Abstract
The diagnostic criteria of Asperger's syndrome (AS), considered a part of the autistic spectrum disorder, are still unclear. A critical marker, which distinguishes AS from autism, is the presence of language. The ability of a child with AS to acquire and use language early results in the fact that AS usually is diagnosed much later than autism. Autism is not usually diagnosed until around the age of 3, whereas AS usually is not diagnosed until the child is 6 or 7 years of age. In the present article, using Eshkol–Wachman movement notation, we present evidence that abnormal movement patterns can be detected in AS in infancy. This finding suggests that AS can be diagnosed very early, independent of the presence of language. As shown earlier by us, almost all of the movement disturbances in autism can be interpreted as infantile reflexes “gone astray”; i.e., some reflexes are not inhibited at the appropriate age in development, whereas others fail to appear when they should. This phenomenon appears to apply to AS as well. Based on preliminary results, a simple test using one such reflex is proposed for the early detection of a subgroup of children with AS or autism.
Classification and diagnostic criteria for autism spectrum disorder and its related syndromes are mostly the result of accumulated clinical observations and lack a uniform nosology (1–4). Frith (3) proposes that this lack of uniformity in classification and diagnostic criteria is caused by errors in defining clinical categories. She states: “In defining clinical categories two kinds of error are common: the categories aimed at are too small and leave the majority of patients unaccounted for, or they are too large and do not differentiate patients who, in most clinicians' opinions, present different types of problems. In autistic spectrum disorders the twin dangers are omnipresent, accounting for pendulum swings between over-inclusion and ultra-specificity.”
Another aspect to be assessed, according to Wing (4), is the relationship between syndromes featuring impaired social interaction, mental retardation, other childhood disorders affecting cognition, and language and social function. This aspect is acutely present when Asperger's syndrome (AS) and autism are considered, because each can be seen as a different limb of the same tree. Klin and Volkmar (2) put it this way: “Designation of Asperger's syndrome as a `variant' or `subtype' of autism (e.g., high-function autism, or adults with autism) would be acceptable, but it would not add to a categorical classification system.” This statement is complicated by the fact that children with a diagnosis of AS often receive fewer services than those with a diagnosis of autism.
In the present article, the comparison of movement patterns in infants diagnosed as autistic versus those diagnosed as AS shows that similar patterns of movement disturbances exist in both syndromes. Thus, the assessment of movement patterns, which are the infant's “first language,” can serve as a common baseline when comparing and studying different syndromes.
Because the infant's movement-behavior is prelingual and presocial, the neural mechanisms at the core of these syndromes can be focused on with the possibility of identifying objective early reflex markers for the detection of AS and autism.
Compared with autism, AS is usually diagnosed rather late. The early severe deficits in social behavior and severe language abnormalities found in children with autism do not occur, thus leaving the AS child undiagnosed until much later (often he/she remains undiagnosed until teenage years and beyond). Frith (3) states: “From the point of view of the diagnostician there is much support for the idea of Asperger's syndrome shading into normality. After all, the diagnosis is, so far, based on behavior and not on tests that clearly identify underlying problems.”
In the present study, we show that abnormal movement patterns (similar to those found in infants with autism) also exist in infants who will later be diagnosed as AS.
Methods
Videos of infants who later received a diagnosis of AS were solicited from parents through conversations at professional conferences, over the Internet, or in advertisements in professional publications. A total of 16 videos of children diagnosed with AS were converted to DVDs by using a Panasonic DVD video recorder (model DMR-E30). Using a Pioneer DVD player (model DVD-V7400), we performed frame-by-frame analysis of the video images. We used Eshkol–Wachman movement notation (EWMN) (5, 6) to analyze the videos.¶
The observations reported in this article were made possible by the use of EWMN, therefore a brief exposition of the basic distinctions of the system follows.
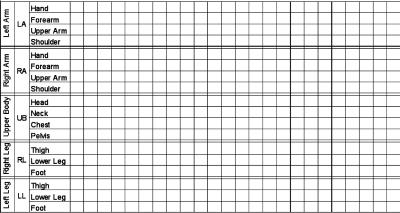
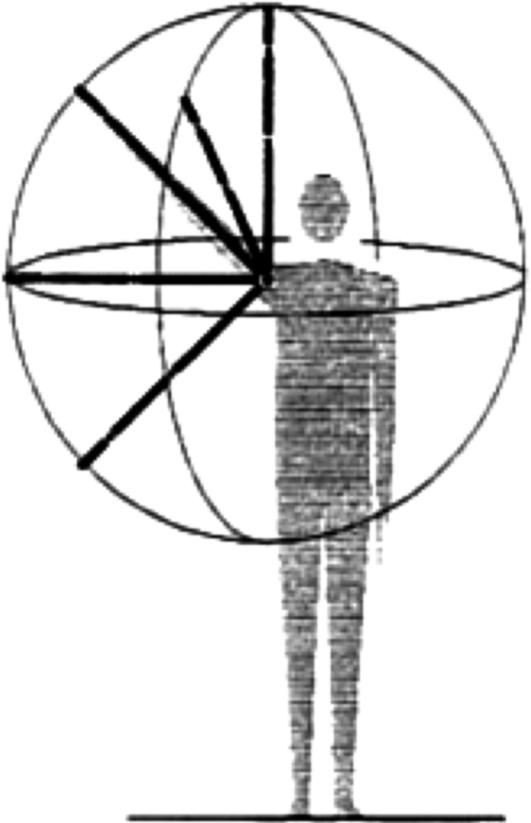
The Body and Manuscript Page. EWMN assumes one general form that will stand conceptually for all bodies. In that form, each limb is reduced to its longitudinal axis, an imaginary straight line of unchanging length. These axes are analogous to the skeletal structure of the body (Fig. 1). The movements of a single axis of constant length free to move about one (fixed) end all will be enclosed by a sphere; the free end will always describe a curved path on the surface of this sphere. Every limb segment in the body can be regarded as such an axis. Typically, the curves described on the surface of the sphere will be circles or parts of circles. The information thus obtained is written in a horizontally ruled page where each segment of the body has its own space. The vertical lines denote units of time (Fig. 2).
Fig. 1.
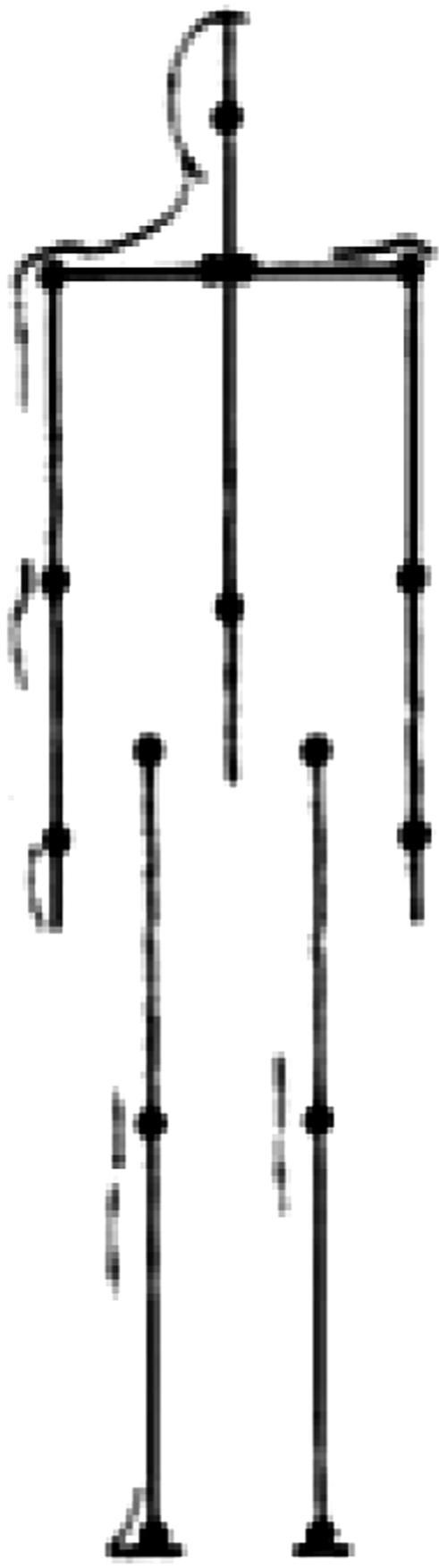
A general form that stands conceptually for all bodies. Each limb's longitudinal axis, an imaginary straight line, is analogous to the skeletal structure of the body.
Fig. 2.
A manuscript page: Each segment of the body has its own space. The vertical lines denote units of time.
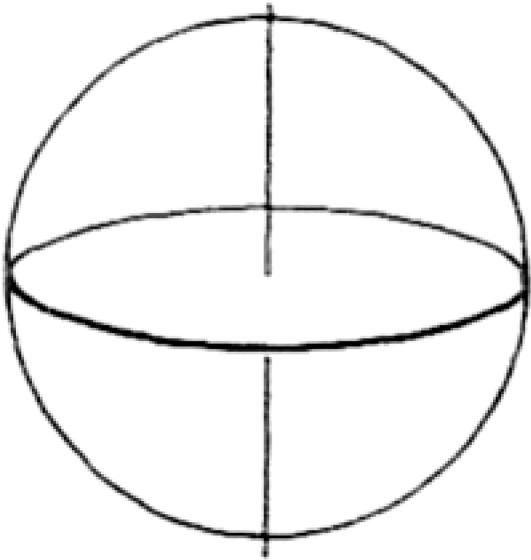
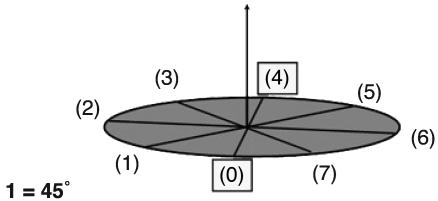
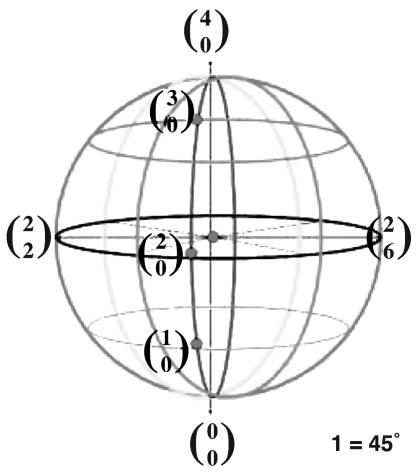
System of Reference (SoR). In EWMN, a coordinate SoR is used in which each limb segment is regarded as the radius of a sphere (Fig. 3). The orientation of the SoR is such that its central axis is perpendicular to the ground; the plane of the equator (the horizontal plane) is parallel to the ground (Fig. 4). One direction on the horizontal plane is selected as a starting position for all measurements. This direction is called 0. Other directions on the horizontal plane are defined in relation to it. Thus, eight directions are obtained when the measuring unit is 1 = 45° (Fig. 5).
Fig. 3.
The curves described on the surface of the sphere, by the movement of the arm, for instance, will be circles or parts of circles.
Fig. 4.
The central axis of the SoR is perpendicular to the ground; the plane of the equator runs parallel to the ground.
Fig. 5.
Eight directions are obtained on the horizontal plane when the measuring unit is 1 = 45°.
Vertical planes are perpendicular to the horizontal plane. They intersect with the positions that result from the division of the horizontal plane. The line in which all vertical planes intersect is the vertical axis of the sphere. When the chosen unit for measurement is 45°, 26 positions (i.e., directions) are obtained. They are expressed in terms of the coordinate grid of the SoR (Fig. 6).
Fig. 6.
Every direction (position) in the SoR is expressed as two numbers. The lower one represents the direction on the horizontal plane, and the upper one represents the amount of movement in the vertical direction from down (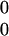 ) to up (
) to up ( ).
).
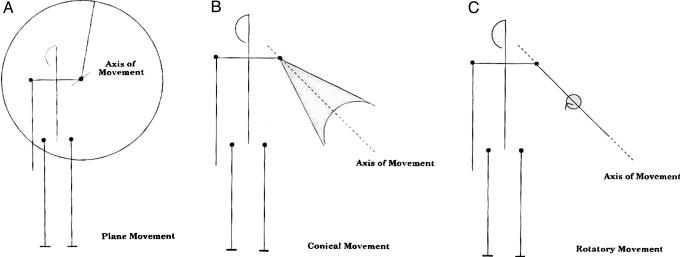
Movement Classification. A type of movement is determined by the angular relation between the axis of movement and the position of the axis of the limb at the beginning of the movement. When the angle of movement is 90°, the surface that results is a plane, and the movement is called plane movement (Fig. 7A). In the case where the angle between the axes is zero (i.e., they coincide), no surface is created, and the axis of the limb simply rotates about itself. This process is referred to as rotatory movement (Fig. 7C). Between these two extremes lie all of the other possible angular relationships of axis of limb to axis of movement. All of these intermediate angles produce conical-shaped surfaces of different sizes. This type of movement is called conical movement (Fig. 7B).
Fig. 7.
There are three types of movement: plane movement (A), rotatory movement (C), and conical movement (B).
Light and Heavy Limb. The limbs of the moving body are characterized as active versus carried. An actively moving limb (heavy) changes the location and modifies the paths of movement of any lighter limb, which is passively being carried along (Fig. 8).
Fig. 8.
The torso is actively moving, while the head, neck, and upper limbs are being carried passively along.
Results and Discussion
Motor Milestones in Development. The development of motor autonomy is the infant's central task in the first year of life. In essence, this development is a transformation from the horizontal dimension (where the infant lies on a horizontal surface, e.g., the bed, the ground) to the vertical (where the infant stands upright and starts to walk). The process unfolds through distinct stages of motor development that are interconnected like rungs of a ladder. The order of their appearance is predetermined (7). Mastering each stage prepares the infant's ascent to the next one. The whole process can be regarded as a series of stages. The stages are evident in the development of every infant. In each stage, complex reflex patterns appear and are integrated with those already existing.
In our earlier article (8), the differences between motor stages of normal infants and infants who would later be diagnosed as autistic were described. In the present article, movement patterns of infants later diagnosed with AS were analyzed. This process allows for a study of the two syndromes and a comparison to the normal infant. Normal infants showed none of the deficits described below.
Moebius Mouth. In the original descriptions of the facial paralysis that have perpetuated his name, Paul J. Moebius described bilateral congenital facial weakness and loss of abduction of the eyes. He also discussed the paralysis of the sixth and seventh nerves. Over the years, the Moebius syndrome's description has been broadened by researchers who added various deformities of the limbs to the original criteria (9, 10). Gillberg and Steffenburg (11) comment that “if Moebius syndrome and autistic disorders tended to coincide in a more than chance fashion, this would have some meaningful theoretical implications because of the assumed underlying brainstem pathology in both conditions.”
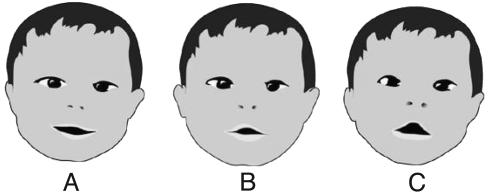
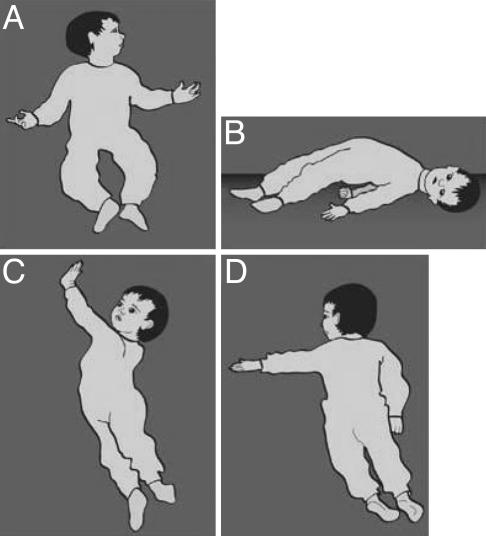
Eight infants from the group of the present study exhibited the characteristic shape of a Moebius mouth. Fig. 9 shows the mouth of an 8-month-old infant later to be diagnosed with AS. When the infant smiles (Fig. 9A), there is no trace of the typical shape of Moebius mouth, but when the smile ceases (Fig. 9 B and C), the tented upper lip and flat lower lip, typical of the Moebius mouth, reappears. Previously, we observed Moebius mouth in children later diagnosed with autism (8). In one case, Moebius mouth was recognizable at birth. As is shown in Fig. 9, the typical shape of a Moebius mouth is sometimes masked by other facial expressions and is present only in passing. (We call this “Moebius en passant.”) Frame-by-frame analysis enables the differentiation of these forms and the detection of the characteristics of the Moebius mouth. The shape is present throughout the infant's motor development, and in some cases, where the infants could be followed into their toddler years, it became even more pronounced. In our experience, Moebius-shaped mouth, albeit an early sign of neurological damage, is not sufficient as a diagnostic criterion for early detection of AS or autism. Nevertheless, it can be a confirming one when it is accompanied by the characteristic cluster of movement disturbances in motor development.
Fig. 9.
In AS, Moebius mouth can be masked by a smile. The smile (A) of an 8-month old infant masks a Moebius-shaped mouth (B–C).
Persistent Asymmetry When Lying. When asymmetry of the posture or movement of the body persists, it is a sign of neurological deficit. At ≈3–4 months, when lying prone, the normal infant is able to support his elevated chest with his forearms (7) and to reach for a toy when lying on his back. As was described (8), persistent asymmetry was present in various forms in all of the infants who would later be diagnosed as autistic. For instance, asymmetry was detected as early as 3 months of age, when the infant failed to assume a symmetrical normal posture when lying prone. The left arm was extended forward supporting his chest while the right arm was trapped underneath his chest. Persistent asymmetry was also present in an infant later diagnosed with AS when lying on his back reaching for his toys. The infant constantly extended only his left arm while manipulating the toys. His right arm usually remained static at the side of his body. When the right arm did participate, it moved with an appreciably slower speed and made fewer attempts to reach, and the movement had significantly smaller amplitude.
Righting: Segmental Rotation from Supine to Prone (“Corkscrew Rotation”). The sequence of righting from supine to prone occurs when the infant reaches ≈4 months of age. Normally, righting has three main phases: (i) The head, keeping its contact with the surface, is turned to one side and dorsally extended. (The direction of the righting will coincide with the direction toward which the face turns.) (ii) Movement of the head releases the occiput-side shoulder from its contact with the surface and initiates the rotation of the pelvis, trunk, and shoulders in a corkscrew fashion. This process is called segmental rotation. The order in which segmental rotation is performed changes as the infant develops. In the infantile form, the segmental rotation starts from the pelvis. In the more mature infant, rotation usually starts from the shoulder (7). (iii) When a corkscrew rotation ends, the infant will be lying prone, his head vertical to the surface and his forearms supporting his elevated chest. He usually brings one of his thighs alongside the stomach in preparation for the next motor stage (crawling). These components were absent in the righting of two fraternal twins later diagnosed as AS. At 8 months of age, the asymmetrical tonic neck reflex (ATNR) was still present in both twins, whereas in normal infants, this reflex is usually inhibited by ≈4 months of age (12). The segmental rotation of the twins was drastically affected by the prolonged presence of the ATNR. First, in keeping with the ATNR pattern, the head turn caused the arm to be extended on the jaw side. Normally the infant would then turn in the direction that the head is facing, i.e., to its left (Fig. 10A). The infant actually turned to its right; the direction of the face therefore was contrary to the direction of the turn. To execute the turn, the infant arched its torso upward, leaving only the head and the toes in contact with the ground (bridge position) (Fig. 10B). From this position, the extended left arm swung up in the air (Fig. 10C). Because the head and the arm are functionally linked in the ATNR, the head followed the movement of the arm (eye gaze fixed on the hand) and rotated to the right as the left arm swung in that direction, causing the infant to roll over en bloc, not segmentally, while maintaining functional contact of the eyes with the left hand. When the infant assumed the prone position, there was no support of the chest (Fig. 10D). The right arm was extended downward on the ground and the left arm (still in the ATNR position) extended sideways at 90°. The head was sustained in a vertical position for only a very short time.
Fig. 10.
In an AS infant, a sequence of righting from supine to prone is being controlled by an uninhibited ATNR.
A different form of abnormal righting, the sideways-upwards pattern, was detected in autistic infants (8) and also observed in two of the infants later diagnosed with AS. A third form of the disintegration of segmental righting was observed when an infant, at 10 months of age, showed the dorsal flexion and rotation of the head with a subsequent rotation of the shoulder but “skipped” the segmental rotation of the torso and pelvis to raise on his arms and hands, assuming a “mermaid posture” of half sitting/half lying. From this posture, the infant easily fell onto his chest and stomach, bringing his legs alongside his torso in a frog-like posture. All of these disintegrated forms of righting lack at least one of the three basic components of normal segmental rotation: (i) the dorsiflexion of the head in the starting position; (ii) segmental (corkscrew) rotation from supine to prone; and (iii) chest supported by forearms and the head vertical in the end position. Corkscrew rotation was missing in all of these abnormal forms.
Sitting and Crawling. With regard to crawling patterns, Freedland and Bertenthal (13) stated that “crawling experience, and specifically experience following the onset of hands-and-knees crawling, contributes to the development and reorganization of a number of other skills, such as spatial orientation, fear of heights, and postural stability.” When considering different crawling patterns, they added “that diagonally opposite limbs (e.g., right arm and left leg) move together and this strategy is most dynamically efficient for human infants because the diagonal coupling of the limbs maintains the most stable center of gravity” (13). Significantly, some infants later diagnosed with AS exhibited crawling patterns that deviated from the basic diagonally opposing limb patterns. Infants with autism exhibited similar asymmetrical crawling: one foot stepped while the other crawled. For instance on the left side, the leg movement was executed as the thigh moved under the belly and the lower leg and foot passively slid on the floor. On the right side, when the leg stepped forward, the foot rather than the knee established contact with the surface and the lower leg reached a vertical position. An identical pattern (one side stepping, one side crawling) was observed in three of the infants later diagnosed with AS.
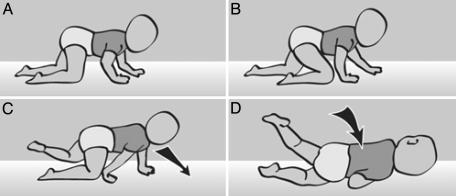
Clumsiness is regarded as one of the characteristics of AS (3, 14) but is usually associated with sport and play activities rather than motor development. An example of clumsiness was detected during the first year of life when an infant later diagnosed with AS attempted to crawl with the diagonally opposite limbs pattern, but instead fell to his right side (Fig. 11). After stepping forward with his left arm and right leg, he failed to release the right arm contact with the ground for the next step (Fig. 11 B and C). Thus, when he stepped with the left thigh his weight was shifted past his center of gravity, and he fell over in the direction of his stuck right arm (Fig. 11D).
Fig. 11.
Failure to synchronize shift of weight with placement of the hand leads to falling over in an AS child. When crawling (A and B), an infant fell to his right side when failing to release the contact of the right arm, thus losing balance (C and D).
Sitting. At ≈6 months, the normally developed infant is able to sit upright without support. Six of the 16 infants did not sit independently by 6 months of age. Two of them were still unable to sit independently as late as 10 months of age, and one of them omitted sitting altogether when, shortly after learning to crawl, he began to stand up and walk. Although the timing of the motor milestones can vary to some extent, when such a marked delay is combined with other signs (like the Moebius mouth, persistent asymmetry, and the absence of segmental rotation), further evaluation of the infant's development is advised. Two of the infants with AS displayed a pattern of falling forward or backward from a sitting position without using protective reflexes of the arms and verticalization of the head (parachute reflex). This abnormal response was also observed in infants later diagnosed with autism (8).
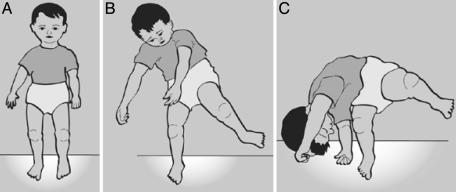
Falling While Walking. For a detailed analysis of normal gait versus deviant patterns of walking of autistic infants, the reader is referred to our earlier article (8). One AS infant of the present group, when making his first attempts to walk at 11 months of age, repeatedly fell to his right side. Although the ATNR was not fully present, the form of the arms suggested to us that the ATNR was still partially uninhibited. When falling, the infant did not use protective reflexes of the arms and fell headfirst onto the floor (Fig. 12).
Fig. 12.
As late as 11 months, protective reflexes failed to appear in the movements of an AS infant. When walking (A), an 11-month-old infant falls (B) and fails to use protective reflexes (C).
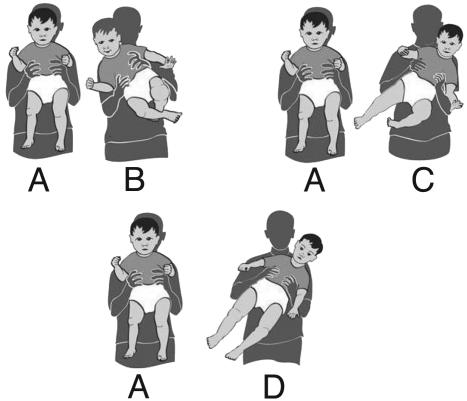
The Tilting Test. The incident described above, of the toddler falling head-on onto the floor, relates to other instances where absence of verticalization of the head was observed and described, one such example being the final posture at the end of segmental rotation; another occurs during the loss of balance from a sitting position (8, 15). In connection with these phenomena, the tilting test is suggested. This test uses the head verticalization reflex (16–18), which can be used as a simple rapid early screening test for a subgroup of infants at risk of autism or AS. The test can be performed by parents and/or pediatricians by holding the infant in the air around the waist (Fig. 13A) and tilting him slowly 45° to one side (Fig. 13B), then back to the erect vertical position (Fig. 13A), repeating the tilt slowly to the other side (Fig. 13C). A typically developing child will maintain his/her head vertical as the body is being tilted, indicating that based on the vestibular signal that is generated during the body tilt, the head is compensating for the tilt by moving itself simultaneously in the direction opposite to the direction of the tilt, thus maintaining itself in the vertical position (Fig. 13 B and C). The initial response can be obtained at ≈6 months of age and, in a more complete form, when the infant is ≈8 months old. A negative response is evident when the infant fails to keep his head vertical when tilted, and instead carries the head in line with the rest of the tilted body (Fig. 13D).
Fig. 13.
The tilting test can be used as an early indicator for possible autism or Asperger's. At 6–8 months a typically developed infant maintains his/her head vertical as the body is being tilted (A–C). A negative response is evident when the infant carries the head in line with the rest of the tilted body (D).
Table 1, which is published as supporting information on the PNAS web site, presents the full details of the movement deficits shown by each subject in the present study.
Discussion
“In infancy the movement disorders present in autism are clearest, not yet masked by other mechanisms that have developed to compensate for them. It is possible that they may vary according to the areas of the brain in which developmental delay or damage has occurred” (8). This statement is clarified when the movement disorders described here are distinguished as infantile reflexes gone astray (15).
The role infantile reflexes play in the psychomotor development of the infant and young child has been well established (12, 15–19), but because autism is traditionally considered to be a behavioral syndrome, namely a triad of impairments in socialization, imagination, and communication (3), it has not been associated with aberrant infantile reflexes. Goddard (17) points to the possible negative outcome of the overly long persistence of infantile reflexes when she states, “Primitive reflexes, retained beyond 6 months of age, may result in immature patterns of behavior or may cause immature systems to remain prevalent, despite the acquisition of later skills.” The stages of development that bring the infant to motor independence can be related to as a reflexive process, one in which the infant gradually matures by the inhibition of more primitive forms. This process enables him to gain control over his posture and mobility. It is generally recognized that early primitive reflexes (e.g., Moro reflex, ATNR, rooting reflex) are gradually inhibited at ≈4 months of age and are replaced by postural reflexes such as the segmental righting reflex, oculo-head-righting reflexes, reflexes that are present into adulthood. Primitive reflexes are assumed to originate in the brainstem, whereas postural reflexes, including sitting and crawling, are presumably based in the midbrain (16, 17).
The aberrant movement patterns seen in infants who were later diagnosed with AS clearly show the characteristics of infantile reflexes gone astray. For instance, as shown in Fig. 10, one abnormal pattern of supine to prone righting described here is an example of the imposition of a primitive reflex, the ATNR, on a postural reflex, the segmental righting reflex. As a result of the ATNR persisting too long, the normal characteristic components of the segmental righting reflex are absent. The head-righting reflex is missing, as is the support of the chest when the infant is lying prone at the end of the process.
When the ATNR persists too long the development of walking also can be disrupted. This was evident when an 11-month-old infant, later diagnosed with AS, maintained the ATNR while trying to walk. He kept falling in the direction of the extended right arm, the one that he was facing, when he tried to get up and walk.
Whereas the above examples are of primitive reflexes that persist too long, the tilting test proposed here can serve as an example of a reflex that does not appear when it should. By the time the normal infant is 6 months of age, he/she is capable of controlling the verticality of the head based on the development of the oculo- and labyrinthine head-righting reflexes (16, 17). The verticalization of the head applies also to lateral tilting. The evidence of the lack of head verticalization response in infants who were later diagnosed with autism is still preliminary, but as we have shown here, several of the reflexes impaired in autism are also impaired in infants soon to be diagnosed with AS. Therefore, the tilting test (head verticalization response) should be routinely performed on all infants beginning at 6 months, particularly if there is a history of autism or AS in the family. This simple noninvasive test takes 20–30 s and can be performed by the infant's pediatrician or parents. The absence of the head verticalization response (even on one side) can be an early warning sign of neurological damage that may be characteristic of autism or AS and indicates the need for additional testing for aberrant movement patterns.
Goddard (17) uses the terms clumsy and poor reorganizational skills when she describes the effect on the motor and psychomotor development of the child following the lack of inhibition of primitive reflexes. Asperger (14) himself pointed out that clumsiness is one of the more evident markers of the children he saw in his clinic. Frith (3) also notes this clumsiness when she says, “One behavioral sign especially in need of clarification concerns the much reported clumsiness of Asperger people. It is tantalizing not to know whether their gaucheness is in fact a motor coordination problem, or will turn out to be a problem not so much with movements as with the use of movements. It is also not yet clear if clumsiness can act as an important discriminating diagnostic sign of Asperger syndrome as opposed to other variants of autism.” The absence of adequate language to describe movement translates into very haphazard descriptions regarding the nature and character of what, for lack of a better name, is referred to as clumsiness in AS. From our preliminary observations, we have a strong tendency to believe that some of the “clumsy” characteristics seen in AS are based on infantile reflexes gone astray.
In summary, infantile reflexes are easy to spot and can be used as early detection signs. When these reflexes persist too long or do not appear when they should, the motor development of the infant and, subsequently, other aspects of his behavior will be affected. Clearly then, they can serve as early detection markers for abnormal neurological development in AS and autism.
Acknowledgments
We are grateful to the families who sent us the videotape material analyzed in this article. Their generosity and goodwill made this work possible. We thank Sheryl Flynn and Caroline Wood for constructive criticisms; Debra Neill-Mareci for drawing the figures; and Helen Horovitz from Kibbutz Merchavia in Israel for assisting us with using the tilt test in normal infants. This work was supported in part by funds provided by the Cure Autism Now Foundation, Los Angeles.
Abbreviations: AS, Asperger's syndrome; SoR, system of reference; ATNR, asymmetrical tonic neck reflex; EWMN, Eshkol–Wachman movement notation.
Footnotes
Because the reader may be interested in using EWMN, we have cited the first book by Eshkol and a recent publication by her. It should be noted that there are several publications by Eshkol in the years between them.
References
- 1.Filipek, P. A., Accardo, P. J., Baranek, G. T., Cook, E. H., Jr., Dawson, G., Gordon, B., Gravel, J. S., Johnson, C. P., Kallen, R. J., Levy, S. E., et al. (1999) J. Autism Dev. Disord. 29, 439-479. [DOI] [PubMed] [Google Scholar]
- 2.Klin, A. & Volkmar, F. R. (1997) in Handbook of Autism and Pervasive Developmental Disorders, eds. Cohen, D. & Volkmar, F. R. (Wiley, New York), 2nd Ed., pp. 94-122.
- 3.Frith, U. (1992) in Autism and Asperger Syndrome, ed. Frith U. (Cambridge Univ. Press, Cambridge, U.K.), pp. 1-36.
- 4.Wing, L. (1992) in Autism and Asperger Syndrome, ed. Frith, U. (Cambridge Univ. Press, Cambridge, U.K.), pp. 93-121.
- 5.Eshkol, N. & Wachman, A. (1958) Movement Notation (Weidenfeld and Nicolson, London).
- 6.Eshkol, N. & Harries, J. (2001) EWMN Part 1 (The Movement Notation Society, Holon, Israel).
- 7.McGraw, M. B. (1989) The Neuromuscular Maturation of the Human Infant (Mac Keith Press, London).
- 8.Teitelbaum, P., Teitelbaum, O., Nye, J., Fryman J. & Maurer R. G. (1998) Proc. Natl. Acad. Sci. USA 95, 13982-13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldblatt, D. & Williams, D. (1986) J. Child Neurol. 1, 71-78. [DOI] [PubMed] [Google Scholar]
- 10.Miller, M. T., Stromland, K., Gillberg, C., Johansson, M. & Nilsson, E. E. (1998) Am. Ophthalmol. Soc. XCVI, 369-385. [PMC free article] [PubMed] [Google Scholar]
- 11.Gillberg, C. & Steffenburg, S. (1989) Acta Pediatr. Scand. 78, 314-316. [DOI] [PubMed] [Google Scholar]
- 12.Paine, R. S., Brazelton, T. B., Donovan, D. E., Drorbaugh, J. E., Hubbell, J. P. & Sears, E. M. (1964) Neurology 14, 1036-1048. [DOI] [PubMed] [Google Scholar]
- 13.Freedland, R. L. & Bertenthal, B. I. (1994) Psychol. Sci. 5, 26-32. [Google Scholar]
- 14.Asperger, H. (1992) in Autism and Asperger Syndrome, ed. Frith, U. (Cambridge Univ. Press, Cambridge, U.K.), pp. 37-92.
- 15.Teitelbaum, P., Teitelbaum, O., Fryman, J. & Maurer, R. G. (2002) J. Dev. Learn. Disord., 6, 15-22. [Google Scholar]
- 16.Fiorentino, M. R. (1980) Normal and Abnormal Development: The Influence of Primitive Reflexes on Motor Development (Charles C. Thomas, Springfield, IL).
- 17.Goddard, S. (1996) A Teacher's Window Into the Child's Mind (Fern Ridge Press, Eugene, OR).
- 18.Peiper, A. (1963) Cerebral Function in Infancy and Childhood (Consultant Bureau Enterprises, New York).
- 19.Fay, T. (1955) Am. J. Psychiatry 111, 644-652. [DOI] [PubMed] [Google Scholar]