Abstract
Introduction. Aim of this study is to determine if HbA1c levels are a reliable predictor of glycemic control in patients with decompensated cirrhosis. Methods. 200 unique patients referred for liver transplantation at University of Tennessee/Methodist University Transplant Institute with a HbA1c result were included. Three glucose levels prior to the “measured” A1c (MA1c) were input into an HbA1c calculator from the American Diabetes Association website to determine the “calculated” A1c (CA1c). The differences between MA1c and CA1c levels were computed. Patients were divided into three groups: group A, difference of <0.5; group B, 0.51–1.5; and group C, >1.5. Results. 97 (49%) patients had hemoglobin A1c of less than 5%. Discordance between calculated and measured HbA1c of >0.5% was seen in 47% (n = 94). Higher level of discordance of greater than >1.5 was in 12% of patients (n = 24). Hemoglobin was an independent predictor for higher discordance (odds ratio 0.77 95%, CI 0.60–0.99, and p value 0.04). HbA1c was an independent predictor of occurrence of HCC (OR 2.69 955, CI 1.38–5.43, and p value 0.008). Conclusion. HbA1c is not a reliable predictor of glycemic control in patients with decompensated cirrhosis, especially in those with severe anemia.
1. Introduction
Hemoglobin A1c (HbA1c) is the gold standard for the measurement of long-range glycemic control in patients with diabetes mellitus. Many studies have reported diabetes mellitus to be a risk factor in patients with alcoholic liver disease and nonalcoholic fatty liver disease (NAFLD) for the development of fibrosis and fibrosis progression [1–3]. In the United States, 2% of adult Americans (5.3 million) are infected with hepatitis B or C [4] and an estimated 31% or more with NAFLD [5, 6].
The presence of CLD is associated with significant impairment in glucose homeostasis. Glucose intolerance is seen in up to 80% of patients with CLD, and frank diabetes is present in 30–60% [7, 8]. Depending on its etiology, CLD has a significant impact on hepatic glucose metabolism.
Among patients with CLD, anemia, portal hypertension, hypersplenism, and variceal bleeding can be common complications. These factors can contribute to longer or shorter red blood cell (RBC) survival [9] and can lead to alteration of the HbA1c. Factors such as nutritional anemia can lead to increased RBC survival and falsely elevated HbA1c levels, whereas bleeding and hemolysis can lower RBC survival time and falsely lower HbA1c values.
Very few studies are dedicated to systematically evaluating the accuracy of HbA1c in CLD patients. In a screening of 20,000 measurements of HbA1c, patients with a very low HbA1c were analyzed further. Of nine abnormally low results, six were the result of cirrhosis, two resulted from hematological neoplasms with anemia, and one was due to hemoglobin F [10]. In a small case series (15 patients with compensated cirrhosis and 20 patients with chronic hepatitis), 40% of cirrhotic patients with diabetes had HbA1c levels below reference range for patients without diabetes [11].
Methodist University Hospital Transplant Institute has been systematically screening its liver transplantation candidates for diabetes with HbA1c levels. Measuring HbA1c is the gold standard for monitoring blood glucose control in diabetes and can now also be used to diagnose diabetes when its results are ≥6.5% [12].
The study objective is to look at glucose and HbA1c levels in cirrhotic patients and determine if HbA1c levels are a reliable predictor of glycemic control in patients who are presented for liver transplantation at Methodist University Hospital Transplant Institute between January 1, 2006, and January 1, 2014.
2. Methods
The University of Tennessee Health Sciences Center institutional review board approved this study. The study population included 200 electronic medical records (EMR) of patients who were evaluated for liver transplantation at the Methodist University Hospital Transplant Institute in Memphis, Tennessee, from July 7, 2010, through September 22, 2011. Case selection was restricted to patients who had HbA1c levels in the EMR. Patients who did not have HbA1c levels in the EMR were excluded from the study. Patients were referred for liver transplantation after they were evaluated by a primary care physician or gastroenterologist; the referral was indicated for them based on their clinical presentation and results of laboratory and diagnostic tests such as computed tomography (CT) scans or magnetic resonance imaging (MRI) or Model of End Stage Liver Disease (MELD) scores.
The relevant clinicopathologic information was logged into a Microsoft Access database. We reviewed comprehensive history and physical examination at time of referral to the Transplant Institute. The collected data included patients' demographics, cigarette smoking and alcohol history, past medical history, whether or not they were transplanted, MELD at time of time transplant, liver alone or simultaneous liver-kidney transplant, etiology of liver disease, HbA1c level reported from laboratory, and three glucose levels at time of transplant referral. Three glucose levels prior to the “measured” HbA1c (MA1c) were averaged, logged, and input into an HbA1c calculator from the American Diabetes Association website (http://professional.diabetes.org/diapro/glucose_calc) to determine the “calculated” HbA1c (CA1c). The difference between MA1c and CA1c levels was computed and the patients were divided into three groups: group 1, difference of <0.5; group 2, 0.51–1.5; and group 3, >1.5.
All analyses were performed using the software SPSS (SPSS, Inc., Chicago, Illinois). Data is presented as mean and SD or median and range. Variables between the three groups were analyzed by one-way ANOVA and Chi-square tests for ordinal and categorical variables. t-test was used for continuous variables and z test for comparison of proportions. A p value <0.05 was considered statistically significant.
3. Results
The medical records of patients who were evaluated for liver transplantation and had HbA1c levels collected at the Methodist University Hospital Transplant Institute in Memphis, Tennessee, from July 7, 2010, through September 22, 2011, were reviewed, yielding a total of 200 cases. The mean age for this cohort was 54 years with a male predominance: 67% were male. Seventy-nine percent were Caucasian. Forty-four percent and 39% of patients had a history of alcohol abuse and smoking history, respectively. Sixty-two patients (31%) in this study were diabetics. Of those 62 diabetic patients, 26 (42%) were on insulin.
Of the 200 patients included in this study, 55.5% (111) underwent liver transplant and of those 111 patients who underwent liver transplant, 10% (11) underwent simultaneous liver and kidney transplant. Average MELD score at time of transplant was 22. Most common etiology of liver disease in our cohort was hepatitis C virus at 41% (82), followed by hepatocellular carcinoma (HCC) 23% (46), alcohol 22% (44), nonalcoholic steatohepatitis (NASH) 16% (32), primary sclerosing cholangitis 6% (12), primary biliary cholangitis 4.5% (9), and hepatitis B virus 4.5% (9). The remaining 14% (28) of patients in this cohort referred to Transplant Institute included patients with cryptogenic cirrhosis, autoimmune hepatitis, alpha-1-antitrypsin deficiency, Wilson's disease, hemochromatosis, polycystic liver disease, cholangiocarcinoma, and neuroendocrine tumor.
The distribution of MA1c and the average glucose levels are depicted in Figure 1 (all patients), Figure 2 (patients without diabetes), and Figure 3 (patients with diabetes). The expected glucose levels for each range of MA1c (x-axis) values were obtained from the following website: http://www.diabeteschart.org/bgc1.html. One can infer from the figure that the MA1c values tend to group towards the lower values and actual average glucose values tend to group towards the higher values. For example, among diabetic patients, 58% of patients had MA1c <6, while 77% of patients had actual average glucose levels of more than 140 mg/dL. Similarly 88% of nondiabetic patients had HbA1c <5.5, but 38% had actual average glucose levels of more than 110 mg/dL.
Figure 1.
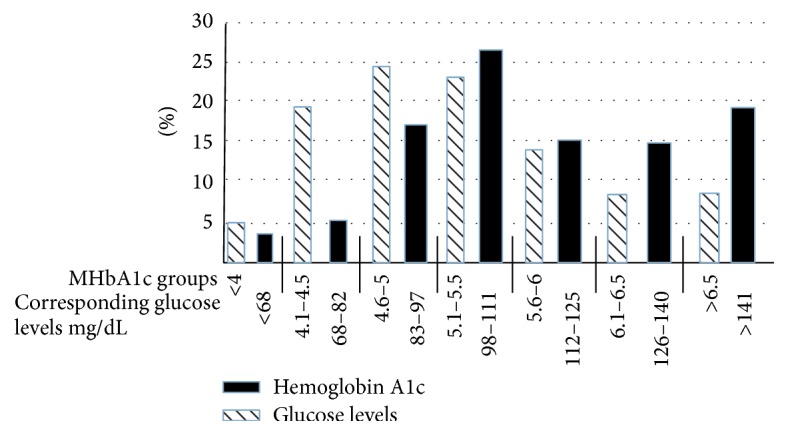
Percentage distribution of HbA1c and average glucose levels in 200 patients. The numbers on x-axis represent the measured HbA1c groups and the expected glucose levels (mg/dL) for each group of measured HbA1c.
Figure 2.
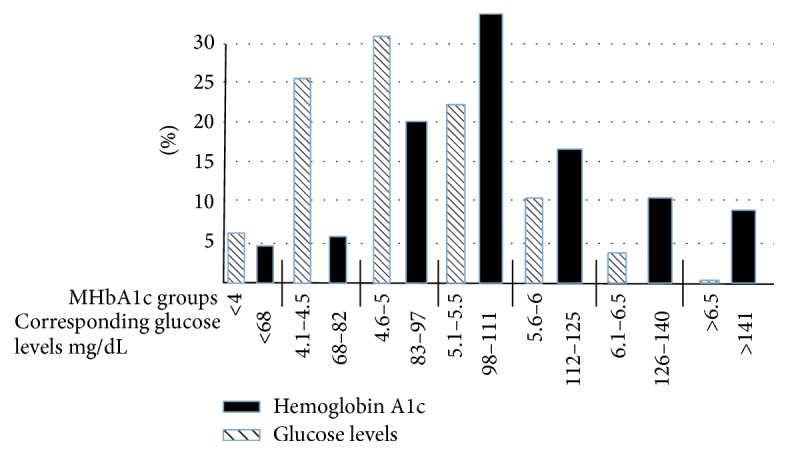
Percentage distribution of HbA1c and average glucose levels in 138 nondiabetic patients. The numbers on x-axis represent the measured HbA1c groups and the expected glucose levels (mg/dL) for each group of measured HbA1c.
Figure 3.
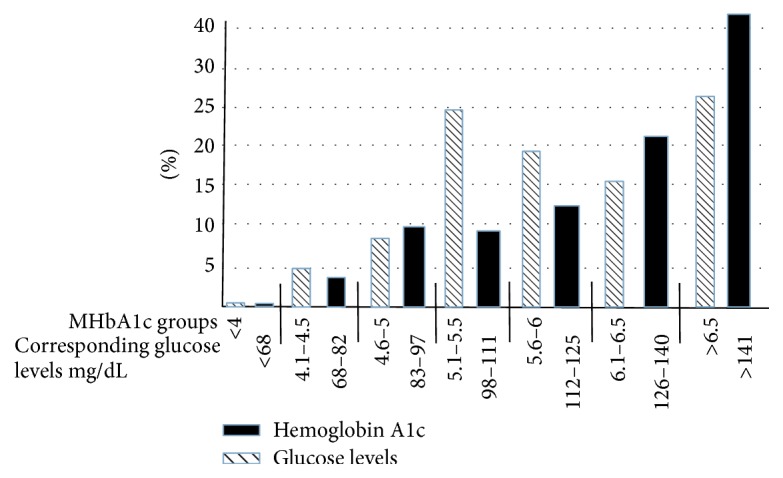
Percentage distribution of HbA1c and average glucose levels in 62 diabetic patients. The numbers on x-axis represent the measured HbA1c groups and the expected glucose levels (mg/dL) for each group of measured HbA1c.
Ninety-seven (49%) patients had HbA1c of less than 5 followed by 72 (36%) patients with hemoglobin A1c of 5-6 and 21 patients (11%) with hemoglobin of more than 7. The discordance between CA1c and MA1c was further categorized into three groups: group 1, the difference between CA1c and MA1c <0.5 (n = 106); group 2, the difference between CA1c and MA1c 0.5–1.5 (n = 70); and group 3, the difference between CA1c and MA1c >1.5 (n = 24). Patients with high discordance were more anemic and had higher incidence of diabetes (Table 1). In a multivariate model including those with high discordance versus those with no discordance, hemoglobin levels were the only independent predictor (odds ratio 0.77 95% CI 0.60–0.99; p value 0.04).
Table 1.
The characteristics of the groups based on difference between the calculated and measured hemoglobin A1c.
| Group 1 (n = 106) | Group 2 (n = 70) | Group 3 (n = 24) | |
|---|---|---|---|
| Age | 53 ± 11 | 56 ± 8 | 52 ± 10 |
| Gender (M/F) | 70/36 | 46/24 | 18/6 |
| BMI | 28.1 ± 5.6 | 28.6 ± 6.0 | 28.6 ± 5.8 |
| Etiology of Liver disease (%) | |||
| HCV%/NASH%/alcohol% | 40/21/20 | 40/20/14 | 50/17/17 |
| DM | 26% | 31% | 50% |
| Hemoglobin (gm/dL) | 12.2 ± 2.3 | 11.6 ± 1.9 | 10.9 ± 2.3∗ |
| Hemoglobin < 10 | 20% | 19% | 37%∗ |
| Hematocrit | 36 ± 6.8 | 34.5 ± 5.6 | 32.3 ± 7.0∗ |
| S. creatinine (mg/dL) | 1.23 ± 1.3 | 1.27 ± 1.08 | 1.23 ± 0.75 |
| Albumin (gm/dL) | 3.0 ± 0.7 | 2.9 ± 0.8 | 2.9 ± 0.7 |
| Platelets | 120 ± 95 | 122 ± 86 | 104 ± 71 |
| HBA1c (measured) | 5.1 ± 0.7 | 5.1 ± 1.1 | 6.0 ± 1.8∗ |
| Calculated HBA1c# | 5.2 ± 0.6 | 6.0 ± 1.13 | 8.6 ± 2.6∗ |
Group 1: the difference between calculated and measured A1c < 1.0, group 2: the difference between calculated and measured A1c 0.5–1.0, group 3: the difference between calculated and measured A1c > 1.5.
∗Indicate a p value < 0.05.
Among the 200 patients included in the study, 46 patients had HCC.(29 in nondiabetics (29%) and 17 in diabetics (27%)). As expected, HCC was most commonly seen in patients with hepatitis C and NASH. The clinical features of patients with HCC and without HCC are highlighted in Table 2. In the multivariate model independent predictors of HCC were measured by HbA1c (OR 2.69 955, CI 1.38–5.43, and p value 0.008) and age (OR 1.07 955, CI 1.02–1.13, and p value 0.004). Female gender was a negative predictor for HCC (OR 0.24 95%, CI 0.94–0.637, and p value 0.004). Presence of diabetes was not predictor for HCC.
Table 2.
Patients with HCC and patients without HCC.
| No HCC (n = 154) | with HCC (n = 46) | p value | |
|---|---|---|---|
| Age | 52 ± 10 | 58 ± 6 | 0.0001 |
| Gender (% males) | 61% | 84% | 0.0001 |
| BMI | 28 ± 5.5 | 28 ± 5.9 | NS |
| Etiology of liver disease | |||
| HCV/NASH/alcohol | 36/20/20 | 59/24/11 | 0.001 |
| DM | 26% | 43% | 0.01 |
| Hemoglobin | 11.5 ± 2.1 | 13.0 ± 2.1 | 0.001 |
| Creatinine | 1.35 ± 1.3 | 0.9 ± 0.3 | 0.029 |
| Albumin | 2.9 ± 0.7 | 3.1 ± 0.8 | NS |
| Platelets | 124 ± 98 | 102 ± 70 | NS |
| HBA1c (measured) | 5.1 ± 0.8 | 5.4 ± 1.6 | 0.002 |
To further study the relationship between HbA1c and HCC we studied the 62 patients with diabetes. Seventeen patients (27%) had HCC. In a multivariate analysis, HbA1c is an independent predictor of HCC (OR 2.14 95%, CI 1.23–3.70, and p value 0.007). There is progressive increase in incidence of HCC with HbA1c levels. Lowest incidence of HCC, 13.4%, is seen in patients with HbA1c being less than 5, followed by 28% for HbA1c levels of 5-6, 38% for patients with HbA1c levels of 6-7, and 50% for patients with HbA1c greater than 7 (p value 0.0001).
4. Discussion
Cirrhosis and advanced liver disease have been associated with an increased risk for hyperglycemia and diabetes mellitus. The diagnostic yield of common tests used to define diabetes in the general population differs from those with liver disease. HbA1c is a reliable test to assess chronic glycemia and recommended both for the diagnosis and monitoring of diabetes mellitus; however, HbA1c is neither accurate nor reliable in patients with cirrhosis [11]. Various kinds of anemia are common in liver disease, such as macrocytic anemia [13]. Liver cirrhosis promotes glucose intolerance and diabetes through various mechanisms including insulin resistance and impaired insulin secretion. Sixty to 80% of patients with liver disease have glucose intolerance and 10–15% eventually develop overt diabetes [14].
Patients with cirrhosis are frequently anemic and therefore HbA1c measurements may not provide accurate measure of glycemic control as shown in our analysis. Most common causes of anemia in patients with liver disease are chronic gastrointestinal (GI) blood loss from portal hypertension [15–17] and aplastic anemia secondary to viral hepatitis [18–21]. In addition, antiviral therapy, especially ribavirin, for hepatitis C [22–24] or immunosuppressive agents used to treat autoimmune liver diseases can lead to anemia. In patients with alcoholic liver disease, several factors such as nutritional deficiency, malabsorption, and direct toxic effect of alcohol on bone marrow can cause anemia [25–27].
Hypersplenism secondary to portal hypertension is another important mechanism of anemia in patients with chronic liver disease. Hypersplenism is associated with pancytopenia and is almost universally present in patients with cirrhosis. Hemolytic anemia occurs because of intrasplenic destruction of erythrocytes [28]. Any condition that shortens erythrocyte survival or decreases mean erythrocyte age (e.g., recovery from acute blood loss, hemolytic anemia) will falsely lower HbA1c test results [29]. HbA1c results must be interpreted with caution given the pathological processes, including anemia, increased red cell turnover, and transfusion requirements, that adversely impact HbA1c as a marker of long-term glycemic control.
Our cohorts were decompensated cirrhotic patients needing transplant. The etiology of anemia is most likely related to hypersplenism or hemolysis associated end stage liver disease or chronic GI blood loss from portal hypertensive gastropathy. Since etiology of anemia in patients with decompensated cirrhosis is multifactorial, our retrospective study could not analyze the effects of different types of anemia on HbA1c levels in these patients.
Cirrhosis and advanced liver disease have been associated with an increased risk for hyperglycemia and type 2 diabetes mellitus (T2DM). The diagnostic yield of common tests used to define diabetes and insulin resistance in the general population differs significantly from the one observed in patients with liver disease. HbA1c is a reliable test to assess chronic glycemia and recommended both for the diagnosis and monitoring of T2DM; however, HbA1c is neither accurate nor reliable in patients with cirrhosis. A validation study has not been performed to define its true usefulness in the setting of cirrhosis.
The results of our analysis suggest there is discordance in HbA1c measurements in patients with decompensated cirrhosis. The patients with the highest difference between HbA1c levels had significantly more anemia. This study indicates that HbA1c levels are not a reliable predictor of glycemic control in patients with decompensated cirrhosis, especially in those with severe anemia. To date there are limited numbers of studies dedicated to systematically evaluating the accuracy of HbA1c levels in cirrhotic patients. It is critical for providers and patients to have evidence-based data to guide their management strategies to optimize individualized care. Therefore, we conducted a comprehensive review of 200 unique patients referred to the Methodist University Hospital Transplant Institute with at least one HbA1c level to help provide insight and help guide physicians decision-making in this uncertain area.
Although diabetes is a major public health problem and the fifth leading cause of death in the United States [30] it is less known to be associated with increased risks of developing HCC [31–33]. Although such an association could be related to the underlying chronic liver diseases that preceded the development of HCC [34–37], there are several lines of evidence suggesting that diabetes is in fact an independent risk factor for HCC development [37–39]. There is also evidence reporting that findings of HCC recurrence after liver resection and transplantation are seen among patients with diabetes [40, 41]. It is interesting that in our data set diabetes was not independent predictor of HCC, while HbA1c was a strong independent predictor for risk of HCC. Our findings could be extrapolated to indicate that glycemic control could lead to a lower risk of HCC in patients with diabetes.
People with established diabetes have an increased risk of developing certain types of cancers over the general population [42–47]. Previous publications have reported the association of HCC with other comorbidities, such as diabetes mellitus [48]. In our study, Table 2 provides evidence that diabetes can increase the risk of HCC in patients with chronic liver disease. Evidence that diabetes can increase the risk of HCC was also seen in a publication written by Li et al. in 2015 [49]. Hyperinsulinemia, insulin resistance, and cytokine production have been shown to cause fat accumulation in the hepatocytes leading to oxidative stress and progressive liver injury and fibrosis [50–52]. There has also been evidence published showing a relationship between HbA1c levels, elevated liver enzymes, and hepatic steatosis [43]. Recent advances in understanding of NASH and HCC have revealed that HCC to be the leading cause of obesity-related cancer deaths in middle aged men [53].
We conclude that HbA1c levels should only be evaluated in context with all liver function parameters as well as a red blood count in patients with liver disease. Although the pathophysiologic reasons have still not been confirmed, our data demonstrate that HbA1c levels are not a reliable predictor of glycemic control in patients with decompensated cirrhosis, especially in those with severe anemia. This interference may be due to alterations in erythrocyte lifespan and altered protein metabolism, but further investigations are needed to elucidate the exact cause of the interference in patients with liver disease.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Wanless I. R., Lentz J. S. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12(5):1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z. M., Gramlich T., Matteoni C. A., Boparai N., McCullough A. J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clinical Gastroenterology and Hepatology. 2004;2(3):262–265. doi: 10.1016/S1542-3565(04)00014-X. [DOI] [PubMed] [Google Scholar]
- 3.Adams L. A., Sanderson S., Lindor K. D., Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. Journal of Hepatology. 2005;42(1):132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Yehia B. R., Schranz A. J., Umscheid C. A., Lo Re V., III The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0101554.e101554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning J. D., Szczepaniak L. S., Dobbins R., et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 6.Blendea M. C., Thompson M. J., Malkani S. Diabetes and chronic liver disease: etiology and pitfalls in monitoring. Clinical Diabetes. 2010;28(4):139–144. doi: 10.2337/diaclin.28.4.139. [DOI] [Google Scholar]
- 7.Petrides A. S., De Fronzo R. A. Failure of glucagon to stimulate hepatic glycogenolysis in well-nourished patients with mild cirrhosis. Metabolism. 1994;43(1):85–89. doi: 10.1016/0026-0495(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 8.García-Compean D., Jaquez-Quintana J. O., Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Annals of Hepatology. 2009;8(1):13–20. [PubMed] [Google Scholar]
- 9.Morse E. E. Mechanisms of hemolysis in liver disease. Annals of Clinical and Laboratory Science. 1990;20(3):169–174. [PubMed] [Google Scholar]
- 10.Schnedl W. J., Lahousen T., Krause R., Wallner S. J., Piswanger-Soelkner C., Lipp R. W. Evaluation of conditions associated with glycated hemoglobin values below the reference range. Clinical Laboratory. 2007;53(3-4):179–181. [PubMed] [Google Scholar]
- 11.Lahousen T., Hegenbarth K., Ille R., et al. Determination of glycated hemoglobin in patients with advanced liver disease. World Journal of Gastroenterology. 2004;10(15):2284–2286. doi: 10.3748/wjg.v10.i15.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(supplement 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruyama S., Hirayama C., Yamamoto S., et al. Red blood cell status in alcoholic and non-alcoholic liver disease. Journal of Laboratory and Clinical Medicine. 2001;138(5):332–337. doi: 10.1067/mlc.2001.119106. [DOI] [PubMed] [Google Scholar]
- 14.Cacciatore L., Cozzolino G., Giardina M. G., et al. Abnormalities of glucose metabolism induced by liver cirrhosis and glycosylated hemoglobin levels in chronic liver disease. Diabetes Research. 1988;7(4):185–188. [PubMed] [Google Scholar]
- 15.Garcia-Pagan J. C., De Gottardi A., Bosch J. Review article: the modern management of portal hypertension—primary and secondary prophylaxis of variceal bleeding in cirrhotic patients. Alimentary Pharmacology and Therapeutics. 2008;28(2):178–186. doi: 10.1111/j.1365-2036.2008.03729.x. [DOI] [PubMed] [Google Scholar]
- 16.Abraldes J. G., Bosch J. The treatment of acute variceal bleeding. Journal of Clinical Gastroenterology. 2007;41(supplement 3):S312–S317. doi: 10.1097/mcg.0b013e318150d3dd. [DOI] [PubMed] [Google Scholar]
- 17.Kravetz D. Prevention of recurrent esophageal variceal hemorrhage: review and current recommendations. Journal of Clinical Gastroenterology. 2007;41(3):S318–S322. doi: 10.1097/mcg.0b013e318157f0a7. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Casas R., Garcia-Buey L., Jones E. A., Gisbert J. P., Moreno-Otero R. Systematic review: hepatitis-associated aplastic anaemia—a syndrome associated with abnormal immunological function. Alimentary Pharmacology and Therapeutics. 2009;30(5):436–443. doi: 10.1111/j.1365-2036.2009.04060.x. [DOI] [PubMed] [Google Scholar]
- 19.Young N. S., Calado R. T., Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies J. K., Guinan E. C. An update on the management of severe idiopathic aplastic anaemia in children. British Journal of Haematology. 2007;136(4):549–564. doi: 10.1111/j.1365-2141.2006.06461.x. [DOI] [PubMed] [Google Scholar]
- 21.Young N. S., Scheinberg P., Calado R. T. Aplastic anemia. Current Opinion in Hematology. 2008;15(3):162–168. doi: 10.1097/MOH.0b013e3282fa7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cariani E., Pelizzari A. M., Rodella A., et al. Immune-mediated hepatitis-associated aplastic anemia caused by the emergence of a mutant hepatitis B virus undetectable by standard assays. Journal of Hepatology. 2007;46(4):743–747. doi: 10.1016/j.jhep.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Ong J. P., Younossi Z. M. Managing the hematologic side effects of antiviral therapy for chronic hepatitis C: anemia, neutropenia, and thrombocytopenia. Cleveland Clinic Journal of Medicine. 2004;71(supplement 3):S17–S21. doi: 10.3949/ccjm.71.suppl_3.s17. [DOI] [PubMed] [Google Scholar]
- 24.Reau N., Hadziyannis S. J., Messinger D., Fried M. W., Jensen D. M. Early predictors of anemia in patients with hepatitis C genotype 1 treated with peginterferon α-2a (40KD) plus ribavirin. American Journal of Gastroenterology. 2008;103(8):1981–1988. doi: 10.1111/j.1572-0241.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno Otero R., Cortés J. R. Nutrition and chronic alcohol abuse. Nutricion Hospitalaria. 2008;23(supplement 2):3–7. [PubMed] [Google Scholar]
- 26.Ioannou G. N., Dominitz J. A., Weiss N. S., Heagerty P. J., Kowdley K. V. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126(5):1293–1301. doi: 10.1053/j.gastro.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Lindenbaum J., Roman M. J. Nutritional anemia in alcoholism. The American Journal of Clinical Nutrition. 1980;33(12):2727–2735. doi: 10.1093/ajcn/33.12.2727. [DOI] [PubMed] [Google Scholar]
- 28.Laffi G., Marra F., Tarquini R., Abbate R. Coagulation defects in cirrhosis—old dogmas not yet ready for burial. Journal of Thrombosis and Haemostasis. 2006;4(9):2068–2069. doi: 10.1111/j.1538-7836.2006.02114.x. [DOI] [PubMed] [Google Scholar]
- 29.Bry L., Chen P. C., Sacks D. B. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clinical Chemistry. 2001;47(2):153–163. [PubMed] [Google Scholar]
- 30.Harris M. I., Flegal K. M., Cowie C. C., et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: the Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21(4):518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 31.Hassan M. M., Hwang L.-Y., Hatten C. J., et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36(5):1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 32.Yuan J.-M., Govindarajan S., Arakawa K., Yu M. C. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101(5):1009–1017. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 33.Davila J. A., Morgan R. O., Shaib Y., McGlynn K. A., El-Serag H. B. Diabetes increases the risk of hepatocellular carcinoma in the United States: A Population Based Case Control Study. Gut. 2005;54(4):533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohki T., Tateishi R., Sato T., et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clinical Gastroenterology and Hepatology. 2008;6(4):459–464. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Torisu Y., Ikeda K., Kobayashi M., et al. Diabetes mellitus increases the risk of hepatocarcinogenesis in patients with alcoholic cirrhosis: A Preliminary Report. Hepatology Research. 2007;37(7):517–523. doi: 10.1111/j.1872-034x.2007.00077.x. [DOI] [PubMed] [Google Scholar]
- 36.Veldt B. J., Chen W., Heathcote E. J., et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47(6):1856–1862. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- 37.El-Serag H. B., Hampel H., Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clinical Gastroenterology and Hepatology. 2006;4(3):369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Harrison S. A. Liver disease in patients with diabetes mellitus. Journal of Clinical Gastroenterology. 2006;40(1):68–76. doi: 10.1097/01.mcg.0000190774.91875.d2. [DOI] [PubMed] [Google Scholar]
- 39.Bell D. S. H., Allbright E. The multifaceted associations of hepatobiliary disease and diabetes. Endocrine Practice. 2007;13(3):300–312. doi: 10.4158/EP.13.3.300. [DOI] [PubMed] [Google Scholar]
- 40.Komura T., Mizukoshi E., Kita Y., et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. American Journal of Gastroenterology. 2007;102(9):1939–1946. doi: 10.1111/j.1572-0241.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda Y., Shimada M., Hasegawa H., et al. Prognosis of hepatocellular carcinoma with diabetes mellitus after hepatic resection. Hepatology. 1998;27(6):1567–1571. doi: 10.1002/hep.510270615. [DOI] [PubMed] [Google Scholar]
- 42.Saydah S., Tao M., Imperatore G., Gregg E. GHb level and subsequent mortality among adults in the U.S. Diabetes Care. 2009;32(8):1440–1446. doi: 10.2337/dc09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christman A. L., Lazo M., Clark J. M., Selvin E. Low glycated hemoglobin and liver disease in the U.S. population. Diabetes Care. 2011;34(12):2548–2550. doi: 10.2337/dc11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma H., Xu C., Xu L., Yu C., Miao M., Li Y. Independent association of HbA1c and nonalcoholic fatty liver disease in an elderly Chinese population. BMC Gastroenterology. 2013;13(1, article 3) doi: 10.1186/1471-230x-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X., Wang Y., Luk A. O. Y., et al. Enhancers and attenuators of risk associations of chronic hepatitis B virus infection with hepatocellular carcinoma in type 2 diabetes. Endocrine-Related Cancer. 2013;20(2):161–171. doi: 10.1530/ERC-12-0290. [DOI] [PubMed] [Google Scholar]
- 46.Donadon V., Balbi M., Valent F., Avogaro A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World Journal of Gastroenterology. 2010;16(24):3025–3032. doi: 10.3748/wjg.v16.i24.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X., Ko G. T. C., So W. Y., et al. Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes. 2010;59(5):1254–1260. doi: 10.2337/db09-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai S.-W., Chen P.-C., Liao K.-F., Muo C.-H., Lin C.-C., Sung F.-C. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. The American Journal of Gastroenterology. 2012;107(1):46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 49.Li C.-I., Chen H.-J., Lai H.-C., et al. Hyperglycemia and chronic liver diseases on risk of hepatocellular carcinoma in Chinese patients with type 2 diabetes—National cohort of Taiwan Diabetes Study. International Journal of Cancer. 2015;136(11):2668–2679. doi: 10.1002/ijc.29321. [DOI] [PubMed] [Google Scholar]
- 50.Parekh S., Anania F. A. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132(6):2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 51.Farrell G. C., Larter C. Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2, supplement 1):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 52.Yang X., So W.-Y., Ma R. C. W., Kong A. P. S., Xu G., Chan J. C. N. Diabetes and cancer: the mechanistic implications of epidemiological analyses from the Hong Kong Diabetes Registry. Diabetes/Metabolism Research and Reviews. 2012;28(5):379–387. doi: 10.1002/dmrr.2287. [DOI] [PubMed] [Google Scholar]
- 53.Michelotti G. A., Machado M. V., Diehl A. M. NAFLD, NASH and liver cancer. Nature Reviews Gastroenterology and Hepatology. 2013;10(11):656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]


