Abstract
The mitochondrial ATP-sensitive K+ (mitoKATP) channel plays a central role in protection of cardiac and neuronal cells against ischemia and apoptosis, but its molecular structure is unknown. Succinate dehydrogenase (SDH) is inhibited by mitoKATP activators, fueling the contrary view that SDH, rather than mitoKATP, is the target of cardioprotective drugs. Here, we report that SDH forms part of mitoKATP functionally and structurally. Four mitochondrial proteins [mitochondrial ATP-binding cassette protein 1 (mABC1), phosphate carrier, adenine nucleotide translocator, and ATP synthase] associate with SDH. A purified IM fraction containing these proteins was reconstituted into proteoliposomes and lipid bilayers and shown to confer mitoKATP channel activity. This channel activity is sensitive not only to mitoKATP activators and blockers but also to SDH inhibitors. These results reconcile the controversy over the basis of ischemic preconditioning by demonstrating that SDH is a component of mitoKATP as part of a macromolecular supercomplex. The findings also provide a tangible clue as to the structural basis of mitoKATP channels.
Although prolonged ischemia results in severe damage to myocardial and neuronal cells and ultimately cell death, brief ischemic episodes can recruit a mechanism that can protect against future ischemic insults. The identification of this endogenous autoprotective mechanism, known as ischemic preconditioning (IPC), has sparked considerable interest given its potential to mitigate cellular injury in heart attack and stroke (1–3). The molecular basis of IPC remains unclear. Recent evidence suggests that the mitochondrial ATP-sensitive K+ channel (mitoKATP) is a key player in the process of IPC and is an inhibitor of apoptosis.
The mitoKATP channel was first identified in 1991 from single-channel recordings of the mitochondrial inner membrane (IM) (4). Subsequent studies (5, 6) revealed that the antihypertensive agent diazoxide is an agonist of mitoKATP channels and protects against cardiac cell death by a mechanism like that of IPC but without the need for conditioning ischemia. These observations led to the notion that mitoKATP channels protect against ischemia by inhibiting apoptosis and necrosis, possibly as a consequence of altered mitochondrial K+ or Ca2+ homeostasis (7–9). Nevertheless, the existence of mitoKATP channels has been questioned (10, 11). Inhibitors of the Krebs cycle enzyme succinate dehydrogenase (SDH) have been shown to mimic the process of IPC (12). Furthermore, some drugs that activate mitoKATP channels also inhibit SDH (13), leading to the alternative hypothesis that respiratory inhibition, rather than channel activity, underlies IPC (10). The controversy has been fostered by the lack of a molecular identity for mitoKATP channels. Such ignorance has also stymied rational drug development of compounds to prevent or delay ischemic injury.
Given the functional and pharmacological overlap between SDH and mitoKATP channels, we hypothesized that SDH either interacts with or forms part of a structure that constitutes the mitoKATP channel. We show that at least four mitochondrial IM proteins, mitochondrial ATP-binding cassette protein 1 (mABC1), phosphate carrier (PIC), adenine nucleotide translocator (ANT), and ATP synthase (ATPase), associate with SDH to form a macromolecular supercomplex in the mitochondrial IM. A highly purified fraction of the mitochondrial IM, containing all five members of this supercomplex, was isolated by using differential centrifugation. This purified fraction was reconstituted into proteoliposomes and lipid bilayers and shown to confer mitoKATP channel activity. This channel activity is sensitive not only to mitoKATP ligands but also to SDH inhibitors, linking the macromolecular supercomplex to the mitoKATP activity. Thus, SDH is a structural and functional component of the mitoKATP channel.
Materials and Methods
Isolation of Rat Liver Mitochondrial IM and the M-Fraction. Mitochondrial IM from rat liver was prepared as described (14). The M-fraction was kindly provided by Peter Pedersen's laboratory (Department of Biological Chemistry, The Johns Hopkins University). This fraction was obtained by several steps of differential centrifugation of the rat liver mitochondrial IMs as described (15). Western blot analysis of the M-fraction and mitochondrial IM with Ab against Kir6.1 did not detect a band. This observation suggests that Kir6.1 is absent in the purified mitochondrial membranes, contrary to a recent observation by Lacza et al. (16). This discrepancy is likely to be due to contamination of the mitochondrial preparations used previously; however, differences related to Abs used cannot be ruled out.
Yeast Two-Hybrid Studies. Yeast two-hybrid methods and results are provided in Supporting Text and Table 1, which are published as supporting information on the PNAS web site.
Coimmunoprecipitation (Co-IP) Experiments. Polyclonal Abs against mABC1 were constructed in our laboratory. Briefly, a rabbit was repeatedly immunized with a mABC1 peptide (spanning the C-terminal 35 aa) linked to keyhole limpet hemocyanin. After three rounds of booster immunization, the serum was isolated and total IgG was purified by using the protein-A purification system (Pierce). mAbs against SDH (both the 30- and 70-kDa components) and ATPase were purchased from Molecular Probes. Polyclonal Ab against ANT was purchased from Santa Cruz Biotechnology. Polyclonal Ab against PIC was a generous gift from Peter Pedersen's laboratory.
Abs against mABC1, PIC, the 30- and 70-kDa components of SDH, and the α-subunit of ATPase and ANT were immobilized on separate columns and incubated with mitochondrial IM, and the eluted proteins were applied to an SDS/PAGE gel. The proteins were transferred to a membrane and probed with different Abs. IM of the mitochondria was used as a control. Suspensions of Dynabeads protein G (Dynal, Oslo) were washed three times in 0.1 M NaPi (pH 8.1). The Abs were applied to the Dynabeads and incubated for 20 min. The Abs were cross linked to the beads by adding 20 mM dimethyl pimelimidate dihydrochloride (Pierce) in 0.2 M triethanolamine (pH 8.2) and incubating for 30 min. The Ab cross-linked beads were then washed extensively with PBS (pH 7.4). IM of mitochondrial preparations (in 300 mM KPi/10% ethylene glycol/5 mM EDTA/4 mM ATP/0.5 mM DTT, pH 7.9) were incubated with 2–3% (octylphenoxy)polyethoxyethanol (IGEPAL; CA-630; Sigma) for 20 min. Also, we used 2% Triton X-100 in place of IGEPAL and yielded similar results. The detergent-treated sample was then applied to the Ab cross-linked beads and incubated at 4°C for 1–2 h. The same amount of mitochondrial IM extract was used for each co-IP experiment. The antigen was eluted off the column by lowering pH by using ≈60 μl of 0.1 M citrate (pH 3.1). Same volumes of eluted proteins were applied on NuPAGE 4–12% Bis·Tris gels and transferred to nitrocellulose membrane (Invitrogen). Silver-stain gel of the eluted proteins from each Ab was examined before Western blot analysis. The membranes were probed with the primary Abs at the following dilutions: 30- and 70-kDa SDH and ATPase at a 1:5,000 dilution, PIC at a 1:1,500 dilution, and ANT and mABC1 at a 1:100 dilution. The secondary Abs were diluted 1:5,000. ANT could also be coimmunoprecipitated with Abs against mABC1, SDH, ATPase, and PIC (data not shown).
Reconstitution of the Protein Complex into Liposomes. The procedure developed for proteoliposome studies is based on the methods developed by Paucek et al. (17). Cardiolipin and l-α-lecithin in a 1:10 ratio were suspended in chloroform, dried under nitrogen (to avoid oxidation), resuspended in ether, and dried again. The lipids were then placed under vacuum overnight to remove all traces of organic solvents. The dried lipids were solubilized at ≈50 mg of lipid per ml in 189 μl of internal medium [100 mM tetraethyl ammonium hydroxide (TEA)·SO4/25 mM TEA·Hepes/1 mM TEA·EDTA, pH 6.8] plus 27 μl of otylpentaoxyethylene detergent. The solubilized protein in 0.5–1% Triton X-100 from the above section plus 300 μM 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10,13-tetraoxa-7,16-diazacyclooctadecane-7,16-diylbis(5-methoxy-6,2-benzofurandiyl)] bis (PBFI) were added to the solubilized lipids. The mixture was added to a 1-ml Bio-beads SM-2 column, which had been preequilibrated with internal medium, to remove detergent and form proteoliposomes. The mixture was incubated two times with Bio-beads SM-2 for 60 min each. The proteoliposome suspensions were then passed three times through 1-ml Sephadex G-25–300 columns, which had been preequilibrated with internal medium without probe. This step removes extravesicular PBFI. The vesicles had an average size of 1.1 μl per mg of lipid.
Fluorescence of PBFI loaded proteoliposomes were measured with a SLM 8000 fluorometer (SLM–Aminco, Urbana, IL) connected to an IBM PS/2 computer. The excitation was set at 343 nm (slit width, 4 nm) and emission was set at 485 nm (slit width, 4 nm). For calibration, 0.5 μM nigericin was added to the proteoliposomes, followed by stepwise addition of KCl, as described (17).
We transferred 15-μl aliquots of the proteoliposome suspensions to a cuvette containing 1.985 ml of external medium (150 mM KCl/25 mM TEA·SO4/1 mM TEA·EDTA, pH 7.2). Fluorescence was measured immediately. The fluorescence emission intensity of PBFI is enhanced in the presence of K+. Fluorescence was plotted against time. Fluorescence increase over the initial 20 s was used in our analysis.
Cation selectivity was confirmed by using the Na-sensitive 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10-trioxa-7,13-diazacyclopentadecane-7,13-diylbis(5-methoxy-6,2-benzofurandiyl)]bis-tetra ammonium salt (SBFI) and replacing K with Na ions.
Reconstitution of the Protein Complex into Lipid Bilayer. For lipid bilayer studies, M-fraction microsomes were suspended in a solution containing 5 mM Tris·maleate, 0.5% NaCl, and 0.3 M sucrose (pH 6.8). Single channels were reconstituted into artificial planar lipid bilayers by fusing native microsomes. Planar bilayers were formed across a 150-μm-diameter aperture in a Teflon partition from a lipid mixture containing phosphatidylethanolamine/phosphatidylcholine (7:3) dissolved in n-decane to a final lipid concentration of 50 mg/ml. Nonpolarizing electrodes (Ag/AgCl pellets) immersed in 3 M KCl were used to connect each side of the bilayer to a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Burlingame, CA) through agar bridges saturated with 3 M KCl. M-fraction microsomes were added to the cis chamber. The trans chamber was connected to the virtual ground of the amplifier. The sidedness of mitoKATP channel was not determined. Initially, the cis and trans chambers contained solutions of the following composition: 30 mM K·Hepes/1 mM EGTA, pH 7.3 (KOH). To favor microsome fusion, cis chamber solution was replaced with a higher-salt-concentration solution (500 mM KCl/1 EGTA, pH 7.3, with KOH) after the bilayer was formed. Under these conditions, the usual channel activity that was recorded corresponded to a Cl- channel with a mean conductance of 150 pS (data not shown). This activity was taken to indicate vesicle fusion. To avoid the recording of Cl- currents that interfered with K+ currents, KCl was then replaced with K·Hepes at the same concentration. The free [Ca2+] was calculated to be <10 nM, and thus, the K+ current is unlikely to be due to Ca2+-activated K+ channel described in ref. 18. Single-channel activity was routinely reconstituted from M-fraction in the presence of K·Hepes. The channel displayed low K+ selectivity in lipid bilayer. Reports have suggested (19) that the mitoKATP channel becomes less selective for K+ in the presence of sulfhydryl-reducing agents. The lipid bilayer studies were performed in the presence of low concentrations of dithiothreitol, which could explain the low selectivity observed in this system.
For pharmacological experiments, single-channel currents were recorded at a holding potential of 0 mV. Unitary conductance was measured by using voltage ramps (from -30 to +30 mV), and when the channel activity was high enough, by using square voltage pulses to different membrane potentials (from -40 to +40 mV). Currents were filtered at 2 kHz and digitized at 5 kHz with a 12-bit AD/DA converter (Digidata 1200, Axon Instruments). Current amplitude was determined by fitting Gaussian functions to the total-amplitude histograms. Open probability (Po) was measured either as relative areas of multiple Gaussian distributions fit to total-amplitude histograms or from idealized records by using the 50% amplitude criteria. Data acquisition, unitary current measurements, statistical analysis, and data processing were performed by using commercially available software packages (pclamp, version 9.0, Axon Instruments; excel xp, Microsoft; and origin, version 7.0, Microcal, Amherst, MA).
Results
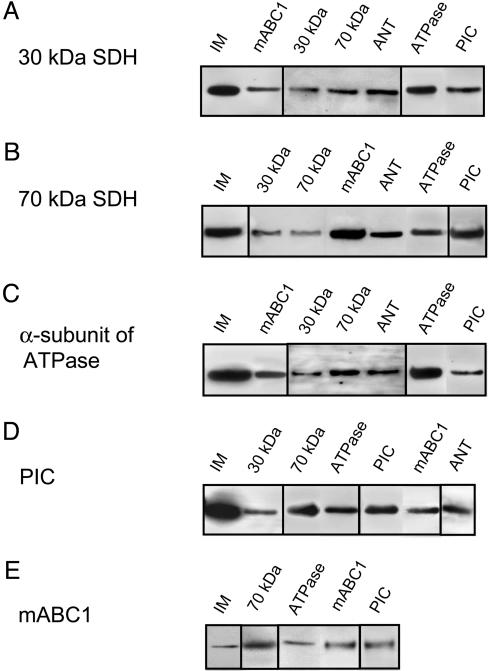
Five Mitochondrial Membrane Proteins Interact to Form a Complex. The controversy of whether mitoKATP or SDH underlies preconditioning motivated us to consider the possibility that SDH either interacts with or forms part of mitoKATP. SDH is composed of two catalytic subunits, a larger 70-kDa flavoprotein and a smaller 30-kDa iron–sulfur protein, which are anchored to the mitochondrial IM by two hydrophobic peptide subunits (20). By using the co-IP technique, we identified four mitochondrial membrane proteins that associate with the following 70- and 30-kDa components of SDH: mABC1, ANT, PIC, and the α-subunit of ATPase (Fig. 1). The function of mABC1 is not known, but the presence of this protein in the complex is intriguing given that the surface KATP channel is a heterooctamer of four K+ channel (Kir6.x) subunits surrounded by four sulfonylurea receptor (SUR) proteins (21, 22), the latter being members of the ABC family. As a consistency check, we used the yeast two-hybrid system to identify proteins that associate with mABC1. SDH and PIC, among several other proteins, associate with mABC1 according to this technique (see Supporting Text). The identification of mABC1 as part of this complex, and reports (23–25) that PIC and ANT can exhibit ion channel activity, prompted us to evaluate the SDH–mABC1–PIC–ANT–ATPase complex for mitoKATP activity.
Fig. 1.
SDH, mABC1, PIC, ANT, and ATPase interact with each other in co-IP studies on rat liver mitochondrial IM. IM of mitochondria was used as a control. (A) Co-IP of the 30-kDa component of SDH with Abs against mABC1, as well as 30- and 70-kDa components of SDH, ANT, ATPase, and PIC. (B) Co-IP of the 70-kDa components of SDH with Abs against 30- and 70-kDa of SDH, mABC1, ANT, ATPase, and PIC. (C) Co-IP of the α-subunit of ATPase with Abs against mABC1, as well as 30- and 70-kDa components of SDH, ANT, ATPase, and PIC. The α-subunit of ATPase has a molecular mass of ≈60 kDa. An Ab against the β-subunit of ATPase coimmunoprecipitated the same proteins. (D) Co-IP of PIC with Abs against 30- and 70-kDa component of SDH, ATPase, PIC, and mABC1. PIC has a molecular mass of ≈30 kDa. (E) Co-IP of mABC1 with Abs against 70-kDa components of SDH, ATPase, mABC1, and PIC. mABC1 has a molecular mass of ≈55 kDa. Negative controls included Abs against complex I and IV of the respiratory pathway, Kir6.1, and cyclophilin D. The IM of mitochondria was prepared in 1.2% digitonin and 6% lubrol WX and was dissolved in 300 mM KPi/10% ethylene glycol/5 mM EDTA/4 mM ATP/0.5 mM DTT, pH 7.9. The columns were also washed with 2–3% (octylphenoxy)polyethoxyethanol to increase the specificity of the interactions between proteins.
First, we studied the crude mitochondrial IM for mitoKATP channel activity. Several different anion and cation channels were noted when the purified IMs were incorporated into lipid bilayers, and a discernible KATP channel activity could not clearly be isolated. Thus, we used a mitochondrial fraction that is enriched in the SDH–mABC1–PIC–ANT–ATPase complex for further functional studies (15). A differential centrifugation technique developed to enrich ATP synthosomes (15) yielded a highly purified fraction of the mitochondrial IM (referred to as the M-fraction herein; Fig. 2A) that contained all five members of the SDH–mABC1–PIC–ANT–ATPase complex (Fig. 2B).
Fig. 2.
Silver staining and Western blot analysis of the purified M-fraction. (A) Silver-stained SDS/PAGE gel of the mitochondrial IM extract and the purified M-fraction. We loaded ≈150 and 40 μg of protein in the IM and M-fraction lanes, respectively. (B) Western blots of the M-fraction with Abs against the 30- and 70-kDa components of SDH, PIC, α-subunit of ATPase, ANT, and mABC1. We loaded 40 μg of the M-fraction in each lane. The Ab against mABC1 also recognizes a smaller ≈30-kDa band.
The M-Fraction Displays K+ Transport Activity in Proteolipososmes. We studied the functional attributes of this fraction by measuring K+ fluxes in reconstituted proteoliposomes. The M-fraction, after solubilization in a detergent, was added to liposomes containing a K+-sensitive fluorescent marker (PBFI, see Materials and Methods) (17). The formed proteoliposomes were tested for K+ transport and compared with nonreconstituted liposomes. Proteoliposomes displayed a rapid rise in K+-mediated fluorescence, which was absent in plain liposomes. On average, the proteoliposomes showed 9- to 10-fold higher initial rates of fluorescence increase than did the nonreconstituted liposomes (Fig. 3A). Cation selectivity was confirmed by using the Na-sensitive 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10-trioxa-7,13-diazacyclopentadecane-7,13-diylbis(5-methoxy-6,2-benzofurandiyl)]bis-tetraammonium salt (SBFI) and replacing K with Na ions. We also studied the response of the reconstituted M-fraction to the known inhibitors and activators of mitoKATP. The baseline K+ transport activity is inhibited significantly by mitoKATP inhibitors, i.e., 5-hydroxydecanote (5-HD), glybenclamide, and ATP (Fig. 3B). Diazoxide (100 μM), an activator of mitoKATP, resulted in a significant increase in K+ transport, whereas 5-HD, glybenclamide, and ATP inhibited diazoxide-activated K+ uptake (Fig. 3B). Pinacidil, a nonselective mitochondrial and sarcolemmal KATP activator, also resulted in an increase in K+ transport (percent increase in K+ transport activity of 194 ± 29, n = 3, P < 0.05), which was reversed by 5-HD. Lower concentrations of diazoxide (i.e., 10 and 50 μM) also resulted in an increase in K+ transport, with 50 μM causing maximal effect (data not shown). HMR1098, a selective surface KATP inhibitor, did not have an effect on the channel activity.
Fig. 3.
Analysis of the M-fraction for mitoKATP channel activity in proteoliposomes and lipid bilayer. (A) Changes in PBFI fluorescence in proteoliposomes containing the M-fraction and nonreconstituted liposomes. Proteins in the M-fraction were reconstituted into liposomes and fluorescence of PBFI marker was measured. There is a higher rate of increase in fluorescence in proteoliposomes compared with nonreconstituted liposomes. The rate of increase in fluorescence is directly proportional to the transport of K+ into the proteoliposomes. Cation selectivity was confirmed by using the Na-sensitive 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10-trioxa-7,13-diazacyclopentadecane-7,13-diylbis(5-methoxy-6,2-benzofurandiyl)]bis-tetraammonium salt (SBFI) and replacing K with Na ions (data not shown). (B) The K+ transport into the proteoliposomes was significantly activated by 100 μM diazoxide. mitoKATP inhibitors, 5-HD (500 μM), glybenclamide (10 μM), and ATP (2 mM), all inhibited diazoxide activated K+ transport activity. Lower concentrations of ATP (i.e., 200 μM) also resulted in a decrease in K+ transport activity. (C) The functional properties of the M-fraction were also studied in lipid bilayers. Unitary K+ currents recorded in lipid planar bilayers after fusion of native microsomes from the M-fraction. Single K+ channel activity was recorded during the application of a continuous voltage ramp from -30 to +30 mV during 5 s. Channel openings are shown as inward deflections and represent K+ movement. Nonspecific leak current was subtracted, and the unitary conductance value was determined as the slope of a linear fit to the open level. (D) K+ channel activity was recorded before and after addition of 100 μM diazoxide. (E) To study the effects of mitoKATP inhibitors, 100 μM diazoxide was added to activate the channel, followed by the addition of 500 μM 5-HD, 10 μM glybenclamide, or 2 mM ATP. These reagents all resulted in a significant inhibition of the diazoxide-activated channel activity. Total-amplitude histograms constructed from 3 min of continuous recording in each condition are also shown. Multipeak Gaussian curves were fitted to the histograms. Mean amplitudes were defined from the difference between peaks. Po values were determined from the open/total area ratio. Top represents a representative experiment in the presence of diazoxide. (F) Summary of the lipid bilayer studies. Each experiment was performed at least three times. ΔF, Change in fluorescence; Gly, glybenclamide; Atr, atractyloside.
The M-Fraction Confers Channel Activity when Reconstituted in Lipid Bilayers. We then evaluated the functional properties of the M-fraction in artificial planar lipid bilayers. Native microsomes from the M-fraction were incorporated into lipid bilayers, and voltage was applied across the membrane. With K+ as the charge carrier, we routinely recorded a channel with predominant conductance levels of ≈200 pS in 500 mM K+ (Fig. 3C) and <100 pS in 100 mM K+ (data not shown). These results agree with those reported for mitoKATP (4, 17). We then evaluated these channels for responses to the known activators and inhibitors of mitoKATP. The basal channel activity was inhibited by the mitoKATP blocker, 5-HD (data not shown). Fig. 3 D and E shows unitary current recordings before and after the addition of different mitoKATP modulators. Diazoxide (a mitoKATP activator) dramatically increased the Po of the channel (Fig. 3D). To evaluate the effect of mitoKATP inhibitors, channel activity was increased with diazoxide before the addition of the inhibitors. Subsequent addition of ATP, glybenclamide, or 5-HD resulted in significant reductions in channel activity (Fig. 3E). Total-amplitude histograms shown for each condition indicate that these agents affect Po, but not conductance. Fig. 3F summarizes our results from the lipid bilayer studies. The results obtained in lipid bilayers are generally consistent with those obtained previously by Zhang et al. (26).
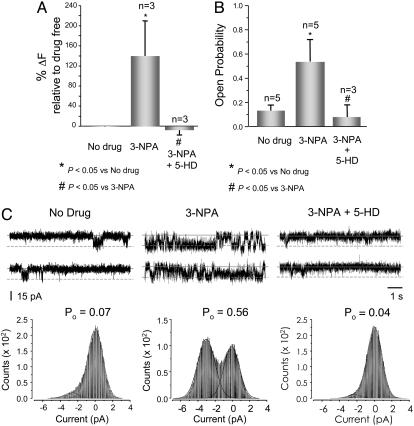
SDH Inhibitors Modulate mitoKATP Activity. Although the M-fraction is highly enriched with SDH, mABC1, PIC, ANT, and ATP synthase, it is still possible that other proteins present in this fraction could underlie the observed K+ channel activity. To better link the five-protein complex to the observed mitoKATP channel activity, we assessed the effects of an SDH inhibitor, 3-nitropropionic acid (3-NPA) (27). 3-NPA is a suicide inactivator of SDH and has been shown to inhibit this enzyme in vitro (27) and in vivo (12, 28, 29). In proteoliposomes, the addition of 3-NPA resulted in a significant increase in K+ transport activity, whereas 5-HD reversed this effect (Fig. 4A). We observed similar behavior in lipid bilayers; 3-NPA led to a significant increase in Po without changes in unitary current amplitude. The activation of the channel by 3-NPA was antagonized by 5-HD (Fig. 4B). Fig. 4C summarizes the results of lipid bilayer studies. The fact that the activation by 3-NPA was reversed by 5-HD in both proteoliposomes and lipid bilayers supports the notion that the 3-NPA response was through mitoKATP and not due to some nonspecific effect. Also, we tested the effect of malonate, another SDH inhibitor. Addition of 1 mM malonate in proteoliposomes resulted in a significant increase in K+ transport activity (percentage of increase in K+ transport activity of 253 ± 3, n = 3, P < 0.05), which was reversed by 5-HD. Thus, SDH, a member of the IM multiprotein complex, influences mitoKATP activity.
Fig. 4.
An inhibitor of SDH (3-NPA) activates the channel, whereas 5-HD reverses this effect. (A) Effects of 3-NPA on K+ transport in proteoliposomes. The K+ transport was significantly activated by addition of 1 mM 3-NPA, whereas 5-HD reversed this effect. (B) A similar response to 3-NPA was noted in lipid bilayer. Microsomes from the M-fraction were incorporated into lipid bilayer, and single K+ channel activity was recorded in the absence of any modulator. After addition of 1 mM of 3-NPA, the Po increased ≈4-fold. Addition of 5-HD reversed this activation significantly. Closed and open current levels for all recordings are indicated by solid and dashed horizontal lines, respectively. (C) Summary of studies on 3-NPA in lipid bilayer.
ANT Modulators Do Not Affect mitoKATP Activity. Several studies (23, 25, 30) have shown that reconstituted purified ANT can behave as a cation-selective ion channel. To study the possibility that ANT is the pore-forming unit of mitoKATP, we used two different modulators of ANT, atractyloside (Atr, an ANT inhibitor that binds to its intermembrane face) and bongkrekic acid (BK, an ANT inhibitor that binds to the matrix face). Addition of these drugs to proteoliposomes did not cause any significant change in the channel activity (percentage of increase in K+ transport activity of 4.25 ± 5.42, n = 4, P = 0.62 for Atr and 1.75 ± 10.34, n = 4, P = 0.92 for BK). Similar results were obtained in the lipid bilayer (Fig. 3F). Thus, these data argue against a role for ANT in the mitoKATP channel pore.
Discussion
The cardioprotective effects of mitoKATP occur by inhibition of apoptosis and necrosis, possibly as a consequence of altered mitochondrial K+ or Ca2+ homeostasis (7–9). However, the very existence of mitoKATP has been questioned (10, 11). The controversy has been fostered by the lack of a molecular identity for mitoKATP. Given the functional and pharmacological overlap between SDH and mitoKATP, we proposed that SDH either interacts with or forms part of mitoKATP. We demonstrated that a complex of (at least) five proteins in the mitochondrial IM transports K+ with characteristics similar to those of native mitoKATP, providing the first tangible clue regarding the molecular identity of such channels. Our results link SDH to mitoKATP and define a multiprotein complex in the IM.
As of now, we do not know precisely how these five proteins interact, nor can we exclude the possible presence of more mitochondrial proteins within this complex. Nevertheless, solubilization of mitochondrial IMs is often incomplete, and immunoprecipitation can then bring down a large number of other proteins that are not specifically interacting but just present in the same partially solubilized membrane fraction. This phenomenon could be particularly true in case of proteins that interact with ATPase because there are several proteins that associate with it through localized lipid rafts. To circumvent this issue, we performed our co-IP experiments under high-salt and detergent conditions. Furthermore, yeast two-hybrid data supported the co-IP results, supporting the view that these interactions are specific. Although our studies have identified a complex of multiple proteins with mitoKATP activity, the pore-forming subunit of the channel remains to be identified.
A complex of multiple unrelated proteins in the mitochondria that fulfills a unique function is not an unfamiliar concept; a similar concept prevails regarding the structure of the mitochondrial permeability transition pore (31). The physical interactions among SDH, mABC1, PIC, ANT, and ATPase in the mitochondrial IM may have functional significance beyond mitoKATP. ATP synthase needs ADP and inorganic phosphate as substrates to synthesize ATP. These substrates are transported into the mitochondria by ANT and PIC, which could rationalize the physical interaction among these proteins (15). Furthermore, bx; 1 the proximity of mABC1 to ATPase may allow it to have preferential access to mitochondrial ATP and the energy source that it needs to carry out its function.
Inhibitors of SDH have been shown to induce cardioprotective effects (12), and diazoxide decreases succinate oxidation in a dose-dependent manner (13), albeit at higher concentrations than those that suffice to activate mitoKATP. Thus, it has been proposed that the cardioprotective effects of diazoxide result from inhibition of SDH and a decrease in respiration, rather than opening of mitoKATP channels (10, 13). Based on our findings that SDH is part of a protein complex capable of transporting K+, we propose that SDH regulates mitoKATP by means of its physical interaction with the ionophore and not by means of its role in oxidative phosphorylation. The fact that SDH modulates mitoKATP activity in bilayers, in the absence of substrates for oxidative phosphorylation, further supports the notion of a regulatory function for SDH in addition to its widely accepted role in the electron transport chain. Such a model would reconcile the disparate roles of SDH and mitoKATP in IPC.
Acknowledgments
We thank Drs. Peter Pedersen, Brian O'Rourke, Jennifer Van Eyk, Jason McDonough, Steven Jones, and Brian Foster for helpful discussions; Craig Semrad and other members of Keith Garlid's laboratory for technical advice on proteoliposomes; and Alma Nani and Kimberly Karko for their technical assistance. This work was supported by National Institutes of Health National Heart Lung and Blood Institute Grant R37 HL36957 (to E.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: co-IP, coimmunoprecipitation; IM, inner membrane; mitoKATP, mitochondrial ATP-sensitive K+ channel; SDH, succinate dehydrogenase; IPC, ischemic preconditioning; ANT, adenine nucleotide translocator; mABC1, mitochondrial ATP-binding cassette protein 1; 5-HD, 5-hydroxydecanote; 3-NPA, 3-nitropropionic acid; PIC, phosphate carrier; TEA, tetraethyl ammonium hydroxide; PBFI, 1,3-benzenedicarboxylic acid, 4,4′-[1,4,10,13-tetraoxa-7,16-diazacyclooctadecane-7,16-diylbis(5-methoxy-6,2-benzofurandiyl)]-bis; Po, open probability.
References
- 1.Gross, G. J. & Fryer, R. M. (1999) Circ. Res. 84, 973-979. [DOI] [PubMed] [Google Scholar]
- 2.Grover, G. J. & Garlid, K. D. (2000) J. Mol. Cell. Cardiol. 32, 677-695. [DOI] [PubMed] [Google Scholar]
- 3.Murry, C. E., Jennings, R. B. & Reimer, K. A. (1986) Circulation 74, 1124-1136. [DOI] [PubMed] [Google Scholar]
- 4.Inoue, I., Nagase, H., Kishi, K. & Higuti, T. (1991) Nature 352, 244-247. [DOI] [PubMed] [Google Scholar]
- 5.Garlid, K. D., Paucek, P., Yarov-Yarovoy, V., Murray, H. N., Darbenzio, R. B., D'Alonzo, A. J., Lodge, N. J., Smith, M. A. & Grover, G. J. (1997) Circ. Res. 81, 1072-1082. [DOI] [PubMed] [Google Scholar]
- 6.Liu, Y., Sato, T., O'Rourke, B. & Marban, E. (1998) Circulation 97, 2463-2469. [DOI] [PubMed] [Google Scholar]
- 7.Garlid, K. D. (2000) Basic Res. Cardiol. 95, 275-279. [DOI] [PubMed] [Google Scholar]
- 8.Akao, M., O'Rourke, B., Teshima, Y., Seharaseyon, J. & Marban, E. (2003) Circ Res 92, 186-194. [DOI] [PubMed] [Google Scholar]
- 9.Murata, M., Akao, M., O'Rourke, B. & Marban, E. (2001) Circ. Res. 89, 891-898. [DOI] [PubMed] [Google Scholar]
- 10.Lim, K. H., Javadov, S. A., Das, M., Clarke, S. J., Suleiman, M. S. & Halestrap, A. P. (2002) J. Physiol. 545, 961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das, M., Parker, J. E. & Halestrap, A. P. (2003) J. Physiol. 31, 893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ockaili, R. A., Bhargava, P. & Kukreja, R. C. (2001) Am. J. Physiol. 280, H2406-H2411. [DOI] [PubMed] [Google Scholar]
- 13.Hanley, P. J., Mickel, M., Loffler, M., Brandt, U. & Daut, J. (2002) J. Physiol. 542, 735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen, P. L. & Hullihen, J. (1978) J. Biol. Chem. 253, 2176-2183. [PubMed] [Google Scholar]
- 15.Ko, Y. H., Delannoy, M., Hullihen, J., Chiu, W. & Pedersen, P. L. (2003) J. Biol. Chem. 278, 12305-12309. [DOI] [PubMed] [Google Scholar]
- 16.Lacza, Z., Snipes, J. A., Miller, A. W., Szabo, C., Grover, G. & Busija, D. W. (2003) J. Mol. Cell. Cardiol. 35, 1339-1347. [DOI] [PubMed] [Google Scholar]
- 17.Paucek, P., Mironova, G., Mahdi, F., Beavis, A. D., Woldegiorgis, G. & Garlid, K. D. (1992) J. Biol. Chem. 267, 26062-26069. [PubMed] [Google Scholar]
- 18.Xu, W., Liu, Y., Wang, S., McDonald, T., Van Eyk, J. E., Sidor, A. & O'Rourke, B. (2002) Science 298, 1029-1033. [DOI] [PubMed] [Google Scholar]
- 19.Mironova, G. D., Skarga, Y. Y., Grigoriev, S. M., Negoda, A. E., Kolomytkin, O. V. & Marinov, B. S. (1999) J. Bioenerg. Biomembr. 31, 159-163. [DOI] [PubMed] [Google Scholar]
- 20.Ackrell, B. A. C., Johnson, M. K., Gunsalus, R. P. & Cecchini, G. (1992) in Chemistry and Biochemistry of Falvoenzymes, ed. Muller, F. (CRC, Boca Raton, FL), Vol. III, pp. 229-297. [Google Scholar]
- 21.Babenko, A. P., Aguilar-Bryan, L. & Bryan, J. (1998) Annu. Rev. Physiol. 60, 667-687. [DOI] [PubMed] [Google Scholar]
- 22.Seino, S. (1999) Annu. Rev. Physiol. 61, 337-362. [DOI] [PubMed] [Google Scholar]
- 23.Brustovetsky, N. & Klingenberg, M. (1996) Biochemistry 35, 8483-8488. [DOI] [PubMed] [Google Scholar]
- 24.Herick, K., Kramer, R. & Luhring, H. (1997) Biochim. Biophys. Acta 1321, 207-220. [DOI] [PubMed] [Google Scholar]
- 25.Brenner, C., Cadiou, H., Vieira, H. L., Zamzami, N., Marzo, I., Xie, Z., Leber, B., Andrews, D., Duclohier, H., Reed, J. C. & Kroemer, G. (2000) Oncogene 19, 329-336. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, D. X., Chen, Y. F., Campbell, W. B., Zou, A. P., Gross, G. J. & Li, P. L. (2001) Circ. Res. 89, 1177-1183. [DOI] [PubMed] [Google Scholar]
- 27.Coles, C. J., Edmondson, D. E. & Singer, T. P. (1979) J. Biol. Chem. 254, 5161-5167. [PubMed] [Google Scholar]
- 28.Wiegand, F., Liao, W., Busch, C., Castell, S., Knapp, F., Lindauer, U., Megow, D., Meisel, A., Redetzky, A., Ruscher, K., et al. (1999) J. Cereb. Blood Flow Metab. 19, 1229-1237. [DOI] [PubMed] [Google Scholar]
- 29.Calabresi, P., Gubellini, P., Picconi, B., Centonze, D., Pisani, A., Bonsi, P., Greengard, P., Hipskind, R. A., Borrelli, E. & Bernardi, G. (2001) J. Neurosci. 21, 5110-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belzacq, A. S., El Hamel, C., Vieira, H. L., Cohen, I., Haouzi, D., Metivier, D., Marchetti, P., Brenner, C. & Kroemer, G. (2001) Oncogene 20, 7579-7587. [DOI] [PubMed] [Google Scholar]
- 31.Weiss, J. N., Korge, P., Honda, H. M. & Ping, P. (2003) Circ. Res. 93, 292-301. [DOI] [PubMed] [Google Scholar]