Strong voluntary contractions of a single limb cause unintended excitatory activity in the opposite limb. However, when contractions are repeated to fatigue, activity in the opposite nonfatigued limb is substantially decreased. Given that repeated single-limb contractions reduced the maximal force-generating capacity in the opposite limb, it appears that inhibitory mechanisms are activated on the opposite side of the body, most likely at the level of the motor cortex.
Keywords: fatigue, ipsilateral motor cortex, crossed effects, motor control
Abstract
The aim of this study was to investigate how maximal intermittent contractions for a hand muscle influence cortical and reflex activity, as well as the ability to voluntarily activate the homologous muscle in the opposite limb. Twelve healthy subjects (age 24 ± 3 yr, all right-hand dominant) performed maximal contractions of the dominant limb first dorsal interosseous (FDI), and activity of the contralateral FDI was examined in a series of experiments. Index finger abduction force, FDI electromyography (EMG), motor evoked potentials, and heteronomous reflexes were obtained from the contralateral limb during brief, nonfatiguing contractions. The same measures, as well as the ability to voluntarily activate the contralateral FDI, were then assessed in an extended intermittent contraction protocol that elicited fatigue. Brief contractions under nonfatigued conditions increased index finger abduction force, FDI EMG, and motor evoked potential amplitude of the contralateral limb. However, when intermittent maximal contractions were continued until fatigue, there was an inability to produce maximal force with the contralateral limb (∼30%), which was coupled to a decrease in the level of voluntary activation (∼20%). These declines were present without changes in reflex activity and regardless of whether cortical or motor point stimulation was used to assess voluntary activation. It is concluded that performing maximal intermittent contractions with a single limb causes an inability of the central nervous system to maximally drive the homologous muscle of the contralateral limb. This is, in part, mediated by mechanisms that involve the motor cortex ipsilateral to the contracting limb.
NEW & NOTEWORTHY
Strong voluntary contractions of a single limb cause unintended excitatory activity in the opposite limb. However, when contractions are repeated to fatigue, activity in the opposite nonfatigued limb is substantially decreased. Given that repeated single-limb contractions reduced the maximal force-generating capacity in the opposite limb, it appears that inhibitory mechanisms are activated on the opposite side of the body, most likely at the level of the motor cortex.
performance of brief strong contractions with a single limb is often accompanied by unintended muscle activity in the contralateral homologous muscle. This unintended activity has been observed for muscles of the upper limb (Christova and Kossev 2001; Kavanagh et al. 2013; Zijdewind et al. 1998, 2006) and, to a lesser extent, the lower limb (Dimitrijevic et al. 1992), and is more likely to emerge when there is no attempt to modulate muscle activity in the contralateral limb (Hortobágyi et al. 2003; Muellbacher et al. 2000; Perez and Cohen 2008). In general, the mechanisms underlying unintended activity in the contralateral limb have primarily been attributed to transcallosal pathways modulating the excitability of one motor cortex when the other is activated (Stedman et al. 1997).
Investigations that use transcranial magnetic stimulation (TMS) to examine motor cortical excitability have observed several important features when performing strong contractions with a single limb. Most notably, excitability of the ipsilateral primary motor cortex (iM1) increases during a brief single-limb contraction (Muellbacher et al. 2000; Perez and Cohen 2008; Zijdewind et al. 2006). This excitability is coupled to a decrease in short-interval intracortical inhibition of iM1 and decreased interhemispheric inhibition from the contralateral motor cortex (cM1) to the iM1 (Perez and Cohen 2008). Overall, it appears that changes in iM1 inhibition are scaled to the intensity of muscle contraction, where higher intensity contractions are associated with the lowest iM1 inhibition (Perez and Cohen 2008) and therefore the greatest unintended activity in the contralateral limb (Hortobágyi et al. 2003; Zijdewind and Kernell 2001).
Although unintended activity has been examined in a variety of contexts (Hortobágyi et al. 2003; Kenway et al. 2014; Morrison et al. 2005; Zijdewind and Kernell 2001), there is a limited understanding of how fatiguing intermittent maximal contractions affect the activation of muscle in the contralateral limb. The majority of investigations have used sustained contractions to induce perturbations in the contralateral limb, and a few notable observations have been made. For example, during 100–120 s of sustained maximal isometric knee extension, the level of voluntary activation (VA) for the opposite leg knee extensor is reduced by up to 10% (Doix et al. 2013; Martin and Rattey 2007; Rattey et al. 2006). Given that a decrease in VA during a contraction task can reflect central fatigue (Gandevia 2001), it may be the case that central nervous system (CNS) properties associated with central fatigue during muscle contractions are transferred to the contralateral limb (Rattey et al. 2006). A surprising finding in these sustained contraction studies is that even though VA was reduced in the contralateral limb, the force-generating capacity of the limb was not necessarily compromised (Rattey et al. 2006; Todd et al. 2003a). This suggests that secondary mechanisms, potentially at subcortical or spinal cord levels, may be present during voluntary contraction to ensure that force in the contralateral limb is not compromised.
The present study was designed to examine how maximal intermittent contractions for a contracting muscle in a single limb influence activity in the homologous muscle of the contralateral limb. Motor cortex, reflex activity, and force generation were examined for the contralateral limb first dorsal interosseous (FDI) when the opposite FDI performed brief maximal contractions. Subsequent experiments were performed to determine how brief and repetitive fatigue-inducing intermittent contractions of the FDI influence motor cortex, reflex activity, and the ability to voluntarily activate the contralateral nonfatigued FDI. It was hypothesized that brief maximal contractions of an unfatigued limb would cause increased unintended force, muscle activity, and cortical excitability in the contralateral homologous muscle. It was also hypothesized that repeated intermittent contractions that cause fatigue would progressively lead to an inability to produce maximal force of the contralateral homologous muscle. Any decrease in the ability to activate the contralateral homologous muscle would be coupled with decreases in level of voluntary activation assessed by either cortical or motor point stimulation techniques.
METHODS
Subjects
A total of 18 healthy subjects (age 24 ± 3 yr, weight 72 ± 12 kg, height 177 ± 8 cm, 3 females, all right handed) participated in the experiments in this study. All subjects performed strong voluntary contractions with their dominant limb, which for the purpose of this study is referred to as the “contracting limb.” Electrophysiological measurements were obtained from the nondominant limb, which is referred to as the “contralateral limb” in this study. The Epworth Sleep Scale was completed before each testing session to confirm that no subject was sleep deprived. Subjects abstained from caffeine, alcohol, and all forms of exercise for at least 6 h before experimental testing, and written informed consent was obtained before testing. All testing procedures were approved by the institutional ethics committee, and experiments were performed in accordance with the Declaration of Helsinki.
Experimental Setup
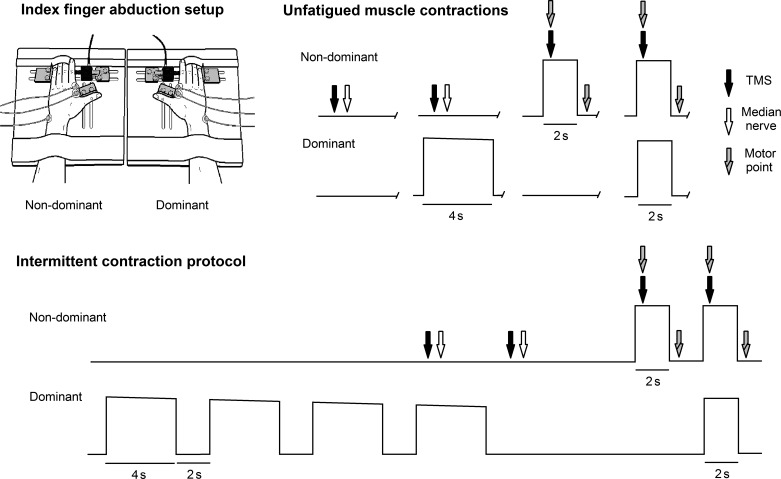
Subjects were seated comfortably in a chair with an upright back support. All participants adopted a posture with the shoulders slightly abducted and elbows flexed to 130°. Each hand was placed flat on adjustable custom-designed devices that measured index finger abduction force (Fig. 1, top left; Kenway et al. 2014). Thumbs were abducted and supported by a solid bracket, and the distal forearm and digits 3–5 were secured with Velcro straps. The index finger metacarpophalangeal joint was positioned at 0° abduction and 0° flexion, and the interphalangeal joints were maintained in extension. Each index finger proximal interphalangeal joint was pressed against a calibrated XTrans S-beam load cell (100 N; Applied Measurements), all of which were connected to the custom hand plates.
Fig. 1.
Top left: the apparatus used to measure bilateral force and EMG responses. Top right: subjects performed combinations of maximal contractions with the dominant (contracting) limb and nondominant (contralateral) limb when muscles were fresh and unfatigued. Bottom: the intermittent contraction protocol used to induce fatigue in a single limb. Maximal contractions were repeated until force-generating capacity of the contracting limb reduced by 50%. In total, one complete “fatigue set” in the contraction protocol was 33 s in duration. Motor cortex and reflex activity were obtained for the contralateral limb in experiments 1 and 2, whereas motor point stimulation of the FDI occurred in experiment 3.
Surface electromyography (EMG) was recorded for the FDI of both hands. Ag-AgCl electrodes (Kendall Arbo) were placed over the muscle belly of the superficial head of FDI and over the distal tendon at the second metacarpophalangeal joint with an interelectrode spacing of 15 mm. Reference electrodes were placed over the ulnar styloid processes. EMG was amplified 1,000 times using NL844 amplifiers and bandpass filtered at 3–400 Hz using NL135 and NL144 filters (Digitimer). Impedance was kept below 5 kΩ at each electrode site. Force and EMG data were acquired at 2,000 Hz using a CED 1401 interface and Signal software (Cambridge Electronic Design, Cambridge, UK).
Transcranial magnetic stimulation.
Motor evoked potentials (MEPs) were elicited in the contralateral limb FDI by stimulating the right motor cortex with a D702 figure-of-eight coil connected to a Magstim 2002 stimulator (Magstim, Dyfed, UK). The optimal stimulation site was determined as the area that elicited the largest and most consistent MEP amplitude that was consistently accompanied with a muscle twitch of the FDI. A microscope floor stand (OpMi-1; Zeiss) was modified to act as a head rest, where the subject's head was restricted from moving, and the 6 degrees-of-freedom microscope arm rigidly held the stimulating coil. This configuration enhanced the consistency of MEP measurement, particularly toward the end of the fatigue task when head and neck tremor may occur. Stimulator output was set at 70% maximum output of the stimulator for each subject. This intensity was selected because large MEPs could be identified during rest and contracting conditions of the contralateral limb. Stimulus intensity was kept constant throughout testing. Stimulus intensities between 60% and 80% of maximum stimulator output have previously shown that a maximal or near-maximal response is elicited from the FDI regarding twitch force and MEP amplitude (Day et al. 1987a, 1987b).
Median nerve stimulation.
A heteronomous reflex of the FDI is able to be elicited by electrical stimulation of the median nerve, which induces a reflex arc resulting in activation of efferent fibers that travel through the ulnar nerve to terminate on the FDI (Duchateau and Hainaut 1993). As such, heteronomous reflexes were generated in the FDI by electrically stimulating the median nerve of the contralateral limb (Baudry et al. 2009; Duchateau and Hainaut 1993; Maluf et al. 2007). The cathode and anode were positioned 4 cm proximal to the wrist joint with an interelectrode distance of 10 mm. Single-pulse stimulation was delivered with a DS7AH constant-current stimulator (Digitimer). Optimal FDI response was obtained with a square-wave pulse of 1-ms duration. Recruitment curves were generated for the FDI response to determine the intensity that produced the maximum reflex amplitude (10–20 mA). The intensity that was used in the experiments was set at 50% of the intensity to elicit the maximum response, since it reduces activation of the homonymous abductor pollicis brevis, which can contaminate force measurements (Duchateau and Hainaut 1993; Maluf et al. 2007), and this intensity maximizes the ability to assess modulatory effects on the motor neuron pool (Maluf et al. 2007).
Motor point stimulation.
Electrical stimulation was employed to activate intramuscular nerve fibers of FDI to evoke twitch responses. Stimulation was delivered with a DS7AH stimulator, with the cathode placed directly over the contralateral limb FDI muscle belly and the anode placed between the first and second metacarpophalangeal joints. Direct muscle stimulation was performed instead of ulnar nerve stimulation because the latter stimulation can decrease index finger abduction forces due to coactivation of the antagonist first palmar interosseous muscle (Zijdewind and Kernell 1994). Superimposed and resting twitches were elicited via double-pulse stimulation (Zijdewind et al. 1998), where 0.1-ms pulses were delivered 11 ms apart. For clarity, peripherally elicited twitches are referred to as “superimposed” or “resting doublet” twitches. Stimulation intensity was set at 120% of the stimulus intensity, which produced a plateau in resting doublet twitch force, which was determined from recruitment curves calculated for each subject.
Experimental Protocol
Twelve subjects participated in the first experiment, which was designed to examine how a brief, nonfatiguing maximal contraction of the FDI affects activity of the contralateral limb homologous muscle. TMS, median nerve stimulation, FDI EMG, and index finger abduction force were examined for the contralateral limb 1) when both limbs were at rest, 2) when the contralateral limb was at rest and the contracting limb index finger was maximally abducting, 3) when the contralateral index finger was maximally abducting and the contracting limb was at rest, and 4) when both index fingers were maximally abducting (Fig. 1, top right). Measurements were made three times for each condition. If the force of a maximal contraction differed by >5% between the three trials in each condition, the contraction was repeated. The order of testing was counterbalanced, and a rest period of no less than 4 min occurred between contractions for each subject. Strong verbal encouragement was given on performance of each maximal voluntary contraction (MVC). Before starting the contraction trials, subjects were instructed to maintain contact with the load cell, so at all times they could feel the device touching against their finger.
The same 12 subjects participated in a second experiment to determine how fatiguing intermittent contractions of the FDI affect cortical and reflex responses of, and the ability to voluntarily activate, the contralateral limb homologous muscle. After baseline measures were obtained, a contraction protocol was implemented. Four maximal index finger abductions of 4-s duration were performed with the active hand, with a 2-s rest period between contractions (Fig. 1, bottom). A resting phase of 3 s in duration then followed where the contralateral limb remained inactive, and no instructions were delivered to the subject to prevent conscious modulation of motor activity in the contralateral limb. A brief 2-s maximal contraction was then performed by the contralateral limb, which was followed by a brief 2-s maximal contraction with both limbs. TMS and median nerve stimulation were delivered at selected points during each set. Similarly to the baseline measurements, this contraction protocol permitted the examination of cortical excitability, reflex activity, FDI EMG, and abduction force for the contralateral limb during all combinations of index finger abductions. The fatigue sets were repeated until the subjects could not maintain 50% of their maximal nonfatigued contraction of the contracting limb.
Seven subjects participated in a third experiment to determine if peripheral mechanisms of fatigue affected the outcome of experiments 1 and 2. One of these subjects completed all three experiments. Superimposed and resting doublet twitches were obtained using motor point stimulation of the contralateral FDI during the same protocols that were used in experiments 1 and 2 (Fig. 1, top right and bottom).
Data Analysis
Voluntary peak abduction force was obtained from the force signal during each maximal contraction. Amplitude of muscle activity was calculated from FDI root-mean-square (RMS) EMG averaged across a 200-ms window immediately before stimulation. Peak-to-peak MEP and peak reflex amplitude were calculated from the raw FDI EMG signal following TMS and median nerve stimulation, respectively. Reflex latency was calculated as the duration between the stimulus artifact and the onset of the reflex response, which was determined as a notable and repeatable deflection in EMG (2 SD above rectified background EMG) in a 25- to 35-ms window poststimulus. A subject's data were only included in the analysis if a complete reflex data set was obtained, i.e., a clear reflex was obtained for every contraction set the subject performed. Total reflex duration and the area under the curve were also calculated. Peak amplitude of the reflex response was calculated from the rectified EMG signal. Given that unintended activity was expected to occur in the contralateral limb in during the contraction protocol and that the magnitude of reflex response is proportional of the level of muscle activity at the time of stimulation (Burke et al. 1989), peak amplitude reflex responses in the contralateral limb were also normalized to FDI RMS EMG.
VA was calculated for the TMS and motor point stimulation procedures used throughout testing. VA was calculated from motor point stimulation as (1 − doublet superimposed twitch/doublet resting twitch) × 100. VA was calculated from cortical stimulation as (1 − cortical superimposed twitch/background force) × 100. Background force was calculated as the average abduction force in a 200-ms window immediately before stimulation. Background force was chosen in preference to cortically evoked resting twitches because the latter procedure demonstrates a nonlinear muscle twitch response in resting conditions (Todd et al. 2003b). Custom-designed MATLAB software (R2010a; The MathWorks) was used for all data analyses.
Statistical Analysis
In the first experiment, one-way ANOVA were used to determine if effects emerged in fresh nonfatigued muscle, whereby cortical, reflex, EMG, and force measures were used as the dependent variables. In the remaining experiments, one-way repeated-measures ANOVA with a factor of time were used for conditions where data were obtained only for the contralateral limb. Bonferroni post hoc comparisons were used to determine if the dependent variables differed between successive fatigue sets, and Dunnett's tests were used to determine if differences occurred from the control condition throughout the contraction protocol. Task failure differs between individuals, and therefore the number of completed fatigue sets, differed between subjects. Therefore, the first six fatigue sets are reported in the results, as well as the final fatigue set, where force output for the contracting limb had reduced by 50%. When measurements were obtained during conditions involving bilateral contractions, two-way repeated-measures analysis of covariance (ANCOVA) with factors of limb and fatigue set were employed. MVC data for the control condition were used as a covariate to account for any between-limb differences in strength that may have occurred in index finger abduction. Bonferroni post hoc comparisons were again used to determine if differences existed between successive fatigue sets, and Dunnett's tests were used to determine if the dependent variables differed from control conditions throughout the contraction protocol. Two-way ANOVA with factors of condition and fatigue set was used to determine if the measurements differed between the unilateral and bilateral testing conditions. All data reported in the text are means ± SD, and the significance level for all tests was set at P < 0.05. All data represented in the figures are means ± SE. All statistical analyses were performed with SPSS (version 21; IBM, Armonk, NY).
RESULTS
Nonfatigued Muscle Contractions
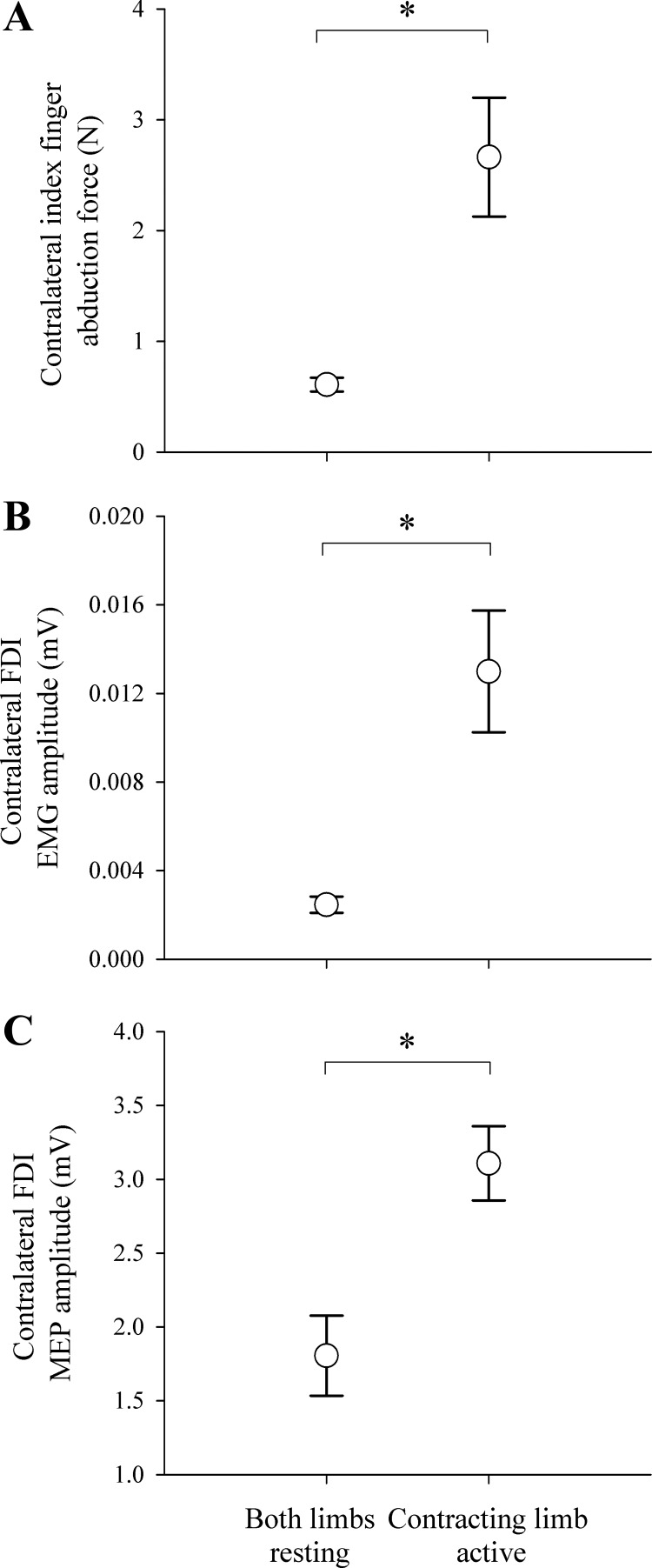
Unintended activity was identified when the contracting limb performed a brief maximal contraction and the contralateral limb remained at rest (Fig. 2). When the contracting limb was active, abduction force (P = 0.002), FDI EMG (P = 0.001), and MEPs (P = 0.003) were all greater in the resting contralateral limb compared with when both limbs were at rest. A clear heteronymous reflex was elicited from the contralateral limb FDI only when the contracting limb was active (not during resting states). Typically, the reflex response of the FDI cannot be elicited without a voluntary contraction of that limb, which potentiates the motoneuron pool (Duchateau and Hainaut 1993; Foltys et al. 2003). As such, unintended activity in the contralateral limb assisted in potentiating the motoneuron pool to elicit the heteronomous reflex. The FDI reflex had a mean onset latency of 26.4 ± 1.56 ms, duration of 9.9 ± 1.2 ms, and peak amplitude of 274 ± 130 μV.
Fig. 2.
Unintended activity in the contralateral limb represented by index finger abduction force (A), FDI EMG amplitude (B), and FDI MEP amplitude (C). Data are presented for unfatigued muscle when both limbs were at rest, as well as when the contracting limb was maximally contracting and the contralateral limb was at rest. *P < 0.05. Error bars represent SE.
Cortical Stimulation During Intermittent Contractions
In the second experiment, subjects performed 9 ± 2 fatigue sets before force output for the contracting limb reduced by 50%. Clear changes were observed in motor output of the contralateral limb during two conditions. These were the conditions when the contralateral limb performed a brief contraction to obtain electrophysiological measurements and when measurements were made for the contralateral limb during bilateral contractions.
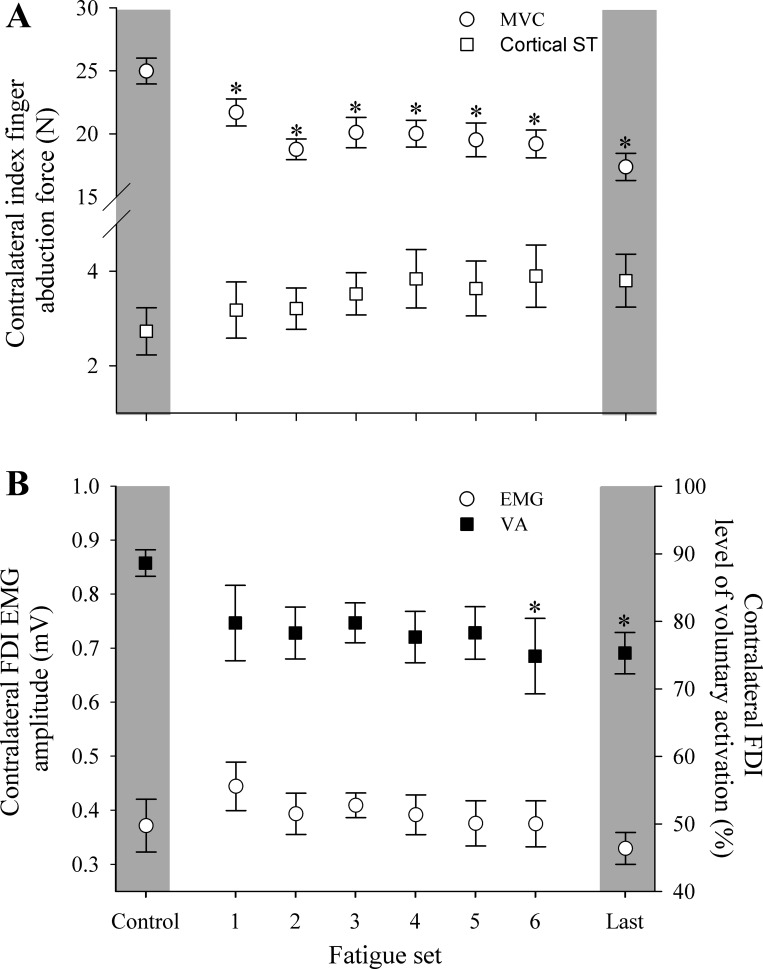
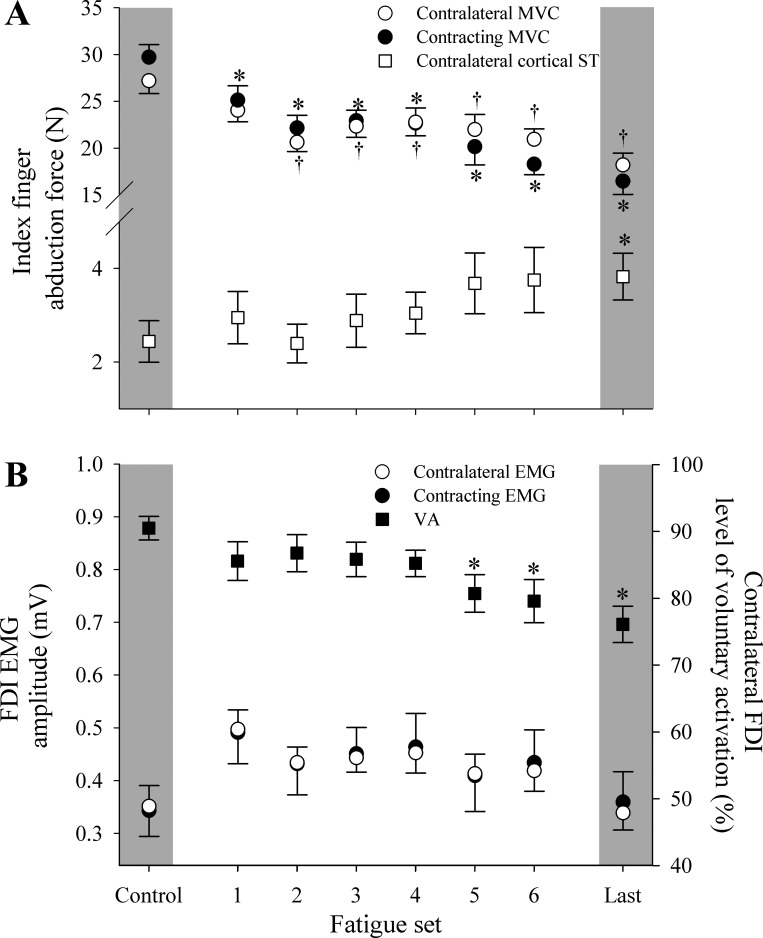
When measurements were made while only the contralateral limb was contracting, significant main effects of fatigue set were identified for abduction force (P < 0.001) and the VA of the contralateral limb (P = 0.033; Figs. 3 and 4). Inducing fatigue for the contracting limb affected the contralateral limb to the extent that force-generating capacity reduced by 31 ± 10%. The onset of differences for abduction force and VA occurred at different times during the contraction protocol. That is, abduction force progressively declined from the first set of the contraction protocol (P = 0.032 to P < 0.001), whereas VA was reduced in the sixth fatigue set (P = 0.04) and in the final contraction set (P = 0.03) compared with control levels.
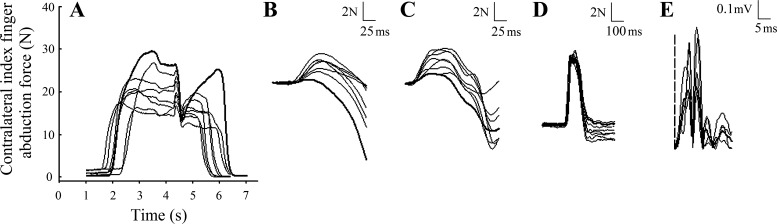
Fig. 3.
Representative data from experiments 2 and 3 where the bold trace represents baseline measures. Raw FDI force progressively reduced throughout the fatigue protocol (A). Cortical superimposed twitch (B) and motor point superimposed twitch (C) progressively increased throughout the fatigue protocol. Resting twitches elicited just after contraction did not change throughout the fatigue protocol (D). Contralateral limb rectified heteronymous reflex responses were only obtained when the contracting limb was maximally contracting and the contralateral limb was at rest (E). Dashed vertical line indicates onset of the reflex, which was ∼28 ms after stimulus.
Fig. 4.
Index finger abduction force and superimposed twitch (ST) of the contralateral limb evoked with TMS (A) and FDI EMG amplitude and level of VA for contralateral limb assessed via cortical stimulation (B) over the course of the contraction protocol. Data are presented for when the contralateral limb was maximally contracting and when the contracting limb was at rest. *P < 0.05, significant difference from control. Error bars represent SE.
Although no significant differences were found between the unilateral and bilateral testing conditions, performance of strong bilateral contractions throughout the contraction protocol revealed marked similarities between limbs. Most notably, declines in motor responses for the contralateral limb were similar to declines observed in the contracting limb. Despite the fact that fatigue-inducing contractions were only performed in one limb, two-way repeated-measures ANCOVA revealed that there were no differences between limbs for abduction forces or FDI EMG throughout the contraction protocol (Fig. 5). However, significant main effects of fatigue set were identified for abduction force (P = 0.035). Compared with baseline, force for the contracting limb decreased from the first set onwards (P < 0.001), and force for the contralateral limb progressively declined from the second set onwards (P < 0.001). Although no main effect of fatigue set was identified for EMG measurements (P = 0.088), EMG for both limbs was elevated for the first four fatigue sets (Fig. 5).
Fig. 5.
Responses throughout the contraction protocol when both limbs were active: abduction forces for both limbs and superimposed twitch of the contralateral limb evoked with TMS (A) and FDI EMG amplitude for both limbs and level of VA for the contralateral limb assessed via cortical stimulation (B). Data are presented for the phases of the fatigue protocol when both limbs were maximally contracting. *P < 0.05, nonfatiguing limb is different from nonfatiguing control. †P < 0.05, fatiguing limb is different from fatiguing limb control. Error bars represent SE.
Main effects were also identified for the VA (P < 0.001) and cortically elicited superimposed twitch (P = 0.046). Compared with control conditions, VA was reduced from the fifth fatigue set onwards (P = 0.005 to P = 0.001), and cortically elicited superimposed twitch was increased for the final fatigue set when force-generation capacity had decreased by 50% (P = 0.019). The added requirements of contracting both FDI muscles while measurements were made caused the VA level to decrease earlier during the protocol (i.e., the 6th set) compared with when measurements were made while only the contralateral limb was contracting. This was also reflected in cortically elicited superimposed twitch, where no differences were identified during the contralateral limb-only contraction, yet a significant decrease was present for the final set when measurements were made while both FDI muscles were contracting.
FDI Reflex Responses During Intermittent Contractions Of a Single Limb
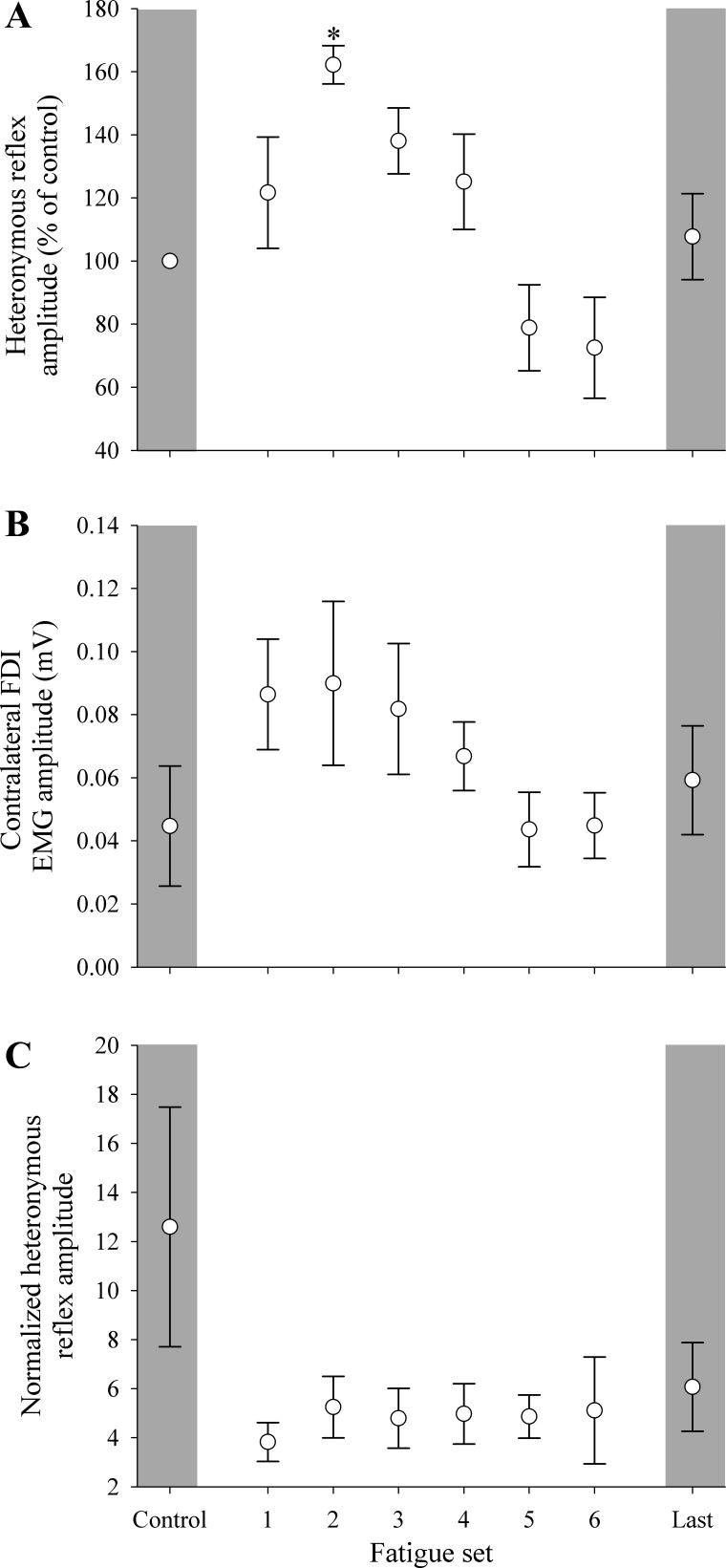
The measurement of heteronomous reflexes was less consistent compared with responses obtained from cortical stimulation. Complete reflex data were only obtained from four subjects, whereby a valid reflex was obtained in every contraction set of the protocol (a completion rate consistent with heteronomous reflex protocols). Moreover, reflexes were only present in the contralateral limb when the opposite limb was contracting. Single-limb studies report that a slight voluntary contraction is necessary to evoke heteronymous reflexes in the FDI (Foltys et al. 2003). When the contracting limb was active in the present study, unintended EMG activity in the contralateral limb seemingly potentiated the motoneuron pool in a similar fashion to a weak voluntary contraction, which enhanced reflexes in that limb (Fig. 3). With the aforementioned increase in EMG activity in the contralateral limb, reflex amplitude was normalized to both the control amplitude and background EMG (Fig. 6). A main effect of fatigue set occurred during the fatigue task (P < 0.05), where reflex amplitude normalized to control increased in the second fatigue set (P < 0.05).
Fig. 6.
Heteronomous reflex responses were obtained when the contracting limb was maximally contracting and the contralateral limb was at rest. Data are presented for reflex amplitude of the contralateral limb normalized to control values (A), level of background EMG activity of the contralateral limb (B), and reflex amplitude of the contralateral limb normalized to background EMG (C). *P < 0.05, significant difference from control. Error bars represent SE.
Motor Point Stimulation During Intermittent Contractions
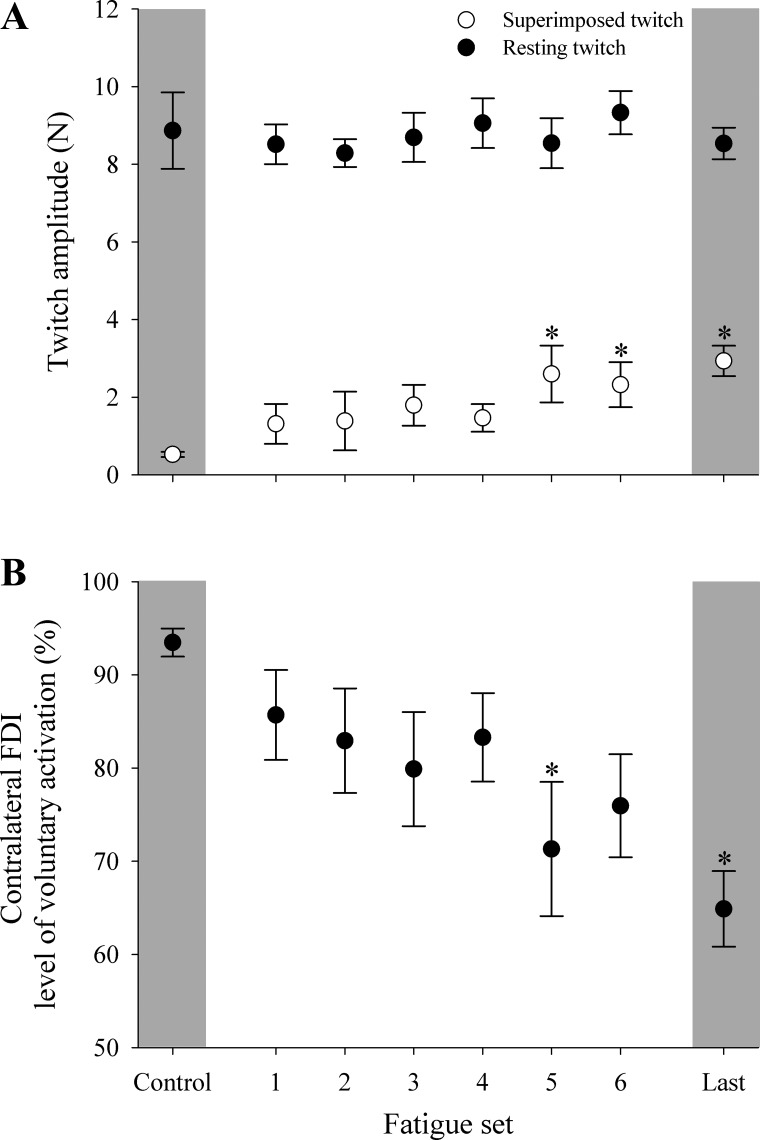
The fatigue-inducing contraction protocol was principally associated with maximal contractions by the contracting limb. However, it must be noted that the contralateral limb was required to perform a small number of contractions to collect measurements for VA. Therefore, the third experiment was performed to verify that changes in the contralateral limb were mediated by CNS mechanisms and not by changes in contractile properties of the muscle. Resting doublet twitches (a common measure of peripheral fatigue) were unchanged from baseline measurements through to the end of the contraction protocol. Instead, main effects were detected for superimposed doublet twitches (P = 0.018) and VA (P = 0.014) similar to TMS, where superimposed doublet twitch increased and level of voluntary activation decreased from the fifth set onwards during motor point stimulation (P = 0.019 to P < 0.001; Figs. 3 and 7).
Fig. 7.
Superimposed and resting doublet twitches for the contralateral limb (A) and level of VA assessed throughout the contraction protocol via direct muscle stimulation (B). Data are presented for when the contralateral limb was maximally contracting and the contracting limb was at rest. *P < 0.05, significant difference from control. Error bars represent SE.
It is noteworthy that superimposed twitches increased as the fatiguing protocol progressed and that cortical or motor point stimulation demonstrated similar relative increases in superimposed twitch force. Absolute amplitude was higher when cortical stimulation was used to elicit twitches in the contralateral limb compared with motor point stimulation. This finding is to be expected, because it has been demonstrated that cortical stimulation produces greater twitch force than motor point or ulnar nerve stimulation due to repetitive firing of alpha-motoneurons (Day et al. 1987a, 1987b).
DISCUSSION
Motor cortex and reflex activity were examined for the FDI while the opposite FDI performed brief maximal contractions. Subsequent experiments were performed to determine how fatigue-inducing intermittent contractions of the FDI influence motor cortex, reflex activity, and the ability to voluntarily activate the contralateral nonfatigued FDI. The main findings were 1) a brief, strong contraction for a single limb caused unintended activity in the contralateral homologous muscle, 2) repeated maximal contractions reduced the force-generating capacity of the contralateral limb, and 3) the level of voluntary activation progressively reduced in the contralateral limb during fatiguing contractions of a single limb.
Strong Contraction of a Single Limb Caused an Increase in Unintended Activity in the Contralateral Homologous Muscle
When a single maximal contraction was performed in a nonfatigued state, a clear increase in abduction force, FDI EMG amplitude, and MEP amplitude was observed in the contralateral resting limb. Increased MEP and EMG activity during maximal contractions are consistent with previously published findings (Post et al. 2008; Zijdewind and Kernell 2001; Zijdewind et al. 2006) and indicate that unintended activity in the contralateral limb is associated with increased iM1 excitability. The current study provides added evidence for cortical mechanisms during brief contractions, because there were no changes in the heteronomous reflex of the contralateral limb when background EMG (unintended muscle activity) was accounted for. Although no study has reported contralateral reflex data for maximal FDI contractions, H-reflexes are reported to be depressed in the contralateral limb during isometric wrist extension at 25–75% MVCs (Hortobágyi et al. 2003). These contrasting findings may be due to the initial conditions of the contralateral limb. The strong presynaptic inhibition of the contralateral wrist extensors described in Hortobágyi et al. (2003) coincided with the muscle remaining electrically silent when measurements were made. In contrast, our study allowed unintended activity to occur in the contralateral limb while measurements were made. This small amount of potentiation in the contralateral limb appeared to be critical to evoke heteronymous reflexes in the FDI (Duchateau and Hainaut 1993; Foltys et al. 2003).
Repeated Contractions Reduced the Force-Generating Capacity of the Contralateral Limb
Although the contraction protocol was designed to induce fatigue in the contracting limb, it was also possible to determine how multiple contractions of nonfatigued muscle influenced activity in the contralateral limb. That is, cortical and reflex activity were compared between baseline and the first fatigue set, where just four contractions were performed. In contrast to a single contraction, performing four maximal contractions caused a reduction the force-generating capacity of the contralateral homologous muscle. Given that resting doublet twitches did not decrease, this decline in force generation was not due to changes in the contractile properties of the muscle (Gandevia 2001). Instead, the reduction in force-generating capacity of the contralateral limb was likely to be mediated by the CNS. The absence of significant differences in FDI reflex amplitude for the first fatigue set (as well as subsequent sets), combined with the clear and gradual decline in EMG and VA throughout the fatigue protocol, suggests that cortical mechanisms mediated the effects seen in the contralateral limb following four maximal contractions of the unfatigued muscle.
Level of Voluntary Activation Decreased in the Contralateral Limb During Fatiguing Contractions
The major finding of the study was that while the contracting limb performed repeated intermittent contractions, the force-generating capacity of the homologous muscle in the contralateral limb progressively decreased. This was caused by a decrease in VA of the FDI, whereby neural drive to the contralateral FDI was suboptimal when the contractions were performed to fatigue. Although minor reductions in contralateral VA have been reported for elbow flexors (∼2.9% decline; Todd et al. 2003a) and knee extensors (∼9.1% decline; Doix et al. 2013) during alternating isometric contractions of each limb, the present study uncovered a greater deficit in the ability to activate the contralateral homologous muscle during single-limb intermittent contractions (∼20%). As such, intermittent contractions may cause greater crossed effects compared with those that have minimal, or no, recovery during contraction protocols.
Although the exact mechanism underlying reduced VA in the contralateral limb during intermittent fatiguing contractions are yet to be identified, functional MRI investigations indicate that interhemispheric activity is strongly influenced by the strength and type of contraction that is performed (Liu 2003; Liu et al. 2002, 2005). Primary sensorimotor area activation of both hemispheres is significantly higher during repetitive intermittent maximal handgrip contractions (2-s contraction dispersed with 1-s rest periods; Liu et al. 2005) compared with cortical activation during a 120-s sustained maximal handgrip contraction (Liu et al. 2002). Irrespective of the total duration of the fatigue protocol, performing intermittent contractions causes a substantial initial increase in iM1 that is then maintained throughout contraction protocols (Liu et al. 2005). If this cortical activity in the iM1 is associated with inhibitory circuits, this may explain why the deficits in VA for the current study are higher than in previous studies that employed sustained contraction protocols. Although the contralateral muscle in the current study was not in itself fatigued, as evidenced by no change in peripheral resting doublet twitches, a differing pattern of cortical activation exhibited during intermittent contraction tasks may contribute to an inability to voluntarily activate the limb.
During a fatiguing single-limb contraction, the FDI heteronymous reflex will typically decline as fatigue accumulates (Duchateau and Hainaut 1993; Foltys et al. 2003). However, this did not occur in our study that examined between-limb effects. Instead, a biphasic response was observed in the contralateral limb, where after an initial facilitation period, the reflex amplitude declined to control values throughout the prolonged contraction protocol. This profile of reflex activity was attributed to the level of background EMG, which has long been known to contribute to the amplitude of reflex responses (Burke et al. 1989). In particular, the pattern of EMG activity and reflex amplitude was similar throughout the fatigue protocol, to the extent that heteronomous reflex activity normalized to background EMG yielded no significant differences. There is limited evidence that monosynaptic (Ia) reflexes play a role in crossed effects in animal or relaxed humans (Mezzarane et al. 2012). However, there is some evidence that the monosynaptic reflex in the contralateral limb can be modified (depressed) at the segmental level with unilateral voluntary contraction (Hortobágyi et al. 2003). This depression of activity of the contralateral limb is not likely because of reduced excitability of the motoneuron pool, but may instead be due to presynaptic inhibition of Ia terminals associated with descending drive to the contralateral motoneuron pool (Hortobágyi et al. 2003). Once again, this mechanism implicates the importance of the motor cortex in unintended activity in the contralateral limb.
Experimental Considerations
It must also be acknowledged that we have based our interpretation within the boundary of our experiment design and our results. Although cortical mechanisms play a critical role in reducing VA in the contralateral limb after intermittent contractions of the opposite limb, it is possible that subcortical mechanisms contributed to the reduction in VA. Although interhemispheric excitatory and inhibitory neural interactions would most likely have caused the behaviors observed in the current study, subcortical outputs that optimize excitability of the primary motor cortex may also have been degraded after intermittent fatigue-inducing contractions.
Although cortical superimposed twitches provide evidence that the inability to activate the contralateral limb is supraspinal in origin, measurements of the compound muscle action potential (Mmax) would provide further insight as to the localization of this effect. Specifically, comparison between motoneuron pool activation using cortical stimulation and peripheral ulnar nerve stimulation would provide insight as to whether these changes are indeed supraspinal or occur at a lower neural site. Future research should also examine cortical activity during different types of voluntary contraction modalities, such as whether repeated intermittent contractions displays different cortical activation patterns compared with the same amount of work performed in a sustained or rhythmical contraction. Some work has been performed in this field, and it has been shown that when 2-Hz rhythmic abduction contractions of the index finger (10% MVC) are compared with force-matched sustained contractions, MEPs are substantially decreased in the contralateral limb FDI (Christova and Kossev 2001; Uehara et al. 2013). This decrease in MEP amplitude is caused by inhibition of the iM1, since subsequent paired-pulse experiments report that long-interval intracortical inhibition and inhibition from the contralateral premotor area to the iM1 are significantly modulated during 2-Hz rhythmic contractions (Uehara et al. 2013).
Conclusions
Unintended activity can be readily observed in the contralateral limb when the opposite limb performs a brief maximal contraction. When repeated maximal intermittent contractions are performed with one limb, the ability to activate the contralateral limbs homologous muscle is impaired. Although the contralateral limb performed a minimal amount of work, this limb and the corresponding motor cortex exhibited signs that are characteristic of central fatigue.
It is apparent that mechanisms in the iM1 play a major role in this response, whereby the iM1 neurons fail to maximally activate the contralateral limb when single-limb intermittent contractions are performed. Further research should investigate cortical mechanisms linked to different modalities of contraction and whether different methods of producing force have effects at the primary motor cortices of both hemispheres.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.K., M.R.F., and M.J.S. conception and design of research; J.J.K. and M.R.F. performed experiments; J.J.K. and M.R.F. analyzed data; J.J.K., M.R.F., and M.J.S. interpreted results of experiments; J.J.K. and M.R.F. prepared figures; J.J.K., M.R.F., and M.J.S. drafted manuscript; J.J.K., M.R.F., and M.J.S. edited and revised manuscript; J.J.K., M.R.F., and M.J.S. approved final version of manuscript.
REFERENCES
- Baudry S, Jordan K, Enoka RM. Heteronymous reflex responses in a hand muscle when maintaining constant finger force or position at different contraction intensities. Clin Neurophysiol 120: 210–217, 2009. [DOI] [PubMed] [Google Scholar]
- Burke D, Adams RW, Skuse NF. The effects of voluntary contraction on the H reflex of human limb muscles. Brain 112: 417–433, 1989. [DOI] [PubMed] [Google Scholar]
- Christova P, Kossev A. Human motor unit recruitment and derecruitment during long lasting intermittent contractions. J Electromyogr Kinesiol 11: 189–196, 2001. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Dick JP, Cowan JM, Berardelli A, Marsden CD. Motor cortex stimulation in intact man. Brain 110: 1191–1209, 1987a. [DOI] [PubMed] [Google Scholar]
- Day BL, Thompson PD, Dick JP, Nakashima K, Marsden CD. Different sites of action of electrical and magnetic stimulation of the human brain. Neurosci Lett 75: 101–106, 1987b. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, McKay WB, Sarjanovic I, Sherwood AM, Svirtlih L, Vrbova G. Co-activation of ipsi- and contralateral muscle groups during contraction of ankle dorsiflexors. J Neurol Sci 109: 49–55, 1992. [DOI] [PubMed] [Google Scholar]
- Doix AC, Lefevre F, Colson SS. Time course of the cross-over effect of fatigue on the contralateral muscle after unilateral exercise. PLoS One 8: e64910, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Behaviour of short and long latency reflexes in fatigued human muscles. J Physiol 471: 787–799, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltys H, Meister IG, Weidemann J, Sparing R, Thron A, Willmes K, Töpper R, Hallett M, Boroojerdi B. Power grip disinhibits the ipsilateral sensorimotor cortex: a TMS and fMRI study. Neuroimage 19: 332–340, 2003. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol 90: 2451–2459, 2003. [DOI] [PubMed] [Google Scholar]
- Kavanagh JJ, Cresswell AG, Sabapathy S, Carroll TJ. Bilateral tremor responses to unilateral loading and fatiguing muscle contractions. J Neurophysiol 110: 431–440, 2013. [DOI] [PubMed] [Google Scholar]
- Kenway LC, Bisset LM, Kavanagh JJ. The effect of isometric contraction on the regulation of force tremor in the contralateral limb. Neurosci Lett 558: 126–131, 2014. [DOI] [PubMed] [Google Scholar]
- Liu JZ. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an fMRI study. J Neurophysiol 90: 300–312, 2003. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Dai TH, Sahgal V, Brown RW, Yue GH. Nonlinear cortical modulation of muscle fatigue a functional MRI study. Brain Res 957: 320–329, 2002. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Zhang L, Yao B, Sahgal V, Yue GH. Fatigue induced by intermittent maximal voluntary contractions is associated with significant losses in muscle output but limited reductions in functional MRI-measured brain activation level. Brain Res 1040: 44–54, 2005. [DOI] [PubMed] [Google Scholar]
- Maluf KS, Barry BK, Riley ZA, Enoka RM. Reflex responsiveness of a human hand muscle when controlling isometric force and joint position. Clin Neurophysiol 118: 2063–2071, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Rattey J. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflügers Arch 454: 957–969, 2007. [DOI] [PubMed] [Google Scholar]
- Mezzarane RA, Kohn AF, Couto-Roldan E, Martinez L, Flores A, Manjarrez E. Absence of effects of contralateral group I muscle afferents on presynaptic inhibition of Ia terminals in humans and cats. J Neurophysiol 108: 1176–1185, 2012. [DOI] [PubMed] [Google Scholar]
- Morrison S, Kavanagh J, Obst SJ, Irwin J, Haseler LJ. The effects of unilateral muscle fatigue on bilateral physiological tremor. Exp Brain Res 167: 609–621, 2005. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111: 344–349, 2000. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post M, Bayrak S, Kernell D, Zijdewind I. Contralateral muscle activity and fatigue in the human first dorsal interosseus muscle. J Appl Physiol 105: 70–82, 2008. [DOI] [PubMed] [Google Scholar]
- Rattey J, Martin PG, Kay D, Cannon J, Marino FE. Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross-over effect. Pflügers Arch 452: 199–207, 2006. [DOI] [PubMed] [Google Scholar]
- Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle Nerve 21: 1033–1039, 1997. [DOI] [PubMed] [Google Scholar]
- Todd G, Petersen NT, Taylor JL, Gandevia SC. The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res 150: 308–313, 2003a. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol 551: 661–671, 2003b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara K, Morishita T, Kubota S, Funase K. Neural mechanisms underlying the changes in ipsilateral primary motor cortex excitability during unilateral rhythmic muscle contraction. Behav Brain Res 240: 33–45, 2013. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res 175: 526–535, 2006. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Index finger position and force of the human first dorsal interosseus and its ulnar nerve antagonist. J Appl Physiol 77: 987–997, 1994. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol 85: 1907–1913, 2001. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Zwarts MJ, Kernell D. Influence of a voluntary fatigue test on the contralateral homologous muscle in humans? Neurosci Lett 253: 41–44, 1998. [DOI] [PubMed] [Google Scholar]