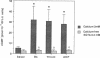Abstract
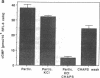
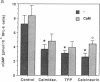
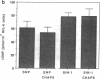
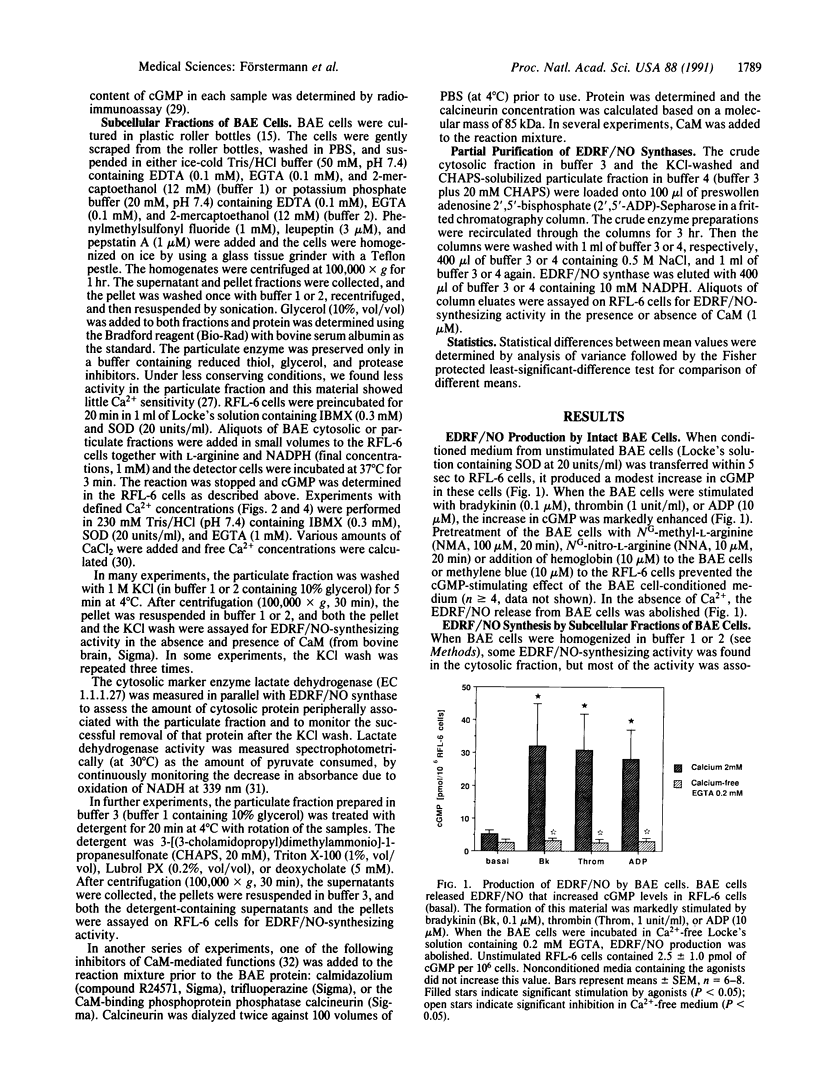
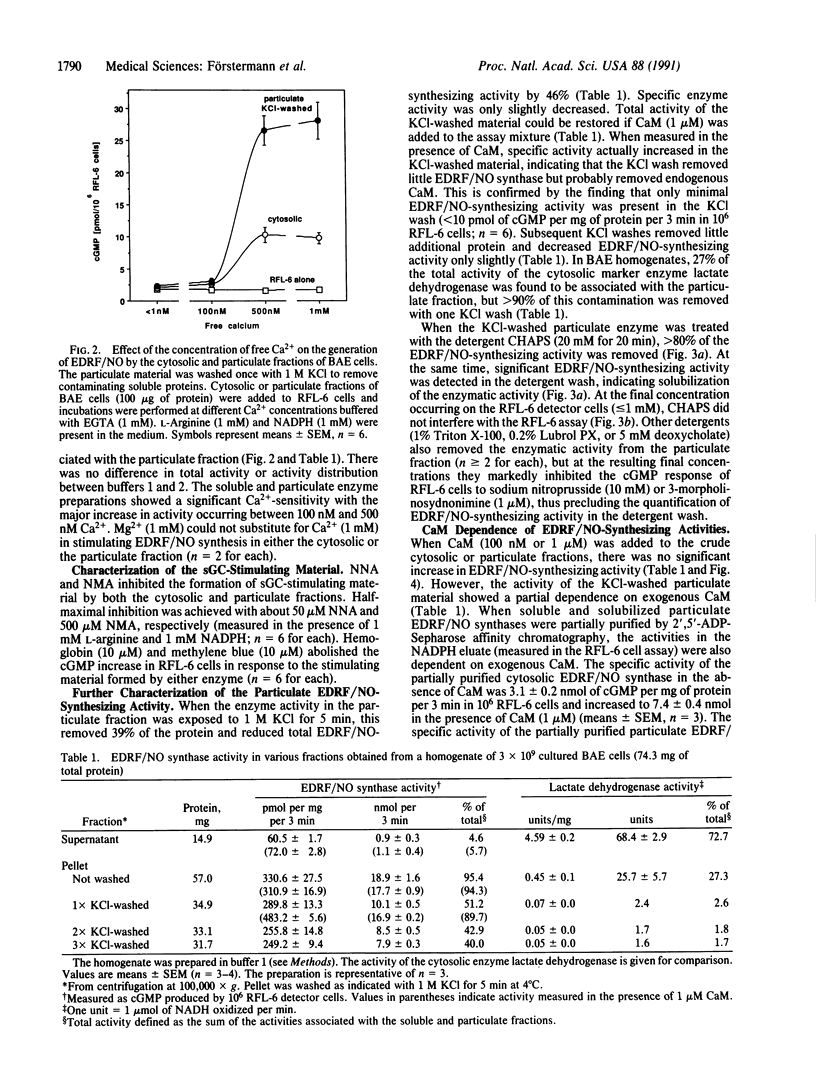
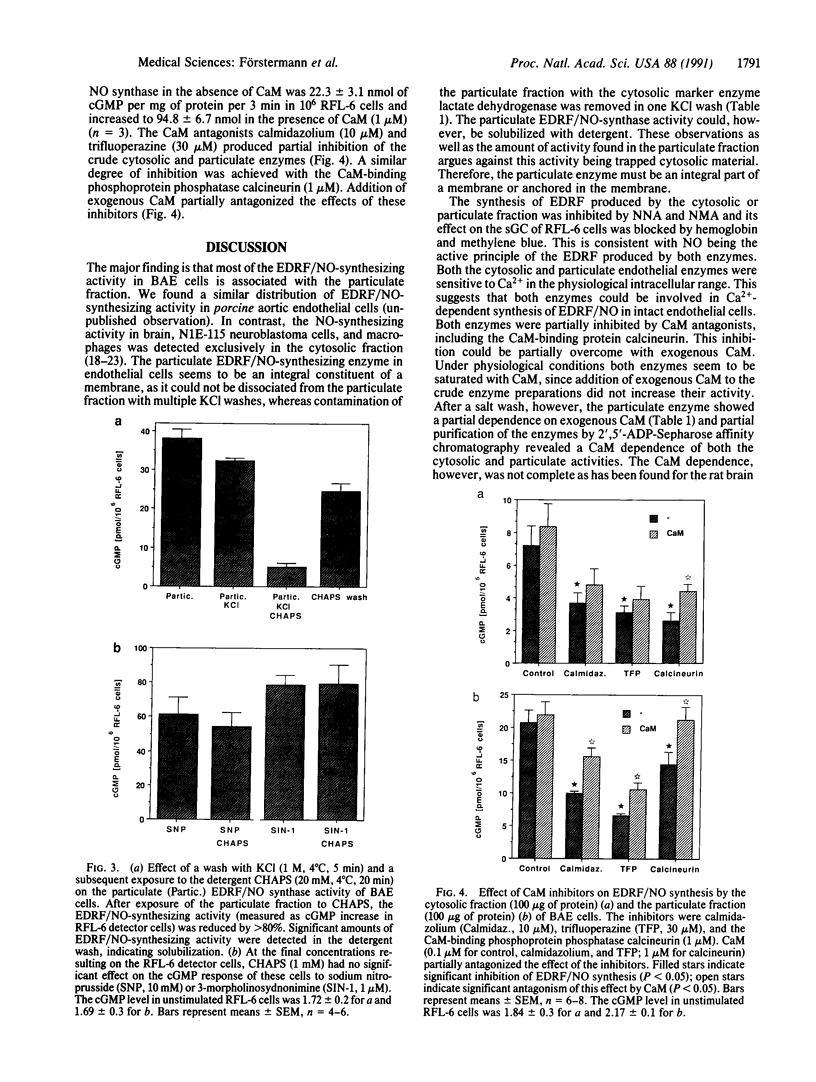
Endothelium-derived relaxing factor/nitric oxide (EDRF/NO) synthesized by bovine aortic endothelial cells and subcellular fractions thereof was assayed by its stimulating effect on soluble guanylyl cyclase of rat fetal lung fibroblasts (RFL-6 cells). The release of EDRF/NO by intact endothelial cells could be stimulated with bradykinin, thrombin, or ADP and was abolished in Ca2(+)-free medium. When subcellular fractions were analyzed, some EDRF/NO-synthesizing activity was found in the cytosolic fraction, but most of the activity was associated with the particulate fraction. Both enzyme activities required L-arginine and NADPH for EDRF/NO synthesis, both were inhibited by NG-nitro-L-arginine and NG-methyl-L-arginine, and hemoglobin or methylene blue abolished the effect of the EDRF/NO produced by both enzymes. Both enzymes were highly sensitive to Ca2+; the major increase in activity occurred between 100 and 500 nM free Ca2+. Exposure of the particulate enzyme activity to 1 M KCl removed 39% of the protein and reduced total activity by 46%, but the activity was restored when exogenous calmodulin (CaM) was added. Further KCl washes caused little further loss of protein or EDRF/NO synthase activity. The KCl-washed particulate enzyme could be solubilized with the detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. The CaM antagonists calmidazolium and trifluoperazine as well as the CaM-binding protein calcineurin inhibited the EDRF/NO synthesis by both the cytosolic and the particulate enzyme. These effects were partially reversed with exogenous CaM. Partial purification of the cytosolic and solubilized particulate enzymes by affinity chromatography on adenosine 2',5'-bisphosphate-Sepharose resulted in EDRF/NO synthase activities dependent on exogenous CaM. We conclude that endothelial cells contain both cytosolic and particulate enzymes that synthesize EDRF/NO. Both enzymes are regulated by free Ca2+ and, at least in part, by CaM.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alheid U., Frölich J. C., Förstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res. 1987 Sep 1;47(5):561–571. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- Amber I. J., Hibbs J. B., Jr, Taintor R. R., Vavrin Z. The L-arginine dependent effector mechanism is induced in murine adenocarcinoma cells by culture supernatant from cytotoxic activated macrophages. J Leukoc Biol. 1988 Feb;43(2):187–192. doi: 10.1002/jlb.43.2.187. [DOI] [PubMed] [Google Scholar]
- Boje K. M., Fung H. L. Endothelial nitric oxide generating enzyme(s) in the bovine aorta: subcellular location and metabolic characterization. J Pharmacol Exp Ther. 1990 Apr;253(1):20–26. [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990 Jun 4;265(1-2):133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Gorsky L. D., Pollock J. S., Ishii K., Schmidt H. H., Heller M., Murad F. Hormone-induced biosynthesis of endothelium-derived relaxing factor/nitric oxide-like material in N1E-115 neuroblastoma cells requires calcium and calmodulin. Mol Pharmacol. 1990 Jul;38(1):7–13. [PubMed] [Google Scholar]
- Förstermann U., Gorsky L. D., Pollock J. S., Schmidt H. H., Heller M., Murad F. Regional distribution of EDRF/NO-synthesizing enzyme(s) in rat brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):727–732. doi: 10.1016/0006-291x(90)92382-a. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Ishii K., Gorsky L. D., Murad F. The cytosol of N1E-115 neuroblastoma cells synthesizes an EDRF-like substance that relaxes rabbit aorta. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):771–774. doi: 10.1007/BF00169689. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Gorsky L. D., Förstermann U., Ishii K., Murad F. Production of an EDRF-like activity in the cytosol of N1E-115 neuroblastoma cells. FASEB J. 1990 Mar;4(5):1494–1500. doi: 10.1096/fasebj.4.5.2155150. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman D. C., Agnost V. L., Tuan J. J., Andresen J. W., Murad F. Atrial natriuretic factor and sodium nitroprusside increase cyclic GMP in cultured rat lung fibroblasts by activating different forms of guanylate cyclase. Biochem J. 1987 May 15;244(1):69–74. doi: 10.1042/bj2440069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rimele T. J., Sturm R. J., Adams L. M., Henry D. E., Heaslip R. J., Weichman B. M., Grimes D. Interaction of neutrophils with vascular smooth muscle: identification of a neutrophil-derived relaxing factor. J Pharmacol Exp Ther. 1988 Apr;245(1):102–111. [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Pollock J. S., Nakane M., Gorsky L. D., Förstermann U., Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Seifert R., Böhme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor and leukotriene B4. FEBS Lett. 1989 Feb 27;244(2):357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- Segal J. Cation chelators and their utilization in the preparation of low concentrations of calcium. Caution of use in biological systems with high affinity to calcium. Biotechnol Appl Biochem. 1986 Oct;8(5):423–429. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Gross S. S., Thiel B. A., Levi R., Nathan C. F. Synthesis of nitrogen oxides from L-arginine by macrophage cytosol: requirement for inducible and constitutive components. Biochem Biophys Res Commun. 1989 Jun 15;161(2):420–426. doi: 10.1016/0006-291x(89)92615-6. [DOI] [PubMed] [Google Scholar]