 227-235. [Google Scholar]
- 4.Hendricks, J. C., Finn, S. M., Panckeri, K. A., Chavkin, J., Williams, J. A., Sehgal, A. & Pack, A. I. (2000) Neuron 25, 129-138. [DOI] [PubMed] [Google Scholar]
- 5.Shaw, P. J., Cirelli, C., Greenspan, R. J. & Tononi, G. (2000) Science 287, 1834-1837. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan, R. J., Tononi, G., Cirelli, C. & Shaw, P. J. (2001) Trends Neurosci. 24, 142-145. [DOI] [PubMed] [Google Scholar]
- 7.Serrato, J., Hernández, O. H. & Ramón, F. (1996) Comp. Biochem. Physiol. 114A, 219-226. [Google Scholar]
- 8.Aréchiga, H. (1995) Newsl. World Fed. Sleep Res. Soc. 4, 7-9. [Google Scholar]
- 9.Ramón, F., Hernández, O. H. & Bullock, T. H. (2001) J. Exp. Biol. 204, 4291-4300. [DOI] [PubMed] [Google Scholar]
- 10.Bullock, T. H. (1945) Yale J. Biol. Med. 17, 657-679. [PMC free article] [PubMed] [Google Scholar]
- 11.Bullock, T. H., Karamürsel, S., Achimowicz, J. Z., McClune, M. C. & Basar-Eroglu, C. (1994) Electroencephalograph. Clin. Neurophysiol. 91, 42-53. [DOI] [PubMed] [Google Scholar]
- 12.Bullock, T.H. & Basar, E. (1988) Brain Res. Rev. 13, 57-75. [DOI] [PubMed] [Google Scholar]