Abstract
Dendritic cells (DCs) utilize pattern recognition receptors to detect microorganisms and activate protective immunity. These cells and receptors are thought to operate in an all-or-none manner, existing in an immunologically active or inactive state. Here, we report that encounters with microbial products and self-encoded oxidized phospholipids (oxPAPC) induce an enhanced DC activation state, which we call “hyperactive.” Hyperactive DCs induce potent adaptive immune responses and are elicited by caspase-11, an enzyme that binds oxPAPC and bacterial lipopolysaccharide (LPS). oxPAPC and LPS bind caspase-11 via distinct domains and elicit different inflammasome-dependent activities. Both lipids induce caspase-11–dependent interleukin-1 release, but only LPS induces pyroptosis. The cells and receptors of the innate immune system can therefore achieve different activation states, which may permit context-dependent responses to infection.
Pattern recognition receptors (PRRs) promote immune defenses upon encountering LPS and other microbial products, which are collectively known as pathogen-associated molecular patterns (PAMPs). PRRs also recognize self-encoded molecules called damage-associated molecular patterns (DAMPs) (1, 2). The existence of self-derived PRR ligands complicates our current understanding of PRRs as determinants of self/nonself discrimination.
Oxidized phospholipids derived from 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine (PAPC), known as oxPAPC, represent one class of DAMPs. oxPAPC is found in dying cells (3), and can reach concentrations of 10-100 μM in damaged tissues (4, 5). oxPAPC is an LPS-mimic that, depending on context, promotes or inhibits Toll-like Receptor 4 (TLR4)–dependent inflammation (6–8). The existence of LPS and a self-derived LPS-mimic provides a model to dissect the activities of PAMPs and DAMPs in innate immunity.
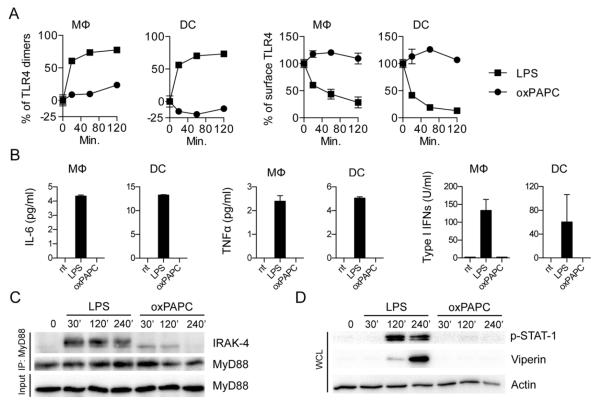
If oxPAPC is truly an LPS-mimic, then LPS and oxPAPC should exhibit similar activities. We therefore determined if oxPAPC activates TLR4 in murine bone marrow-derived macrophages (MΦ) and dendritic cells (DCs). LPS, but not oxPAPC, induced TLR4 dimerization and endocytosis, MyD88-IRAK4 interactions (i.e., myddosome formation), and expression of the cytokines interleukin (IL)-6, tumor necrosis factor (TNF)α, IL-1β, and interferon (IFN)β (Fig. 1, A to C, and fig. S1, A to C). Furthermore, oxPAPC-treated cells contained undetectable viperin or phosphorylated STAT1, both of which were abundant upon LPS treatment (Fig. 1D and fig. S1D). These data indicate that oxPAPC cannot activate TLR4.
Fig. 1. oxPAPC does not induce TLR4 signaling.
(A) MΦs or DCs were treated with LPS or oxPAPC for the indicated time points. TLR4 dimerization and endocytosis was measured by flow cytometry. Line graph represents means and standard deviations of four replicates. (B) MΦs or DCs were treated with LPS or oxPAPC. Cytokine production was analyzed 18 hours later. Means and standard deviations of four replicates are shown. (C) Myddosome formation in iMΦs was assessed at the indicated time points after treatment with LPS or oxPAPC by co-immunoprecipitation (IP) of IRAK4 with MyD88 followed by western analysis of the proteins indicated. (D) Whole cell lysates (WCL) were collected and DCs were monitored for STAT-1 phosphorylation and viperin expression after treatment with LPS oxPAPC. (C and D) One experiment representative of three is shown.
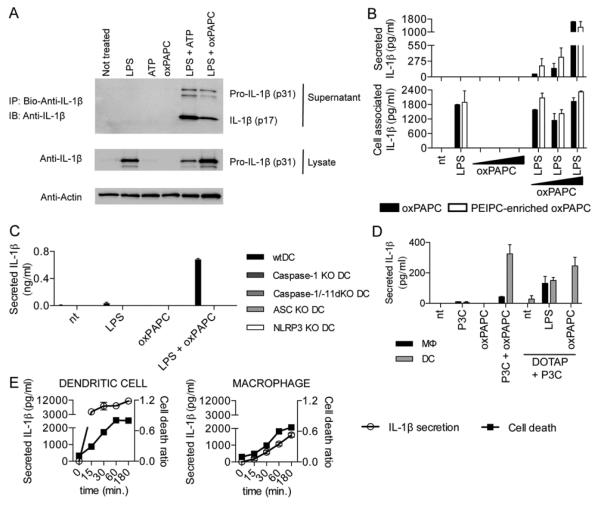
Some DAMPs only induce cytokine release from cells previously exposed to microbial products. For example, ATP activates IL-1β release from cells primed with TLR ligands (9). We therefore examined IL-1β release from LPS-primed DCs. Interestingly, oxPAPC, similar to ATP, induced the release of cleaved IL-1β from LPS-primed DCs (Fig. 2, A and B, and fig. S2, A and B). oxPAPC also elicited IL-1β release from primed DCs isolated from the spleens of mice (fig. S2D).
Fig. 2. oxPAPC induces the activation of the NLRP3 inflammasome in DCs.
(A) DCs primed with LPS, followed by ATP or oxPAPC treatment. Cell culture supernatant from DCs subjected to indicated treatments were collected, and processed IL-1β (p17) production was assessed. One experiment representative of three is shown. (B) DCs were treated with LPS alone, 10, 50, or 120μM oxPAPC or were primed with LPS for 3 hours and then treated with oxPAPC. For this experiment, commercially available oxPAPC and an oxPAPC enriched in PEIPC were used. 18 hours after LPS administration, secreted (left panel) and cell associated (right panel) IL-1β were measured by ELISA. Means and standard deviations of four replicates are shown. (C) DCs of the genotypes indicated were treated with LPS alone, oxPAPC alone or were primed with LPS for 3 hours and then treated with oxPAPC. 18 hours after LPS administration, IL-1β and secretion was measured by ELISA. Means and standard deviations of four replicates are shown. (D) MΦs and DCs were treated with Pam3CSK (P3C) alone, oxPAPC alone, or were primed with Pam3CSK for 3 hours and then treated with oxPAPC, DOTAP alone, and LPS or oxPAPC encapsulated in DOTAP. 18 hours after P3C administration, IL-1β was measured by ELISA. Means and standard deviations of four replicates are shown. (E) DCs (left panel) or MΦs (right panel) were primed with LPS for three hours and treated with ATP. At indicated time points, IL-1β was measured by ELISA and cell death was measured by PI permeabilization assay. Means and standard deviations of four replicates are shown.
Oxidation of PAPC to oxPAPC generates a heterogeneous mixture of lipids (fig. S4, A and B). To determine if alternative sources of oxPAPC have similar activities, we generated oxPAPC enriched in PEIPC (1-palmitoyl-2-(5,6 epoxyisoprostanoyl)-sn-glycero-3-phosphocholine) (fig. S4C), an active component of oxPAPC (10). Like oxPAPC, PEIPC induced IL-1β release from LPS-primed DCs (Fig. 2B).
In contrast to the effects on IL-1β release, neither ATP nor oxPAPC influenced the abundance of cell-associated IL-1β (Fig. 2B and fig. S2C), or the secretion of TNFα (fig. S2, D and E). Additionally, when DCs were treated simultaneously with LPS/ATP or LPS/oxPAPC (i.e., no priming), IL-1β release was only induced by LPS/oxPAPC (fig. S3B), suggesting differences in how these DAMPs promote IL-1β release. When the phosphocholine variant, 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), was used, it could not elicit IL-1β release (fig. S3C). In contrast, purified components of oxPAPC (KOdiA-PC, POVPC, or PGPC) elicited IL-1β release (fig. S3C). In all cases, LPS-induced TNFα secretion was unaffected (fig. S3C). Individual lipids within oxPAPC therefore promote IL-1β release.
Inflammasomes are cytoplasmic protein complexes that trigger IL-1β release (9). To determine if IL-1β release is in flammasome-dependent, we examined DCs from ASC knockout (KO), caspase-1 KO, caspase-1/caspase-11 double (d)KO or NLRP3 KO mice, each of which are defective for inflammasome functions (11, 12). All of these factors were required for oxPAPC-induced IL-1β release (Fig. 2C), where as no inflammasome regulator was required for LPS-induced TNFα secretion (fig. S2F).
Interestingly, oxPAPC could not elicit IL-1β release from MΦs (fig. S3A). To explain this finding, we considered that DCs are better “primed” than MΦs, as they produce more TNFα than MΦs in response to LPS (fig. S2E). However, IFNγ-treated MΦs were primed as well as DCs, yet they could not respond to oxPAPC (fig. S3D). Transfection of oxPAPC elicited IL-1β release from DCs primed with the TLR2 ligand Pam3CSK, but not MΦs, whereas LPS transfection of MΦs elicited IL-1β release (Fig. 2D). ATP treatments also revealed differences between MΦs and DCs. DCs and MΦs die upon LPS/ATP with similar kinetics, but release different amounts of IL-1β (Fig. 2E) and express different levels of ASC (fig. S3, E and F), but not other inflammasome components (fig. S3F). oxPAPC therefore revealed differences in inflammasome-related activities in bone marrow derived MΦs and DCs (fig. S5). We do note, however, that populations of DCs and MΦs may exist that exhibit different responses to oxPAPC than those described above.
Further analysis of the mechanisms of inflammasome activation revealed that potassium efflux promoted ATP-induced, but not oxPAPC-induced, IL-1β release (fig. S6, A to C). Additionally, oxPAPC did not alter mitochondrial functions (fig. S6D).
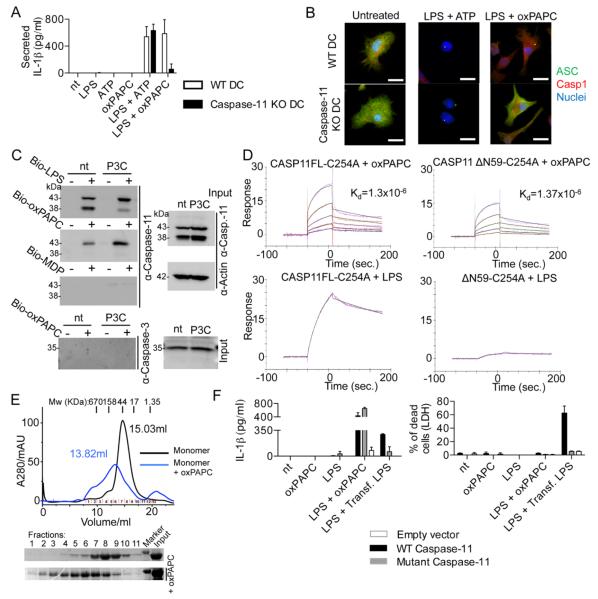
Caspase-11 is an LPS receptor that promotes IL-1β release by noncanonical inflammasomes (13). Interestingly, oxPAPC-mediated IL-1β release was abolished in caspase-11 KO DCs (Fig. 3A), whereas ATP-mediated IL-1β release remained intact. TNFα secretion was unaffected by caspase-11 deficiency (fig. S6E). Microscopic analysis revealed that oxPAPC and ATP induced the formation of ASC and caspase-1 containing “specks” in LPS-pretreated DCs (Fig. 3B), albeit with different kinetics (fig. S6F). These structures are recognized as individual inflammasomes (14), and in the specific case of oxPAPC stimulations, speck formation was caspase-11 dependent (Fig. 3B and fig. S6G). Caspase-11 is therefore likely required for oxPAPC induced IL-1β release because it promotes inflammasome assembly in DCs.
Fig. 3. oxPAPC promotes noncanonical inflammasome activation.
(A) WT DC and caspase-11 KO DC were treated with LPS alone, oxPAPC alone or were primed with LPS for 3 hours and then treated with oxPAPC. 18 hours after LPS administration, IL-1β secretion was measured by ELISA. Means and standard deviations of four replicates are shown. (B) DCs were left untreated or primed with LPS and then stimulated with ATP or oxPAPC. Specks containing ASC (green) and caspase-1 (Casp1, red) were analyzed 18 hours after LPS stimulation. Nuclei are shown in blue. Panels are representative of four independent experiments. Scale bar: 10 μm. (C) S100 fractions of nontreated (nt) or P3C-primed (P3C) MΦs were incubated with biotin-LPS (Bio-LPS), biotin-oxPAPC (Bio-oxPAPC) or biotin-MDP (Bio-MDP). Endogenous proteins associated with biotinylated-ligands were captured by streptavidin beads and revealed by western analysis. Shown is a representative blot out of three independent experiments. (D) SPR analysis of the interactions between the proteins and lipids indicated. (E) Gel filtration analysis of the size of caspase-11 complexes before and after exposure to oxPAPC. Complex size was monitored by A280 or western analysis, as indicated. Shown is a representative blot out of three independent experiments. (F) Bone marrow cells were infected with the pMSCV2.2-IRES-GFP vector (empty), the pMSCV2.2-IRES-GFP vector encoding WT caspase-11 (WT caspase-11) or the same vector containing a catalytic mutant caspase-11 (C254A). DCs were primed or not with LPS and then stimulated with oxPAPC, or transfected with LPS-containing FuGENE (Trans. LPS). 18 hours after LPS priming, supernatant were collected and IL-1β was measured by ELISA. Cell viability was assessed by measuring LDH release. Means and standard deviations of four replicates are shown.
Interestingly, multiple TLR ligands primed DCs for oxPAPC responsiveness, as Pam3CSK-primed DCs induced IL-1β release in response to oxPAPC (fig. S6H), by an NLRP3-, ASC- and caspase-11–dependent process (fig. S6H). The TLR9 ligand CpG-DNA also primed DCs for oxPAPC responsiveness (fig. S6I). Similarly, oxPAPC, but not DMPC, elicited IL-1β release from an LPS or CpG-DNA primed splenic DC line called D1 (15) (fig. S6J). oxPAPC therefore activates multiple DCs upon encounters with diverse TLR ligands. The finding that multiple TLR ligands prime DCs for oxPAPC responsiveness eliminates the possibility that oxPAPC acts as an LPS-carrier to caspase-11.
We considered that oxPAPC interacts with caspase-11, like LPS (13). Endogenous caspase-11 (but not caspase-3) was captured from DC or immortal bone marrow-derived MΦ (iMΦ) lysates through interactions with biotin-LPS or biotin-oxPAPC (figs. S4D and S7A and Fig. 3C). Caspase-11 was not captured by the biotinylated NOD2 ligand MDP (Fig. 3C). oxPAPC displayed a dose-dependent signal with immobilized catalytically inactive caspase-11(C254A) using surface plasmon resonance (SPR), as did LPS (Fig. 3D). In contrast, DMPC did not bind caspase-11, and oxPAPC did not bind IgG (fig. S7B). The dissociation constant (Kd) between caspase-11 and oxPAPC was calculated as 1.3 × 10−6 M, whereas the Kd for interactions with LPS is 3.78 × 10−8 M (13). Gel filtration chromatography revealed that oxPAPC also promoted caspase-11 oligomerization (Fig. 3E), with monomers eluting at 15.03 ml, dimers at 13.82 ml, and higher order oligomers earlier.
Mutation of lysine residues within the caspase-11 CARD prevent interactions with LPS (13), as assessed by the ability of biotin-LPS to capture caspase-11 produced in 293T cells (fig. S7C). Interestingly, these mutations did not prevent interactions with biotin-oxPAPC (fig. S7C). Moreover, the isolated caspase-11 catalytic domain (but not the CARD) retained the ability to bind biotin-oxPAPC (fig. S7D). SPR analysis verified these results, as nearly identical affinities of oxPAPC for caspase-11 or the catalytic domain (noted as δN59) were calculated (Fig. 3D). LPS could not bind the caspase-11 catalytic domain (Fig. 3D), as expected (13). These data establish that distinct domains within caspase-11 bind LPS and oxPAPC.
The interaction of oxPAPC with the catalytic domain prompted us to examine caspase-11 enzymatic activity. Whereas LPS strongly increased activity of caspase-11 monomers, oxPAPC displayed minimal activity (fig. S7E). We also examined pre-existing caspase-11 oligomers, where intrinsic activity is high (fig. S7E). Interestingly, whereas LPS stimulated this activity further, oxPAPC suppressed intrinsic activity (fig. S7E). Moreover, oxPAPC blocked LPS-induced caspase-11 activity, in a dose-dependent manner (fig. S7F). These data indicate that LPS promotes, but oxPAPC prevents, caspase-11 activity.
To determine if caspase-11 activity is required for oxPAPC-induced IL-1β release, we reconstituted caspase-11 KO DCs with WT or catalytic mutant (C254A) caspase-11, or empty vector. LPS elicited IL-1β release from cells expressing WT caspase-11, but not empty vector or mutant caspase-11 (Fig. 3F). These data confirm that caspase-11 activity promotes LPS-induced IL-1β release (16, 17). Interestingly, WT and mutant-reconstituted DCs released IL-1β in response to oxPAPC (Fig. 3F). TNFα release was unaffected under all conditions (fig. S7G). Two modes of caspase-11 mediated IL-1β release therefore exist, with catalytic activity only being necessary for LPS responses.
In addition to caspase-11, oxPAPC-induced IL-1β release requires caspase-1 (Figs. 2C and 3A). Interestingly, independent of caspase-11, biotin-oxPAPC captured endogenous caspase-1 from cell lysates, whereas biotin-LPS could not (fig. S7, H and I). These data support a model whereby oxPAPC and LPS promote inflammasome formation via distinct mechanisms, with oxPAPC specifically forming a caspase-1/11 hetero-complex that may promote IL-1β release. The precise mechanisms that govern oxPAPC-caspase interactions, and how these interactions promote inflammasome activities, await further investigation.
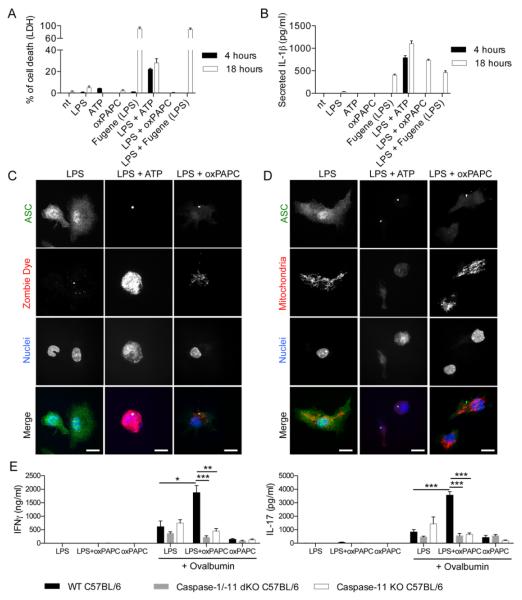
Pyroptosis, another inflammasome-dependent activity (18), is characterized by loss of plasma membrane integrity, and the release cytoplasmic proteins and organelles. Caspase-11 activity was necessary for transfected LPS to induce pyroptosis, as assessed by LDH release from the cytosol (Fig. 3F). Surprisingly, oxPAPC did not elicit pyroptosis (Fig. 3F). We explored this observation further in WT DCs, where LPS/ATP or transfected LPS induced pyroptosis with differing kinetics (Fig. 4A). Interestingly, while LPS transfection or oxPAPC treatment induced similar amounts of IL-1β release (Fig. 4B), only LPS transfection caused pyroptosis (Fig. 4A and fig. S8A).
Fig. 4. oxPAPC prevents DC death and potentiates adaptive immune responses.
(A and B) DCs were treated with LPS alone, ATP alone, oxPAPC alone or FuGENE complexed LPS [Fugene (LPS)], or were primed for three hours with LPS and then treated with the indicated stimuli. Cell death was measured by LDH release (A) or IL-1β secretion was measured by ELISA (B). Means and standard deviations of four replicates are shown. (C and D) DCs were pretreated with LPS for 3 hours and then activated with ATP or oxPAPC. 18 hours later, cells were stained for ASC (green), nuclei (blue) Zombie Dye (red) (C) or active mitochondria (red). Scale bar: 10 μm. (D). Panels are representative of three independent experiments. (E) CD4+ T-cells were isolated from the draining lymph nodes 40 days after immunization with OVA + LPS in IFA (LPS), OVA + LPS + oxPAPC in IFA (LPS+oxPAPC) or OVA + oxPAPC in IFA (oxPAPC) of WT, caspase-1/-11 dKO or caspase-11 KO mice. CD4+ T-cells were restimulated or not with OVA in the presence of DCs. IFNγ (left panel) and IL-17 (right panel) secretion was measured 5 days later by ELISA. Bar graphs represent means and standard errors of two experiments with five animals per group. *P < 0.05; **P < 0.01; ***P < 0.005.
To corroborate these observations, we examined plasma membrane integrity of individual cells containing ASC-specks. Cells treated with LPS/ATP contained specks, and these cells lost mitochondria and stained positive for Zombie dye, a cytoplasmic stain (Fig. 4, C and D). In contrast, cells treated with LPS/oxPAPC contained specks, but retained functional mitochondria and displayed minimal Zombie staining (Fig. 4, C and D). These data indicate that oxPAPC-induced inflammasomes do not promote pyroptosis, and suggest that oxPAPC promotes IL-1β release from living cells. Moreover, not only does oxPAPC not induce pyroptosis, this lipid counteracted the slow-acting death pathways activated by LPS (19) (fig. S8B).
Since oxPAPC promotes DC viability and IL-1β promotes T-cell activation (20, 21), we examined if oxPAPC displayed adjuvant activity. WT, caspase-11 and caspase-1/-11 dKO mice were injected subcutaneously with LPS, ovalbumin (OVA) and/or oxPAPC that was emulsified in incomplete Freund’s adjuvant. After 40 days, CD4+ T-cells were isolated from draining lymph nodes and exposed to DCs that were pulsed (or not) with OVA. T-cell activation was examined by measuring IL-2, IL-17 and IFNγ secretion. Interestingly, LPS/oxPAPC immunizations yielded substantially higher levels of all cytokines examined, as compared to immunizations with LPS alone (Fig. 4E and fig. S8C). The ability of oxPAPC to enhance T-cell activation was lost in caspase-11 or caspase-1/-11 dKO mice (Fig. 4E and fig. S8C). Similar results were obtained measuring T-cell responses 7 days after immunization (fig. S8D). oxPAPC therefore potentiates LPS-mediated T-cell activation in a caspase-11–dependent manner.
In this study, we report two states of DC activation. The first state results from encounters with PAMPs, which induce TLRs to upregulate several factors that promote T-cell activation (22). The second state of DCs is “hyperactive” and results from coincident encounters with PAMPs and oxPAPC, an abundant lipid at sites of tissue damage. The co-detection of PAMPs and oxPAPC promotes activities elicited by the classical DC activation state, but also promotes DC survival and IL-1β release. As such, hyperactive DCs are superb inducers of T-cell mediated immunity. We speculate that promoting DC hyperactivation may benefit vaccination regimens, and may naturally be important during highly infectious encounters, where tissue damage and microbial products are abundant.
Our analysis also revealed caspase-11 to be an unusual PRR, which binds PAMPs and DAMPs via distinct domains and has distinct modes of activation. We consider CARD engagement by LPS to be an anti-microbial mode of caspase-11 activation, designed to expose intracellular bacteria to infiltrating neutrophils after pyroptosis (23). In contrast, catalytic domain engagement by oxPAPC may be an immuno-regulatory mode of caspase-11 activation, designed to promote T-cell activation, specifically in DCs (fig. S5).
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Kagan laboratory for helpful discussions. The results reported in this manuscript are tabulated in the main paper and in the supplementary materials. J.C.K. and Boston Children’s Hospital has filed an international patent application (PCT/US2016/012994) that relates to the adjuvant activity of oxPAPC. C.K. is supported by NIH grants AI093589, AI072955, and P30 DK34854, and a gift from Mead Johnson & Company. J.C.K. holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. I.Z. is supported by NIH grant 1R01AI121066-01A1, HDDC P30 DK034854 grant, and the Cariplo Foundation. Y.T. is supported by a fellowship of the Jane Coffin Childs Fund (the Merck Fellow). J.S. is supported by NIH grant 1R15HL121770-01A1. J.S. and F.S. performed the experiments in Fig. 3D but were not involved in the experimental design or interpretation of data.
Footnotes
www.sciencemag.org/cgi/content/full/science.aaf3036/DC1
Materials and Methods
Figs. S1 to S8
References (24–26)
REFERENCES AND NOTES
- 1.Pradeu T, Cooper EL. The danger theory: 20 years later. Front. Immunol. 2012;3:287. doi: 10.3389/fimmu.2012.00287. Medline doi:10.3389/fimmu.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. Medline doi:10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. Medline doi:10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berliner JA, Watson AD. A role for oxidized phospholipids in atherosclerosis. N. Engl. J. Med. 2005;353:9–11. doi: 10.1056/NEJMp058118. Medline doi:10.1056/NEJMp058118. [DOI] [PubMed] [Google Scholar]
- 5.Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr. Opin. Lipidol. 2003;14:421–430. doi: 10.1097/00041433-200310000-00002. Medline doi:10.1097/00041433-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. Medline doi:10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, Chen WH, Ernst RK, Rossignol DP, Gusovsky F, Blanco JC, Vogel SN. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. Medline doi:10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. Medline doi:10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 9.Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: A danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. Medline doi:10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Springstead JR, Gugiu BG, Lee S, Cha S, Watson AD, Berliner JA. Evidence for the importance of OxPAPC interaction with cysteines in regulating endothelial cell function. J. Lipid Res. 2012;53:1304–1315. doi: 10.1194/jlr.M025320. Medline doi:10.1194/jlr.M025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. Medline doi:10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 12.Ye Z, Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr. Opin. Immunol. 2008;20:3–9. doi: 10.1016/j.coi.2008.01.003. Medline doi:10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. Medline. [DOI] [PubMed] [Google Scholar]
- 14.Stutz A, Horvath GL, Monks BG, Latz E. ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol. 2013;1040:91–101. doi: 10.1007/978-1-62703-523-1_8. Medline doi:10.1007/978-1-62703-523-1_8. [DOI] [PubMed] [Google Scholar]
- 15.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. Medline doi:10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. Medline doi:10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. Medline. [DOI] [PubMed] [Google Scholar]
- 18.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, Costa B, Zaza A, Ricciardi-Castagnoli P, Granucci F. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. Medline doi:10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 20.Sims JE, Smith DE. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. Medline doi:10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 21.Schenten D, Nish SA, Yu S, Yan X, Lee HK, Brodsky I, Pasman L, Yordy B, Wunderlich FT, Brüning JC, Zhao H, Medzhitov R. Signaling through the adaptor molecule MyD88 in CD4+ T cells is required to overcome suppression by regulatory T cells. Immunity. 2014;40:78–90. doi: 10.1016/j.immuni.2013.10.023. Medline doi:10.1016/j.immuni.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. Medline doi:10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015;265:130–142. doi: 10.1111/imr.12287. Medline doi:10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat. Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. Medline doi:10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 25.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. Medline doi:10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 26.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. Medline doi:10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.