Abstract
BCR-ABL tyrosine kinase inhibitors (TKIs) are effective against chronic myeloid leukemia (CML), but they rarely eliminate CML stem cells. Disease relapse is common upon therapy cessation, even in patients with complete molecular responses. Furthermore, once CML progresses to blast crisis (BC), treatment outcomes are dismal. We hypothesized that concomitant targeting of BCL-2 and BCR-ABL tyrosine kinase could overcome these limitations. We demonstrate increased BCL-2 expression at the protein level in bone marrow cells, particularly in Lin−Sca-1+cKit+ cells of inducible CML in mice as determined by CyTOF mass cytometry. Further, selective inhibition of BCL-2, aided by TKI-mediated MCL-1 and BCL-XL inhibition, markedly decreased leukemic Lin−Sca-1+cKit+ cell numbers and long-term stem cell frequency, and prolonged survival in a murine CML model. Additionally, this combination effectively eradicated CD34+CD38−, CD34+CD38+, and quiescent stem/progenitor CD34+ cells from BC CML patient samples. Our results suggest that BCL-2 is a key survival factor for CML stem/progenitor cells and that combined inhibition of BCL-2 and BCR-ABL tyrosine kinase has the potential to significantly improve depth of response and cure rates of chronic phase and BC CML.
Introduction
Chronic myeloid leukemia (CML) is characterized by the t(9;22) Philadelphia translocation in hematopoietic stem cells, which results in constitutive activation of BCR-ABL tyrosine kinase and aberrant myeloid cell proliferation. BCR-ABL tyrosine kinase inhibitors (TKIs) are the most successful class of molecular targeted therapy of any malignant disease and, as such, they are the first-line therapy for newly diagnosed CML. However, they are inactive against CML stem cells (1–3). Hence, cures of CML with TKIs are rare (4).
CML stem cells are quiescent, and this likely accounts for the lack of disease eradication by TKIs in most patients (1–8). CML stem cells can accumulate additional mutations, including those in BCR-ABL, and thus may provide a reservoir for the emergence of drug-resistant clones. With additional mutations, activation of alternative signaling pathways, and mechanisms that are not fully understood, a subset of CML progresses to blast crisis (BC). Not surprisingly, TKIs are essentially ineffective against BC CML (9), and treatment options for these patients are limited. Furthermore, in these patients leukemia stem cells can also originate from progenitor cells that acquire self-renewal capacity (10). Therefore, strategies that eradicate CML stem cells and target BC CML are needed to improve true cure rates in chronic phase CML and treatment outcomes in BC CML.
BCL-2 family proteins are key regulators of mitochondrial-mediated apoptosis (11, 12) and critical for the survival of leukemia and leukemia stem cells (13–16). BCL-2 anti-apoptotic protein expression is higher in CML than in normal hematopoietic stem cells, and is further increased in BC CML (17, 18). BCR-ABL signaling supports CML cell survival, in part, by upregulating anti-apoptotic BCL-2 proteins including BCL-XL and MCL-1 (19, 20). We and others have reported that combined treatments with dual BCL-2/BCL-XL or pan-BCL-2 inhibitors and TKIs can eliminate CML stem/progenitor cells (17, 21–23). Recently, we demonstrated that activation of p53 in combination with BCL-2/BCL-XL or BCR-ABL inhibition has synergistic effects in CD34+ proliferating and quiescent BC CML cells, in part by inducing pro- and suppressing anti-apoptotic BCL-2 proteins (24). However, it is not entirely clear which BCL-2 members are indispensable for CML stem cell survival. ABT-263, a potent BCL-2/BCL-XL inhibitor, has entered clinical trials for hematological malignancies. Although it has shown effectiveness, it causes pronounced thrombocytopenia resulting from the depletion of BCL-XL-dependent platelets (25). Thus, for the future development of this targeting concept, it is critical to determine if inhibition of BCL-2 alone is sufficient to sensitize CML stem cells to TKIs.
ABT-199/GDC-0199 (venetoclax) is a potent and highly selective BCL-2 inhibitor with strong antitumor activity (26, 27). Preclinical studies demonstrated that ABT-199 has activity against various hematological malignancies (28–32). ABT-199 was recently approved by FDA for chronic lymphocytic leukemia and has entered clinical testing for acute myeloid leukemia (AML), lymphoma, and multiple myeloma. A recent study showed that ABT-199 enhanced imatinib-induced CML progenitor cell death in vitro (33). However, the effect of ABT-199 alone and in combination with TKIs on CML stem cells in vivo and stem/progenitor cells from patients with TKI-resistant BC has not been investigated.
Using CyTOF mass cytometry, a BCR-ABL transgenic murine model, and cells from patients with BC, we determined the role of BCL-2 in CML and the effect of its inhibition by ABT-199 alone and in combination with TKIs. We demonstrate the critical role of BCL-2 in CML cells and stem/progenitor cells and show that selective inhibition of BCL-2, aided by TKI-mediated BCL-XL/MCL-1 inhibition, has the potential to cure CML by eliminating CML stem cells.
Results
Targeting of BCL-2 and BCR-ABL exerts potent anti-leukemia activity in BCR-ABL transgenic mice
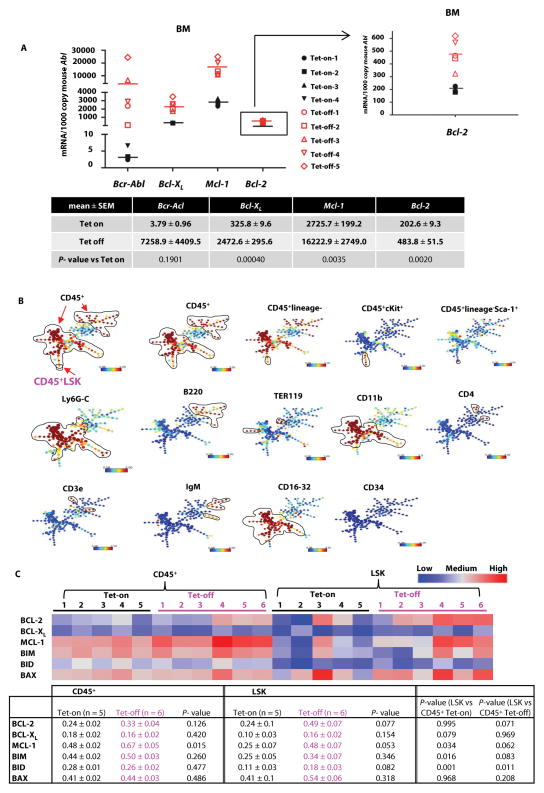
To assess the anti-leukemia activity of ABT-199 and TKI combinations in CML, we used an inducible transgenic CML mouse model (Scl-tTa-BCR-ABL) in a FVB/N background as previously described (34). We withdrew tetracycline (Tet-off), and the mice developed a myeloproliferative disease as evidenced by increased white blood cell (WBC) and neutrophil counts and enlarged spleens (fig. S1), reminiscent of human CML as previously described for this model. We collected bone marrow (BM) cells from these mice (Tet-off, n = 5; Tet-on, n = 4) and quantified Bcr-Abl, Bcl-XL, Mcl-1, and Bcl-2 as well as Bax, Bim, and Bid mRNA. Induction of Bcr-Abl expression was associated with markedly increased expression of Bcl-XL (Tet-off/Tet-on = 7.6-fold) and Mcl-1 (6.0-fold) and increased Bcl-2 expression (2.4-fold) (Fig. 1A), consistent with previous reports for the regulation of anti-apoptotic BCL-2 proteins by BCR-ABL (19, 20). Induction was also observed to a lesser degree for pro-apoptotic BCL-2 proteins (fig. S2A).
Fig. 1. Expression of BCL-2 proteins in Tet-off/on CML mice.
(A) BM cells were collected from Tet-off/on mice, and the mRNA expression of Bcr-Abl, Bcl-XL, Mcl-1, and Bcl-2 was determined by real-time RT-PCR. Horizontal bars indicate the mean values. (B) SPADE tree analysis of mouse BM cell populations determined by CyTOF. (C) BCL-2, BCL-XL, MCL-1, BIM, BID, and BAX protein expression in BM cells from Tet-off and Tet-on mice determined and quantified by CyTOF.
To determine whether this transcriptional regulation translated into protein changes, we determined the expression of BCL-2 family proteins in the Tet-off (n = 6) and Tet-on (n = 5) mouse BM hematopoietic cells (CD45+) and also in Lin−Sca-1+cKit+ (LSK) cell population by CyTOF and SPADE analysis. CyTOF can simultaneously measure the expression of cell surface and intracellular proteins at single-cell resolution, therefore determine protein expression in phenotypically defined rare cell populations. With SPADE, cell populations from all samples are clustered hierarchically according to the expression of surface markers and displayed in single minimal spanning tree, where nodes can be annotated for further analysis. Fig. 1B shows the LSK population (identified as a single node in the tree) and the expression levels of individual surface markers in the SPADE tree of mouse BM cell populations. As shown in Fig. 1C, although not statistically significant, we observed overall increases in BCL-2 and MCL-1, but not BCL-XL protein expression in CD45+ cells in Tet-off compared to Tet-on mice. This increases were also detected in LSK cells for BCL-2, BCL-XL, and MCL-1. Only the BCL-2 protein expression was higher in LSK compared to CD45+ cells in Tet-off mice, and this difference was not observed in Tet-on mice. Although there were no major differences in pro-apoptotic proteins in CD45+ cells, BIM, BID, and BAX protein expression was increased in BM LSK cells from Tet-off compared to Tet-on mice. These data suggest a critical role for BCL-2 in the survival of CML bulk and stem/progenitor cells and imply that CML stem cells may be sensitive to apoptosis induction by BCL-2 inhibition.
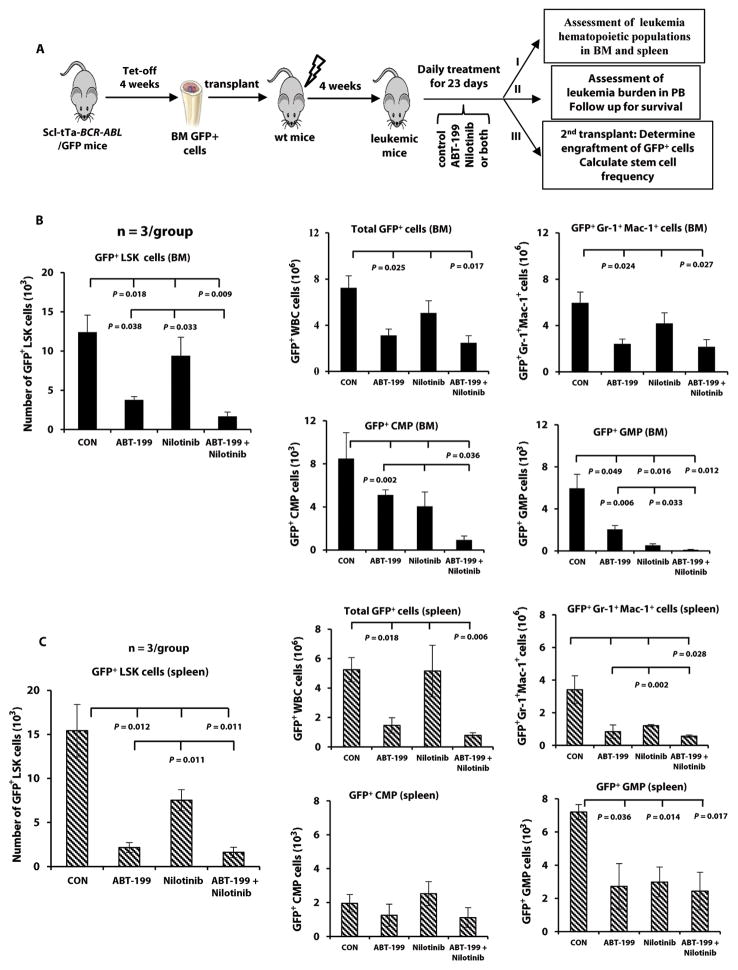
After demonstrating the effectiveness of ABT-199 and its combination with nilotinib in vitro (fig. S2B), we performed an in vivo study. We crossed Scl-tTa-BCR-ABL mice with transgenic green fluorescent protein (GFP)-expressing mice. Four weeks after Tet-off, BM cells from these mice were collected. GFP+ cells were FACS-sorted and injected into irradiated wild-type FVB/N mice (34). Upon development of neutrophilic leukocytosis in the recipient mice, they were randomized into four groups (n = 14–16/group) and treatment was initiated. At the end of the 23-day treatment period, three mice per group were sacrificed, and BM and spleen cells were collected (Fig. 2A). Treatment with ABT-199 significantly decreased the number of highly immature BM LSK cells (GFP+LSK) (P = 0.018, Fig. 2B), whereas nilotinib had only modest effect. However, the activity of ABT-199 was enhanced by its combination with nilotinib (P = 0.009), and the combination was significantly more effective than either agent alone (P = 0.038 and 0.033 compared to ABT-199 and nilotinib, respectively). ABT-199 alone and in combination with nilotinib significantly decreased the number of BM GFP+ WBCs and mature myeloid cells (GFP+Gr-1+Mac-1+) (Fig. 2B). In addition, there was a significant reduction in the number of leukemic common myeloid progenitor (CMP) cells (GFP+Lin−Sca-1−cKit+CD34+FcγRII/IIIlo) (P = 0.036) in the combination treatment group. Although the number of leukemic granulocyte-macrophage progenitor (GMP) cells (GFP+Lin−Sca-1−cKit+CD34+FcγRII/IIIhi) in the BM was significantly lower in the three treatment groups as compared to the control group, the combination was significantly more effective than ABT-199 or nilotinib alone (Fig. 2B). The most pronounced effects were seen in LSK, CMP, and GMP cells, and less so in mature myeloid cells.
Fig. 2. Targeting leukemic cells and leukemic LSK cells in BCR-ABL–expressing mice by inhibiting BCL-2 and BCR-ABL.
(A) BM cells obtained from Tet-off Scl-tTa-BCR-ABL/GFP CML mice were transplanted into wild-type (wt) recipient mice (0.6 × 106 cells/mouse) irradiated at 900 cGy. Mice were randomized after flow cytometric confirmation of the development of myeloproliferative disease and given a vehicle control (n = 14), ABT-199 (100 mg/kg) (n = 15), nilotinib (50 mg/kg) (n = 16), or both agents (n = 16) daily by oral gavage. At the end of a 23-day treatment, three mice per group were sacrificed. BM and spleen cells were collected (arm I). The rest of mice was followed for leukemia burden and survival (arm II). A separate set of mice were treated for second transplant (arm III). (B) BM and (C) spleen (n = 3): numbers of leukemia LSK cells (GFP+Lin−Sca-1+cKit+) in each treatment group are shown on the left and numbers of leukemia cells (GFP+ WBCs), mature myeloid leukemia cells (GFP+Gr-1+Mac-1+), and leukemia progenitor cells (CMPs: GFP+Lin−Sca-1−cKit+CD34+FcγRII/IIIlo; GMPs: GFP+Lin−Sca-1−cKit+CD34+FcγRII/IIIhi) in each treatment group on the right. CON, control.
Both ABT-199 and the combination were highly active against spleen LSK cells, and enhanced effect was discernible in mature myeloid cells (Fig. 2C). We also analyzed BM and spleen GFP−LSK cells from treated mice because they may reflect the effects of treatments on normal stem/progenitor cells. Although the number of GFP−LSK cells decreased in the combination treatment group compared to controls, the effect was not significant (fig. S3), suggesting selectivity of the treatment of CML cells.
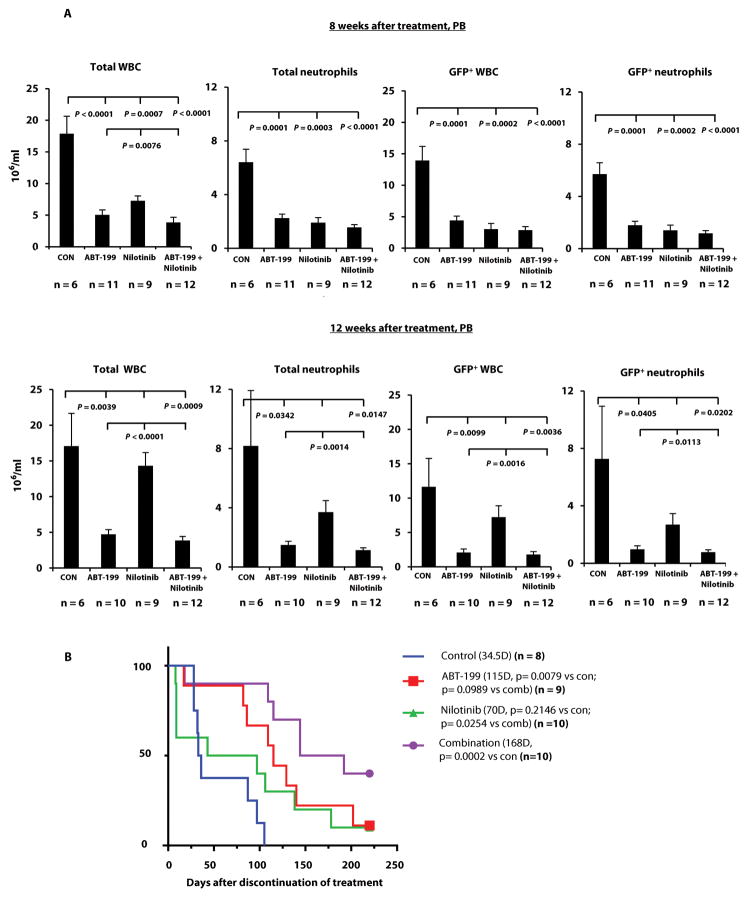
At eight weeks after cessation of treatment, we still observed strong anti-leukemia activity, which consisted of significantly fewer total and leukemic WBCs and neutrophils in peripheral blood (PB) of all treatment groups relative to controls (Fig. 3A, top). However, at 12 weeks, the mice treated with nilotinib alone showed re-emergence of total and GFP+ leukemia cells resembling clinical disease relapse after TKI cessation. Mice treated with ABT-199 alone or in combination with nilotinib still demonstrated a statistically significant reduction of leukemic WBCs and neutrophils (Fig. 3A, bottom), indicating a persistent anti-leukemic activity.
Fig. 3. Effect of targeting BCL-2 and BCR-ABL on leukemia in transgenic BCR-ABL–expressing mice.
A subset of mice from Fig. 2A (arm II) underwent follow-up for examination of leukemia burden and survival. (A) Leukemia burden was assessed according to the number of total and GFP+ WBCs and neutrophils in mouse PB samples by flow cytometry at 8 and 12 weeks after treatment. CON, control. (B) Survival curves for mice in the vehicle control, ABT-199, nilotinib, and combined treatment groups. D, days. Note: at the end of 12-week post treatments, 3 mice per group were sacrificed for additional experiments which were not conducted. These mice were not included in the survival curves.
The explanation for the similar leukemia burden observed in controls at eight and 12 weeks was likely that mice with higher leukemia burden had died before the earlier time point. The nilotinib-treated mice (median survival 70 days) tended to live longer than controls (median survival 34.5 days) after discontinuation of therapy (Fig. 3B) but the difference did not reach statistical significance (P = 0.2146). The median survival after ABT-199 treatment was 115 days, which was significantly longer than controls (P = 0.0079). This survival benefit was even more pronounced for the combination therapy group (median survival 168 days, P = 0.0002, Fig. 3B).
Inhibition of BCL-2 and BCR-ABL targets CML stem cells in BCR-ABL transgenic mice
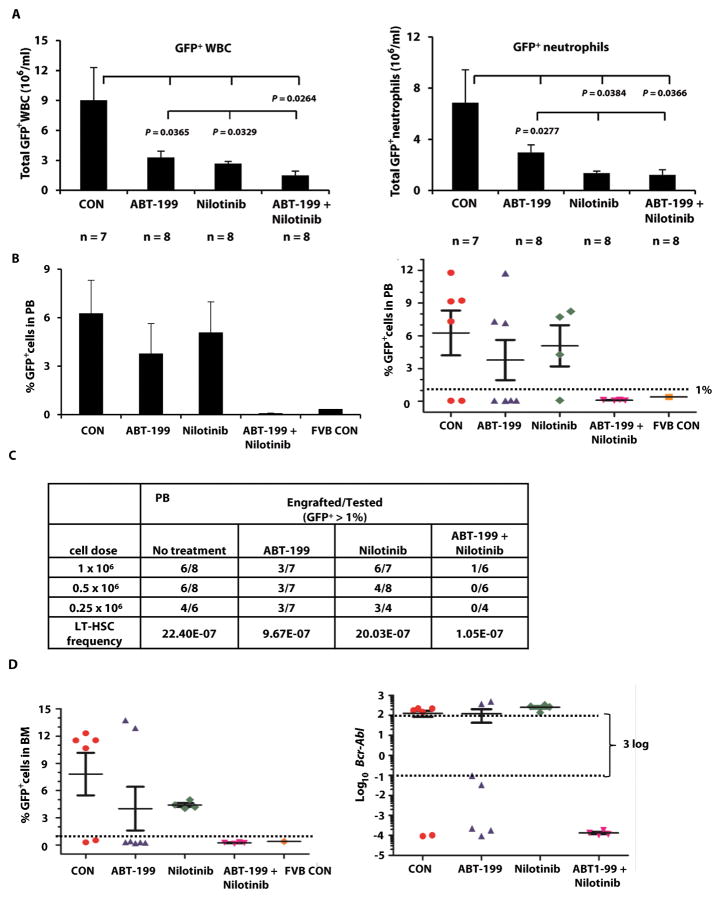
In a separate experiment, leukemic WBCs and neutrophils in each group were reduced to the same degree as in the first study at the end of the 23-day treatment period (Fig. 4A, n = 7–8/group). BM cells from each treatment group were collected and transplanted at 1 × 106, 0.5 × 106, or 0.25 × 106 cells plus 0.2 × 106 normal BM cells into each irradiated wild-type recipient mouse (Fig. 2A). At 16 weeks after transplant, we detected markedly fewer PB GFP+ cells in mice transplanted with cells from the ABT-199/nilotinib co-treatment group than in any other group. Fig. 4B shows the percentages of GFP+ PB cells in each group (left) and in individual mice (right) after a secondary transplant at 0.25 × 106 BM cells/mouse. We defined non-engraftment as PB GFP-positivity of less than 1%, which was considered background (equivalent to the amount seen in sham transplanted FVB mouse). Fig. 4C shows the numbers of engrafted versus tested mice in all groups, and the long-term leukemia stem cell (LT-HSC) frequency, which differed significantly between groups (P = 3.51 × 10−5). Nilotinib treatment alone did not affect engraftment or LT-HSC frequency (P = 0.78) compared to controls, in agreement with previous published data suggesting that TKIs are inactive against CML stem cells (1–3). Treatment with ABT-199 decreased the number of engrafted mice and LT-HSC frequency compared to controls (P = 0.049). Moreover, the combination treatment significantly decreased secondary engraftment and LT-HSC frequency (P = 9.38 × 10−6 compared to controls and P = 0.006 compared to the ABT-199 treated group). For every 22.4 LT-HSCs detected in mice transplanted with BM cells from untreated CML mice, 9.67, 20.03, and only 1.05 such cells were found in mice transplanted with BM cells obtained from animals treated with ABT-199, nilotinib, and their combination, respectively.
Fig. 4. Combined inhibition of BCL-2 and BCR-ABL on leukemia LT-HSC frequency.
A separate experiment was performed as described in Fig. 2A. At the end of treatment, BM cells from each group (1 × 106, 0.5 × 106, and 0.25 × 106 cells/mouse) plus 0.2 × 106 wild-type BM cells were transplanted into wild-type FBV/N recipient mice irradiated at 900 cGy (Fig. 2A, arm III). (A) PB leukemia burden at the end of treatments. (B) PB GFP+ cells obtained from mice 16 weeks after secondary transplant at 0.25 × 106 cells/mouse (left, plotted as mean ± SEM and right as individual animals). (C) PB engrafted/total transplanted mice and LT-HSC frequency in mice transplanted with BM cells from each treatment group. (D) BM GFP+ cells and Bcr-Abl mRNA expression in BM cells in mice 16 weeks after secondary transplant at 0.25 × 106 cells/mouse. CON, control.
In addition, we obtained BM cells from mice that had received a secondary transplant with 0.25 × 106 BM cells from each treatment group and determined the percentage of GFP positivity and extent of Bcr-Abl expression using real-time reverse transcription (RT)-polymerase chain reaction (PCR). Mice with ≥ 3 log reduction in BM Bcr-Abl mRNA also had ≤ 1% BM GFP positivity, and mice transplanted with BM cells from combination-treated donors had only background levels of GFP+ cells and negligible Bcr-Abl mRNA expression (Fig. 4D).
BCL-2 and BCR-ABL inhibition induces apoptosis in bulk and stem/progenitor BC CML cells
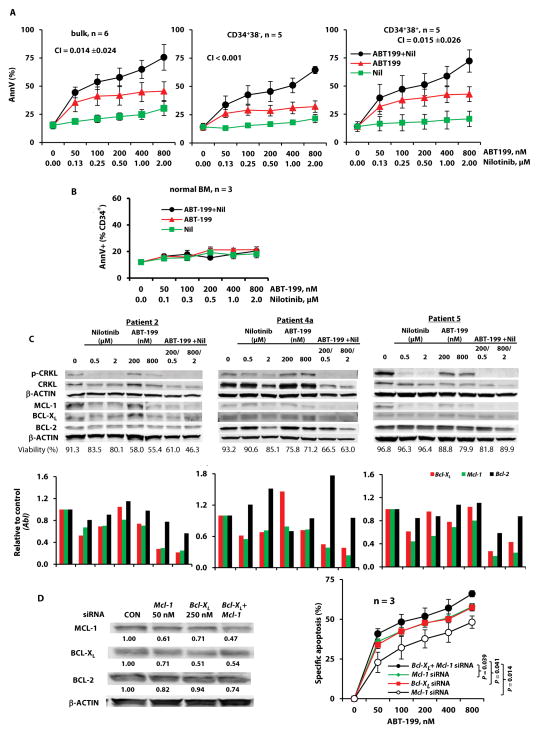
To determine the effects of combined inhibition of BCL-2 and BCR-ABL on BC CML, cells from BC CML patients (n = 6) were treated with ABT-199, nilotinib, or the combination. Some of the patient samples had T315I or other mutations of BCR-ABL, and all six had experienced treatment failures with one or more TKIs (Table 1, samples 1–3, 4a, 5, 6). Apoptosis in bulk and CD34+CD38− stem/progenitor cells was determined. Because CML stem cells in BC can derive from progenitors acquiring self-renewal capacity, we also determined treatment effects in CD34+CD38+ cells. As expected, neither bulk, CD34+CD38−, or CD34+CD38+ cells were sensitive to nilotinib (Fig. 5A). Although ABT-199 induced apoptosis, its combination with nilotinib synergistically enhanced this effect in bulk (mean combination index CI [± SEM], 0.014 ± 0.024), CD34+CD38− (CI < 0.001), and CD34+CD38+ (mean CI [± SEM], 0.015 ± 0.026) cells obtained from these patients regardless of their previous clinical responses to TKIs. The responses of these six samples to treatments plotted individually are shown in fig. S4.
Table 1.
Patient characteristics and in vitro treatments.
| Pt. No. | source | Blast (%) | BCR-ABL status | Treatments and responses | In vitro treatment and assays
|
% Q Cells | Days of co-culture with MSC | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cells in which apoptosis was determined | WB/PCR | Bcl-XL/Mcl-1 KD | ||||||||
| 1 | PB | 91 | T315I, E255K | Resistant to imatinib; treated with chemotherapy and dasatinib | P/Q | bulk/CD34+38−/CD34+38+ | 9.4 | 6 | ||
| 2 | PB | 89 | T315I, E255V | Resistant to imatinib, dasatinib, nilotinib, and ponatinib | P/Q | bulk/CD34+38− CD34+38+ |
protein/RNA | siRNAs | 15.9 | 7 |
| 3 | PB | 83 | H396R | Failed imatinib, dasatinib, and nilotinib. | P/Q | bulk/CD34+38− CD34+38+ |
siRNAs | 22.1 | 10 | |
| 4a | PB | 89 | T315I, E255B | Resistant to imatinib, nilotinib, dasatinib, and ponatinib; treated with CECA, ponatinib, and dasatinib | bulk/CD34+38− CD34+38+ |
protein/RNA | siRNAs | |||
| 5 | PB | 80 | No mutation | Resistant to imatinib, and dasatinib | bulk | protein/RNA | ||||
| 6 | BM | 93 | No mutation | Failed imatinib; treated with nilotinib | bulk/CD34+38− CD34+38+ |
|||||
| 4b | PB | 62 | T315I, E255V | Resistant to imatinib, dasatinib, and ponatinib; treated with nilotinib, decitabine, and dasatinib | P/Q | 7.7 | 6 | |||
| 7 | PB | 24 | No mutation | Resistant to imatinib; treated with nilotinib | P/Q | 6.8 | 8 | |||
| 8 | PB | 11 | No mutation | Resistant to imatinib, dasatinib, nilotinib, and ponatinib; treated with decitabine, dasatinib, and bosutinib | P/Q | 14.4 | 7 | |||
Abbreviations: Pt, patients; No, number; CECA, cyclophosphamide, etoposide, carboplatin, and cytosine arabinoside; P/Q, apoptosis in proliferating and quiescent cells; WB, western blot; % Q cells, % quiescent cells over total CD34+ cells.
Fig. 5. Targeting of BCL-2 and BCR-ABL in bulk, CD34+CD38−, and CD34+CD38+ leukemia cells from BC CML patients.
(A) Cells from TKI-resistant BC CML patients (Table 1, n = 6) were treated with ABT-199, nilotinib, or both. Apoptosis in bulk, CD34+CD38−, and CD34+CD38+ cells was assessed after 48 hours. (B) Normal BM cells were treated with ABT-199, nilotinib, or both. Apoptosis in CD34+ cells was assessed after 48 hours. (C) Cells from TKI-resistant BC CML patients were treated with ABT-199, nilotinib, or both for 24 hours. Protein expression in the cells was examined using immunoblot, and mRNA expression using real-time RT-PCR analysis. (D) Cells from TKI-resistant BC CML patients were treated with siRNAs against Bcl-XL, Mcl-1, or both for 24 hours and then with ABT-199 for 48 hours. Cell death was then assessed. CON, control; Nil, nilotinib; AnnV, annexin V.
To better understand anti-apoptotic BCL-2 protein expression and ABT-199 sensitivity in CML cells, we determined the IC50 of ABT-199 and the expression of BCL-2, BCL-XL, and MCL-1 by immunoblot in these samples (fig. S4). Samples 1 and 6 had the lowest amounts of BCL-2 and the highest IC50. Sample 4a had the highest BCL-2, but also the highest BCL-XL and high MCL-1, which made it relatively resistant to ABT-199. These data support ABT-199 specificity for BCL-2 and establish MCL-1 and BCL-XL as resistance factors for ABT-199. The combination therapy had minimal activity against normal BM (n = 3) CD34+ cells (Fig. 5B). Similar results were obtained when ABT-199 was combined with other TKIs (fig. S5A).
We assessed protein and gene expression of BCL-2 family members in selected samples (Table 1: patients 2, 4a, 5). Overall, ABT-199 treatment did not markedly decrease BCL-2 protein, as we expected. Nilotinib treatment partially decreased BCR-ABL signaling, as measured by the amount of phosphorylated CRKL (p-CRKL), and reduced MCL-1, BCL-XL, or both, while having only a minimal effect on BCL-2 expression. The combination greatly decreased p-CRKL expression, and enhanced the reduction of all three BCL-2 family proteins, particularly MCL-1, regardless of whether mutations in BCR-ABL were detected in these samples (patients 2 and 4a) or not (patient 5) (Fig. 5C).
Real-time RT-PCR analysis demonstrated that nilotinib treatment decreased Bcl-XL and Mcl-1 mRNA expression, further confirming their transcriptional regulation by BCR-ABL. Mcl-1 and Bcl-XL mRNA expression was further reduced by the combination treatment, but Bcl-2 mRNA expression was less affected (Fig. 5C). Although the individual agents and the combination did not show marked reduction in mRNA of pro-apoptotic BCL-2 proteins, some decreases in BAX and BID proteins were observed, especially with the combination treatment (fig. S5B). However, BIM protein expression was largely unchanged, and the basal amount of BAX protein was high and was still detectable after treatment. This is likely sufficient for induction of apoptosis, because the combination treatment greatly diminished MCL-1, partially decreased BCL-XL expression and antagonized BCL-2 function.
To determine whether the synergy of nilotinib and ABT-199 treatment was caused, at least in part, by TKI-mediated BCL-XL/MCL-1 inhibition, we knocked down the expression of BCL-XL, MCL-1, or both in cells obtained from BC patients (Table 1: patients 2, 3, and 4a) by specific siRNAs. Twenty-four hours later, the cells were treated with ABT-199 for additional 48 hours. A partial inhibition of BCL-XL and MCL-1 slightly increased the sensitivity of CML cells to ABT-199 (Fig. 5D), suggesting that the cooperative inhibition of BCL-2 and BCL-XL/MCL-1 contributed to the observed synergy.
Targeting of BCL-2 and BCR-ABL induces apoptosis in quiescent CD34+ BC CML cells
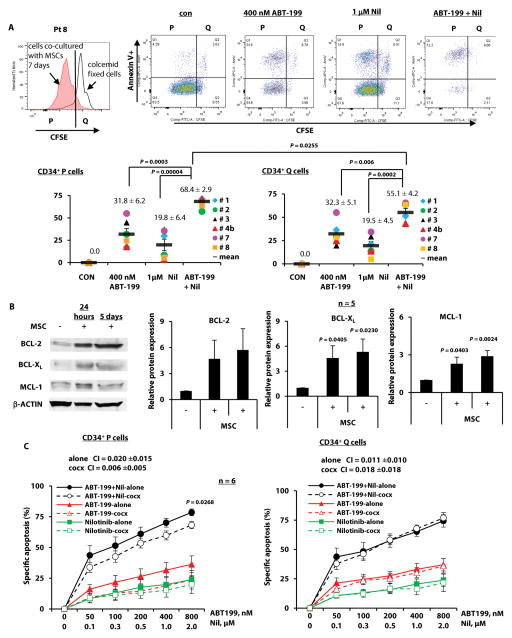
To evaluate the effects of ABT-199 and TKI combinations on TKI-insensitive quiescent CML stem/progenitor cells, we labeled cells from BC CML patients with the cell division-tracking dye carboxyfluorescein succinimidyl ester (CFSE) and co-cultured them with human BM-derived mesenchymal stromal cells (MSCs). Once proliferating and quiescent cells were distinguishable by flow cytometry, they were treated with ABT-199, TKIs, and combinations for 48 hours. Apoptosis was determined in proliferating (CD34+CFSEdim) and quiescent (CD34+CFSEbright) progenitor cells. Fig. 6A shows the flow cytometric profiles of one patient’s cells before and after treatments (upper panel; patient 8) and of six patients after treatments (lower panel; patients 1–3, 4b, 7, 8, Table 1). The combination was significantly more effective than either agent alone in inducing apoptosis in proliferating CD34+ cells from all six patients and although it was less effective than in proliferating cells (P = 0.0255), it was also significantly more effective than either agent alone in quiescent CD34+ cells.
Fig. 6. Targeting of BCL-2 and BCR-ABL in proliferating and quiescent CD34+ cells from TKI-resistant BC CML patients.
(A) CFSE-stained cells were treated with ABT-199, nilotinib, or both. Apoptosis in proliferating (P) and quiescent (Q) CD34+ cells was assessed after 48 hours. Upper panel shows flow cytometric profiles of cells from patient 8 (Pt 8) before and after treatment. Lower panel shows the results of six treated patient samples, where each dot represents the results for one patient sample. (B) Expression of BCL-2, BCL-XL, and MCL-1 in cells from CML patient samples co-cultured with MSCs (cocx) for 24 hours or 5 days was examined using immunoblot. (C) CFSE-labeled cells (Table 1, n = 6) were treated with ABT-199, nilotinib, or both with or without MSC co-culture. Apoptosis was assessed in proliferating and quiescent CD34+ cells after 48 hours. con, control; Nil, nilotinib.
We previously demonstrated that MSCs in the BM microenvironment protect AML cells in part by inducing the expression of anti-apoptotic proteins (35, 36). Here, we found that co-culture of BC CML cells with MSCs also increased the expression of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins. Fig. 6B illustrates a representative result from one and quantitative results from five patient samples. These increases are unlikely due to MSC contamination, because negligible amounts of MSCs were found in the collected leukemia cells (fig. S6A) and we did not see similar changes in pro-apoptotic BCL2 proteins (fig. S6B). Co-cultures with MSCs partially protected proliferating, but not quiescent CD34+ cells from ABT-199/nilotinib–induced apoptosis (Fig. 6C). The combination treatment was highly synergistic in inducing apoptosis in both proliferating (mean CI [± SEM], 0.020 ± 0.015) and quiescent (mean CI [± SEM], 0.011 ± 0.010) leukemia cells, even when these cells were co-cultured with MSCs (mean CI [± SEM]: 0.006 ± 0.005 for proliferating and 0.018 ± 0.018 for quiescent cells) (Fig. 6C). We obtained similar results with ABT-199 combined with other TKIs (fig. S6C) (Table 1, samples, 1–3, 4b, 7, 8).
Discussion
Although TKIs have dramatically improved long-term survival for CML patients, the vast majority of them has residual disease as determined by Bcr-Abl RT-PCR. Relapse occurs in >50% of these patients upon discontinuation of treatment, even in patients who achieved complete molecular responses. Thus, to control the disease, patients need to be treated with TKIs continuously. Consequently, the number of CML survivors on continuous treatment is ever increasing and there will be over 100,000 patients with CML in 2020 in the United States alone (37). Long-term treatment with TKIs for these patients comes at a high cost, both in terms of side effects (see (38) for a review) and financially. Worldwide, most CML patients cannot afford the extraordinary expenses associated with TKI-based therapy. Furthermore, acquired BCR-ABL mutations that render TKIs ineffective can develop, and in patients progressing to BC, there are no meaningful responses to TKIs and survival is counted in weeks or months.
We and others identified BCL-2 as a key survival factor for myeloid leukemia cells (16, 17, 31). Anti-apoptotic BCL-2 proteins were reported to be regulated by BCR-ABL tyrosine kinase (19, 20). However, their induction at the protein level, especially in stem/progenitor cells has not been not well investigated. Although several studies (17, 21–23) have shown that BH3 mimetics synergize with TKIs to target CML cells and stem/progenitor cells, none has established a critical role of BCL-2 in CML stem cell survival.
We determined BCL-2 protein expression and examined whether selective BCL-2 inhibition could render CML cells, including stem cells, more senstive to TKIs. To mimic human chronic-phase CML, we used a validated inducible transgenic CML mouse model (39, 40). We observed higher Bcl-XL and Mcl-1 and to a lesser degree Bcl-2 mRNA expression in BM cells in Tet-off as compared to Tet-on BCR-ABL–transgenic mice, supporting the transcriptional regulation of these proteins by BCR-ABL. Aided by CyTOF, we found BCL-2 not only induced in CML cells but also differentially overexpressed in LSK vs. bulk CML cells at the protein level, suggesting its critical role in CML stem cells. Treatment of the CML mice with ABT-199 decreased leukemia burden and LSK leukemic cells in the BM, which greatly prolonged the survival of CML mice. It also decreased engraftment and leukemia LT-HSC frequency in mice upon secondary transplantation, supporting a critical role of BCL-2 in CML cell/stem cell survival.
Nilotinib showed stronger anti-leukemia activity in circulating blood than in the BM. Although nilotinib tended to decrease the numbers of various CML cell populations, it failed to significantly affect leukemic LSK cells. Like ABT-199, nilotinib was highly effective in controlling leukemia burden in PB even eight weeks after treatment discontinuation. However, at 12 weeks, in contrast to ABT-199, the anti-leukemia effect of nilotinib was lost, which mirrored the relapses observed in CML patients upon cessation of TKI therapy. When ABT-199 and TKIs were combined, the enhanced anti-leukemia activity was reflected in reduced leukemia burden and increased survival of CML mice after only 23 days of treatment. The combination was highly effective in preventing secondary engraftment and reducing leukemia LT-HSC frequency, suggesting the eradication of leukemia stem cells and potential of curing CML with an optimized treatment schedule.
It has been reported that cellular BCL-2 protein expression correlates with cell sensitivity to ABT-199 (31). The amount of anti-apoptotic BCL-2 proteins increases in CML patients as they progress to BC, as determined by reverse-phase protein arrays (18) and qRT-PCR (17). Samples that were examined in vitro were obtained from TKI-resistant patients with BC CML, and we showed that treatment with nanomolar concentrations of ABT-199 induced apoptosis in these cells. These concentrations of ABT-199 have been achieved systemically in clinical trials in chronic lymphocytic leukemia and AML (41).
Apoptosis was synergistically enhanced by combining ABT-199 with TKIs in BC CML, likely because of the partial inhibition of MCL-1 and, to a lesser degree, BCL-XL by TKIs. The fact that exposure to Mcl-1/Bcl-XL siRNAs sensitized cells to ABT-199, even with insufficient knockdown of MCL-1/BCL-XL in patient samples, supports this notion. We found that combined inhibition of BCL-2 and BCR-ABL cooperatively targeted anti-apoptotic BCL-2 proteins. Although we cannot exclude the possibility that some samples may consist of more than one clone and that some of the clones were sensitive to one or more TKIs, clinical PCR monitoring showed that patient 4 had a T315I mutation in 100% of the PCR product. Nilotinib had minimal effect on p-CRKL and no effect on MCL-1 and BCL-XL in this patient. When nilotinib was combined with ABT-199, p-CRKL and MCL-1 were greatly reduced and BCL-XL also decreased.
We and others have reported that TKI activity can be enhanced by multiple agents other than ABT-199, even in TKI-resistant cells (24, 34, 42). For ABT-199 and TKI combinations, in addition to the proposed mechanisms, we cannot rule out other mechanisms, particularly because we observed further decreases of not only phosphorylated but also total CRKL in all samples with the combination treatment. Cellular functions are dynamically regulated by multiple interacting pathways to determine their outcomes. Combination therapies can modify protein dynamics and change cell fates, providing new therapeutic opportunities (43), likely also the case for combinations of TKIs and ABT-199.
Collectively, our results demonstrate that combined blockade of pro-survival BCL-2 and an oncogenic tyrosine kinase has the potential of curing CML and improving outcomes in patients with BC, and, as such, it warrants clinical testing. This combination strategy may also apply to other malignancies that depend on kinase signaling for progression and maintenance.
Materials and Methods
Study design
This study was designed to test the hypothesis that inhibition of BCL-2 in combination with TKIs that affect MCL-1/BCL-XL inhibition has the potential to eradicate CML stem cells and BC CML cells. We demonstrated in chronic phase CML, using an established inducible transgenic mouse model, that the combination eliminates leukemic LSK cells, prolongs survival, and decreases leukemia stem cell frequency in secondary transplant experiments. In BC CML, using TKI-resistant samples from patients, we showed that the combination is effective in killing bulk, CD34+CD38+, CD34+CD38−, and both proliferating and quiescent CD34+ stem/progenitor cells. Sample sizes and P values are indicated in the text, figure legends, or in figures.
In vivo study
Mouse care and experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center. All mice are on a FVB/N background. Inducible transgenic Scl-tTa-BCR-ABL/GFP mice were generated as described previously (34, 39, 40). Four weeks after Tet-off induction of Bcr-Abl expression in the mice, GFP-expressing cells in mouse BM were sorted by flow cytometry and transplanted by tail vein injection (0.6 × 106 cells/mouse) into wild-type FVB/N recipient mice (The Jackson Laboratory) irradiated at 900 cGy. After they developed neutrophilic leukocytosis, the mice were randomized and given ABT-199 (100 mg/kg; vehicle: 60% Phosal 50 PG, 30% PEG400, and 10% ethanol) (27, 31), nilotinib (50 mg/kg; vehicle: 10% N-methyl-2-pyrrolidone and 90% PEG300) (44, 45), ABT-199 plus nilotinib, or a vehicle control (1:1 volume of each vehicle) daily by oral gavage (n = 14–16/group). At the end of the 23-day treatment, GFP+ LSK hematopoietic cells, myeloid progenitor cells, and myeloid cells in BM collected from femurs and spleen samples (n = 3/group) were counted after cells were stained with a lineage cocktail and antibodies against Sca-1, cKit (CD117), CD34, FcγRII/III, Gr-1 (Ly6 G), and Mac-1 (CD11b) (all from BioLegend). Leukemia LSK cells were defined as GFP+Lin−Sca-1+cKit+ cells; total leukemia WBC cells as GFP+CD45+ cells; leukemic CMP and GMP cells as GFP+Lin−Sca-1−cKit+CD34+FcγRII/IIIlo and GFP+Lin−Sca-1−cKit+CD34+FcγRII/IIIhi cells, respectively; and myeloid leukemia cells as GFP+Gr-1+Mac-1+ cells. The leukemia burden in PB samples was monitored by total and GFP+ WBC (CD45+) and total and GFP+ neutrophil (Ly6 G+) cells using flow cytometry. Mouse survival was recorded. BM cells obtained from another set of treated mice were pooled and diluted for secondary transplant (1 × 106, 0.5 × 106, or 0.25 × 106 cells/mouse plus 0.2 × 106 normal BM cells/mouse ) as described previously (34). Engraftment of leukemia cells was monitored using flow cytometry-based determination of the percentage of GFP+ cells in PB and BM samples. The number of mice with evidence of engraftment in PB at 16 weeks after secondary transplant was determined, and the leukemia LT-HSC frequency was calculated.
Cells, cell culture, and cell treatment
Samples were acquired from BC CML patients and normal controls after informed consent following the MD Anderson Cancer Center IRB approved protocol. The patients’ characteristics are listed in Table 1. Mononuclear cells from these samples and BM cells from Tet-off (3 to 4 weeks) and Tet-on transgenic Scl-tTa-BCR-ABL mice were cultured in α minimal essential medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin and treated with ABT-199, TKIs, or their combinations. Doxycycline (1 μg/ml) was included in the medium for cells from Tet-on mice.
Identification and treatment of proliferating and quiescent CML cells
Proliferating and quiescent cells were identified by staining mononuclear cells obtained from BC CML patients with CFSE and co-culturing them with human BM-derived MSCs as described previously (21, 46). Cell proliferation was monitored using flow cytometric measurement of cellular CFSE intensity, which halved with cell division, and compared with that of Colcemid-treated (100 ng/ml) cells. Once proliferating and quiescent cells became distinguishable (co-culture days and % quiescent CD34+ cells for each sample are shown in Table 1), cells were treated with ABT-199, TKIs, and the combinations with or without MSC co-culture. MSCs were isolated from BM samples from healthy subjects as described previously (47). Quiescent cells are defined as those in a region of CFSE fluorescence of Colcemid-treated cells (CFSEbright) and proliferating cells as those with a fluorescent intensity less than the control cells (CFSEdim).
Apoptosis assay
Apoptosis was estimated by flow cytometry measurement of phosphatidylserine externalization with annexin V staining (BD Biosciences) in bulk, CD34+CD38+, CD34+CD38−, and proliferating (CD34+CFSEdim) and quiescent (CD34+CFSEbright) CML progenitor cells. To assess apoptosis in leukemia cells co-cultured with MSCs, leukemia cells were collected by combining cells in the supernatant and after two washes with PBS. Apoptotic cells were defined as annexin V-positive CD45+ cells. Specific apoptosis was defined using the following formula (21):
Immunoblot analysis
Proteins were quantified by immunoblot analysis as described previously (21, 24). For co-culture experiments, leukemia cells were collected by combining cells in the supernatant and after two washes with PBS. Antibodies against p-CRKL, BID, and BCL-XL were purchased from Cell Signaling Technology, MCL-1 from Santa Cruz Biotechnology Inc., BIM and CRKL from Abcam, BAX from Sigma, and BCL-2 from Dako. Signals were detected using an Odyssey infrared imaging system and quantitated using the Odyssey software program (version 3.0; LI-COR Biotechnology). β-ACTIN (Sigma) was used as a loading control.
Real-time RT-PCR
Cell pellets were lysed in Trizol solution (Thermo Fisher Scientific), and RNA was isolated as described previously (48). cDNA was prepared from total RNA in a mixture containing dNTP, random hexamers, SuperScript III reverse transcriptase, and Superase In RNase Inhibitor (Thermo Fisher Scientific) at 50°C for 1 hour and 70°C for 15 minutes. The relative abundance of target mRNAs was measured by an ABI 7900HT Fast Real Time PCR System (Thermo Fisher Scientific) using TaqMan Gene Expression Assays and TaqMan Fast Universal Master Mix as directed by the manufacturer (Thermo Fisher Scientific). Primer sets used for the study are shown in table S1. Abl RNA was used as an internal control. The abundance of each transcript relative to that of Abl was calculated using the 2−ΔCt method, in which ΔCt is the mean Ct of the transcript of interest minus the mean Ct of Abl transcript.
Protein determination by CyTOF mass cytometry
BM cells collected from Tet-on (n = 5) and Tet-off (n = 6) mice were stained with a panel of metal-tagged antibodies (table S2) against cell surface and BCL-2 family proteins and subjected to CyTOF analysis as previously described (49). FCS files from individual samples were normalized against EQ-beads loaded with each sample, using v.2 beads passport in CyTOF software (Fluidigm). Viable (cisplatin low) single cells were gated with FlowJo software (v10.1r5, FlowJo LLC) and exported as FCS data for subsequent analysis in SPADE (v3.0, http://pengqiu.gatech.edu/software/SPADE/). A SPADE tree was generated using all surface markers (table S2). The LSK node(s) were identified by negative expression of lineage markers (table S2) and positive expressions of Sca-1 and cKit. ArcSinh-transformed counts for each marker were then exported and visualized with heat maps to show differences in bulk and LSK populations and between Tet-off and Tet-on mice.
Knockdown of protein expression
To transiently knock down the expression of BCL-XL, MCL-1, or both, PB cells from CML patient samples (2, 3, 4a, Table 1) were transfected with siRNAs for Bcl-XL, Mcl-1, or both (on TARGETplus SMARTpool; Thermo Scientific Dharmacon) via electroporation using an Amaxa apparatus (Amaxa Biosystems) following the manufacturer’s instructions.
Statistical analyses
The results were expressed as mean ± SEM. Statistical significance was set at P < 0.05 using the two-sided Student t-test. The CI, determined using the Chou-Talalay method (50), was expressed as the mean of CI values obtained at the effective doses in 50%, 75%, and 90% of the population exposed to the agents: CI < 1, synergistic; CI = 1, additive; and CI > 1, antagonistic. Mouse survival data were analyzed using the log-rank test. LT-HSC frequency was calculated using the Extreme Limiting Dilution Analysis software program (http://bioinf.wehi.edu.au/software/elda/) (51).
Supplementary Material
Fig. S1. Characteristics of Scl-tTa-BCR-ABL transgenic mice.
Fig. S2. Expression of pro-apoptotic Bcl-2 mRNAs in BM cells of Tet-off/on CML mice and in vitro treatment of mouse BM cells.
Fig. S3. Enumeration of BM and spleen GFP−LSK cells in each group after treatment.
Fig. S4. Targeting of BCL-2 and BCR-ABL in bulk, CD34+38−, and CD34+38+ leukemia cells from TKI-resistant BC CML patients.
Fig. S5. Targeting of BCL-2 and BCR-ABL with TKIs in bulk, CD34+38−, and CD34+38+ leukemia cells from TKI-resistant BC CML patients.
Fig. S6. Targeting of BCL-2 and BCR-ABL in proliferating and quiescent CD34+ leukemia cells from TKI-resistant BC CML patients.
Table S1. Primers used for real-time PCR.
Table S2. Antibody panel for CyTOF analysis.
Acknowledgments
We thank Donald R. Norwood, Numsen Hail, Jr., and Deanna A. Alexander for assisting with the manuscript preparation.
Funding: Supported in part by National Institutes of Health grants P01CA49639 and P30CA016672 and the Paul and Mary Haas Chair in Genetics (to M.A.) and by research funding from AbbVie Inc. (to B.Z.C.).
Footnotes
Author contributions: B.Z.C. conceptualized the study, designed the experiments, analyzed data, wrote the manuscript, and provided funding; P.Y.M. and H.M. performed experiments and analyzed the data; H.Z., D.H.M., and W.S. performed experiments; J.D.L. provided material and proofed the manuscript; B.Z. and R.B. helped with the mouse study; X.H. assisted with statistical analysis; J.C, H.K, and M.K. identified and provided patient samples; and M.A. designed experiments, provided funding, and edited the manuscript.
Competing interests: Joel Leverson is an employee of AbbVie Inc, which developed ABT-199. The study was in part supported by research funding from AbbVie Inc.
References and notes
- 1.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, Barow M, Mountford JC, Holyoake TL. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML, but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 2.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. The Journal of clinical investigation. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 4.Elrick LJ, Jorgensen HG, Mountford JC, Holyoake TL. Punish the parent not the progeny. Blood. 2005;105:1862–1866. doi: 10.1182/blood-2004-08-3373. [DOI] [PubMed] [Google Scholar]
- 5.Holtz MS, Forman SJ, Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19:1034–1041. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- 6.Heaney NB, Holyoake TL. Therapeutic targets in chronic myeloid leukaemia. Hematological oncology. 2007;25:66–75. doi: 10.1002/hon.813. [DOI] [PubMed] [Google Scholar]
- 7.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- 8.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, Dang P, Goyne JM, Slader C, Filshie RJ, Mills AK, Melo JV, White DL, Grigg AP, Hughes TP. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 9.Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MW, Fischer T, O’Brien SG, Stone RM, Gambacorti-Passerini CB, Russell NH, Reiffers JJ, Shea TC, Chapuis B, Coutre S, Tura S, Morra E, Larson RA, Saven A, Peschel C, Gratwohl A, Mandelli F, Ben-Am M, Gathmann I, Capdeville R, Paquette RL, Druker BJ. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. The New England journal of medicine. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 11.Bhola PD, Letai A. Mitochondria-Judges and Executioners of Cell Death Sentences. Mol Cell. 2016;61:695–704. doi: 10.1016/j.molcel.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata AN, Engelman JA, Faber AC. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer discovery. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beurlet S, Omidvar N, Gorombei P, Krief P, Le Pogam C, Setterblad N, de la Grange P, Leboeuf C, Janin A, Noguera ME, Hervatin F, Sarda-Mantel L, Konopleva M, Andreeff M, Tu AW, Fan AC, Felsher DW, Whetton A, Pla M, West R, Fenaux P, Chomienne C, Padua RA. BCL-2 inhibition with ABT-737 prolongs survival in an NRAS/BCL-2 mouse model of AML by targeting primitive LSK and progenitor cells. Blood. 2013;122:2864–2876. doi: 10.1182/blood-2012-07-445635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, Mori Y, Shima T, Iwasaki H, Takenaka K, Nagafuji K, Mizuno S, Niiro H, Gilliland GD, Akashi K. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114:5034–5043. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell stem cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goff DJ, Court Recart A, Sadarangani A, Chun HJ, Barrett CL, Krajewska M, Leu H, Low-Marchelli J, Ma W, Shih AY, Wei J, Zhai D, Geron I, Pu M, Bao L, Chuang R, Balaian L, Gotlib J, Minden M, Martinelli G, Rusert J, Dao KH, Shazand K, Wentworth P, Smith KM, Jamieson CA, Morris SR, Messer K, Goldstein LS, Hudson TJ, Marra M, Frazer KA, Pellecchia M, Reed JC, Jamieson CH. A Pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell stem cell. 2013;12:316–328. doi: 10.1016/j.stem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintas-Cardama A, Qiu YH, Post SM, Zhang Y, Creighton CJ, Cortes J, Kornblau SM. Reverse phase protein array profiling reveals distinct proteomic signatures associated with chronic myeloid leukemia progression and with chronic phase in the CD34-positive compartment. Cancer. 2012;118:5283–5292. doi: 10.1002/cncr.27568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H, Florian S, Sonneck K, Akgul C, Derdak S, Pickl WF, Wacheck V, Selzer E, Monia BP, Moriggl R, Valent P, Sillaber C. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105:3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 20.Horita M, Andreu EJ, Benito A, Arbona C, Sanz C, Benet I, Prosper F, Fernandez-Luna JL. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. The Journal of experimental medicine. 2000;191:977–984. doi: 10.1084/jem.191.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak DH, Wang RY, Schober WD, Konopleva M, Cortes J, Kantarjian H, Andreeff M, Carter BZ. Activation of apoptosis signaling eliminates CD34+ progenitor cells in blast crisis CML independent of response to tyrosine kinase inhibitors. Leukemia. 2012;26:788–794. doi: 10.1038/leu.2011.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuroda J, Kimura S, Andreeff M, Ashihara E, Kamitsuji Y, Yokota A, Kawata E, Takeuchi M, Tanaka R, Murotani Y, Matsumoto Y, Tanaka H, Strasser A, Taniwaki M, Maekawa T. ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drug-resistance mechanisms. British journal of haematology. 2008;140:181–190. doi: 10.1111/j.1365-2141.2007.06899.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda J, Kimura S, Strasser A, Andreeff M, O’Reilly LA, Ashihara E, Kamitsuji Y, Yokota A, Kawata E, Takeuchi M, Tanaka R, Tabe Y, Taniwaki M, Maekawa T. Apoptosis-based dual molecular targeting by INNO-406, a second-generation Bcr-Abl inhibitor, and ABT-737, an inhibitor of antiapoptotic Bcl-2 proteins, against Bcr-Abl-positive leukemia. Cell death and differentiation. 2007;14:1667–1677. doi: 10.1038/sj.cdd.4402168. [DOI] [PubMed] [Google Scholar]
- 24.Carter BZ, Mak PY, Mak DH, Ruvolo VR, Schober W, McQueen T, Cortes J, Kantarjian HM, Champlin RE, Konopleva M, Andreeff M. Synergistic effects of p53 activation via MDM2 inhibition in combination with inhibition of Bcl-2 or Bcr-Abl in CD34+ proliferating and quiescent chronic myeloid leukemia blast crisis cells. Oncotarget. 2015;6:30487–30499. doi: 10.18632/oncotarget.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, Josefsson EC, Alwis I, Ono A, Willcox A, Andrews RK, Mason KD, Salem HH, Huang DC, Kile BT, Roberts AW, Jackson SP. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 26.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 27.Vandenberg CJ, Cory S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood. 2013;121:2285–2288. doi: 10.1182/blood-2013-01-475855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson NM, Harrold I, Mansour MR, Sanda T, McKeown M, Nagykary N, Bradner JE, Lan Zhang G, Look AT, Feng H. BCL2-specific inhibitor ABT-199 synergizes strongly with cytarabine against the early immature LOUCY cell line but not more-differentiated T-ALL cell lines. Leukemia. 2014;28:1145–1148. doi: 10.1038/leu.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khaw SL, Merino D, Anderson MA, Glaser SP, Bouillet P, Roberts AW, Huang DC. Both leukaemic and normal peripheral B lymphoid cells are highly sensitive to the selective pharmacological inhibition of prosurvival Bcl-2 with ABT-199. Leukemia. 2014;28:1207–1215. doi: 10.1038/leu.2014.1. [DOI] [PubMed] [Google Scholar]
- 30.Niu X, Wang G, Wang Y, Caldwell JT, Edwards H, Xie C, Taub JW, Li C, Lin H, Ge Y. Acute myeloid leukemia cells harboring MLL fusion genes or with the acute promyelocytic leukemia phenotype are sensitive to the Bcl-2-selective inhibitor ABT-199. Leukemia. 2014;28:1557–1560. doi: 10.1038/leu.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, Cortes J, DeAngelo DJ, Debose L, Mu H, Dohner H, Gaidzik VI, Galinsky I, Golfman LS, Haferlach T, Harutyunyan KG, Hu J, Leverson JD, Marcucci G, Muschen M, Newman R, Park E, Ruvolo PP, Ruvolo V, Ryan J, Schindela S, Zweidler-McKay P, Stone RM, Kantarjian H, Andreeff M, Konopleva M, Letai AG. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer discovery. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. ABT-199 selectively inhibits BCL2 but not BCL2L1 and efficiently induces apoptosis of chronic lymphocytic leukaemic cells but not platelets. British journal of haematology. 2013;163:139–142. doi: 10.1111/bjh.12457. [DOI] [PubMed] [Google Scholar]
- 33.Ko TK, Chuah CT, Huang JW, Ng KP, Ong ST. The BCL2 inhibitor ABT-199 significantly enhances imatinib-induced cell death in chronic myeloid leukemia progenitors. Oncotarget. 2014;5:9033–9038. doi: 10.18632/oncotarget.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B, Strauss AC, Chu S, Li M, Ho Y, Shiang KD, Snyder DS, Huettner CS, Shultz L, Holyoake T, Bhatia R. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer cell. 2010;17:427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 36.Mak PY, Mak DH, Mu H, Shi Y, Ruvolo P, Ruvolo V, Jacamo R, Burks JK, Wei W, Huang X, Kornblau SM, Andreeff M, Carter BZ. Apoptosis repressor with caspase recruitment domain is regulated by MAPK/PI3K and confers drug resistance and survival advantage to AML. Apoptosis: an international journal on programmed cell death. 2014;19:698–707. doi: 10.1007/s10495-013-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123–3127. doi: 10.1002/cncr.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rea D. Management of adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Ann Hematol. 2015;94(Suppl 2):S149–158. doi: 10.1007/s00277-015-2318-y. [DOI] [PubMed] [Google Scholar]
- 39.Huettner CS, Koschmieder S, Iwasaki H, Iwasaki-Arai J, Radomska HS, Akashi K, Tenen DG. Inducible expression of BCR/ABL using human CD34 regulatory elements results in a megakaryocytic myeloproliferative syndrome. Blood. 2003;102:3363–3370. doi: 10.1182/blood-2003-03-0768. [DOI] [PubMed] [Google Scholar]
- 40.Koschmieder S, Gottgens B, Zhang P, Iwasaki-Arai J, Akashi K, Kutok JL, Dayaram T, Geary K, Green AR, Tenen DG, Huettner CS. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 2005;105:324–334. doi: 10.1182/blood-2003-12-4369. [DOI] [PubMed] [Google Scholar]
- 41.Konopleva MPD, Potluri J, McKeegan BJ, Salem A, Zhu M, Ricker JL, Dunbar M, Kirby R, Falotico N, Leverson JD, Stone RM, Kantarjian HM, Letai AG. A Phase 2 Study of ABT-199 (GDC-0199) in Patients with Acute Myelogenous Leukemia (AML) Blood. 2014;124 [Google Scholar]
- 42.Naka K, Ishihara K, Jomen Y, Jin CH, Kim DH, Gu YK, Jeong ES, Li S, Krause DS, Kim DW, Bae E, Takihara Y, Hirao A, Oshima H, Oshima M, Ooshima A, Sheen YY, Kim SJ, Kim DK. Novel oral transforming growth factor-beta signaling inhibitor EW-7197 eradicates CML-initiating cells. Cancer Sci. 2016;107:140–148. doi: 10.1111/cas.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behar M, Barken D, Werner SL, Hoffmann A. The dynamics of signaling as a pharmacological target. Cell. 2013;155:448–461. doi: 10.1016/j.cell.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Packer LM, Rana S, Hayward R, O’Hare T, Eide CA, Rebocho A, Heidorn S, Zabriskie MS, Niculescu-Duvaz I, Druker BJ, Springer C, Marais R. Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer cell. 2011;20:715–727. doi: 10.1016/j.ccr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisberg E, Azab AK, Manley PW, Kung AL, Christie AL, Bronson R, Ghobrial IM, Griffin JD. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia. 2012;26:985–990. doi: 10.1038/leu.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mak DH, Schober WD, Chen W, Konopleva M, Cortes J, Kantarjian HM, Andreeff M, Carter BZ. Triptolide induces cell death independent of cellular responses to imatinib in blast crisis chronic myelogenous leukemia cells including quiescent CD34+ primitive progenitor cells. Molecular cancer therapeutics. 2009;8:2509–2516. doi: 10.1158/1535-7163.MCT-09-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 48.Carter BZ, Mak DH, Wang Z, Ma W, Mak PY, Andreeff M, Davis RE. XIAP downregulation promotes caspase-dependent inhibition of proteasome activity in AML cells. Leukemia research. 2013;37:974–979. doi: 10.1016/j.leukres.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han L, Qiu P, Zeng Z, Jorgensen JL, Mak DH, Burks JK, Schober W, McQueen TJ, Cortes J, Tanner SD, Roboz GJ, Kantarjian HM, Kornblau SM, Guzman ML, Andreeff M, Konopleva M. Single-cell mass cytometry reveals intracellular survival/proliferative signaling in FLT3-ITD-mutated AML stem/progenitor cells. Cytometry A. 2015;87:346–356. doi: 10.1002/cyto.a.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 51.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. Journal of immunological methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Characteristics of Scl-tTa-BCR-ABL transgenic mice.
Fig. S2. Expression of pro-apoptotic Bcl-2 mRNAs in BM cells of Tet-off/on CML mice and in vitro treatment of mouse BM cells.
Fig. S3. Enumeration of BM and spleen GFP−LSK cells in each group after treatment.
Fig. S4. Targeting of BCL-2 and BCR-ABL in bulk, CD34+38−, and CD34+38+ leukemia cells from TKI-resistant BC CML patients.
Fig. S5. Targeting of BCL-2 and BCR-ABL with TKIs in bulk, CD34+38−, and CD34+38+ leukemia cells from TKI-resistant BC CML patients.
Fig. S6. Targeting of BCL-2 and BCR-ABL in proliferating and quiescent CD34+ leukemia cells from TKI-resistant BC CML patients.
Table S1. Primers used for real-time PCR.
Table S2. Antibody panel for CyTOF analysis.