Abstract
Background
Phytotherapy is a popular treatment option in cases of benign prostatic hyperplasia (BPH), with many different herbal products being used for the treatment of this condition. Withania coagulans (WC) is an herbal medicine that has shown anti-tumoral, anti-inflammatory, and antioxidant effects.
Objectives
This study examined the effect of Withania coagulans extract (WCE) on prostatic cell apoptosis and cyclooxygenase-2 (COX-2) expression in cases of benign prostatic hyperplasia (BPH) in rats.
Methods
Forty Wistar rats were equally divided into five groups: control, sham, BPH, BPH + WCE, and BPH + CLX (celecoxib) as a positive control group. The induction of BPH was achieved via the subcutaneous injection of 3 mg/kg of testosterone propionate (TP) daily for 28 days. The animals received WCE, celecoxib, or distilled water by oral gavage accompanied by the TP injection. After four weeks, the prostate glands of the rats were weighed to measure the prostatic index (PI). The ventral lobes of the prostates were dissected and processed with paraffin blocks in order to study the number of mast cells. A TUNEL analysis was performed to evaluate the cell apoptosis, while the expression of COX-2 was examined using immunohistochemistry.
Results
BPH was obvious in the ventral lobe of the prostate, and the administration of WCE markedly decreased the PI and the number of mast cells (P < 0.001) in the BPH rats. Additionally, the WCE treatment induced prostatic cell apoptosis when compared to the BPH group. Furthermore, following the WCE treatment, the expression of COX-2 in the prostatic tissues was significantly decreased when compared to the BPH groups.
Conclusions
According to the results of this study, WCE was effective in the treatment of BPH in rats. It may therefore have beneficial effects in the treatment of patients with BPH.
Keywords: Benign Prostatic Hyperplasia, Withania coagulans, Apoptosis, Cyclooxygenase-2
1. Background
Benign prostatic hyperplasia (BPH) is an age-related disease that is characterized by the progressive proliferation of prostatic glandular and stromal tissues (1). Senility and androgens are the two main factors believed to influence the progression of BPH, although some research studies have suggested a relation between inflammation of the prostate and BPH development (2). It has been reported that chronic prostatic inflammation was observed in 79% of patients with severe BPH (3). α1-adrenoceptor antagonists and 5α-reductase inhibitors are used for the treatment of BPH (4). New treatments for inflammatory diseases can result in the inhibition of the inflammation that is induced by cytokines (5). Some of the pro-inflammatory cytokines stimulate the expression of cyclooxygenase-2 (COX-2), which is associated with an increasing proliferative rate and the inhibition of cell death (6). The COX-2 enzyme converts arachidonic acid into prostaglandins, which play a main role in the inflammatory responses in the body (6).
Due to the upregulation of anti-apoptotic protein Bcl-2 expression, COX-2 causes a reduction in prostate tissue cell death (7). Many researchers have revealed that the human prostatic tissue expresses the COX-2 mRNA (6, 8). Some studies have shown that treatment with non-steroidal anti-inflammatory drugs (NSAIDs) inhibits BPH-1 cell line growth (9). It is feasible that by restraining the pro-inflammatory pathways created by COX-2 with such NSAIDs, the symptoms associated with BPH may be ameliorated (10). Herbal medicines have fewer negative effects and hence they are often preferred for the management of BPH (11). Many natural products stemming from plants have been shown to restrain cell proliferation and induce apoptosis, as well as promoting the management of BPH (11, 12). Withania coagulans (WC) is a traditional medicine from the Solanaceae family that is used for the treatment of various health problems and diseases in South Asia and the southeast regions of Iran (13). WC is well known for its antioxidant, anti-inflammatory, antiproliferative, and anti-cancerous properties (14-16). It has been reported that Withaferin A, one of the bioactive components of Withania, induces apoptosis in prostate cancer cells (17). Withanolides isolated from WC are known to be plant hormones that interact with COX-2 as well as being better inhibitors of COX-2 than NSAIDs (16, 18).
2. Objectives
In our previous study, we showed that WC extract (WCE) improved the prostate antioxidant status and decreased the stromal cell proliferation in a rat BPH model (14). Our aim in the present study was to evaluate the effects of WCE on COX-2 expression and apoptosis in a rat model of BPH.
3. Methods
3.1. Plant Extract Preparation
The WC plant was prepared at the herbarium center of Sistan and Baluchestan university. The whole plant was washed with deionized water. Then, the shadow air-dried plants were ground and the powder was subjected to extraction via the immersion of 1 g of plant powder into 20 mL of hydro-methanol (3:1 v/v) solution. The mixture was kept at room temperature overnight. The samples were then centrifuged at 18,000 × g for 30 minutes. The supernatants were filtered, evaporated to dryness, freeze dried, and then stored under refrigeration.
3.2. Animals
Forty male Wistar rats (weight range of 200 - 250 g) were obtained from the research center of the animal lab of Zahedan University of Medical Sciences. The animals were maintained under standard conditions of humidity, temperature (22 ± 2°C), and light/dark cycle (12 hours:12 hours). They had free access to the laboratory diet and water. The study was approved by the institutional animal care and ethical committee of Zahedan University of Medical Sciences (EC/93/7446). The BPH condition was induced via the daily subcutaneous (s.c) injection of 3 mg/kg of testosterone propionate (TP) for 28 days (19). The animals were then assigned to the following five groups (n = 8 per group):
Group I: Control (did not receive any treatment).
Group II: Sham (received distilled water by gavage in combination with s.c corn oil as a vehicle).
Group III: BPH (received distilled water by gavage in combination with s.c TP).
Group IV: BPH+WCE (received 1000 mg/kg WCE by gavage in combination with s.c TP).
Group V: BPH+CLX as a positive control (received 10 mg/kg celecoxib in combination with TP s.c by gavage) (20).
3.3. Tissue Samples
After four weeks, the animals were sacrificed under deep anesthesia with chloroform. The prostate of each rat was collected and weighed. The ventral prostate was carefully separated and then fixed in 10% neutral-buffered formalin, followed by embedding in paraffin. Seven micrometer (μm) sections were obtained for histopathological examination and immunohis-tochemical (IHC) analysis.
3.4. Prostate Weight
After the prostates had been weighed, the percentage of inhibition (PI) was measured as follows: 100 - [treated group - control) / (BPH - control) × 100] (21).
3.5. Histological Studies
Following the histological process, the tissues’ paraffin blocks were prepared and cut into 7 μm sections. Some of the samples were stained with toluidine blue (Sigma, T3260) in order to determine the level of inflammation by mast cell staining, while the other sections were prepared for immunohistochemical staining (TUNEL staining and COX-2 expression).
3.6. Toluidine Blue Staining
The tissue sections were embedded in xylene for paraffin removal, hydrated through an ethanol series, and rinsed in distilled water. The sections were then incubated with toluidine blue solution for 2 - 3 minutes. Next, the slides were rinsed in distilled water, dehydrated in alcohol, cleared in xylene, and mounted. The number of mast cells (toluidine blue positive cells) was counted at an x 400 field magnification using 15 random fields from five different rats in each group (22).
3.7. TUNEL Staining
The TUNEL staining method was performed according to the Roche protocol (Roche molecular biochemicals kit, Germany). Briefly, dewaxed tissue sections were treated with proteinase K for 30 minutes. In order to block the endogenous peroxidase activity, the sections were incubated with 3% H2O2 for 10 minutes, and then the TUNEL reaction mixture was added and the sections were kept for 1 hours at 37°C. They were incubated with a converter-POD for 30 minutes at 37°C and then incubated with diaminobenzidine substrate solution (DAB) for 15 minutes. The counterstaining was performed with hematoxylin. The apoptotic cells in ten random microscopic (× 400) fields per section were counted. The apoptotic index (AI) was estimated as follows: the number of TUNEL-positive cells / the total number of epithelial cells × 100 (23).
3.8. Immunohistochemical Detection of COX 2
The dewaxed sections were embedded in a 10 mM citrate buffer (pH 6.0) and then autoclaved for 5 minutes for antigen retrieval. The sections were incubated with 5% bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 2 hours. Then, anti COX 2 rabbit monoclonal antibodies (Abcam, SP21, USA) were added to the samples and they were kept overnight at 4°C. The slides were washed with PBS and incubated with the corresponding HRP-conjugated secondary antibody (goat anti-rabbit IgG (HRP), Abcam, USA) for 1 hours. The samples were washed again with PBS and then incubated with DAB for 10 minutes. Counterstaining was applied using hematoxylin, and the slides were observed under a light microscope. Diffuse brown cytoplasm staining in the prostate epithelial cells was considered to be positive staining with COX-2. The slides were evaluated using the immunohistochemical score (IHS), which was calculated by combining an estimate of the percentage of immunoreactive cells (quantity score) with an estimate of the staining intensity (staining intensity score), as previously described (24).
3.9. Statistical Analysis
The data are expressed as the mean ± SEM. All data analyses were performed using a one way ANOVA of variance and Tukey’s test. The IHS scores were compared using the Kruskal-Wallis test. P values < 0.05 were considered to be significant.
4. Results
4.1. Effect of W. coagulans on Prostate Weight and Growth Inhibition in BPH
As shown in Table 1, there were no significant differences in the prostate weights between the control and sham groups. However, in the BPH group, the prostate weight (2.55 ± 0.15 g) was significantly elevated when compare to the weight in the control and sham groups. The WCE significantly decreased the prostate weight (1.67 ± 0.2 g) and growth (62.8%) when compared with the BPH group. Additionally, in the CLX group, a significant decrease in both the prostate weight (1.86 ± 0.03 g) and growth (49.3%) was observed in comparison to the BPH group.
Table 1. Effects of WCE on Prostate Weight and Growth Inhibition in BPH.
| Group | Prostate Weight, gr, Mean ± SEM | Growth Inhibition, (%) |
|---|---|---|
| Control | 1.15 ± 0.5 | - |
| Sham | 1.18 ± 0.6 | - |
| BPH | 2.55 ± 0.1 | - |
| BPH + WCE | 1.67 ± 02a,b | 62.8 |
| BPH + CLX | 1.86 ± 0.03a,b | 49.3 |
aP < 0.005 vs. control group.
bP < 0.001 vs. BPH group.
4.2. Effect of W. coagulans on Mast Cell Infiltration in BPH
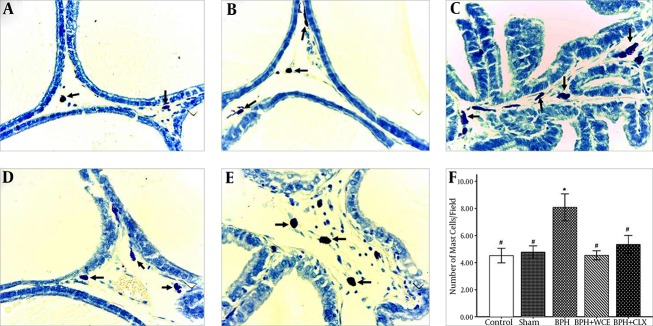
Toluidine blue staining was used to visualize the mast cell infiltration in the stromal cells of all the experimental groups. BPH resulted in an increased number of mast cells (violet cells) when compared to the control and sham groups (Figure 1A, B, and C ). The administration of WCE resulted in a decrease in mast cell infiltration when compared to the BPH group (Figure 1D and F). The CLX group also showed lower mast cell infiltration when compared to the BPH group, although the infiltration was higher when compared to that in the WCE group (Figure 1E and F).
Figure 1. Toluidine Blue Staining of the Rat Ventral Prostate in the Experimental Groups.
Representative pictures of the control, A; sham, B; BPH, C; BPH + WCE, D; and BPH + CLX, E groups (× 400). The histogram, F shows the number of mast cells in the stromal cells of the prostate. * P < 0.001 vs. control group; # P < 0.001 vs. BPH group.
4.3. Effect of W. coagulans on Apoptosis in BPH
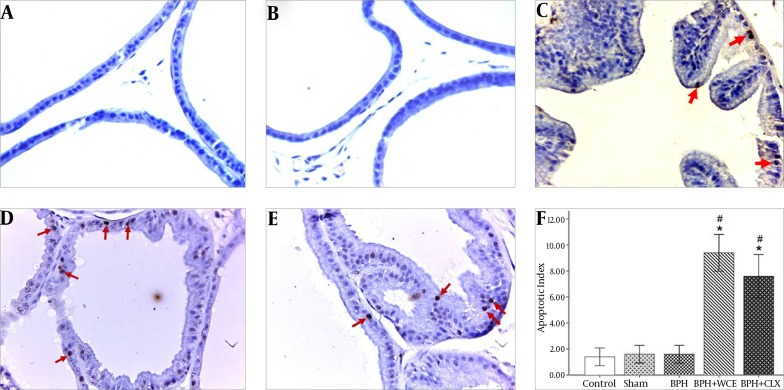
We investigated whether WCE could decrease the proliferation of prostate cells in BPH via immunohistochemical staining for TUNEL. TUNEL-positive cells were rarely detected in the prostate in the control, sham, and BPH groups (Figure 2A, B, and C). Treatment with WCE and CLX increased the apoptotic index when compared to the BPH group (P < 0.001) (Figure 2D and E). The number of TUNEL-positive (apoptotic) cells in the WCE group was higher than that in the CLX group, although the difference was not statistically significant (P > 0.05) (Figure 2F).
Figure 2. TUNEL Staining of the Prostate Tissues of Different Groups of Rats, Magnification × 400).
Namely the control, A; sham, B; BPH, C; BPH + WCE, D and BPH + CLX, E groups. The histogram, F shows the number of apoptotic cells. * P < 0.001 vs. control group; # P < 0.001 vs. BPH group. Red arrows indicate the apoptotic cells.
4.4. Effect of W. coagulans on COX-2 Expression in BPH
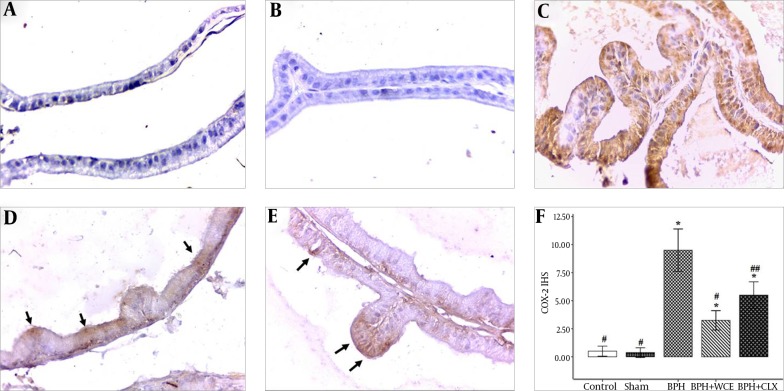
The COX-2 expression was negligible in the control and sham groups, but it was upregulated in the prostate epithelium of the BPH rats (Figure 3C). In addition, the upregulation of COX-2 was suppressed in the WCE group (Figure 3D). The administration of CLX also resulted in a decrease in COX-2 expression, albeit lower than the decrease observed on the WCE group (Figure 3F).
Figure 3. Immunohistochemical Staining and Immunohistochemical Score (IHS) for COX-2 in the Prostate Tissues of Different Groups of Rats, Magnification × 400.
Namely the control, A; sham, B; BPH, C; BPH + WCE, D and BPH + CLX, E groups. The IHS was equal to the quantity scores multiplied by the staining intensity scores, F. * P < 0.001 vs. control group; # P < 0.001 and ## P < 0.05 vs. BPH group. Black arrows indicate the COX-2 cytoplasm staining (in brown).
5. Discussion
In the present study, we evaluated the effects of the W. coagulans extract on cell apoptosis and COX-2 inhibition in BPH induced by testosterone in rats. Treatment with WCE decreased the prostate weight, and COX-2 expression, as well as increasing cell apoptosis, in the prostate when compared to the BPH group. The histopathological study also revealed that WCE caused a decrease in the pro-inflammatory cells (mast cells) in the BPH model.
Although medical therapy is helpful in the treatment of BPH, plant extracts are known to be useful for the relief of BPH symptoms without resulting in side effects (11).
WCE has anti-inflammatory, antiproliferative, and apoptotic effects that have been demonstrated by us and other researchers (14, 25, 26). In our previous study, we demonstrated the antiproliferative and antioxidant effects of WCE in different doses on a BPH rat model (14). A 1000 mg/kg WCE dose was found to be effective and therefore we applied that dose in the present study.
BPH is a pathologic disease and many factors are involved in the condition. In addition to aging, androgens play a principal role in the pathogenesis of BPH, with testosterone being the main androgen associated with prostate growth and the sustenance of its structural and functional integrity (27). An alteration in the testosterone level is usually accompanied by changes in the growth and weight of the prostate (28).
About 90% of testosterone (TE) is converted into dihydrotestosterone (DHT) by the prostate cells. The DHT then binds to the androgen receptors and stimulates protein synthesis, differentiation, and prostate cell growth (29).
Researchers have reported that there is an increase in the serum testosterone and DHT in BPH patients, and these levels are directly related to the size of the prostate (30). Some previous studies have reported that an increase in the prostatic weight is the main marker of the development of BPH (31, 32).
In this study, we found an increase in the prostate weight in the BPH model. The animals treated with WCE showed a decrease in prostate weight when compared to the BPH group. This finding was more prominent in the WCE group than the CLX group. The two main active components of Withania plants are Withaferin A and withanolides. Withanolides are also known as plant hormones; when there is an excess of a certain hormone, withanolides acting as cell membrane receptors are able to prevent animal hormones from attaching to these sites and can hence engage in their true activities (16). In this study, the withanolides from WCE probably did not permit exogenous testosterone to bind to the testosterone receptors and prevent its effects, which caused proliferation and an increase in the weight of the prostate.
The pathogenesis of BPH remains obscure, although several studies have demonstrated the role of inflammatory infiltrates and their mediators in the development of BPH (33). Increasing evidence indicates that mast cells play a main role in the pathogenesis of inflammatory diseases (34). Mast cells also play an important role in the development of many tumors, including prostate cancer, and they are now discussed as the main regulators of inflammatory diseases (35).
Huang and colleagues showed that mast cells blocked the apoptosis in tumor cells via the secretion of proinflammatory factors such as tumor necrosis factor-α (TNF-α) (36).
Mast cells are a variety of inflammatory cells that release proteases, angiogenic factors, and cytokines. The enhancement of the cytokines induces cell survival, growth, differentiation, and cyclooxygenase-2 expression (6).
Pittoni et al. confirmed that mast cells play an essential role in the initial and progression of prostate cancer, and also that mast cell inactivation may prove useful for antitumor therapy (37). Papadoukakis et al. showed a remarkable enhancement in mast cell numbers in the stromal area of the ventral prostate in a BPH rat model with a significant number of intracytoplasmic granules and degranulation (38).
The present work showed that BPH induction caused an increase in mast cell infiltration, which was in accordance with the findings reported by Bahey et al. (22). Treatment with WCE decreased the number of mast cells significantly when compared to the BPH group. This might be attributed to the anti-inflammatory property of Withania coagulans, which has been previously described by several researchers (26, 39).
Various studies have shown that pro-inflammatory cytokines induce COX-2 expression, which leads to an increase in the proliferation rate in tissues (5, 40). Via the conversion of arachidonic acid into prostaglandins, the COX-2 enzyme is responsible for inflammatory responses in the body (41). The upregulation of COX-2 increases the cell proliferation and inhibits cell death (42). Wang et al. indicated the over-expression of COX-2 in patients with BPH and prostatitis (6).
Other studies have also indicated that COX-2 inhibition causes a decrease in proliferation in cases of prostate cancer and BPH (43).
Altavilla et al. showed that Flavocoxid, a COX-2 inhibitor, reduced the prostate weight in a BPH model in mice via the induction of Bax expression and the decrease of Bcl-2 (anti-apoptotic) (10).
Further, Jayaprakasam et al. reported that the withanolides isolated from the Withania plant possess excellent selective COX-2 inhibitory activity (25).
In another study, Min et al. demonstrated that Withaferin A, the main constitute of the Withania plant, could regulate the inflammatory reactions in microglia by decreasing COX-2 expression and prostaglandin E2 (PGE2) production (44).
Moreover, Withaferin A revealed that WCE inhibits TNF-α and nuclear factor-κB (NF-κB) activation (45). TNF-α acts as a stimulator of COX-2 expression in normal and malignant prostate cells (46). The study by Ichikawa et al. showed that withanolides downregulated the expression of COX-2 (47).
COX-2 enzyme receptors have a high affinity for being occupied by plant steroid hormones (48). In the present study, we found an enhancement in COX-2 activity in the prostate tissues in BPH rats, which was in agreement with the results obtained by Wang and colleagues, who found that COX-2 was upregulated in the prostate cells in both prostate cancer and BPH tissue samples (40). The present study demonstrated that the COX 2 levels in the WCE treatment group significantly decreased when compared to those in the BPH group. This finding indicates that WCE restrains the development of BPH in rats, which is related to a decrease in COX 2 expression. However, the expression of this enzyme was better suppressed in the WCE treatment group’s prostate tissue than in the CLX treatment group, which indicates that the withanolides revealed themselves to be better inhibitors of COX-2 than NSAIDs. Therefore, withanolides could serve as substitutes for NSAIDs (16).
Previous studies have shown that an increase in cell proliferation and a decrease in apoptosis causes pathologic prostate enlargement (49). Apoptosis or programmed cell death is a protective mechanism against the accumulation and spread of defective cells (50). In our previous study, we evaluated the anti-proliferative effects of WCE in a BPH rat model using PCNA immunohistochemistry staining. The results showed that WCE decreases the epithelial proliferation in prostate tissue. Withaferin A from Withania showed an inhibition of the G1/S cell cycle and cell proliferation, as well as the induction of apoptosis, in prostate cancer cells. In addition, Withaferin A prevented the DNA binding activity of NF-κB, a main regulator of the cellular processes involved in the differentiation, cellular proliferation, and apoptosis (17).
In the present investigation, we evaluated the effect of WCE on apoptosis in prostate epithelial cells using the TUNEL staining method. We found that WCE increases apoptosis in the epithelial prostate cells in a BPH model of rats, which might be attributed to the pro-apoptotic effects of WCE through the inhibition of both the G1/S cell cycle and NF-κB expression. This result is consistent with the findings reported by Roy et al. concerning prostate cancer cells (17).
In conclusion, our results suggested that WCE might inhibit oxidative stress, inflammation, COX-2 expression, and the induction of apoptosis, which has a therapeutic effect in BPH. Therefore, this plant should be considered as a novel phytomedicine against BPH in patients. However, more studies are necessary to determine the potential therapeutic effects of Withania coagulans in BPH patients.
Acknowledgments
The authors wish to thank Dr. Soroush Dabiri for his kind cooperation.
Footnotes
Authors’ Contribution:All the authors played an equal role in this study.
Funding/Support:This project was part of an MSc thesis supported by the research deputy of Zahedan University of Medical Sciences (project number 7446).
References
- 1.De Nunzio C, Albisinni S, Gacci M, Tubaro A. The Role of Inflammation in the Progression of Benign Prostatic Hyperplasia. Current Bladder Dysfunction Reports. 2013;8(2):142–9. doi: 10.1007/s11884-013-0179-6. [DOI] [Google Scholar]
- 2.Briganti A, Capitanio U, Suardi N, Gallina A, Salonia A, Bianchi M, et al. Benign Prostatic Hyperplasia and Its Aetiologies. Eur Urol Supplements. 2009;8(13):865–71. doi: 10.1016/j.eursup.2009.11.002. [DOI] [Google Scholar]
- 3.Robert G, Descazeaud A, Nicolaiew N, Terry S, Sirab N, Vacherot F, et al. Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. Prostate. 2009;69(16):1774–80. doi: 10.1002/pros.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McVary KT. A review of combination therapy in patients with benign prostatic hyperplasia. Clin Ther. 2007;29(3):387–98. doi: 10.1016/s0149-2918(07)80077-4. [DOI] [PubMed] [Google Scholar]
- 5.Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol. 2006;16(1):25–9. [PubMed] [Google Scholar]
- 6.Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61(1):60–72. doi: 10.1002/pros.20061. [DOI] [PubMed] [Google Scholar]
- 7.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83(3):493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 8.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191(2):125–35. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 9.Kahokehr A, Vather R, Nixon A, Hill AG. Non-steroidal anti-inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. BJU Int. 2013;111(2):304–11. doi: 10.1111/j.1464-410X.2012.11559.x. [DOI] [PubMed] [Google Scholar]
- 10.Altavilla D, Minutoli L, Polito F, Irrera N, Arena S, Magno C, et al. Effects of flavocoxid, a dual inhibitor of COX and 5-lipoxygenase enzymes, on benign prostatic hyperplasia. Br J Pharmacol. 2012;167(1):95–108. doi: 10.1111/j.1476-5381.2012.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Allkanjari O, Vitalone A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015;126:42–56. doi: 10.1016/j.lfs.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 12.van Breemen RB, Sharifi R, Viana M, Pajkovic N, Zhu D, Yuan L, et al. Antioxidant effects of lycopene in African American men with prostate cancer or benign prostate hyperplasia: a randomized, controlled trial. Cancer Prev Res (Phila). 2011;4(5):711–8. doi: 10.1158/1940-6207.CAPR-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigomi M, Mohammadifar MA, Hashemi M, Senthil K, Valizadeh M. Biochemical and rheological characterization of a protease from fruits of Withania coagulans with a milk-clotting activity. Food Sci Biotechnol. 2014;23(6):1805–13. [Google Scholar]
- 14.Sarbishegi M, Khani M, Salimi S, Valizadeh M, Sargolzaei Aval F. Antiproliferative and Antioxidant Effects of Withania coagulans Extract on Benign Prostatic Hyperplasia in Rats. Nephrourol Mon. 2016;8(1):33180. doi: 10.5812/numonthly.33180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarbishegi M, Heidari Z, Mahmoudzadeh-Sagheb H, Valizadeh M, Doostkami M. Neuroprotective effects of Withania coagulans root extract on CA1 hippocampus following cerebral ischemia in rats. Avicenna J Phytomed. 2016;6(4):399–409. [PMC free article] [PubMed] [Google Scholar]
- 16.Khodaei M, Jafari M, Noori M. Remedial use of withanolides from Withania coagolans (Stocks) Dunal. Adv Life Sci. 2012;2(1):6–19. [Google Scholar]
- 17.Roy RV, Suman S, Das TP, Luevano JE, Damodaran C. Withaferin A, a steroidal lactone from Withania somnifera, induces mitotic catastrophe and growth arrest in prostate cancer cells. J Nat Prod. 2013;76(10):1909–15. doi: 10.1021/np400441f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayaprakasam B, Nair MG. Cyclooxygenase-2 enzyme inhibitory withanolides from Withania somnifera leaves. Tetrahedron. 2003;59(6):841–9. [Google Scholar]
- 19.Hardik S, Hardik M, Deepti J, Ghanashyam P. Pharmacological investigation of an Ayurvedic formulation on testosterone propionate-induced benign prostatic hyperplasia rats. J Exp Integr Med. 2014;4(2):131–6. [Google Scholar]
- 20.Funahashi Y, O'Malley KJ, Kawamorita N, Tyagi P, DeFranco DB, Takahashi R, et al. Upregulation of androgen-responsive genes and transforming growth factor-beta1 cascade genes in a rat model of non-bacterial prostatic inflammation. Prostate. 2014;74(4):337–45. doi: 10.1002/pros.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veeresh Babu SV, Veeresh B, Patil AA, Warke YB. Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. Eur J Pharmacol. 2010;626(2-3):262–5. doi: 10.1016/j.ejphar.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Bahey NG, Soliman GM, El-Deeb TA, El-Drieny EA. Influence of insulin and testosterone on diabetic rat ventral prostate: Histological, morphometric and immunohistochemical study. J Micros Ultra. 2014;2(3):151–60. doi: 10.1016/j.jmau.2014.06.002. [DOI] [Google Scholar]
- 23.Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomarkers Prev. 2003;12(9):933–9. [PubMed] [Google Scholar]
- 24.Zheng H, Xu W, Lin J, Peng J, Hong Z. Qianliening capsule treats benign prostatic hyperplasia via induction of prostatic cell apoptosis. Mol Med Rep. 2013;7(3):848–54. doi: 10.3892/mmr.2013.1265. [DOI] [PubMed] [Google Scholar]
- 25.Jayaprakasam B, Zhang Y, Seeram NP, Nair MG. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003;74(1):125–32. doi: 10.1016/j.lfs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Shukla K, Dikshit P, Shukla R, Sharma S, Gambhir JK. Hypolipidemic and antioxidant activity of aqueous extract of fruit of Withania coagulans (Stocks) Dunal in cholesterol-fed hyperlipidemic rabbit model. Indian J Exp Biol. 2014;52(9):870–5. [PubMed] [Google Scholar]
- 27.Jarvis TR, Chughtai B, Kaplan SA. Testosterone and benign prostatic hyperplasia. Asian J Androl. 2015;17(2):212–6. doi: 10.4103/1008-682X.140966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales A. Androgen replacement therapy and prostate safety. Eur Urol. 2002;41(2):113–20. doi: 10.1016/s0302-2838(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JB, Roehrborn CG, Schalken JA, Emberton M. The progression of benign prostatic hyperplasia: examining the evidence and determining the risk. Eur Urol. 2001;39(4):390–9. doi: 10.1159/000052475. [DOI] [PubMed] [Google Scholar]
- 30.Carson C, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61(4 Suppl 1):2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 31.Hutchison A, Farmer R, Verhamme K, Berges R, Navarrete RV. The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries. Eur Urol. 2007;51(1):207–15. doi: 10.1016/j.eururo.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Al-Ansari AA, Shokeir AA. Noninvasive treatment of benign prostatic hyperplasia. Where do we stand in 2005. Saudi Med J. 2006;27(3):299–304. [PubMed] [Google Scholar]
- 33.Robert G, Salagierski M, Schalken JA, de La Taille A. Inflammation and benign prostatic hyperplasia: cause or consequence? [in French]. Prog Urol. 2010;20(6):402–7. doi: 10.1016/j.purol.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146(1-2):1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 35.Taverna G, Giusti G, Seveso M, Hurle R, Colombo P, Stifter S, et al. Mast cells as a potential prognostic marker in prostate cancer. Dis Markers. 2013;35(6):711–20. doi: 10.1155/2013/478303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112(4):1269–79. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittoni P, Tripodo C, Piconese S, Mauri G, Parenza M, Rigoni A, et al. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res. 2011;71(18):5987–97. doi: 10.1158/0008-5472.CAN-11-1637. [DOI] [PubMed] [Google Scholar]
- 38.Papadoukakis S, Kyroudi-Voulgari A, Truss MC, Perea D, Mitropoulos D. Quantitative study of mast cells in experimentally induced benign prostatic hyperplasia. Urol Int. 2010;84(1):100–4. doi: 10.1159/000273475. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad MK, Mahdi AA, Shukla KK, Islam N, Rajender S, Madhukar D, et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril. 2010;94(3):989–96. doi: 10.1016/j.fertnstert.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005;11(9):3250–6. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- 41.Kirschenbaum A, Klausner AP, Lee R, Unger P, Yao S, Liu XH, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56(4):671–6. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 42.Fujita H, Koshida K, Keller ET, Takahashi Y, Yoshimito T, Namiki M, et al. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53(3):232–40. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- 43.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58(19):4245–9. [PubMed] [Google Scholar]
- 44.Min KJ, Choi K, Kwon TK. Withaferin A down-regulates lipopolysaccharide-induced cyclooxygenase-2 expression and PGE2 production through the inhibition of STAT1/3 activation in microglial cells. Int Immunopharmacol. 2011;11(8):1137–42. doi: 10.1016/j.intimp.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Int Immunopharmacol. 2009;9(5):614–9. doi: 10.1016/j.intimp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Subbarayan V, Sabichi AL, Llansa N, Lippman SM, Menter DG. Differential expression of cyclooxygenase-2 and its regulation by tumor necrosis factor-alpha in normal and malignant prostate cells. Cancer Res. 2001;61(6):2720–6. [PubMed] [Google Scholar]
- 47.Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006;5(6):1434–45. doi: 10.1158/1535-7163.MCT-06-0096. [DOI] [PubMed] [Google Scholar]
- 48.Mulabagal V, Subbaraju GV, Rao CV, Sivaramakrishna C, Dewitt DL, Holmes D, et al. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother Res. 2009;23(7):987–92. doi: 10.1002/ptr.2736. [DOI] [PubMed] [Google Scholar]
- 49.Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol. 1996;27(7):668–75. doi: 10.1016/s0046-8177(96)90396-2. [DOI] [PubMed] [Google Scholar]
- 50.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]