Abstract
Consumption of olives (Olea europaea L.) is associated with a low incidence of inflammation-related diseases. Olive fruit is rich in bioactive pentacyclic triterpenoids, mainly maslinic acid. This study, a randomized, double-blind, and placebo-controlled trial, examined the effects of an orally administered maslinic acid supplement, olive fruit extract, on 20 middle-aged and elderly volunteers with mild knee joint pain. Each subject (58 ± 7 years) received either olive fruit extract, containing 50 mg maslinic acid (n = 12), or placebo (n = 8) daily for 12 weeks and evaluated for pain and physical functions as primary outcome measures. Secondary outcome measures included body composition and inflammatory biomarkers in serum. Although both groups exhibited improved pain visual analogue scale score and quality of life after supplementation, symptoms were better in the maslinic acid group than in the placebo group. After 12 weeks, maslinic acid group exhibited significant decrease in body weight and body mass index suggesting that maslinic acid affected the weight of volunteers with mild knee joint pain. Therefore, olive products containing maslinic acid may be useful as a new preventive and therapeutic food ingredient for arthritic diseases. Since this clinical study is a preliminary study, it was not registered in a publicly accessible database.
Keywords: olive fruit extract, maslinic acid, knee joint pain, weight loss, anti-inflammation
Introduction
Aging is associated with an increased risk of problems related to the locomotive organs, such as fractures and joint disorders. The Japanese government has promoted improvement of locomotive ability in the elderly as a means of preventing geriatric and disuse syndromes. In spite of these countermeasures, there seems to be a lack of awareness among the general public that diseases of the locomotive organs can cause a person to become bedridden and to require long-term nursing care.(1) In 2007, the Japanese Orthopaedic Association (JOA) planned to develop simple pretest to assess an individual’s locomotive ability in order to identify those who are at risk and are highly likely to need nursing care. Moreover, in Japan, JOA proposed a new concept of a “locomotive syndrome” which is defined as having difficulty in the ability to walk or lead a normal life owing to a dysfunction in one part or more of the locomotive system such as cartilage, bone, and muscles.(1)
It is a well-accepted fact that the Mediterranean diet, with its high content of olive oil, fiber, fruit, vegetables, and fish, and traditionally tied to areas that grow olives, has beneficial effect on health. Consumption of table olives and olive oil can help prevent inflammation-associated diseases.(2,3) In addition, olive pomace, or the residues after extraction of olive oil made up of olives flesh, skin, and seeds, is a rich source of a variety of pentacyclic triterpenes, which mainly include maslinic acid (MA) (2α, 3β-dihydroxyolean-12-en-28-oic acid) (Fig. 1). In our previous study, we identified MA as the main active component of olive pomace extract (OPE) in addition to the pentacyclic triterpenes such as oleanolic acid, uvaol and erythrodiol,(4) and obtained evidence in vitro and in vivo that MA is an effective anti-inflammation and anti-arthritis component of olives.(5) Recently, MA has been reported to have several biological and therapeutic properties against diseases, including the ability to low hepatocyte membranes’ lipid susceptibility to peroxidation, offering advantages in the resistance to oxidative stress.(5) In addition, MA suppressed osteoclastogenesis and prevented ovariectomy-induced bone loss and MA can improve kidney function in diabetes mellitus patients.(5) Although olive MA is likely to have beneficial effects on inflammation-related diseases, such as swelling and arthritis in mice as shown in our previous study,(5) there are not enough scientific evidence of its anti-inflammatory effect in human clinical trials has not yet been obtained.
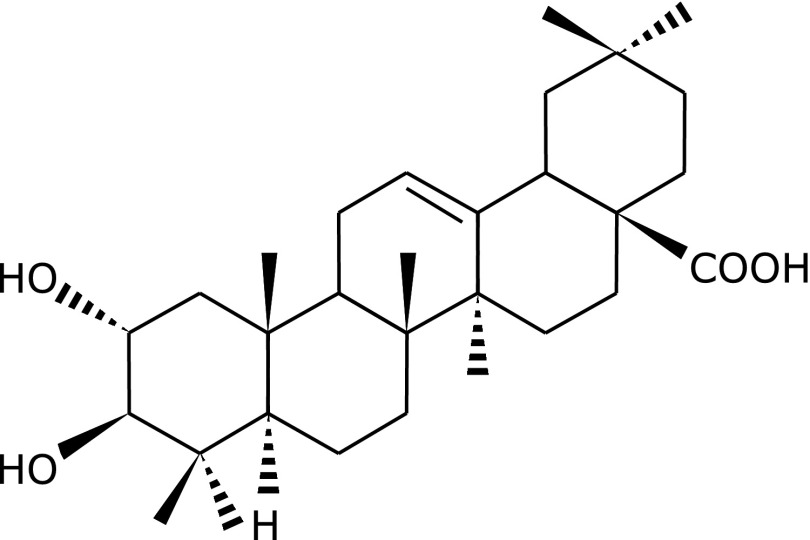
Fig. 1.
Chemical structure of maslinic acid (2α, 3β-dihydroxyolean-12-en-28-oic acid).
In this study, we investigated the effects of an orally administered MA-containing olive fruit extract supplement over a period of 12 weeks in randomized, double-blind, placebo-controlled trial involving middle-aged and elderly subjects with mild knee joint pain, particularly, those who experience knee pain when climbing stairs. In detail, in this study the measurements of visual analogue scale (VAS) to measure spontaneous pain and Short Form-8 (SF-8) questionnaire to measure general aspect of health-related quality of life (QOL) were considered as the primary outcomes. The secondary outcomes included the measurement of body composition and serum biomarkers associated with cartilage metabolism and joint inflammation, as well as safety of MA for daily consumption.
Material and Methods
Participants
Observations for this clinical trial were performed at Sakura Clinic of the Medical Corporation in Tokyo, from May 2014 to October 2014. Ethical approval was obtained from the Ethical Committee at Suda Clinic of Medical Corporation according to the Declaration of Helsinki. All participants gave written informed consent at the time of enrolment.
The inclusion criteria were, (1) male or female participants are at least 40 years old and not more than 75 years old, (2) suffering from knee pain when ascending or descending stairs, and (3) diagnosed with slight degree of knee arthritis after medical examination.
Exclusion criteria were (1) moderate to severe knee osteoarthritis, (2) non-knee osteoarthritis patients with trauma, germ or gout, (3) cardiovascular disease, liver failure and kidney failure, and (4) taking supplements (hyaluronic acid, collagen, glucosamine, and similar supplements in particular) for joint pain for two weeks prior to joining the study, (5) consuming products that contain MA such as extra virgin olive oil, table olives, (6) participating in or has participated in other clinical trials in the past 3 months. Participants were allowed to take drugs not related to knee pain relief, and were instructed not to make any changes in their existing (background) exercise and diet during the study. After recruitment, the participants were randomly distributed into two groups.
Test supplement for improvement of joint support
Subjects took either 3 placebo capsules or 50 mg of MA (500 mg as olive fruit extract) capsules of the same shape and color once a day, depending on the group they belong to. Every day, each subject recorded whether (or not) he/she took the capsule during breakfast, their general physical condition, and the degree of knee pain. The medical staff checked the records once a month and gave the participants comments to help motivate them in following the required schedule of taking the supplement and recording the relevant data.
Olive fruit extract was analyzed and was found to contain 10.7% MA, 57% gamma-cyclodextrin, 22.8% protein, 1.5% fat, 2.5% ash, 3.4% moisture, and other minor components such as oleanolic acid. Test capsules contain olive fruit extract, cornstarch, and calcium stearate with each capsule containing either 0 or 16.7 mg of MA. The placebo capsules on the other hand contained cornstarch instead of olive fruit extract. The MA content of the test capsules was determined using HPLC as described previously.(5) Briefly, HPLC separation was performed using Shimadzu Prominence HPLC system (Shimadzu Corporation, Kyoto, Japan) equipped with Inertsil ODS-3 column (150 mm × 4.6 mm, 5.6 µm; GL sciences, Inc. Tokyo, Japan). The mobile phase consisted of acetonitrile/methanol/water/phosphoric acid (500:400:100:0.5; v/v/v/v) at a flow rate of 1 ml/min for 15 min. The column was set at 30°C. Typical retention time of MA is about 4.8 min.
Study design
This study is a randomized, double-blind, placebo-controlled trial with two groups. A total of 20 selected participants with a mild degree of knee arthritis were recruited then employed a third party to randomly assign each participant into one of the two groups. The participants were randomly distributed into one of the following groups: olive fruit extract intake group (50 mg/day of MA) (n = 12) and placebo intake group (n = 8). Test capsules and placebo capsules were also administered during 12-week period.
Outcome assessment
The main recorded parameters were a VAS to measure spontaneous pain (from 0 = no pain to 100 = maximum pain) and the SF-8 questionnaire to measure the general aspect of health-related QOL.(6,7) VAS and SF-8 were performed before and after the 8-week and 12-week period. Secondary outcome measures were obtained at 0 and 12 weeks using body composition and blood parameters such as high-sensitivity C-reactive protein (hs-CRP) for inflammation,(8) and N-propeptide of collagen IIA (PIIANP) and collagen type-II cleavage (C2C) for cartilage metabolism.(9,10)
General clinical test
Blood samples were collected from the participants at the imeQ Inc. (Tokyo, Japan) after overnight fasting for measurement of the following in peripheral blood: WBC, RBC, Hb, Ht, PLT, total protein, albumin, urea nitrogen, creatinine, AST, ALT, LDH, ALP, γ-GTP, Na, Cl, and K. Measurement of the hs-CRP, PIIANP, and C2C was performed by Biomarker Science Co., Ltd (Kyoto, Japan). Measurement of the urine samples (specific gravity, pH, protein, sugar, urobilinogen, and occult blood) was performed by imeQ Inc. (Tokyo, Japan).
Baseline characteristics
We recorded the participants’ age, height, body weight, BMI, VAS for pain, and SF-8 for health-related QOL, and considered them as the baseline characteristics (Table 1).
Table 1.
Demographic data and baseline characteristics of the subjects
| Characteristics | MA (n = 12) | Placebo (n = 8) |
|---|---|---|
| Age (year) | 57.4 ± 7.5 | 59.1 ± 5.5 |
| Height (cm) | 156.4 ± 5.3 | 154.0 ± 5.5 |
| Body weight (kg) | 55.2 ± 9.2 | 51.2 ± 5.2 |
| Body mass index (kg/m2) | 22.6 ± 3.5 | 21.6 ± 1.8 |
| Visual analogue scale (mm) | 23.2 ± 8.3 | 25.5 ± 9.1 |
| Short Form-8 | ||
| Physical components | 43.1 ± 2.7 | 43.0 ± 4.3 |
| Mental components | 45.8 ± 4.0* | 49.8 ± 2.4 |
Values are expressed as means ± SD. Mean values with their SD, *p<0.05 vs placebo group. MA, maslinic acid.
Safety
Information on all the adverse events, any adverse clinical symptoms, syndrome, or illness that occurs or worsens were collected. In addition, abnormal value and worsened value for medical examination of clinical studies were considered adverse events. All the data on adverse events that occurred during the study were collected after test supplementation.
Statistical analysis
Data analysis was performed after the 20 subjects have successfully completed the study. Data are expressed as mean ± SE. A paired t test was used to compare data on subjects within a group (between before and after treatment). Student’s t test was used to compare between MA group and placebo groups. The level of significance considered was 5% for both sides.
Results
Baseline characteristics and compliance with the protocol
In order to obtain the data and verify the effects of olive fruit extract intake in humans for the first time, participants were allocated either as MA intake group (12 subjects) or placebo intake group (8 subjects).
All the recruited 20 participants of the study completed all the aspects of the study for 12 weeks. The participants’ baseline characteristics for both groups are shown in Table 1. There was no request from any of the participants for dropping or withdrawal from the study, protocol deviations, and other reasons.
Endpoints
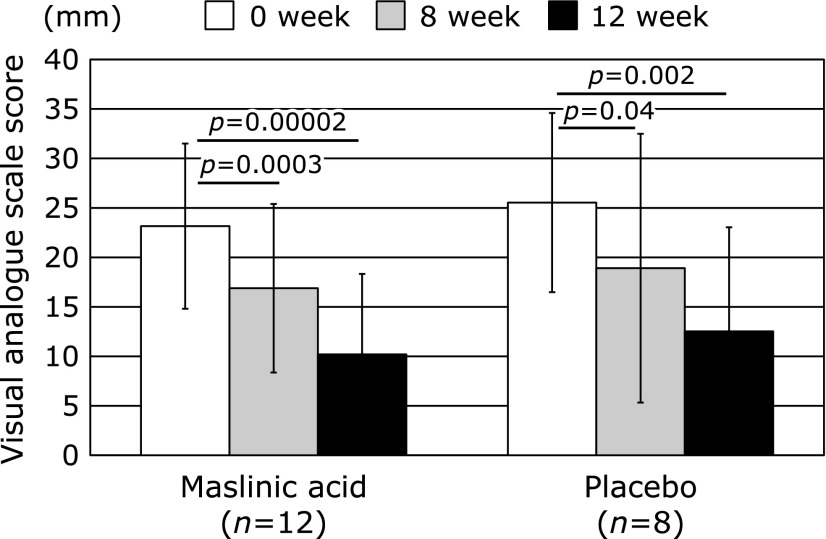
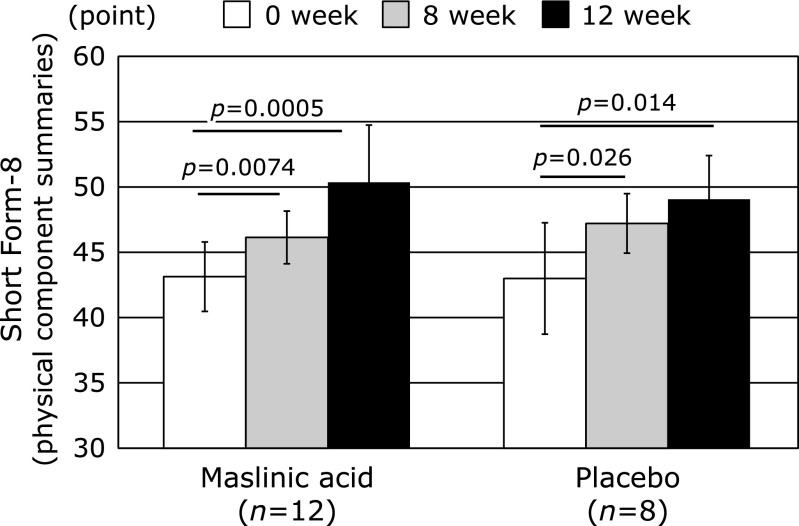
The primary endpoint as VAS score and SF-8 are shown in Fig. 2 and 3. As shown in Fig. 2, although both the MA and placebo groups exhibited improved pain scores based on the VAS of the primary outcome at 8th and 12th week after treatment compared with those of baseline, the MA group exhibited slightly more improved symptoms, as shown by the improvement rate (%) and p value, compared to those of the placebo group at the end of the study (↓56% vs ↓51%, p = 0.00002 vs p = 0.002, respectively). VAS pain did not differ significantly between groups during the 12-week period (MA: 23.2 ± 8.3 to 10.2 ± 8.1 points, placebo: 25.5 ± 9.1 to 12.5 ± 10.5 points, p = 0.65) (Fig. 2). In addition, as shown in Fig. 3, although both the MA and placebo groups also exhibited improved QOL as indicated by the physical component summaries on the SF-8 of the primary outcome at 8th and 12th week after treatment compared with those of baseline, the MA group exhibited slightly more improved symptoms, improvement rate (%) and p value, compared to those of the placebo group at the end of the study (↑117% vs ↑115%, p = 0.0005 vs p = 0.014, respectively) (Fig. 3). The QOL as indicated by the physical component summaries did not differ significantly between groups during the 12-week study (MA: 43.1 ± 2.7 to 50.4 ± 4.4 points, placebo: 43.0 ± 4.3 to 49.1 ± 3.3 points, p = 0.63). On the other hand, both the MA and placebo groups exhibited improved QOL based on the mental component summaries on the SF-8 of the primary outcome at 8th and 12th week after treatment compared with those of baseline, with the MA group exhibiting slightly more improved symptoms compared to those of the placebo group at the end of study (↑109% vs ↑106%, respectively, data not shown). As shown in Table 1, since the mental component score on SF-8 comparison between two groups at baseline were statistically significant (MA: 45.8 ± 4.0, placebo: 49.8 ± 2.4, p = 0.022), this indicator is as a reference value. QOL of mental component did not differ significantly between groups during the 12 weeks study (MA: 45.8 ± 4.0 to 49.7 ± 4.2 points, placebo: 49.8 ± 2.8 to 53.0 ± 2.6 points, p = 0.73).
Fig. 2.
VAS joint pain in maslinic acid group compared to the placebo group during the 12-week study. The p values at week 8 and 12 in the graph represent paired t test contrast within groups compared to week 0.
Fig. 3.
SF-8 physical component in maslinic acid group compared to the placebo group during the 12-week study. The p values at week 8 and 12 in the graph represent paired t test contrast within groups compared to week 0.
The secondary endpoints as body composition such as body weight, body mass index (BMI), and serum biochemical markers are shown in Table 2 and 3. The MA group exhibited significantly decreased body weight and BMI in the subjects with mild joint pain at week 12 from before to after supplementation (body weight: p = 0.019 vs p = 0.109, BMI: p = 0.019 vs 0.095, respectively), which was not observed in the placebo group (Table 2). Both body weight and BMI did not differ significantly between groups (Table 2). Furthermore, we examined that hs-CRP and other biochemical markers (PIIANP and C2C) of cartilage turnover in serum and the results are presented in Table 3. hs-CRP (percentage change of before and after: MA ↓15.2% vs placebo ↑11.8%), PIIANP (percentage change of before and after: MA ↑6% vs placebo ↓1.3%) and C2C (percentage change of before and after: MA ↑8.1% vs placebo ↑2.0%) values were not significant both from before to after supplementation of each group and between groups (Table 3). In addition, C2C/PIIANP ratio of each group was not changed after treatment compared with those of baseline (MA: 0.13 ± 0.03 to 0.13 ± 0.04, placebo: 0.11 ± 0.02 to 0.11 ± 0.03) (Table 3).
Table 2.
Body compositions before and after 12 week of daily ingestion of maslinic acid or placebo
| MA (n = 12) |
Placebo (n = 8) |
||||
|---|---|---|---|---|---|
| 0 week | 12 week | 0 week | 12 week | ||
| Body weight (kg) | 55.2 ± 9.2 | 54.7 ± 9.4* | 51.2 ± 5.2 | 50.4 ± 5.2 | |
| BMI (kg/m2) | 22.6 ± 3.5 | 22.3 ± 3.6* | 21.6 ± 1.8 | 21.3 ± 1.8 | |
Mean values with their SD, *p<0.05 vs 0 week. BMI, Body mass index; MA, maslinic acid.
Table 3.
Serum hs-CRP and other biochemical markers of cartilage turnover before and after 12 week of daily ingestion of maslinic acid or placebo
| MA (n = 12) |
Placebo (n = 8) |
||||
|---|---|---|---|---|---|
| 0 week | 12 week | 0 week | 12 week | ||
| hs-CRP (mg/L) | 0.061 ± 0.07 | 0.052 ± 0.07 | 0.038 ± 0.03 | 0.043 ± 0.043 | |
| PIIANP (ng/ml) | 1,660 ± 402 | 1,759 ± 399 | 1,877 ± 314 | 1,852 ± 323 | |
| C2C (ng/ml) | 202 ± 29 | 218 ± 18 | 197 ± 16 | 201 ± 42 | |
| C2C/PIIANP | 0.13 ± 0.03 | 0.13 ± 0.04 | 0.11 ± 0.02 | 0.11 ± 0.03 | |
Mean values with their SD. MA, maslinic acid.
Adverse events
At week 12, there were no statistically significant changes observed on the baseline data in terms of the levels of WBC, RBC, Hb, Ht, PLT, total protein, urea nitrogen, creatinine, AST, ALT, LDH, γ-GTP, and K in MA and placebo groups (Table 4). The concentrations of albumin and Na at week 12 were significantly higher than at baseline in both MA and placebo groups. On the other hand, those of concentration of ALP and Cl in MA group and/or placebo group at week 12 were significantly lower than at baseline. There were however, no abnormal changes reported by the participants that were not related to the MA intake. Furthermore, no adverse effect due to the intake of MA was reported by any of the groups. One objective symptom was reported as adverse events in MA group during the study, but in this case, it was found to be not related to MA supplementation.
Table 4.
Blood parameters before and after 12 week of daily ingestion of maslinic acid or placebo
| MA (n = 12) |
Placebo (n = 8) |
||||
|---|---|---|---|---|---|
| 0 week | 12 week | 0 week | 12 week | ||
| WBC (104/µl) | 552.0 ± 867.7 | 5,566.7 ± 678.7 | 4,575.0 ± 341.2 | 4,500.0 ± 537.2 | |
| RBC (104/µl) | 452.7 ± 52.7 | 449.4 ± 49.0 | 425.1 ± 38.0 | 427.6 ± 42.6 | |
| Hb (g/dl) | 13.3 ± 1.3 | 13.5 ± 1.5 | 12.5 ± 1.4 | 12.8 ± 1.3 | |
| Ht (%) | 40.7 ± 3.8 | 40.4 ± 3.6 | 38.6 ± 4.2 | 39.0 ± 3.8 | |
| PLT (104/µl) | 22.6 ± 4.9 | 23.0 ± 5.2 | 23.3 ± 3.7 | 23.2 ± 4.0 | |
| Total protein (g/dl) | 7.3 ± 0.3 | 7.2 ± 0.3 | 7.7 ± 0.3 | 7.6 ± 0.5 | |
| Albumin (g/dl) | 4.4 ± 0.2 | 4.5 ± 0.2** | 4.5 ± 0.3 | 4.7 ± 0.3 | |
| Urea nitrogen (mg/dl) | 16.0 ± 3.9 | 14.2 ± 3.1 | 15.0 ± 2.8 | 14.8 ± 2.4 | |
| Creatinine (mg/dl) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | |
| AST(GOT) (U/L) | 19.5 ± 4.1 | 18.9 ± 3.2 | 20.4 ± 3.9 | 19.9 ± 5.6 | |
| ALT(GPT) (U/L) | 15.7 ± 4.1 | 15.6 ± 4.1 | 17.8 ± 4.5 | 16.9 ± 5.1 | |
| LDH (U/L) | 181.2 ± 17.4 | 180.7 ± 19.1 | 183.1 ± 29.5 | 193.9 ± 62.8 | |
| ALP (U/L) | 241.3 ± 56.0 | 228.4 ± 56.1* | 216.1 ± 59.8 | 203.4 ± 60.7** | |
| γ-GTP (U/L) | 24.4 ± 16.7 | 24.2 ± 13.6 | 18.6 ± 6.4 | 17.9 ± 6.8 | |
| Na (mEq/L) | 142.5 ± 1.5 | 141.9 ± 1.1 | 141.3 ± 1.3 | 142.6 ± 2.0* | |
| Cl (mEq/L) | 104.4 ± 1.3 | 103.5 ± 1.6* | 102.5 ± 1.4 | 103.1 ± 1.0 | |
| K (mEq/L) | 4.0 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.3 ± 0.4 | |
Mean values with their SD, *p<0.05 vs 0 week, **p<0.01 vs 0 week. MA, maslinic acid.
Discussion
This is the first report on a randomized, double-blind, placebo-controlled, with two groups on the effects of intake of olive fruit extract containing MA on middle-aged and elderly subjects with mild knee joint pain.
In the primary outcome, although both the MA and placebo groups exhibited significantly improved VAS pain scores and QOL after supplementation, symptoms were better in the MA group than in the placebo group (Fig. 2 and 3). In the secondary outcome, the MA group alone exhibited significant decrease in body weight and BMI at week 12 compared to the baseline level (Table 2). Liu et al.(11) has reported that oral administration of MA can reduce the epididymal fat pads weight of KK-Ay mice, and similarly, in this human clinical study, the participants belonging to the MA group also exhibited significant decreased body weight (Table 2). From these findings, we can also deduce that MA intake has anti-obesity effect that likely reduced the weight putting burden of knee arthritis.
It has been reported that MA is one of the pentacyclic triterpenes, besides other pentacyclic triterpenes such as oleanolic acid, uvaol, and erythrodiol, and main active component of olive fruit.(4) In our previous study, we isolated MA from olive pomace, a by-product of olive oil production, which can alleviate LPS-induced TNF-α production and regulate the genes involved in inflammatory responses in RAW 264.7 cells. In addition, MA, based on other studies, regulates the inflammation-related NF-κB signaling pathway.(12,13) Furthermore, MA exerted anti-inflammatory and anti-arthritis effects as shown by the suppression of paw edema, inflammatory cells, and destruction of synovium in knee joints, as well as improvement of arthritis score.(5) In this clinical study, although the differences in serum hs-CRP level were not statistically significant between groups at 12 weeks of treatment, the decrease in the level of serum hs-CRP of the MA intake group was greater than the those of the placebo group’s (percentage change of before and after treatment: MA ↓15.2% vs placebo ↑11.8%) (Table 3). Based on these findings, MA is most likely to improve joint condition and QOL by reducing pain and inflammatory response in this study.
Furthermore, it has been reported that MA suppresses osteoclastogenesis and prevents bone loss.(12) It has been reported that cartilage turnover is slow in elderly patients and in individuals at the onset of osteoarthritis.(14,15) The principal components of articular cartilage are collagen type II and proteoglycan (aggrecan), which are estimated to be have a half-life of over a one hundred years and approximately twenty years, respectively.(16) Accumulation of advanced glycation end products (AGEs), which are known to adversely affect cartilage turnover and mechanical properties, is the mechanism by which aging contributes to the development of osteoarthritis.(15) In addition, AGEs are known to induce the production of several inflammatory cytokines.(17) Ohno et al.(18) have reported that mangosteen pericarp extract inhibits the formation of AGEs such as pentosidine. Given this information, it is considered likely that MA is an AGEs inhibitor and plays an important role in preventing joint pain pathogenesis. In this clinical study, although the differences in serum PIIANP level were not statistically significant between groups at 12 week of supplementation compared with those of baseline, at the end of the study, the average level of serum PIIANP of the MA intake group was higher than the values at the beginning of the study (before supplementation) compared to that of that placebo group’s (percentage change of before and after: MA ↑6% vs placebo ↓1.3%) (Table 3). And yet at the same time, the average levels of serum C2C before and after MA or placebo intake of each group (MA vs Placebo) were both increased (percentage change of before and. after treatment: MA ↑8.1% vs placebo ↑2.0%), but not are not significantly different. Furthermore, the ratio of C2C/PIIANP (the ratio of degradation and synthesis of joint cartilage) of both groups (MA vs Placebo) were the same after treatment compared with those of baseline (MA: 0.13 ± 0.03 to 0.13 ± 0.04, placebo: 0.11 ± 0.02 to 0.11 ± 0.03) (Table 3). Therefore, we consider that olive fruit MA likely regulates and activates cartilage turnover but further investigation with long-term use of MA will be needed to confirm the direct effects of MA on joint cartilage as well as determine the effective dose when consumed daily. Based on further studies of the inhibitory effects of MA on the formation of AGEs, it is possible that MA is effective against various age-related diseases such as diabetic complications and atherosclerosis as well as osteoarthritis.
MA has been shown to act as a growth factor of protein turnover and synthesis rates in liver and white muscles when added to a standard trout diet.(19,20) We consider that MA is likely to be an effective therapy against sarcopenia or loss of skeletal muscle mass and function.(21) From these discussions, MA in olive fruit extract is a potential food ingredient that can support in the eradication of “locomotive syndrome” defined by the JOA such as osteoarthritis and muscle deterioration.
Oleanolic acid (3β-hydroxyolean-12-en-28-oic acid) is a pentacyclic triterpene from the oleanane family that is also present in olive pomace that modulate different signaling pathways and showing a wide range of pharmacological activities against inflammation, cancer, and cardiovascular diseases.(22,23) Since the olive fruit extract used in this clinical study contains a small amount of oleanolic acid, approximately 2.5%, amelioration of pain and improved QOL might be observed. However, this human clinical study has a relatively small number of healthy subjects and there is a bias in the number of people [olive fruit extract (n = 12), placebo (n = 8)] so that the verification of the beneficial effects and obtaining of the data on MA-containing olive fruit extract intake in humans will need larger cohort of osteoarthritic participants.
In the Mediterranean region, where diets are olive-based, the average intake of triterpenes, including MA, could reach 400 mg/kg/day.(24) This seemingly high concentration of MA has no cytotoxic effect, as demonstrated in our previous study, on the oral toxicity of MA using rat, which confirmed that administration of MA is considered safe.(5) Therefore, MA is safe and non-toxic for daily consumption. Regular intake of MA-containing table olives and olive oil, which also contain other triterpenes and other phenolic compounds, has likely contributed to the high adult life expectancy and low chronic disease rates of people in the Mediterranean region. Our findings further suggest that MA in olive fruit and MA derived from olive products have potential as a food fortification for “anti-locomotive syndrome” in today’s aging Japanese society.
Conclusions
In this study, we investigated the effects of an orally administered 500 mg olive fruit extract/day (50 mg as MA) on middle-aged and elderly healthy human volunteers/subjects with mild knee joint pain, particularly, those who experience or suffer from pain in their knees when climbing stairs, done over a period of 12 weeks in randomized, double-blind, placebo-controlled trial. Our results demonstrate that MA in olive fruit is likely to improve joint pain and physical components of QOL by promoting weight loss, having an anti-inflammation effects, as well as reduction in the pain and QOL burden in humans with mild knee joint pain.
Author’s Contributions
Satoshi Fukumitsu and Myra O. Villareal co-write draft of the manuscript. Kazuhiko Aida helped the clinical setting of olive fruit extract capsule. Akihiro Hino participated in the design of the study. Noriya Hori analyzed clinical data. Hiroko Isoda and Yuji Naito provided overall supervision. All authors read and approved the final manuscript.
Abbreviations
- AGE
advanced glycation end product
- JOA
Japanese Orthopaedic Association
- MA
maslinic acid
- OPE
olive pomace extract
- QOL
quality of life
- SF-8
short Form 8
- VAS
visual analogue scale
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nakamura K. A “super-aged” society and the “locomotive syndrome”. J Orthop Sci. 2008;13:1–2. doi: 10.1007/s00776-007-1202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keys A. Mediterranean diet and public health: personal reflections. Am J Clin Nutr. 1995;61 (6 Suppl):1321S–1323S. doi: 10.1093/ajcn/61.6.1321S. [DOI] [PubMed] [Google Scholar]
- 3.Rosillo MA, Sánchez-Hidalgo M, Sánchez-Fidalgo S, Aparicio-Soto M, Villegas I, Alarcón-de-la-Lastra C. Dietary extra-virgin olive oil prevents inflammatory response and cartilage matrix degradation in murine collagen-induced arthritis. Eur J Nutr. 2016;55:315–325. doi: 10.1007/s00394-015-0850-0. [DOI] [PubMed] [Google Scholar]
- 4.Márquez Martin A, de la Puerta Vázquez R, Fernández-Arche A, Ruiz-Gutiérrez V. Supressive effect of maslinic acid from pomace olive oil on oxidative stress and cytokine production in stimulated murine macrophages. Free Radic Res. 2006;40:295–302. doi: 10.1080/10715760500467935. [DOI] [PubMed] [Google Scholar]
- 5.Fukumitsu S, Villareal MO, Fujitsuka T, Aida K, Isoda H. Anti-inflammatory and anti-arthritic effects of pentacyclic triterpenoids maslinic acid through NF-κB inactivation. Mol Nutr Food Res. 2016;60:399–409. doi: 10.1002/mnfr.201500465. [DOI] [PubMed] [Google Scholar]
- 6.Torrance GW, Feeny D, Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med Decis Making. 2001;21:329–334. doi: 10.1177/0272989X0102100408. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa H, Itokazu M, Ito Y, Matsumoto K, Takigami I. Quality of life evaluated by Short Form-8 in patients with rheumatoid arthritis who were receiving infusion of infliximab. Mod Rheumatol. 2009;19:27–32. doi: 10.1007/s10165-008-0113-5. [DOI] [PubMed] [Google Scholar]
- 8.Pearle AD, Scanzello CR, George S, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:516–523. doi: 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau JC, Zhu Y, Miossec P, et al. Serum levels of type IIA procollagen amino terminal propeptide (PIIANP) are decreased in patients with knee osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2004;12:440–447. doi: 10.1016/j.joca.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Dejica VM, Mort JS, Laverty S, et al. Increased type II collagen cleavage by cathepsin K and collagenase activities with aging and osteoarthritis in human articular cartilage. Arthritis Res Ther. 2012;14:R113. doi: 10.1186/ar3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Sun H, Duan W, Mu D, Zhang L. Maslinic acid reduces blood glucose in KK-Ay mice. Biol Pharm Bull. 2007;30:2075–2078. doi: 10.1248/bpb.30.2075. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Yang Z, Li Z, et al. Maslinic acid suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by regulating RANKL-mediated NF-κB and MAPK signaling pathways. J Bone Miner Res. 2011;26:644–656. doi: 10.1002/jbmr.242. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Yang Z, Zhai C, et al. Maslinic acid potentiates the anti-tumor activity of tumor necrosis factor alpha by inhibiting NF-kappaB signaling pathway. Mol Cancer. 2010;9:73. doi: 10.1186/1476-4598-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeGroot J, Verzijl N, Bank RA, Lafeber FP, Bijlsma JW, TeKoppele JM. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.DeGroot J, Verzijl N, Wenting-van Wijk MJ, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50:1207–1215. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- 16.Verzijl N, DeGroot J, Bank RA, et al. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 17.Nagai R, Shirakawa J, Fujiwara Y, et al. Detection of AGEs as markers for carbohydrate metabolism and protein denaturation. J Clin Biochem Nutr. 2014;55:1–6. doi: 10.3164/jcbn.13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohno R, Moroishi N, Sugawa H, et al. Mangosteen pericarp extract inhibits the formation of pentosidine and ameliorates skin elasticity. J Clin Biochem Nutr. 2015;57:27–32. doi: 10.3164/jcbn.15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Navarro M, Peragón J, Amores V, De La Higuera M, Lupiáñez JA. Maslinic acid added to the diet increases growth and protein-turnover rates in the white muscle of rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:158–167. doi: 10.1016/j.cbpc.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Navarro M, Peragón J, Esteban FJ, de la, Lupiáñez JA. Maslinic acid as a feed additive to stimulate growth and hepatic protein-turnover rates in rainbow trout (Onchorhynchus mykiss) Comp Biochem Physiol C Toxicol Pharmacol. 2006;144:130–140. doi: 10.1016/j.cbpc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Bosaeus I, Rothenberg E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc Nutr Soc. 2016;75:174–180. doi: 10.1017/S002966511500422X. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Rodriguez R. Oleanolic acid and related triterpenoids from olives on vascular function: molecular mechanisms and therapeutic perspectives. Cur Med Chem. 2015;22:1414–1425. doi: 10.2174/0929867322666141212122921. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Quesada C, López-Biedma A, Gaforio JJ. Oleanolic acid, a compound present in grapes and olives, protects against genotoxicity in human mammary epithelial cells. Molecules. 2015;20:13670–13688. doi: 10.3390/molecules200813670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babalola TI, Shode OF. Ubiquitous ursolic acid: a potential pentacyclic triterpene natural product. J Pharm Phytochem. 2013;2:214–222. [Google Scholar]