Abstract
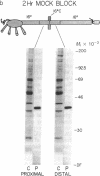
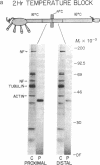
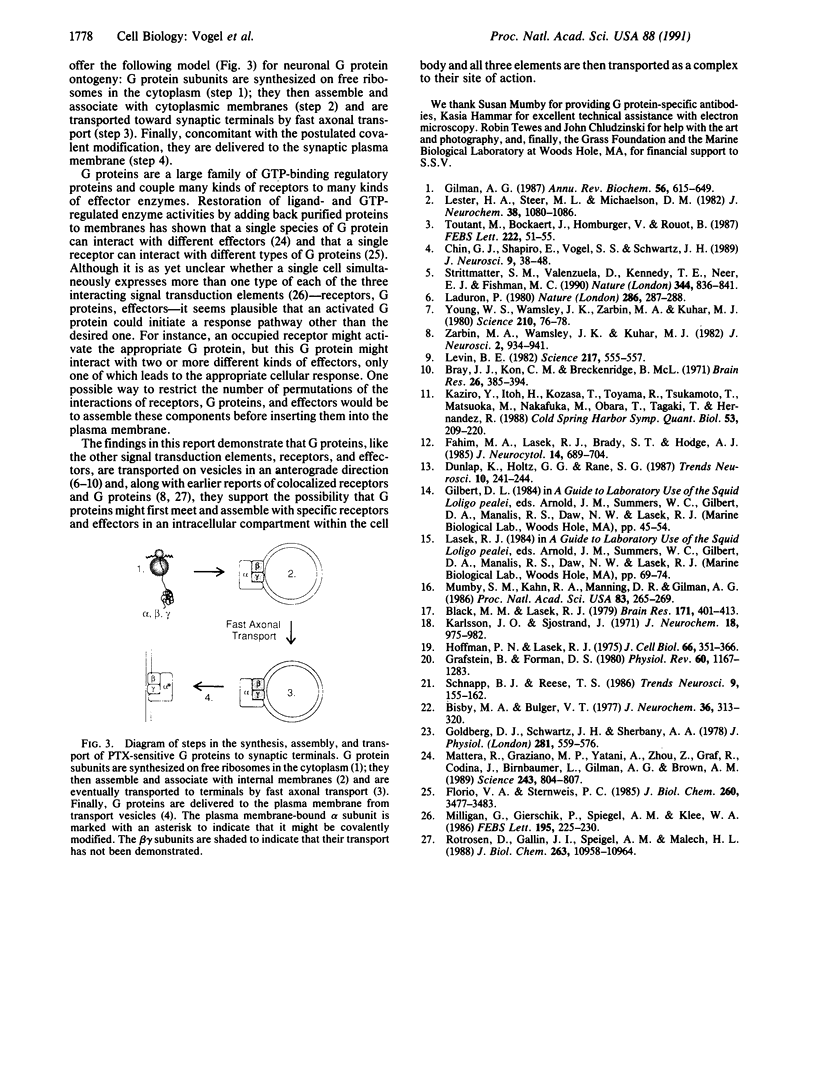
We find that half of the pertussis toxin-sensitive guanine nucleotide-binding protein (G protein) in the squid (Loligo pealei) giant axon is cytoplasmic and that this species of G protein is intermediate in size between the two forms present in axolemma. This G protein is transported toward synaptic terminals at 44 mm/day. Moreover, these data are consistent with there being two additional steps leading to the maturation of G proteins: (i) association with and transport on intracellular organelles and (ii) modification at the time of transfer to the plasmalemma resulting in a molecular weight shift. Since the other two components of G protein-mediated signal transduction pathways, receptors and effector enzymes, are known to be delivered to the synaptic terminals by fast axonal transport, our findings introduce the possibility that these three macromolecules are assembled as a complex in the cell body and delivered together to the plasma membrane of the axon and synaptic terminals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisby M. A., Bulger V. T. Reversal of axonal transport at a nerve crush. J Neurochem. 1977 Aug;29(2):313–320. doi: 10.1111/j.1471-4159.1977.tb09624.x. [DOI] [PubMed] [Google Scholar]
- Black M. M., Lasek R. J. Axonal transport of actin: slow component b is the principal source of actin for the axon. Brain Res. 1979 Aug 10;171(3):401–413. doi: 10.1016/0006-8993(79)91045-x. [DOI] [PubMed] [Google Scholar]
- Bray J. J., Kon C. M., Breckenridge B. M. Adenyl cyclase, cyclic nucleotide phosphodiesterase and axoplasmic flow. Brain Res. 1971 Mar 5;26(2):385–394. [PubMed] [Google Scholar]
- Chin G. J., Shapiro E., Vogel S. S., Schwartz J. H. Aplysia synaptosomes. I. Preparation and biochemical and morphological characterization of subcellular membrane fractions. J Neurosci. 1989 Jan;9(1):38–48. doi: 10.1523/JNEUROSCI.09-01-00038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim M. A., Lasek R. J., Brady S. T., Hodge A. J. AVEC-DIC and electron microscopic analyses of axonally transported particles in cold-blocked squid giant axons. J Neurocytol. 1985 Oct;14(5):689–704. doi: 10.1007/BF01170822. [DOI] [PubMed] [Google Scholar]
- Florio V. A., Sternweis P. C. Reconstitution of resolved muscarinic cholinergic receptors with purified GTP-binding proteins. J Biol Chem. 1985 Mar 25;260(6):3477–3483. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldberg D. J., Schwartz J. H., Sherbany A. A. Kinetic properties of normal and perturbed axonal transport of serotonin in a single identified axon. J Physiol. 1978 Aug;281:559–579. doi: 10.1113/jphysiol.1978.sp012439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S. Intracellular transport in neurons. Physiol Rev. 1980 Oct;60(4):1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975 Aug;66(2):351–366. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. O., Sjöstrand J. Transport of microtubular protein in axons of retinal ganglion cells. J Neurochem. 1971 Jun;18(6):975–982. doi: 10.1111/j.1471-4159.1971.tb12027.x. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Toyama R., Tsukamoto T., Matsuoka M., Nakafuku M., Obara T., Takagi T., Hernandez R. Structures of the genes coding for G-protein alpha subunits from mammalian and yeast cells. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):209–220. doi: 10.1101/sqb.1988.053.01.027. [DOI] [PubMed] [Google Scholar]
- Laduron P. Axoplasmic transport of muscarinic receptors. Nature. 1980 Jul 17;286(5770):287–288. doi: 10.1038/286287a0. [DOI] [PubMed] [Google Scholar]
- Lester H. A., Steer M. L., Michaelson D. M. ADP-ribosylation of membrane proteins in cholinergic nerve terminals. J Neurochem. 1982 Apr;38(4):1080–1086. doi: 10.1111/j.1471-4159.1982.tb05351.x. [DOI] [PubMed] [Google Scholar]
- Levin B. E. Presynaptic location and axonal transport of beta 1-adrenoreceptors in the rat brain. Science. 1982 Aug 6;217(4559):555–557. doi: 10.1126/science.6178165. [DOI] [PubMed] [Google Scholar]
- Mattera R., Graziano M. P., Yatani A., Zhou Z., Graf R., Codina J., Birnbaumer L., Gilman A. G., Brown A. M. Splice variants of the alpha subunit of the G protein Gs activate both adenylyl cyclase and calcium channels. Science. 1989 Feb 10;243(4892):804–807. doi: 10.1126/science.2536957. [DOI] [PubMed] [Google Scholar]
- Milligan G., Gierschik P., Spiegel A. M., Klee W. A. The GTP-binding regulatory proteins of neuroblastoma x glioma, NG108-15, and glioma, C6, cells. Immunochemical evidence of a pertussis toxin substrate that is neither Ni nor No. FEBS Lett. 1986 Jan 20;195(1-2):225–230. doi: 10.1016/0014-5793(86)80165-x. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I., Spiegel A. M., Malech H. L. Subcellular localization of Gi alpha in human neutrophils. J Biol Chem. 1988 Aug 5;263(22):10958–10964. [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Toutant M., Bockaert J., Homburger V., Rouot B. G-proteins in Torpedo marmorata electric organ. Differential distribution in pre- and post-synaptic membranes and synaptic vesicles. FEBS Lett. 1987 Sep 28;222(1):51–55. doi: 10.1016/0014-5793(87)80190-4. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Wamsley J. K., Zarbin M. A., Kuhar M. J. Opioid receptors undergo axonal flow. Science. 1980 Oct 3;210(4465):76–78. doi: 10.1126/science.6158097. [DOI] [PubMed] [Google Scholar]
- Zarbin M. A., Wamsley J. K., Kuhar M. J. Axonal transport of muscarinic cholinergic receptors in rat vagus nerve: high and low affinity agonist receptors move in opposite directions and differ in nucleotide sensitivity. J Neurosci. 1982 Jul;2(7):934–941. doi: 10.1523/JNEUROSCI.02-07-00934.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]