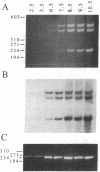
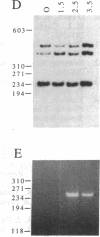
Abstract
The amyloid precursor protein (APP) is thought to be processed aberrantly to yield the major constituent of the amyloid plaques observed in the brains of patients with Alzheimer disease and Down syndrome. However, the gene encoding APP is expressed widely in normal human tissues and in adult and fetal mouse tissues and is alternatively spliced in a tissue-specific pattern in the adult. There is evidence that APP may function as a growth factor and as a mediator of cell adhesion and in these roles could be important in morphogenesis. As a step toward determining the role of APP in development and in determining how the adult pattern of tissue-specific splicing is established, we have used reverse transcription and the polymerase chain reaction to demonstrate APP expression in mouse oocytes, preimplantation embryos, and postimplantation embryonic stages to the late embryonic period. All three splicing forms described in mouse were present at each stage, although there were changes in the ratios of the splicing forms at different stages. Screens for APP clones in embryonic cDNA libraries from the egg cylinder stage and the early somite stage were used to confirm the results of the polymerase chain reaction, and APP clone abundance was found to increase 10-fold between the two stages.
Full text
PDF

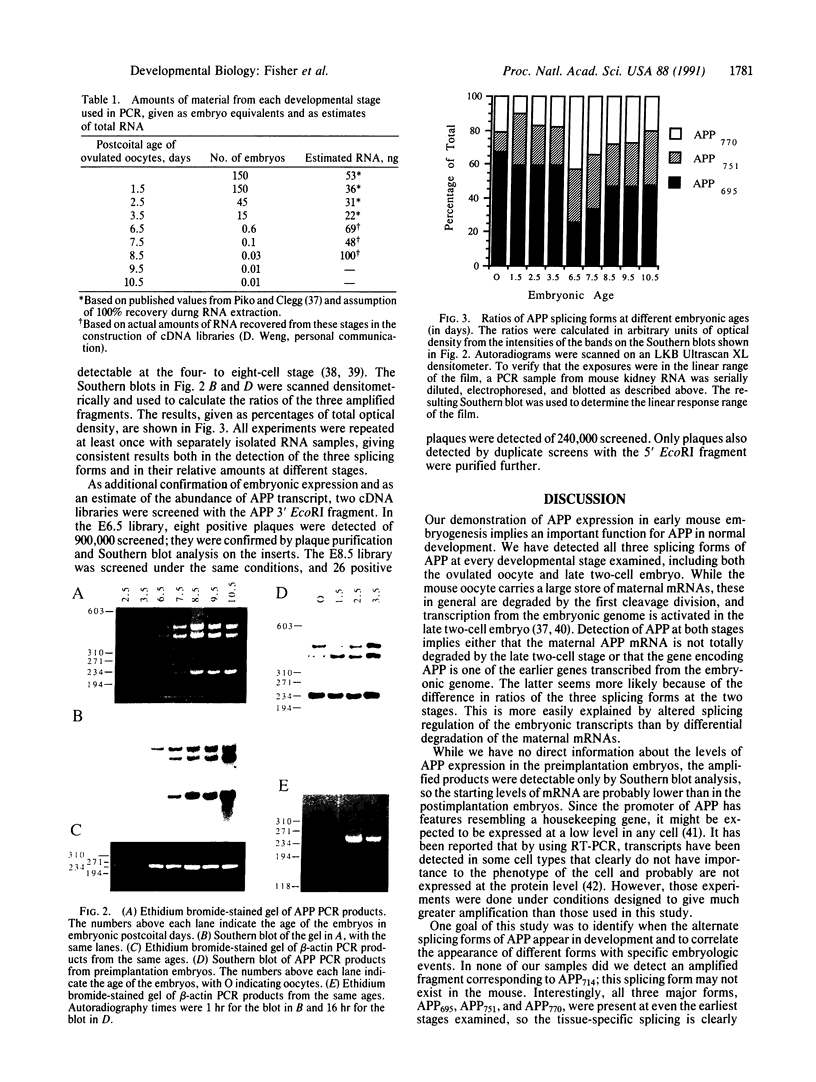

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachvarova R., Cohen E. M., De Leon V., Tokunaga K., Sakiyama S., Paynton B. V. Amounts and modulation of actin mRNAs in mouse oocytes and embryos. Development. 1989 Jul;106(3):561–565. doi: 10.1242/dev.106.3.561. [DOI] [PubMed] [Google Scholar]
- Bachvarova R., De Leon V. Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev Biol. 1980 Jan;74(1):1–8. doi: 10.1016/0012-1606(80)90048-2. [DOI] [PubMed] [Google Scholar]
- Brenner C. A., Adler R. R., Rappolee D. A., Pedersen R. A., Werb Z. Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev. 1989 Jun;3(6):848–859. doi: 10.1101/gad.3.6.848. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Usiak M., Younkin L. H., Younkin S. G. Expression of beta amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer's disease using PCR. Neuron. 1990 Feb;4(2):253–267. doi: 10.1016/0896-6273(90)90100-t. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Higgins G. A., Lewis D. A., Bahmanyar S., Goldgaber D., Gajdusek D. C., Young W. G., Morrison J. H., Wilson M. C. Differential regulation of amyloid-beta-protein mRNA expression within hippocampal neuronal subpopulations in Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1297–1301. doi: 10.1073/pnas.85.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Cork L. C., Unterbeck A., Bayney R. M., Price D. L. Differential expression of amyloid precursor protein mRNAs in cases of Alzheimer's disease and in aged nonhuman primates. Neuron. 1990 Jan;4(1):97–104. doi: 10.1016/0896-6273(90)90446-m. [DOI] [PubMed] [Google Scholar]
- Lovett M., Goldgaber D., Ashley P., Cox D. R., Gajdusek D. C., Epstein C. J. The mouse homolog of the human amyloid beta protein (AD-AP) gene is located on the distal end of mouse chromosome 16: further extension of the homology between human chromosome 21 and mouse chromosome 16. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1069–1075. doi: 10.1016/s0006-291x(87)80073-6. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Finch E. A., Dawes L. R. Expression of the Alzheimer amyloid precursor gene transcripts in the human brain. Neuron. 1988 Oct;1(8):669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- O'Hara B. F., Bendotti C., Reeves R. H., Oster-Granite M. L., Coyle J. T., Gearhart J. D. Genetic mapping and analysis of somatostatin expression in Snell dwarf mice. Brain Res. 1988 Dec;464(4):283–292. doi: 10.1016/0169-328x(88)90037-x. [DOI] [PubMed] [Google Scholar]
- O'Hara B. F., Fisher S., Oster-Granite M. L., Gearhart J. D., Reeves R. H. Developmental expression of the amyloid precursor protein, growth-associated protein 43, and somatostatin in normal and trisomy 16 mice. Brain Res Dev Brain Res. 1989 Oct 1;49(2):300–304. doi: 10.1016/0165-3806(89)90031-x. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Golde T. E., Cohen M. L., Kovacs D. M., Tanzi R. E., Gusella J. F., Usiak M. F., Younkin L. H., Younkin S. G. Amyloid protein precursor messenger RNAs: differential expression in Alzheimer's disease. Science. 1988 Aug 26;241(4869):1080–1084. doi: 10.1126/science.2457949. [DOI] [PubMed] [Google Scholar]
- Paynton B. V., Rempel R., Bachvarova R. Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev Biol. 1988 Oct;129(2):304–314. doi: 10.1016/0012-1606(88)90377-6. [DOI] [PubMed] [Google Scholar]
- Pikó L., Clegg K. B. Quantitative changes in total RNA, total poly(A), and ribosomes in early mouse embryos. Dev Biol. 1982 Feb;89(2):362–378. doi: 10.1016/0012-1606(82)90325-6. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Brenner C. A., Schultz R., Mark D., Werb Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988 Sep 30;241(4874):1823–1825. doi: 10.1126/science.3175624. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Robakis N. K., Oster-Granite M. L., Wisniewski H. M., Coyle J. T., Gearhart J. D. Genetic linkage in the mouse of genes involved in Down syndrome and Alzheimer's disease in man. Brain Res. 1987 Sep;388(3):215–221. doi: 10.1016/0169-328x(87)90028-3. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Sundsmo M., Roch J. M., Kimura N., Cole G., Schubert D., Oltersdorf T., Schenk D. B. Secreted form of amyloid beta protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989 Aug 25;58(4):615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Salbaum J. M., Weidemann A., Lemaire H. G., Masters C. L., Beyreuther K. The promoter of Alzheimer's disease amyloid A4 precursor gene. EMBO J. 1988 Sep;7(9):2807–2813. doi: 10.1002/j.1460-2075.1988.tb03136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Access to a messenger RNA sequence or its protein product is not limited by tissue or species specificity. Science. 1989 Apr 21;244(4902):331–334. doi: 10.1126/science.2565599. [DOI] [PubMed] [Google Scholar]
- Schubert D., Jin L. W., Saitoh T., Cole G. The regulation of amyloid beta protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989 Dec;3(6):689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Saitoh T., Cole G. Characterization of an amyloid beta precursor protein that binds heparin and contains tyrosine sulfate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2066–2069. doi: 10.1073/pnas.86.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Schroeder R., LaCorbiere M., Saitoh T., Cole G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science. 1988 Jul 8;241(4862):223–226. doi: 10.1126/science.2968652. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Smith R. P., Higuchi D. A., Broze G. J., Jr Platelet coagulation factor XIa-inhibitor, a form of Alzheimer amyloid precursor protein. Science. 1990 Jun 1;248(4959):1126–1128. doi: 10.1126/science.2111585. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Yamada T., Sasaki H., Dohura K., Goto I., Sakaki Y. Structure and expression of the alternatively-spliced forms of mRNA for the mouse homolog of Alzheimer's disease amyloid beta protein precursor. Biochem Biophys Res Commun. 1989 Feb 15;158(3):906–912. doi: 10.1016/0006-291x(89)92808-8. [DOI] [PubMed] [Google Scholar]
- Yamada T., Sasaki H., Furuya H., Miyata T., Goto I., Sakaki Y. Complementary DNA for the mouse homolog of the human amyloid beta protein precursor. Biochem Biophys Res Commun. 1987 Dec 16;149(2):665–671. doi: 10.1016/0006-291x(87)90419-0. [DOI] [PubMed] [Google Scholar]
- de Sauvage F., Octave J. N. A novel mRNA of the A4 amyloid precursor gene coding for a possibly secreted protein. Science. 1989 Aug 11;245(4918):651–653. doi: 10.1126/science.2569763. [DOI] [PubMed] [Google Scholar]