Abstract
Small intestinal mucosal injury caused by low-dose aspirin is a common cause of obscure gastrointestinal bleeding. We aimed to investigate the protective effects and optimal dose of rebamipide for low-dose aspirin-induced gastrointestinal mucosal injury. In this prospective randomized trial, 45 healthy volunteers (aged 20–65 years) were included and divided into three groups. The groups received enteric-coated aspirin 100 mg (low-dose aspirin) plus omeprazole 10 mg (Group A: proton pump inhibitor group), low-dose aspirin plus rebamipide 300 mg (Group B: standard-dose group), or low-dose aspirin plus rebamipide 900 mg (Group C: high-dose group). Esophagogastroduodenoscopy and video capsule endoscopy were performed, and the fecal occult blood reaction and fecal calprotectin levels were measured before and two weeks after drug administration. Although the fecal calprotectin levels increased significantly in Group A, they did not increase in Groups B and C. The esophagogastroduodenoscopic and video capsule endoscopic findings and the fecal occult blood test findings did not differ significantly among the three groups. In conclusion, standard-dose rebamipide is sufficient for preventing mucosal injury of the small intestine induced by low-dose aspirin, indicating that high-dose rebamipide is not necessary.
Keywords: rebamipide, low-dose aspirin, gastrointestinal mucosal injury, fecal calprotectin, capsule endoscopy
Introduction
Long-term use of low-dose aspirin (LDA; 100 mg) is associated with the development of peptic ulcers, and deaths due to these peptic ulcers have been reported.(1–6) For prevention of LDA-induced gastroduodenal mucosal injury, proton pump inhibitors (PPIs) are the first-choice drug according to several guidelines.(7–11) However, gastric acid suppressants, like proton pump inhibitors and histamine H2-receptor antagonists, do not prevent small intestinal mucosal injury because there is no acid in the intestine. In recent years, the gastrointestinal mucosal injury induced by LDA has attracted attention not only in the upper gastrointestinal tract but also in the lower gastrointestinal tract, and Lanas et al.(12) reported that LDA was associated with increased risk of both upper and lower gastrointestinal bleeding.
Several gastric mucoprotective drugs other than PPIs have been found to prevent LDA-induced small intestinal mucosal injury to some degree;(13–18) however, one study found no preventive effect of such drugs on LDA-induced small intestinal mucosal injury.(19) Of note, in the aforementioned reports, the dosages of gastric mucoprotective drugs were those recommended for the treatment of gastric/duodenal ulcers, the so-called “standard dosage”. However, these recommended dosages have not been firmly established to be adequate for the small intestine as well. It can be speculated that high-dose gastric mucoprotective drugs are more effective for the small intestine, although no trials on whether high doses of gastric mucoprotective drugs are necessary for preventing small intestinal mucosal injury have yet been reported.
Rebamipide, 2-(4-chlorobenzylamino)-3-[2(1H)-quinolinone-4-yl] propionic acid (Otsuka Pharmaceutical Co., Tokyo, Japan) is a gastric mucoprotective drug that stimulates the production of prostaglandins and epidermal growth factor, thereby preventing Helicobacter pylori-elicited neutrophil-induced mucosal injury and decreasing free radical levels.(20–22) Clinically, the efficacy against non-steroidal anti-inflammatory drug (NSAID)-induced gastric mucosal injury has been reported to be comparable to that of famotidine (10 mg two times a day), a histamine H2-receptor antagonist,(23) with an effective dose of 300 mg (standard dose) of rebamipide for preventing LDA-induced gastroduodenal mucosal injury.(20) A few clinical trials have investigated the effects of rebamipide (300 mg/day) plus PPIs vs placebo plus PPIs. There were a few reports on the protective effects of rebamipide against NSAID-induced small intestinal mucosal injury.(17,24) However, the required dosage of rebamipide for effective prevention of LDA-induced small intestinal mucosal injury is unclear, with 900 mg being the maximum safe dose of rebamipide, as confirmed in a phase I study.(25) In addition, Wallace et al.(26) reported that PPIs exacerbate NSAID-induced small intestinal mucosal injury. There are currently no reported studies comparing rebamipide and PPIs directly for the prevention of LDA-induced gastrointestinal mucosal injury.
Based on the above reports, we devised this clinical study with the aim of comparing the efficacy in three groups (300 mg of rebamipide, 900 mg of rebamipide, and 10 mg of omeprazole) for the prevention of LDA-induced mucosal injury from the esophagus to the small intestine.
Methods
Subjects
The study was conducted prospectively at Osaka Medical College Hospital. Subjects eligible for inclusion were healthy adults who: 1) were aged between 20 and 65 years at the time of providing consent, 2) had freely provided informed consent based on their full understanding of the study protocol, and 3) had no history of medication use during the month prior to enrolment in the study. The exclusion criteria were as follows: 1) a history of peptic ulcer or gastrointestinal bleeding; 2) significant hepatic, renal, heart, or respiratory disease; 3) a history of gastrointestinal surgery other than appendectomy; 4) oral use or planned oral use of a drug other than an anti-ulcer drug (H2 receptor antagonists, misoprostol, gastrointestinal kinetic agents, etc.), which may affect healing of small intestinal injury; 5) alcohol or chemical dependency; 6) a history of intestinal obstruction or suspected gastrointestinal obstruction on other tests; 7) refusal to consent to the surgery that would be required if the capsule endoscope was retained in the body; and 8) determination by the investigator, at his discretion, that a subject was ineligible for participation in the study for any other reason. All criteria were met by 45 subjects who were included in the study. All subjects received oral and written explanations of the study prior to participation and provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki of 1975 (as revised in 1983), and the protocol was approved by the Ethics Review Committee of Osaka Medical College (No. 0777; May 10, 2010) and registered in UMIN (000013845).
Study design
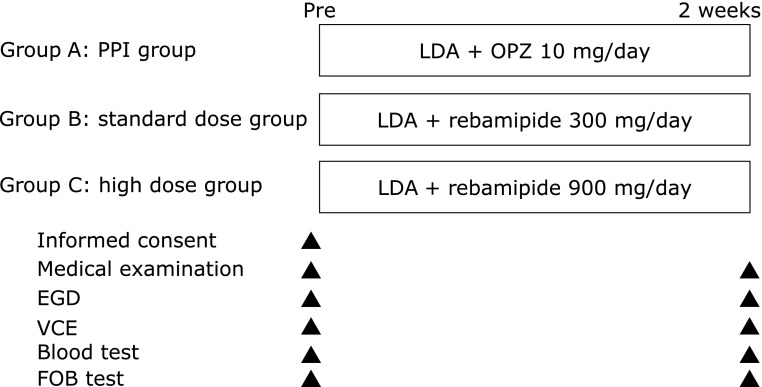
The present study was a prospective, randomized trial comparing the effects of a PPI and two dosages of a gastric mucoprotective drug on the esophagus, stomach, duodenum, small intestine, and colon. The subjects (n = 45) were divided into three groups (Groups A, B, and C; n = 15 each) and instructed to take the study drugs as directed for two weeks. Group A [the control (PPI) group] received low-dose (100 mg) enteric-coated aspirin (LDA) once a day plus omeprazole 10 mg once a day, Group B (standard-dose) received LDA plus rebamipide 300 mg (100 mg three times a day), and Group C (high-dose) received LDA plus rebamipide 900 mg (300 mg three times daily). The dosage of aspirin was determined based on the dosage recommended for antithrombotic activity in cardiovascular and cerebrovascular diseases.(1–3) In Japan, the dosage of a PPI used for the prevention of LDA-induced gastric ulcers is half the dosage used for the treatment of gastric ulcers. On this basis, we determined that the appropriate dosage of omeprazole should be 10 mg/day. Both esophagogastroduodenoscopy (EGD) and video capsule endoscopy (VCE) were performed before and two weeks after drug administration. In addition, we measured the fecal occult blood reactions and fecal calprotectin levels of the subjects before and two weeks after to assess the level of inflammation in the lower gastrointestinal mucosa (Fig. 1).
Fig. 1.
Study design. Forty-five subjects were divided into three groups (Groups A, B, and C; n = 15 each) and randomized to receive either enteric-coated aspirin 100 mg (low-dose aspirin; LDA) plus omeprazole (OPZ) 10 mg (Group A), LDA plus rebamipide 300 mg (Group B), or LDA plus rebamipide 900 mg (Group C). EGD, esophagogastroduodenoscopy; FOB, fecal occult blood; LDA, low-dose aspirin; VCE, video capsule endoscopy.
Sample size
The sample size was calculated based on the results of a review of the incidence of NSAID-induced small intestinal mucosal injury examined by capsule endoscopy. According to several studies, the rate of NSAID-induced small intestinal mucosal injury ranges from 50 to 70%.(27–29) Furthermore, Niwa et al.(24) previously investigated the use of rebamipide for NSAID-induced small intestinal mucosal injury, and reported that the incidence of mucosal injury in the placebo and rebamipide groups was 80% and 20%, respectively. Assuming that omeprazole, which was employed as the control agent in this study, does not influence the small intestinal mucosa, the incidence of LDA-induced small intestinal mucosal injury in the omeprazole and rebamipide groups was estimated to be 70% and 20%, respectively. The number of patients required for each group to reach a significance level (paired) of 5% and detection power of 80% was 14.3, which was determined using the chi-square test; therefore, we enrolled 15 patients in each group.
Randomization
A coordinator performed a simple fixed-allocation randomization using a block-randomization scheme. Random numbers were generated by SAS software (SAS Institute, Cary, NC).
Evaluation of small intestinal lesions by VCE findings
Evaluation of small intestinal lesions was performed using a PillCamSB2 (Given Imaging, Yokneam, Israel), a VCE device specifically designed for the small intestine, after pre-treatment using the method described by Nouda et al.(30) The subjects received 1 L of polyethylene glycol solution (Niflec®; Ajinomoto Pharma Co., Ltd., Tokyo, Japan) containing 200 mg of dimethylpolysiloxane (Baros®; Horii Pharmaceutical Ind., Ltd., Osaka, Japan) over one hour starting at 6:00 a.m. on the day of the examination, which was followed by VCE at 9:00 a.m. The small intestine was examined 8 h after capsule administration. Images were analyzed using RAPID® Reader 6.5 software (Given Imaging).
The investigators responsible for evaluating the results of the capsule endoscopy of the small intestine were required to attend a standardized training session on the use of the Given Diagnostic System. These two investigators (S.N., T.K.) independently assessed the capsule endoscopic images under blinded conditions. If the observers recorded different findings, they discussed the case until an agreement was reached.
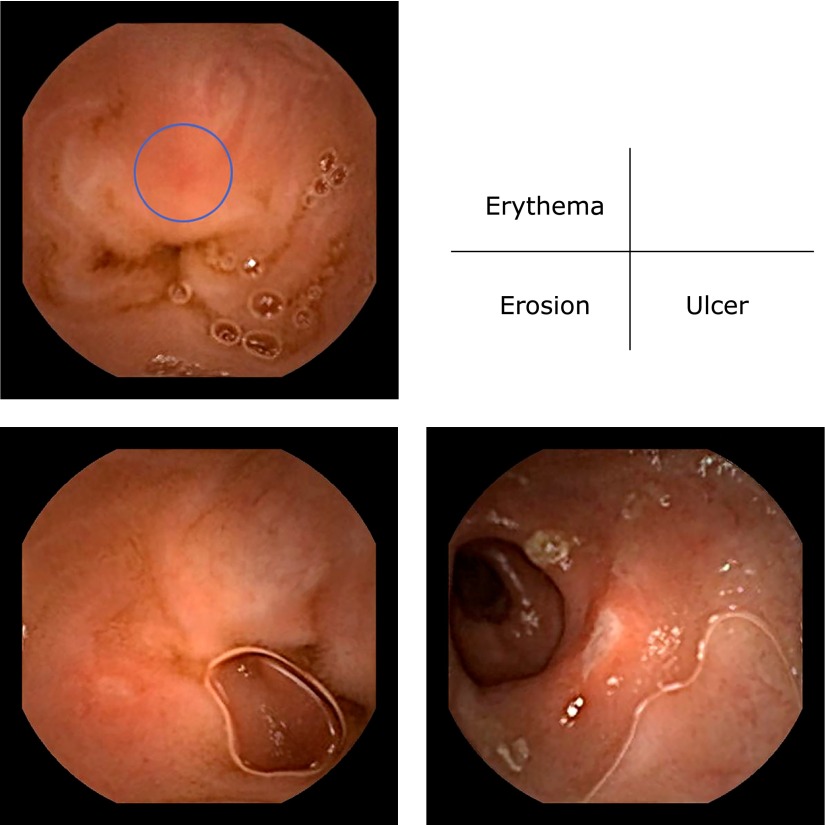
We evaluated the small intestinal mucosal injury based on the presence and degree of bleeding, erythema, erosions, ulcers, and stenosis. Erythema was defined as a red region with a border extending from the peripheral normal mucosa, erosion as a defect in the normal villus mucosa, and ulcers as mucosal defects covered with a white coat based on the classifications reported by Fujimori et al.(31) and Niwa et al.(24) with slight modifications. Additionally, inflammatory changes in the small intestinal mucosa were evaluated using the Lewis score.(32)
Evaluation of upper gastrointestinal lesions using EGD
We evaluated the improvement rate of reflux esophagitis and the modified Lanza score (MLS) for the gastroduodenal mucosal injury.(33) EGD was performed by one of four endoscopists (K.O., S.N., T.K., and T.T.) who were blinded to information regarding the subjects and the allocated treatment.
Evaluation of fecal calprotectin
Fecal calprotectin is a biomarker for inflammation of the digestive tract.(34) Stool samples were collected and frozen within 12 h of receipt and stored at −20°C for subsequent analysis by enzyme-linked immunosorbent assay (Immundiagnostik, Bensheim, Germany) as previously described.(35) The calprotectin level was expressed as micrograms of calprotectin per gram of feces, and a cut-off value of 50 µg/g was used as recommended by the manufacturer.(27)
Fecal occult blood test
The presence of fecal occult blood was assessed with an OC-Micro analyzer (Eiken, Tokyo, Japan) and defined as either positive or negative.
Statistical analysis
For continuous and categorical variables, the statistical significance of the differences between the groups was determined using the t test or Mann-Whitney U test, and the statistical significance of differences within a group was determined by the Wilcoxon signed-rank test.
For binary variables, the statistical significance of differences between groups was determined using the Fisher’s exact test (EGD findings), and the statistical significance of differences within a group before and two weeks after drug administration was determined using the paired t test (VCE findings) and Wilcoxon signed-rank test (fecal calprotectin levels).
All reported p values are two-sided, and values <0.05 were considered statistically significant.
Results
Baseline characteristics
The subjects ranged in age from 20 to 65 years. There were no significant differences in background factors such as age, height, weight, smoking rate, alcohol consumption rate, incidence of Helicobacter pylori infection, and degree of gastric mucosa atrophy (Kimura-Takemoto classification) between the groups (Table 1). Furthermore, the time required for the VCE to pass through the stomach and small intestine was almost identical in the three groups (Table 2).
Table 1.
Baseline characteristics
| Characteristic | Group A | Group B | Group C | p |
|---|---|---|---|---|
| Omeprazole 10 mg | Rebamipide 300 mg | Rebamipide 900 mg | ||
| (n = 15) | (n = 15) | (n = 15) | ||
| Age (years) | 35.2 ± 8.9 | 36.5 ± 9.1 | 35.6 ± 7.7 | NS |
| Height (cm) | 173.5 ± 5.1 | 173.0 ± 4.8 | 173.3 ± 4.9 | NS |
| Weight (kg) | 70.1 ± 9.7 | 70.9 ± 8.2 | 76.5 ± 16.0 | NS |
| Smoking history | 5/15 (33.3%) | 6/15 (40%) | 6/15 (40%) | NS |
| Drinking history | 10/15 (66.7%) | 11/15 (73.3%) | 12/15 (80%) | NS |
| H. pylori infection | 1/15 (6.7%) | 3/15 (20.0%) | 1/15 (6.7%) | NS |
| History of ulcer | 0/15 (0%) | 0/15 (0%) | 0/15 (0%) | NS |
| Kimura-Takemoto classification | ||||
| C1 | 7 | 7 | 8 | NS |
| C2 | 3 | 2 | 1 | |
| C3 | 0 | 0 | 1 | |
| O1 | 0 | 2 | 1 |
Data are presented as the mean ± SD or as n (%). NS, not significant.
Table 2.
Capsule endoscope transit times (min)
| Group A | Group B | Group C | p | |
|---|---|---|---|---|
| Omeprazole 10 mg | Rebamipide 300 mg | Rebamipide 900 mg | ||
| (n = 15) | (n = 15) | (n = 15) | ||
| Stomach | ||||
| Baseline | 69.3 ± 59.1 | 57.2 ± 88.2 | 35.1 ± 31.1 | NS |
| Post-treatment | 53.6 ± 45.5 | 39.9 ± 31.8 | 37.3 ± 41.0 | NS |
| Small intestine | ||||
| Baseline | 199.8 ± 108.8 | 223.8 ± 91.9 | 145.7 ± 90.1 | NS |
| Post-treatment | 219.6 ± 67.7 | 224.9 ± 84.1 | 193.5 ± 88.5 | NS |
Data are presented as the mean ± SD. NS, not significant.
Evaluation of upper gastrointestinal lesions before and two weeks after drug administration
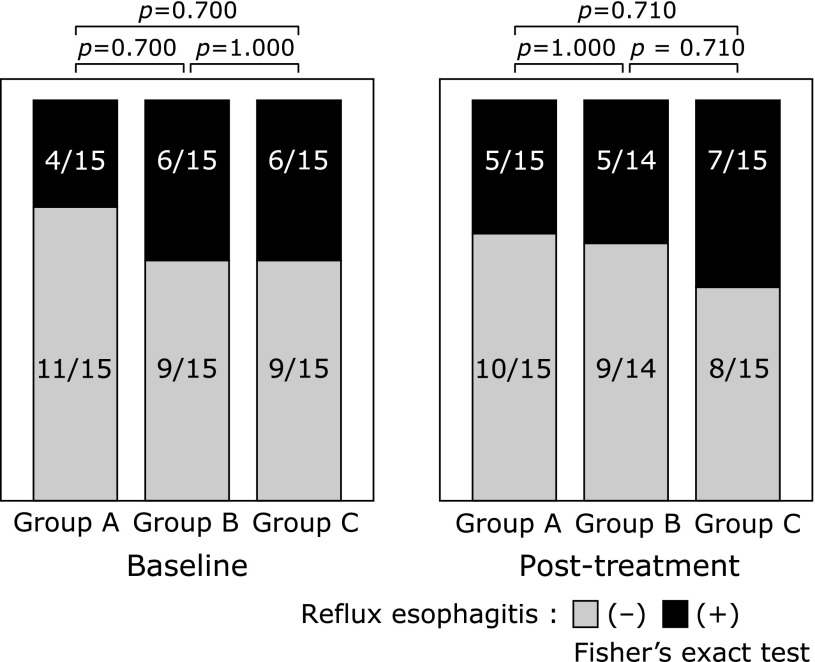
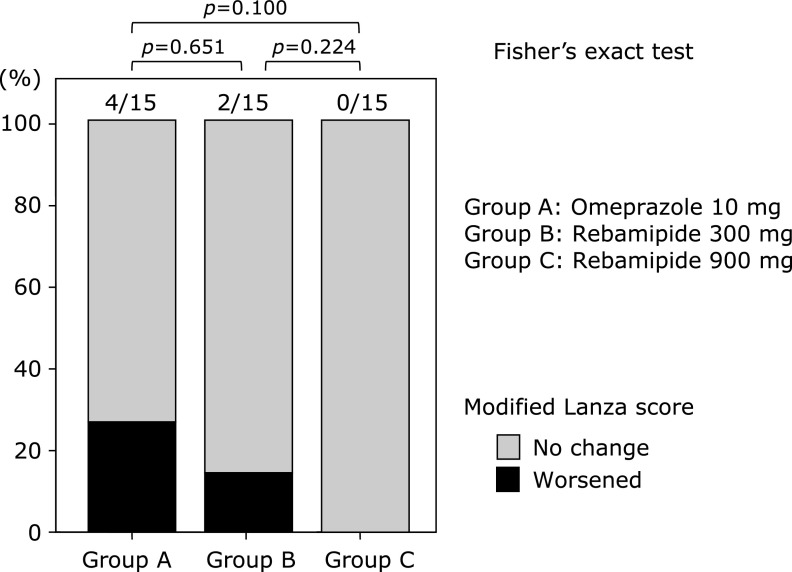
There were no significant differences in the prevalence rates of reflux esophagitis among the three groups both before and two weeks after drug administration (Fig. 2). Changes in the MLS following drug administration determined by EGD in each group are shown in Fig. 3. There were no significant differences in the rate of worsening of the MLS between the groups; however, there was a trend toward improvement in MLS scores in the treatment groups, which became more evident as the dosage of rebamipide increased: Group A vs B: p = 0.651, B vs C: p = 0.224, and A vs C: p = 0.100. One subject in Group B did not undergo the second EGD.
Fig. 2.
Esophageal lesions. There were no significant differences in the improvement rate of reflux esophagitis among the three groups.
Fig. 3.
Gastroduodenal lesions. No significant differences in the rate of modified Lanza score worsening were observed. However, there was trend toward prevention of low-dose aspirin-induced mucosal injuries in the stomach/duodenum in the rebamipide groups, and this tendency was dose-dependent, with a stronger response observed with high-dose rebamipide. NS, not significant.
Evaluation of small intestinal lesions before and two weeks after drug administration
There were no significant differences in the numbers of small intestinal lesions in each group before and two weeks after drug administration (Table 3). Fig. 4 shows typical capsule endoscopic views of the small intestinal mucosal injuries observed in this study. Bleeding and stenosis were not found in any subject.
Table 3.
The average numbers of small intestinal lesions before and two weeks after drug administration (paired t test)
| Small intestinal lesions | Baseline (mean ± SD) |
Post-treatment (mean ± SD) |
p | |
|---|---|---|---|---|
| Group A Omeprazole 10 mg (n = 15) |
Erythema | 1.5 ± 1.685 | 2.7 ± 4.818 | 0.247 |
| Erosion | 0.2 ± 0.775 | 2.9 ± 7.049 | 0.167 | |
| Ulcer | 0 ± 0.000 | 0.5 ± 1.060 | 0.110 | |
| Group B Rebamipide 300 mg (n = 15) |
Erythema | 0.8 ± 1.320 | 2.3 ± 3.411 | 0.087 |
| Erosion | 0.2 ± 0.561 | 1.4 ± 2.444 | 0.095 | |
| Ulcer | 0 ± 0.000 | 0.2 ± 0.775 | 0.334 | |
| Group C Rebamipide 900 mg (n = 15) |
Erythema | 1.3 ± 2.225 | 2.1 ± 4.758 | 0.408 |
| Erosion | 0 ± 0.000 | 0.8 ± 1.612 | 0.075 | |
| Ulcer | 0 ± 0.000 | 0.1 ± 0.352 | 0.164 |
Data are presented as the mean ± SD.
Fig. 4.
Representative video capsule endoscopy findings of small intestinal mucosal injuries after treatment
Evaluation of fecal calprotectin before and two weeks after drug administration
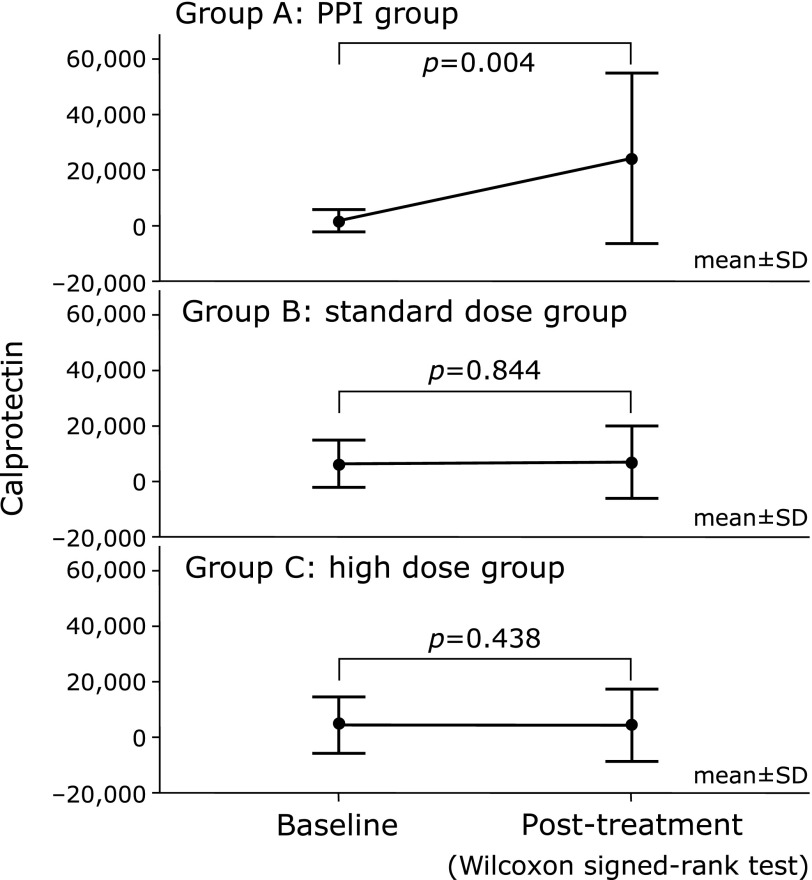
The fecal calprotectin levels in Group A worsened two weeks after drug administration (from 2,665 ± 4,245 at baseline to 22,192 ± 32,481 ng/g post-treatment; p = 0.004), whereas those in Groups B and C remained unchanged (from 5,785 ± 8,316 to 7,781 ± 14,754 ng/g; p = 0.844 and from 5,877 ± 11,168 to 2,484 ± 4,197 ng/g; p = 0.438, respectively) (Fig. 5).
Fig. 5.
Fecal calprotectin levels before and after treatment. A significant increase in the fecal calprotectin levels was observed between baseline and post-treatment in Group A (p = 0.004). Conversely, there were no significant differences in the calprotectin levels between the baseline and post-treatment in Group B (p = 0.844) or Group C (p = 0.438).
Presence of fecal occult blood before and two weeks after drug administration
There were no significant differences in the presence of fecal occult blood before and two weeks after drug administration in any of the groups (Table 4).
Table 4.
Presence of fecal occult blood before and two weeks after drug administration
| Group A Omeprazole |
Group B Rebamipide |
Group C Rebamipide |
p | ||
|---|---|---|---|---|---|
| 10 mg (n = 15) | 300 mg (n = 15) | 900 mg (n = 15) | |||
| Fecal occult blood | Baseline | 1/15 (6.7%) | 1/15 (6.7%) | 0/13 (0%) | NS |
| Post-treatment | 1/14 (7.1%) | 0/15 (0%) | 0/14 (0%) | NS |
Data are presented as n (%). NS, not significant.
Discussion
This study revealed that 300 mg of rebamipide can prevent LDA-induced small intestinal mucosal injury with an efficacy similar to that of 900 mg in healthy volunteers. Certainly, there were no significant differences in the prevalence rates of lesions in the upper gastrointestinal tract among the three groups at two weeks after drug administration.
The main effect of LDA is suppression of cyclooxygenase-1 activity, and it is believed that the mechanism of LDA-induced small intestinal mucosal injury is similar to that of other NSAIDs, which also involves suppressing cyclooxygenase activity. The pathology and prophylaxis of NSAID-induced small intestinal mucosal injury have recently been investigated in animal models.(36) It has been speculated that NSAID-induced small intestinal mucosal injury occurs due to reduced production of prostaglandins, which in turn causes microcirculatory disturbance by reducing mucus production and accelerating peristalsis, and this activates inflammatory cytokines resulting in mucosal injury. In addition, it has been reported that enterobacteria may cause inflammation via Toll-like receptor-4.(37)
The use of gastric mucoprotective drugs for the prevention of NSAID- or LDA-induced small intestinal mucosal injuries has been evaluated in several studies. Gastromucoprotective drugs can be classified as effective(9–11,13–17,24,28) or ineffective(19) in preventing NSAID- or LDA-induced small intestinal mucosal injury. Each drug has already been proven to prevent NSAID- or LDA-induced small intestinal mucosal injury in animal models. However, the results of these studies are insufficient to confirm that the ineffective drug is indeed ineffective in preventing small intestinal mucosal injury because the dosages used in these studies were those used for treating gastric ulcers. By increasing the dosage, a gastromucoprotective effect may have been obtained. However, the appropriate dosage of gastromucoprotective drugs for the prevention of small intestinal mucosal injuries is not yet known. Therefore, we investigated the protective effects and optimal dosage of rebamipide for LDA-induced gastrointestinal mucosal injury. There are no studies comparing high-dose with standard-dose rebamipide for the prevention of LDA-induced small intestinal mucosal injury, although the dosage to treat gastric ulcers may be sufficient to prevent LDA-induced small intestinal mucosal injury.
There are a few reports regarding rebamipide use in the prevention of NSAID- or LDA-induced gastrointestinal mucosal injury. Fujimori et al.(38) reported that the combination of 300 mg rebamipide and 20 mg omeprazole has higher potential for reducing the injury severity of diclofenac sodium-induced small intestinal mucosal injury than 20 mg of omeprazole alone. However, some analyses in their study were inappropriate: The two groups were compared without excluding the obvious outliers. Their study does not provide conclusive evidence for the preventive effect of rebamipide against NSAID-induced small intestinal injury. Mizukami et al.(17) reported that the preventive effect of rebamipide plus omeprazole was significantly higher than that of placebo plus omeprazole; however, they their study did not confirm the damaging effect of omeprazole on the small intestine. In the present study, the standard dosage of rebamipide significantly inhibited the onset of small intestinal mucosal injuries as well as that with high dose. On the contrary, Watanabe et al.(39) reported 900 mg of rebamipide, not the standard dosage one, was necessary to treat LDA-induced moderate-to-severe small intestinal mucosal injury.
The mechanisms underlying the effect of rebamipide on the small intestine are not clear. The effect of rebamipide in the small intestine could be the same as that in the stomach: increasing mucus secretion and scavenging free radicals. Recently, Tanigawa et al.(40) reported that intestinal microbiota modulation by upregulation of α-defensin 5 by rebamipide might be one of the mechanisms underlying its preventive effect against NSAID-induced small intestinal mucosal injury. Kurata et al.(41) reported that rebamipide regulates the small intestinal microbiota, in particular decreasing the number of Enterobacretiaceae induced by indomethacin administration, and decreases the gene expression of TNFα and Duox2 upregulated by indomethacin treatment.
Calprotectin is a major protein of the neutrophil cytoplasm, and the amount of fecal calprotectin reflects the degree of inflammation of the lower digestive tract and is a highly sensitive and specific marker for inflammatory bowel diseases.(35,42) The difference in the degree of LDA-induced small intestinal injury observed upon capsule endoscopy is unclear, owing to the fact that LDA does not cause as significant of a gastrointestinal mucosal injury as other NSAIDs. Hence, it is necessary to measure the calprotectin levels to clarify the difference in the degree of LDA-induced small intestinal injury. Accordingly, we verified LDA-induced gastrointestinal mucosal injury biochemically by measuring fecal calprotectin. We found that, while the fecal calprotectin levels increased in the PPI group, they did not increase in the rebamipide groups suggesting that the PPI did not prevent LDA-induced small intestinal mucosal injury. However, the presence of fecal occult blood, another well-established marker for colonic mucosal injury, was not significantly different between the groups.
We previously reported that capsule endoscopic findings correlated with fecal calprotectin levels in two studies using diclofenac sodium 75 mg.(9,10) In these two reports, the mean number of small intestinal mucosal injuries by capsule endoscopy and fecal calprotectin levels per subject who took diclofenac sodium 75 mg plus omeprazole 10 mg, or famotidine 20 mg for two weeks, increased significantly. In the present study, although there were no significant differences in the capsule endoscopic findings between the three groups, there was a significant increase in the fecal calprotectin levels in the PPI group between baseline and post-treatment, which was not seen in the standard- or high-dose rebamipide groups. There are two possibilities. One is that mucosal injury induced by LDA may be milder than that by diclofenac sodium. The other is that the fecal calprotectin levels may have a higher sensitivity than endoscopic findings. In the present study, VCE revealed no significant differences between the groups and the fecal calprotectin level was significantly higher in the PPI group than in the standard- and high-dose rebamipide groups.
This study has some important limitations, including the short duration, the inclusion of healthy volunteers, and the lack of colonoscopic evaluation. Accordingly, large-scale, long-term, prospective studies in different patient populations are warranted to confirm our results.
In conclusion, the present study revealed that both 300 mg and 900 mg of rebamipide were superior to 10 mg of omeprazole for preventing LDA-induced small intestinal mucosal injury. Based on these findings, we recommend that long-term LDA users without a history of peptic ulcers or gastrointestinal bleeding should be simultaneously treated with 300 mg of rebamipide instead of a PPI for total gastrointestinal management. High-dose rebamipide, such as 900 mg, is not necessary for preventing LDA-induced gastrointestinal mucosal injury.
Conflict of Interest
KH has received research grants and speaker’s fees from Otsuka Pharmaceutical Co., Ltd. The other authors declare no conflicts of interest associated with this manuscript.
References
- 1.CAPRIE Steering Committee. A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 2.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 3.Belch J, MacCuish A, Campbell I, et al. ; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taha AS, Angerson WJ, Knill-Jones RP, Blatchford O. Clinical outcome in upper gastrointestinal bleeding complicating low-dose aspirin and antithrombotic drugs. Aliment Pharmacol Ther. 2006;24:633–636. doi: 10.1111/j.1365-2036.2006.03017.x. [DOI] [PubMed] [Google Scholar]
- 5.Taha AS, Angerson WJ, Prasad R, McCloskey C, Blatchford O. Upper gastrointestinal bleeding and the changing use of COX-2 non-steroidal anti-inflammatory drugs and low-dose aspirin. Aliment Pharmacol Ther. 2007;26:1171–1178. doi: 10.1111/j.1365-2036.2007.03458.x. [DOI] [PubMed] [Google Scholar]
- 6.Taha AS, Angerson WJ, Prasad R, McCloskey C, Gilmour D, Morran CG. Clinical trial: the incidence and early mortality after peptic ulcer perforation, and the use of low-dose aspirin and nonsteroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;28:878–885. doi: 10.1111/j.1365-2036.2008.03808.x. [DOI] [PubMed] [Google Scholar]
- 7.Chan FK, Ching JY, Hung LC, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med. 2005;352:238–244. doi: 10.1056/NEJMoa042087. [DOI] [PubMed] [Google Scholar]
- 8.Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:119–125. doi: 10.1016/S0140-6736(09)61246-0. [DOI] [PubMed] [Google Scholar]
- 9.Umegaki E, Kuramoto T, Kojima Y, et al. Geranylgeranylacetone, a gastromucoprotective drug, protects against NSAID-induced esophageal, gastroduodenal and small intestinal mucosal injury in healthy subjects: a prospective randomized study involving a comparison with famotidine. Intern Med. 2014;53:283–290. doi: 10.2169/internalmedicine.53.1572. [DOI] [PubMed] [Google Scholar]
- 10.Kuramoto T, Umegaki E, Nouda S, et al. Preventive effect of irsogladine or omeprazole on non-steroidal anti-inflammatory drug-induced esophagitis, peptic ulcers, and small intestinal lesions in humans, a prospective randomized controlled study. BMC Gastroenterol. 2013;13:85. doi: 10.1186/1471-230X-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima Y, Takeuchi T, Ota K, et al. Effect of long-term proton pump inhibitor therapy and healing effect of irsogladine on nonsteroidal anti-inflammatory drug-induced small-intestinal lesions in healthy volunteers. J Clin Biochem Nutr. 2015;57:60–65. doi: 10.3164/jcbn.15-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanas Á, Carrera-Lasfuentes P, Arguedas Y, et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants Clin Gastroenterol Hepatol 201513906–912..e2. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Sugimori S, Kameda N, et al. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6:1279–1282. doi: 10.1016/j.cgh.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Watari I, Oka S, Tanaka S, et al. Effectiveness of polaprezinc for low-dose aspirin-induced small-bowel mucosal injuries as evaluated by capsule endoscopy: a pilot randomized controlled study. BMC Gastroenterol. 2013;13:108. doi: 10.1186/1471-230X-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tozawa K, Oshima T, Okugawa T, et al. A randomized, double-blind, placebo-controlled study of rebamipide for gastric mucosal injury taking aspirin with or without clopidogrel. Dig Dis Sci. 2014;59:1885–1890. doi: 10.1007/s10620-014-3108-4. [DOI] [PubMed] [Google Scholar]
- 16.Mizukami K, Murakami K, Hirashita Y, et al. Efficacy of rebamipide for low-dose aspirin-related gastrointestinal symptoms. J Clin Biochem Nutr. 2012;51:216–220. doi: 10.3164/jcbn.12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizukami K, Murakami K, Abe T, et al. Aspirin-induced small bowel injuries and the preventive effect of rebamipide. World J Gastroenterol. 2011;17:5117–5122. doi: 10.3748/wjg.v17.i46.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto T, Isono A, Mishina Y, et al. Gastroduodenal mucosal injury in patients taking low-dose aspirin and the role of gastric mucoprotective drugs: possible effect of rebamipide. J Clin Biochem Nutr. 2010;47:27–31. doi: 10.3164/jcbn.09-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiotani A, Haruma K, Nishi R, et al. Randomized, double-blind, pilot study of geranylgeranylacetone versus placebo in patients taking low-dose enteric-coated aspirin. Low-dose aspirin-induced small bowel damage. Scand J Gastroenterol. 2010;45:292–298. doi: 10.3109/00365520903453182. [DOI] [PubMed] [Google Scholar]
- 20.Terano A, Arakawa T, Sugiyama T, et al. ; Rebamipide Clinical Study Group Rebamipide, a gastro-protective and anti-inflammatory drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori in a Japanese population: a randomized, double-blind, placebo-controlled trial. J Gastroenterol. 2007;42:690–693. doi: 10.1007/s00535-007-2076-2. [DOI] [PubMed] [Google Scholar]
- 21.Nishizawa T, Suzuki H, Nakagawa I, et al. Rebamipide-promoted restoration of gastric mucosal sonic hedgehog expression after early Helicobacter pylori eradication. Digestion. 2009;79:259–262. doi: 10.1159/000213241. [DOI] [PubMed] [Google Scholar]
- 22.Nishizawa T, Suzuki H, Kanai T, Yahagi N. Proton pump inhibitor alone vs proton pump inhibitor plus mucosal protective agents for endoscopic submucosal dissection-induced ulcer: a systematic review and meta-analysis. J Clin Biochem Nutr. 2015;56:85–90. doi: 10.3164/jcbn.14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naito Y, Iinuma S, Yagi N, et al. Prevention of indomethacin-induced gastric mucosal injury in Helicobacter pylori-negative healthy volunteers: a comparison study rebamipide vs famotidine. J Clin Biochem Nutr. 2008;43:34–40. doi: 10.3164/jcbn.2008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niwa Y, Nakamura M, Ohmiya N, et al. Efficacy of rebamipide for diclofenac-induced small-intestinal mucosal injuries in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled, cross-over study. J Gastroenterol. 2008;43:270–276. doi: 10.1007/s00535-007-2155-4. [DOI] [PubMed] [Google Scholar]
- 25.Kishi H, Ogawa N. Phase I trial of anti-ulcer drug, Proamipide (OPC-12759) Jpn J Clin Pharmacol Ther. 1989;3:355–363. [Google Scholar]
- 26.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 27.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein JL, Eisen GM, Lewis B, Grainek M, Ziotnick S, Fort JG, ; Investigators Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 29.Maiden L, Thjodleifsson B, Seigal A, et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5:1040–1045. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Nouda S, Morita E, Murano M, et al. Usefulness of polyethylene glycol solution with dimethylpolysiloxanes for bowel preparation before capsule endoscopy. J Gastroenterol Hepatol. 2010;25:70–74. doi: 10.1111/j.1440-1746.2009.05968.x. [DOI] [PubMed] [Google Scholar]
- 31.Fujimori S, Gudis K, Takahashi Y, et al. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur J Clin Invest. 2010;40:504–510. doi: 10.1111/j.1365-2362.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 32.Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146–154. doi: 10.1111/j.1365-2036.2007.03556.x. [DOI] [PubMed] [Google Scholar]
- 33.Lanza FL, Graham DY, Davis RE, Rack MF. Endoscopic comparison of cimetidine and sucralfate for prevention of naproxen-induced acute gastroduodenal injury. Effect of scoring method. Dig Dis Sci. 1990;35:1494–1499. doi: 10.1007/BF01540567. [DOI] [PubMed] [Google Scholar]
- 34.Tøn H, Brandsnes O, Dale S, et al. Improved assay for fecal calprotectin. Clin Chim Acta. 2000;292:41–54. doi: 10.1016/s0009-8981(99)00206-5. [DOI] [PubMed] [Google Scholar]
- 35.Langhorst J, Elsenbruch S, Mueller T, et al. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11:1085–1091. doi: 10.1097/01.mib.0000187980.08686.18. [DOI] [PubMed] [Google Scholar]
- 36.Higuchi K, Umegaki E, Watanabe T, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–888. doi: 10.1007/s00535-009-0102-2. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe T, Higuchi K, Kobata A, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181–187. doi: 10.1136/gut.2007.125963. [DOI] [PubMed] [Google Scholar]
- 38.Fujimori S, Takahashi Y, Gudis K, et al. Rebamipide has the potential to reduce the intensity of NSAID-induced small intestinal injury: a double-blind, randomized, controlled trial evaluated by capsule endoscopy. J Gastroenterol. 2011;46:57–64. doi: 10.1007/s00535-010-0332-3. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe T, Takeuchi T, Handa O, et al. A multicenter, randomized, double-blind, placebo-controlled trial of high-dose rebamipide treatment for low-dose aspirin-induced moderate-to-severe small intestinal damage. PLoS One. 2015;10:e0122330. doi: 10.1371/journal.pone.0122330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanigawa T, Watanabe T, Otani K, et al. Rebamipide inhibits indomethacin-induced small intestinal injury: possible involvement of intestinal microbiota modulation by upregulation of α-defensin 5. Eur J Pharmacol. 2013;704:64–69. doi: 10.1016/j.ejphar.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Kurata S, Nakashima T, Osaki T, et al. Rebamipide protects small intestinal mucosal injuries caused by indomethacin by modulating intestinal microbiota and the gene expression in intestinal mucosa in a rat model. J Clin Biochem Nutr. 2015;56:20–27. doi: 10.3164/jcbn.14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vrakas G, O'Connor M, Matsou A, et al. Markers of malnutrition after intestinal transplantation: the role of IGF-1 and calprotectin. J Clin Biochem Nutr. 2015;56:64–65. doi: 10.3164/jcbn.14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]