Abstract
Olfaction is a key insect adaptation to a wide range of habitats. In the last thirty years, the detection of octenol by blood-feeding insects has been primarily understood in the context of animal host-seeking. The recent discovery of a conserved octenol receptor gene in the strictly nectar-feeding elephant mosquito Toxorhynchites amboinensis (TaOr8) suggests a different biological role. Here, we show that TaOR8 is a functional ortholog of its counterparts in blood-feeding mosquitoes displaying selectivity towards the (R)-enantiomer of octenol and susceptibility to the insect repellent DEET. These findings suggest that while the function of OR8 has been maintained throughout mosquito evolution, the context in which this receptor is operating has diverged in blood and nectar-feeding mosquitoes.
The molecular mechanisms by which insects detect odor cues involve many gene families of which odorant receptors (Ors) play a major role1. The interactions between ORs and their ligands (biochemical function) and the ecological contexts in which these interactions take place are fascinating relationships to be explored. With the exception of pheromone receptors, whose roles are well understood, there is little knowledge of how insects detect and process specific olfactory cues that are important to their life histories.
The survival of most mosquitoes depends on locating animal hosts, resting and oviposition sites, as well as suitable sources of nectar (Fig. 1a)2. The role of (R)-(−)-1-octen-3-ol (thereafter termed (R)-octenol) is of particular interest for several reasons. First identified as a tsetse attractant released by cattle3, octenol has also been shown to be present in human sweat4 and to attract mosquitoes5. The identification of a labeled pathway for this compound in all blood feeding mosquitoes6,7,8 suggests a role in animal host seeking as well2. However, the behavioral significance of octenol is complex, species specific5,9,10 and generally poorly understood11. Octenol may require the concomitant presence of other cues such as CO25. Octenol seems to be an attractant for Anopheles and Aedes but in Culex, octenol elicits little to no attractive effects12 or repels this mosquito in a host-feeding context13.
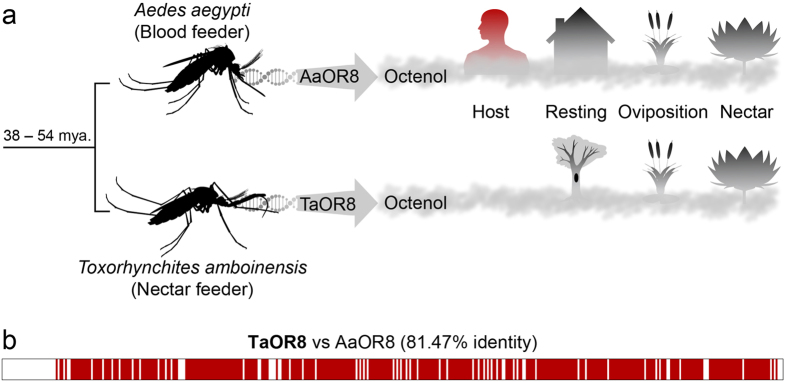
Figure 1. Ecological context of OR8 function.
(a) Aedes aegypti and Toxorhynchites diverged 40 million years (MY) ago. Both insects may use octenol in overlapping and different contexts. (b) TaOR8 and AaOR8 share 81.47% amino-acid sequence identity (red).
Octenol may play a role in nectar-seeking as proposed by earlier authors14. For instance, leaves and flowers of the wild sage Lantana camara release (R)-octenol15 and are known to attract mosquitoes16. Other observations suggest that octenol is involved in behaviors other than animal host-seeking. Non-blood-feeding male Ae. aegypti exhibit octenol-sensitive basconic sensilla17 and express the Or8 gene (AaOr8)18. The role of OR8 in Culex quinquefasciatus mosquitoes is unclear as multiple OR8 paralogs are activated by high doses of octenol13,19. This mosquito also shows a marked preference for birds20, which are not known to release octenol21. However, these observations may be consistent with animal host-seeking if one considers that male Ae. aegypti have been found in proximity to hosts, perhaps as a means to increase their likelihood to locate a mate, and that octenol has not been excluded as a bird emanation. In any case, the 145–200 million years old conservation of the octenol receptor OR8, in Culicinae and Anophelinae mosquitoes18,22 underscores its importance perhaps in multiple ecological contexts.
The recent discovery of the OR repertoire in the Elephant mosquitoes Toxorhynchites might be valuable to explore the role of octenol since animal host-seeking is not part of their behavior23,24 (Fig. 1a). The Toxorhynchites group, which belongs to the Culicinae subfamily, separated from the Aedes and Culex lineages about 38–54 million years ago22,25. T. amboinensis belong to a small group of nectar-feeding mosquitoes26, which share a majority of Or homologs with Ae. aegypti including Or8 (TaOr8)27. Additionally, T. amboinensis and Ae. aegypti express Or8 at similar levels in the maxillary palps suggesting a conserved key role in the life cycle of adult mosquitoes. Resolving the tuning properties of TaOR8 may clarify the role of this receptor outside animal host-seeking paradigms. Using a cell-based functional assay, we show that TaOR8’s response to octenol is highly sensitive, enantioselective, inhibited by the insect repellent DEET and odorant specific. Common ancestral origin and functional conservation support TaOR8 as a functional ortholog of the Ae. Aegypti, Anopheles gambiae and Culex quinquefasciatus OR8s. These features provide evidence that TaOR8 is an octenol receptor whose ecological role is unknown but excludes animal host-seeking. These findings question the ecological role traditionally ascribed to the OR8/(R)-octenol partner in blood-feeding mosquitoes and suggest that octenol may be useful to mosquitoes in multiple contexts beyond animal host-seeking.
Results
High amino-acid sequence conservation of OR8 in blood and nectar feeding mosquitoes
Despite 38–54 million years of evolution since the Aedes-Toxorhynchites split and different ecological requirements (Fig. 1a), TaOR8 and AaOR8 exhibit high peptide sequence conservation (Fig. 1b). TaOr8 encodes a 394 amino-acid protein (1185 nucleotides including the stop codon) sharing 81% overall amino-acid identity with AaOR828 (Fig. 1b). Previous functional analysis of AaOR8 was carried out with a gene (1269 nucleotides including stop codon) encoding a 422 amino-acid protein. While amino-acid divergence is evenly distributed throughout the peptide sequence, highest amino-acid diversity is highest on the N-terminus of AaOR8, which exhibits an extra 26 amino-acids.
TaOR8 is enantioselective
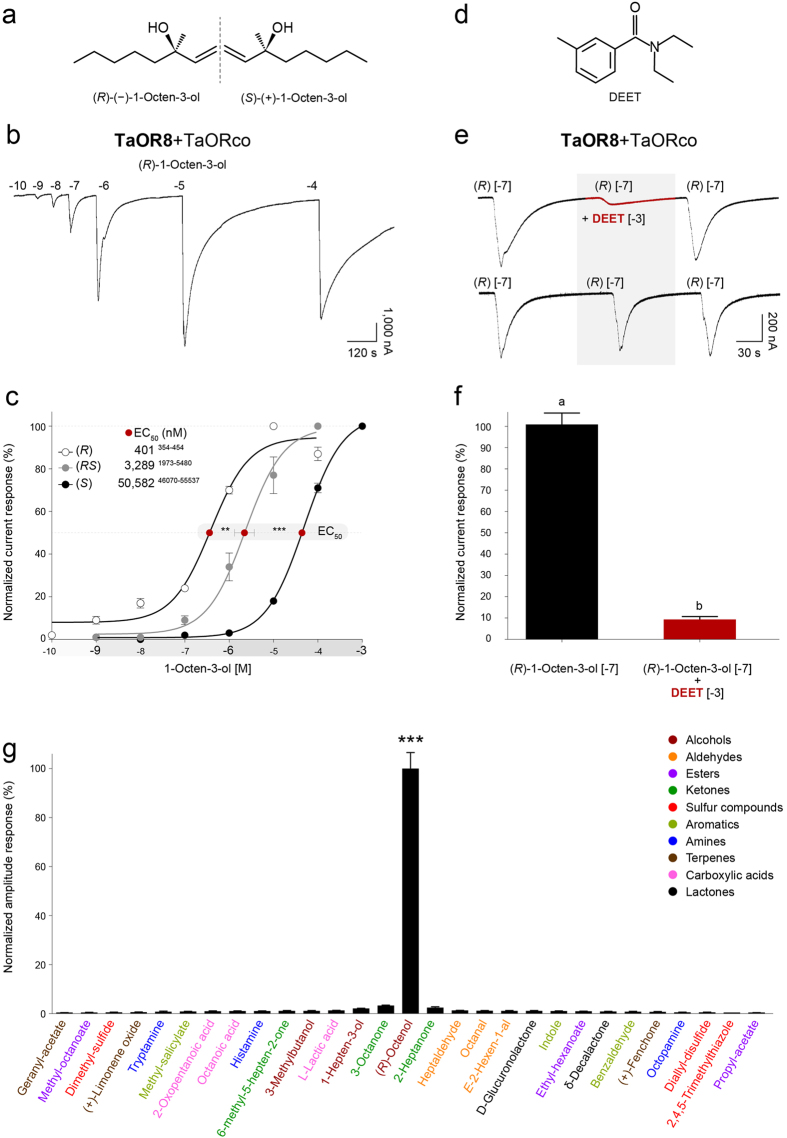
Octenol is a chiral compound composed of the (R)-(−)-1-octen-3-ol and (S)-(+)-1-octen-3-ol enantiomers (Fig. 2a). The (R) enantiomer is the predominant form15,29,30 found in nature. In order to investigate whether TaOR8 is a functional ortholog of AaOR8, we expressed TaOR8 in combination with TaORco in Xenopus laevis oocytes and recorded the responses of this receptor complex to the (R), (S) and racemic mixture (RS) of 1-octen-3-ol using the two-microelectrode voltage clamp technique. An example of a current trace is shown in Fig. 2b. The resulting electrophysiological responses were fitted to sigmoid curves (Fig. 2c). Extrapolated EC50 values show that the (R) enantiomer (EC50: 401 nM) is approximately 8 and 126 times more potent than the (RS) mixture (EC50: 3,289 nM) and the (S) enantiomer (EC50: 50,582 nM), respectively. Although the (S) contained <0.1% or no (R) at all, we cannot exclude the possibility that TaOR8 response was elicited by trace amount of the (R) enantiomer. Sensitivity in the nanomolar range for (R)-octenol supports a cognate receptor ligand relationship, which is comparable to pheromone receptor-pheromone pairs31.
Figure 2. Functional analysis of TaOR8.
(a) 1-Octen-3-ol occurs in two enantiomeric forms, (R)-(−)-1-octen-3-ol and (S)-(+)-1-octen-3-ol. (b) Representative current trace elicited by increasing concentrations of (R)-(−)-1-octen-3-ol recorded from Xenopus oocytes co-expressing the TaOr8 and TaORco receptor complex. (c) Concentration-response relationships of TaOR8+TaORco elicited by (R)-(−)-1-octen-3-ol [(R), open circle, n = 5], (S)-(+)-1-octen-3-ol [(S), grey circles, n = 5) and (RS)-1-octen-3-ol [(RS), black circles, n = 6]. Responses were normalized to the maximum response. Extrapolated EC50 values are shown with red circles. Lower and upper EC50 values (standard error) are in upper case. Asterisks represent statistically significant differences of the OR responses (one-way ANOVA followed by Tukey’s post test; **P < 0.01 and ***P < 0.001). Odorant concentrations were plotted on a logarithmic scale. Each point represents the mean and error bars indicate s.e.m. (d) N,N-Diethyl-meta-toluamide, commonly called DEET, is a synthetic insect repellent. (e) DEET inhibits the response of OR8 to octenol: Representative current traces of oocytes expressing TaOR8+TaORco elicited by 10−7 M (R)-(−)-1-octen-3-ol alone or in combination with 10−3 M DEET. (f) Normalized responses of TaOR8+TaORco to 10−7 M (R)-(−)-1-octen-3-ol alone or in combination with 10−3 M DEET. DEET’s effect was statistically significant (Student’s t-test, P < 0.01, n = 5–7). (g) (R)-(−)-1-octen-3-ol is a potent TaOR8 activator (one-way ANOVA followed by Tukey’s post test; ***P < 0.0001). Mean responses (±s.e.m., n = 6) to 400 nM of 28 odorants were normalized to (R)-octenol.
DEET inhibits TaOR8’s response to (R)-octenol
To further confirm that both mosquito receptors are functional orthologs, we tested the inhibitory effect of DEET (10−3 M) (Fig. 2a) on the TaOR8 response to a non-saturating concentration of (R)-octenol (10−7 M), as carried out previously with AaOR832. Despite the alleged masking effect of DEET on octenol shown in single cell recordings from olfactory receptor neurons33, we have previously shown that no such effects occur in solution34. An example of a current trace is shown in Fig. 2e. DEET reduced TaOR8 activation by 90% (Fig. 2f). Following DEET exposure, TaOR8 response to octenol returned to baseline. Response to (R)-octenol alone did not differ before and after exposure to the (R)-octenol-DEET mix, indicating no adaptive effect (Fig. 2f). Applying three consecutive doses of 10−7 M (R)-octenol elicited identical TaOR8 responses, excluding a potential position effect.
TaOR8 is narrowly tuned to (R)-octenol
We used a panel of 29 compounds including (R)-octenol) belonging to 10 classes of organic compounds (alcohols, aldehydes, esters, ketones, sulfur compounds, aromatics, amines, terpenes, carboxylic acids and lactones) to explore the odor space of TaOR8 (Fig. 2g). All compounds were delivered for 8 s at a concentration of 400 nM, which corresponds to the EC50 value of (R)-octenol. (R)-octenol was the most potent ligand eliciting a response 30 times higher than the next most potent chemical 3-octanone, also an 8-carbon aliphatic compound, and 46 times higher than 1-hepten-3-ol, which is identical to octenol except for one carbon shorter.
Discussion
Our major objective was to functionally characterize OR8 from a non-blood feeding mosquito as a means to explore its potential biological role in blood-feeding mosquitoes. This interest was motivated by the recent discovery of a conserved octenol receptor in T. amboinensis solely expressed in the maxillary palp27, which suggested that this receptor might be a functional octenol receptor operating in a context other than animal host-seeking.
This study shows that TaOR8 and AaOR8 are functional orthologs as both (i) share a high level of sequence identity, (ii) are expressed in the maxillary palps, (iii) exhibit high sensitivity (nanomolar range) towards (R)-octenol, (iv) feature a susceptibility to DEET inhibition, (v) are narrowly tuned to (R)-octenol. Such evolutionarily conservation of receptor biochemical function in Toxorhynchites is surprising since this species does not animal host-seek23,24. Indeed, considering that 95% of mosquito species are blood-feeders and assuming that octenol plays a role in animal host-seeking, expecting a tuning shift in TaOR8 would have been a reasonable expectation. Maintenance of the octenol-receptor phenotype in a non-animal host-seeking mosquito supports a role in locating resting/oviposition sites, nectar sources or other contexts (Fig. 1a). Perhaps more intriguing is the possibility that OR8 in blood-feeding mosquitoes may also play a role outside animal host-seeking14. These findings suggest that conservation of biochemical function does not necessarily translate into conserved behaviors, which underscores the role of the brain in determining their ecological contexts.
Our results support a role of TaOR8 outside an animal host-seeking context. Since TaOR8 requires ORco, this is consistent with the discovery that orco is not only involved with animal host selection but also with the detection of honey, which contains nectar metabolites (DeGennaro et al., 2013). Identifying other contexts in which this compound is used by mosquitoes will be challenging, as octenol is a common environmental volatile that may serve multiple roles in mosquito behavior. Octenol is synthesized by fungi35,36, plants15,37,38,39 and is also released by vertebrates3,40,41. However, whether animals possess a biosynthetic pathway to produce this chemical is unknown and it is possible that its occurrence in animal secretions results from microbial activity.
Octenol is used as an aggregation pheromone in the sawtoothed grain beetle, Oryzaephilus surinamensis42 and as a plant attractant in the black blowfly, Phormia regina43, the legume pod borer, Maruca vitrata44, the Grapevine Moth, Lobesia botrana45, the European grape berry moths, Eupoecilia ambiguella46 and the sandfly Lutzomyia longipalpis47. It is also used as a compost attractant for the phorid fly, Megaselia halterata48 and as an avoidance cue for the parasitoid Lariophagus distinguendus49.
In Diptera, octenol has been suggested to act as an oviposition attractant. First mentioned as a potential oviposition cue for Ae. aegypti50 and later for T. amboinensis51, it has also been implicated as an oviposition cue in other dipterans including the oriental fruit fly, Bactrocera dorsalis52 and the bean seed fly, Delia platura53. It therefore appears that octenol detection may be an ancestral cue in insects and an oviposition cue in Dipterans53.
What is the role of OR8 in Ae. aegypti? The ecological context in which AaOR8 operates may be restricted to animal host-seeking, but perhaps more intriguing is the possibility that AaOR8 is involved in eliciting multiple behaviors. As a ubiquitous cue, octenol may be used by mosquitoes in combination with other cues, olfactory or otherwise54 to detect a variety of resources important for the life cycle of adult mosquitoes. For example, octenol in combination with CO2 and other volatiles may be used as an animal host attractant5. But more generally, these insects may use octenol as a proxy chemical cue for detecting humid microhabitats55, signaled by the presence of microorganisms such as fungi or bacteria, including oviposition/resting sites and nectar sources (Fig. 1a). Additional studies exploring the behavioral influence of (R)-octenol on blood-feeding mosquitoes will be necessary to reveal the entire ecological contexts of its biochemical function.
Methods
Gene cloning and sequencing of TaOr8 and TaORco
RNA was isolated from antennae or maxillary palps of adult female Toxorhynchites amboinensis by trizol extraction. First strand cDNA synthesis was carried out using the TranscriptorTM kit (Roche Diagnostics, Indianapolis, IN, USA), according to the manufacturers protocol. PCR amplification of full-length TaOr8 or TaORco coding sequences was performed with antennal or maxillary palp-derived cDNA templates and the following primers: TaORco forward: 5′CACCATGAATGTTCAACCAACCAAG3′; TaORco reverse: TTACTTCAGCTGCACCAGCAC; TaOr8 forward: 5′CACCATGAGACTCAGAAAGATGAACG3′; TaOr8 reverse: 5′CTATTTCGGTCCATACATTGTT3′. Amplicons were cloned into the pENTRTM vector using the GatewayR directional cloning system (Invitrogen Corp., Carlsbad, CA, USA) and subcloned into the Xenopus laevis expression destination vector, pSP64t RFA.
Plasmids were purified using the The ZR Plasmid Miniprep™-Classic (Zymo Research, Irvine, CA, USA) and sequenced by Macrogen Europe (Amsterdam, the Netherland). DNA and amino-acid sequences for TaOr8 and TaORco have previously been published27 and can be accessed here (http://dx.doi.org/10.6084/m9.figshare.1092617).
Chemical reagents
For establishing the tuning curve, we used the following 29 chemicals, including (R)-octenol (described below): 17 compounds from Sigma-Aldrich (Milwaukee, WI, USA), including 1-hepten-3-ol (CAS 4938-52-7), 3-methylbutanol (CAS 123-51-3), E-2-hexen-1-al (CAS 6728-26-3), heptaldehyde (CAS 111-71-7), octanal (CAS 124-13-0), propyl-acetate (CAS 109-60-4), 3-octanone (CAS 106-68-3), 6-methyl-5-hepten-2-one (CAS 110-93-0), 2,4,5-trimethylthiazole (CAS 13623-11-5), diallyl-sulfide (CAS 2179-57-9), benzaldehyde (CAS 100-52-7), indole (CAS 83-34-1), histamine (CAS 51-45-6), (+)-limonene oxide (CAS 203719-54-4), geranyl-acetate (CAS 105-87-3), (+)-fenchone (CAS 4695-62-9), 2-oxopentanoic acid (CAS 1821-02-9); 7 compounds from Merck (Darmstadt, Germany), including methyloctanoate (CAS 111-11-5), ethyl-hexanoate (CAS 123-66-0), 2-heptanone (CAS 110-43-0), dimethyl-sulfide (CAS 2179-57-9), tryptamine (CAS 61-54-1), octanoic-acid (CAS 124-07-2) and D-glucuronolactone (CAS 32449-92-6); 2 compounds from Acros Organics (Thermo Fisher Scientific, Waltham, MA, USA), including methyl-salicylate (CAS 119-36-8) and octopamine (CAS 770-05-8); and 2 compounds from Alfa-Aesar (Ward Hill, MA, USA), including L-lactic acid (CAS 79-33-4) and δ-Decalactone (CAS 705-86-2).
Racemic octenol (CAS number 3391-86-4) and N,N-Diethyl-m-toluamide (DEET; CAS number 134-62-3) were obtained from Sigma. (R)-(−)-1-octen-3-ol (CAS 3687-48-7, 98.2%) and (S)-(+)-1-octen-3-ol (CAS 24587-53-9, >99.9%) chiral compounds were gifts from Bedoukian Research Inc.
Two-electrode voltage clamp electrophysiological recording of Xenopus oocytes expressing TaOR8 and TaORco
The methodologies and protocols used in this study have been described elsewhere28. TaOr8 and TaORco cRNA were synthesized using the mMESSAGE mMACHINE® SP6 Transcription Kit (ThermoFisher Scientific) and linearized pSP64tRFA expression vectors. Stage V-VI oocytes were manually separated and enzymatically defolliculated using a 2 mg/mL collagenase (Sigma-Aldrich, Milwaukee, WI, USA) solution (calcium-free ND96 buffer, [pH 7.6]) for 30 min at 18 °C. Oocytes were then successively washed in calcium-free ND96 and gentamycin-supplemented (10 mg/mL, Sigma-Aldrich, Milwaukee, WI, USA) calcium-free ND96. Oocytes were then washed and incubated in ND96 buffer supplemented with calcium (0.1 M), 5% heat-inactivated horse serum (ThermoFisher Scientific), 50 mg/ml tetracycline (Carl Roth GmbH), 100 mg/ml streptomycin (Sigma-Aldrich, Milwaukee, WI, USA) and 550 mg/ml sodium pyruvate (Sigma-Aldrich, Milwaukee, WI, USA) for four to five days. Oocytes were injected with 27.6 nL (27.6 ng of each cRNA) of RNA using the Nanoliter 2010 injector (World Precision Instruments, Inc., Sarasota, FL, USA). Odorant-induced currents of oocytes expressing TaOr8 and TaORco were recorded using the two-microelectrode voltage-clamp technique (TEVC). The OC-725C oocyte clamp (Warner Instruments, LLC, Hamden, CT, USA) maintained a −80 mV holding potential.
For the establishment of concentration-response curves, oocytes were exposed to (R), (S) or (RS)-octenol alone (10−10 M to 10−3 M). To measure the effect of DEET on TaOR8, we used (10−7 M) (R)-octenol or a combination of (10−7 M) (R)-octenol and DEET (10−3 M) in 1% DMSO for 8 s. Current was allowed to return to baseline between drug administrations. Data acquisition and analysis were carried out with the Digidata 1550 A digitizer and pCLAMP10 software (Molecular Devices, Sunnyvale, CA, USA).
The tuning curve was generated using a panel 29 odorants including (R)-octenol and known to elicit physiological or behavioral responses in mosquitoes (see list of chemicals above). All chemicals used were administered at 400 nM, which corresponds to the EC50 of (R)-octenol. All the data analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA).
Additional Information
How to cite this article: Dekel, A. et al. Evolutionarily conserved odorant receptor function questions ecological context of octenol role in mosquitoes. Sci. Rep. 6, 37330; doi: 10.1038/srep37330 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This research was supported by the ISRAEL SCIENCE FOUNDATION (grant No. 1990/16). Our sincere thanks to the reviewers for their valuable comments. We are grateful to Elisha Shalgi and Shachar Koren for their help with the frog oocytes. We also thank Bedoukian Research for providing the octenol enantiomers. We thank Prof. Richard G. Vogt and Dr. Jackson Sparks for their review of the manuscript.
Footnotes
Author Contributions J.D.B. designed and supervised this study, performed the data analysis and experiments, prepared figures and wrote the manuscript. A.D. performed experiments, performed the data analysis and co-wrote the manuscript. R.J.P. provided helpful discussions and comments. R.J.P. annotated and cloned TaOr8. E.Y. provided technical support. All the authors critically read and approved the manuscript. J.D.B. finalized the manuscript.
References
- Suh E., Bohbot J. & Zwiebel L. J. Peripheral olfactory signaling in insects. Current Opinion in Insect Science 6, 86–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W. & Knols B. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol 44, 131–157 (1999). [DOI] [PubMed] [Google Scholar]
- Hall D., Beevor P., Cork A., Nesbitt B. & Vale G. 1-octen-3-ol, a potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Insect Sci. Appl. 5, 335–339 (1984). [Google Scholar]
- Cork A. & PARK K. C. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol 10, 269–276 (1996). [DOI] [PubMed] [Google Scholar]
- Takken W. & Kline D. L. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J Am Mosq Control Assoc 5, 311–316 (1989). [PubMed] [Google Scholar]
- Lu T. et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol 17, 1533–1544 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. J. & O′Connell R. J. Electrophysiological responses from receptor neurons in mosquito maxillary palp sensilla in Olfaction in Mosquito-Host Interactions (eds Bock G. R. et al.) 233–253 (John Wiley & Sons 1996). [DOI] [PubMed] [Google Scholar]
- Grant A. J. & Dickens J. C. Functional characterization of the octenol receptor neuron on the maxillary palps of the yellow fever mosquito, Aedes aegypti. PLoS ONE 6, e21785 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D. Attractants for mosquito surveillance and control: 1-Octen-3-ol. Journal of the American Mosquito Control Association 10, 280–287 (1994). [PubMed] [Google Scholar]
- Takken W., Dekker T. & Wijnholds Y. Odor-mediated flight behavior of Anopheles gambiae Giles Sensu Stricto and An. stephensi Liston in response to CO2, acetone, and 1-octen-3-ol (Diptera: Culicidae). Journal of Insect Behavior 10, 395–407 (1997). [Google Scholar]
- Torr S. J. Dose responses of tsetse flies (Glossina) to carbon dioxide, acetone and octenol in the field. Physiological Entomology 15, 93–103 (1990). [Google Scholar]
- Kline D. L., Allan S. A., Bernier U. R. & Welch C. H. Evaluation of the enantiomers of 1-octen-3-ol and 1-octyn-3-ol as attractants for mosquitoes associated with a freshwater swamp in Florida, USA. Med Vet Entomol 21, 323–331 (2007). [DOI] [PubMed] [Google Scholar]
- Xu P., Zhu F., Buss G. K. & Leal W. S. 1-Octen-3-ol – the attractant that repels. F1000Res 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z. & Leal W. S. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chem Senses 32, 727–738 (2007). [DOI] [PubMed] [Google Scholar]
- Syed Z. & Guerin P. M. Tsetse flies are attracted to the invasive plant Lantana camara. Journal of Insect Physiology 50, 43–50 (2004). [DOI] [PubMed] [Google Scholar]
- Impoinvil D. et al. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Med Vet Entomol 18, 108–115 (2004). [DOI] [PubMed] [Google Scholar]
- McIver S. Comparative studies on the sense organs on the antennae and maxillary palps of selected male culicine mosquitoes. Can J Zool 49, 235–239 (1971). [DOI] [PubMed] [Google Scholar]
- Bohbot J. et al. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol. BIol. 16, 525–537 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. R., Majeed S. & Ignell R. Molecular basis for odorant receptor tuning: a short C-terminal sequence is necessary and sufficient for selectivity of mosquito Or8. Insect Mol. Biol. 24, 491–501 (2015). [DOI] [PubMed] [Google Scholar]
- Kline D., Takken W., Wood J. & Carlson D. Field studies on the potential of butanone, carbon dioxide, honey extract, 1-octen-3-ol, L-lactic acid and phenols as attractants for mosquitoes. Med Vet Entomol 4, 383–391 (1990). [DOI] [PubMed] [Google Scholar]
- Cook J. I. et al. Enantiomeric selectivity in behavioural and electrophysiological responses of Aedes aegypti and Culex quinquefasciatus mosquitoes. Bulletin of Entomological Research 101, 541–550 (2011). [DOI] [PubMed] [Google Scholar]
- Arensburger P. et al. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330, 86–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan W. A. & Evenhuis N. L. Biology of Toxorhynchites. Annual Review of Entomology 26, 159–181 (1981). [Google Scholar]
- Collins L. & Blackwell A. Colour cues for oviposition behaviour in Toxorhynchites moctezuma and Toxorhynchites amboinensis mosquitoes. J Vector Ecol 25, 127–135 (2000). [PubMed] [Google Scholar]
- Besansky N. & Fahey G. Utility of the white gene in estimating phylogenetic relationships among mosquitoes (Diptera: Culicidae). Mol Biol Evol 14, 442–454 (1997). [DOI] [PubMed] [Google Scholar]
- Colledge W. R. Notes on a brush-tongued mosquito. Brisbane Proceedings of the Royal Society of Queensland 23, 121–131 (1911).
- Zhou X., Rinker D. C., Pitts R. J., Rokas A. & Zwiebel L. J. Divergent and conserved elements comprise the chemoreceptive repertoire of the non-blood feeding mosquito Toxorhynchites amboinensis. Genome Biol Evol 6, 2883–2896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot J. D. & Dickens J. C. Characterization of an enantioselective odorant receptor in the yellow fever mosquito Aedes aegypti. PLoS ONE 4, e7032 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag W. & Ney K. H. [Contribution on the occurrence of 1-octen-3-ol]. Eur J Biochem 4, 315–318 (1968). [DOI] [PubMed] [Google Scholar]
- Wurzenberger M. & Grosch W. The Enzymic Oxidative Breakdown of Linoleic Acid in Mushrooms (Psalliota bispora). Z Lebensm Unters Forch 175, 186–190 (1982). [Google Scholar]
- Bohbot J. D. & Pitts R. J. The narrowing olfactory landscape of insect odorant receptors. Frontiers in Ecology and Evolution 3, 39 (2015). [Google Scholar]
- Bohbot J. D. et al. Multiple activities of insect repellents on odorant receptors in mosquitoes. Med Vet Entomol 25, 436–444 (2011). [DOI] [PubMed] [Google Scholar]
- Syed Z. & Leal W. Mosquitoes smell and avoid the insect repellent DEET. Proceedings of the National Academy of Sciences 36, 13598–13603 (2008). [DOI] [PMC free article] [PubMed]
- Bohbot J. & Dickens J. Insect repellents: modulators of mosquito odorant receptor activity. PLoS ONE 5, e12138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiński E., Stawicki S. & Wasowicz E. Volatile flavor compounds produced by molds of Aspergillus, Penicillium, and Fungi imperfecti. Appl Microbiol 27, 1001–1004 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra F. Y. & Wikén T. O. Studies on mushroom flavours. 1. Organoleptic significance of constituents of the cultivated mushroom, agaricus bisporus. Z Lebensm Unters Forsch 160, 255–262 (1976). [DOI] [PubMed] [Google Scholar]
- Honkanen E. & Moisio T. On the occurrence of oct-1-en-3-ol in clover plants. Acta Chemica Scandinavica 17, 858 (1963). [Google Scholar]
- Lumen B. O., Stone E. J., Kazeniac S. J. & Forsythe R. H. Formation of volatile flavor compounds in green beans from linoleic and linolenic acids. Journal of Food Science 43, 698–702 (1978). [Google Scholar]
- Buttery R. G. & Kamm J. A. Volatile components of alfalfa: possible insect host plant attractants. Journal of agricultural and food chemistry 28, 978–981 (1980). [Google Scholar]
- Cork A. & Park K. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol 10, 269–276 (1996). [DOI] [PubMed] [Google Scholar]
- Bernier U. R. U., Kline D. L. D., Barnard D. R. D., Schreck C. E. C. & Yost R. A. R. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal Chem 72, 747–756 (2000). [DOI] [PubMed] [Google Scholar]
- Pierce A. M., Pierce H. D., Borden J. H. & Oehlschlager A. C. Production dynamics of cucujolide pheromones and identification of 1-octen-3-ol as a new aggregation pheromone for Oryzaephilus surinamensis and O. mercator (Coleoptera: Cucujidae). Environmental Entomology 18, 747–755 (1989). [Google Scholar]
- Maeda T. et al. Neuronal projections and putative interaction of multimodal inputs in the subesophageal ganglion in the blowfly, Phormia regina. Chem Senses 39, 391–401 (2014). [DOI] [PubMed] [Google Scholar]
- Bendera M., Ekesi S., Ndung′u M., Srinivasan R. & Torto B. A major host plant volatile, 1-octen-3-ol, contributes to mating in the legume pod borer, Maruca vitrata (Fabricius) (Lepidoptera: Crambidae). Naturwissenschaften 102, 47–47 (2015). [DOI] [PubMed] [Google Scholar]
- Arx von M., Schmidt-Büsser D. & Guerin P. M. Plant volatiles enhance behavioral responses of grapevine moth males, Lobesia botrana to sex pheromone. J Chem Ecol 38, 222–225 (2012). [DOI] [PubMed] [Google Scholar]
- Schmidt-Büsser D., Arx, von M. & Guerin P. M. Host plant volatiles serve to increase the response of male European grape berry moths, Eupoecilia ambiguella, to their sex pheromone. J Comp Physiol A 195, 853–864 (2009). [DOI] [PubMed] [Google Scholar]
- Magalhães-Junior J. T. et al. A laboratory evaluation of alcohols as attractants for the sandfly Lutzomyia longipalpis (Diptera:Psychodidae). Parasites & vectors 7, 60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeil R. M. & Mumma R. O. Bioassay for evaluating attraction of the phorid fly, Megaselia halterata to compost colonized by the commercial mushroom, Agaricus bisporus and to 1-octen-3-ol and 3-octanone. Entomologia Experimentalis et Applicata 69, 137–144 (2011). [Google Scholar]
- Steiner S., Erdmann D., Steidle J. L. M. & Ruther J. Host habitat assessment by a parasitoid using fungal volatiles. Front. Zool. 4, 3 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. E. Response of the antennal receptors of the male Aedes aegypti mosquito. Journal of Insect Physiology 23, 613–617 (1977). [Google Scholar]
- Collins L. E. & Blackwell A. Electroantennogram studies of potential oviposition attractants for Toxorhynchites moctezuma and T. amboinensis mosquitoes. Physiological Entomology 23, 214–219 (1998). [Google Scholar]
- Kamala Jayanthi P. D. et al. Specific volatile compounds from mango elicit oviposition in gravid Bactrocera dorsalis females. J Chem Ecol 40, 259–266 (2014). [DOI] [PubMed] [Google Scholar]
- Gouinguené S. P. & Städler E. Oviposition in Delia platura (Diptera, Anthomyiidae): the role of volatile and contact cues of bean. J Chem Ecol 32, 1399–1413 (2006). [DOI] [PubMed] [Google Scholar]
- van Breugel F., Riffell J., Fairhall A. & Dickinson M. H. Mosquitoes use vision to associate odor plumes with thermal targets. Curr Biol 25, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen J. T. & Tollsten L. Floral scent in bat-pollinated plants: a case of convergent evolution. Botanical Journal of the Linnean Society 119, 45–57 (1995). [Google Scholar]