Abstract
The leaf venation architecture is an ideal, highly structured and efficient irrigation system in plant leaves. Leaf vein density (LVD) and vein thickness are the two major properties of this system. Leaf laminae carry out photosynthesis to harvest the maximum biological yield. It is still unknown whether the LVD and/or leaf vein thickness determines the plant hydraulic conductance (Kplant) and leaf photosynthetic rate (A). To investigate this topic, the current study was conducted with two varieties under three PEG-induced water deficit stress (PEG-IWDS) levels. The results showed that PEG-IWDS significantly decreased A, stomatal conductance (gs), and Kplant in both cultivars, though the IR-64 strain showed more severe decreases than the Hanyou-3 strain. PEG-IWDS significantly decreased the major vein thickness, while it had no significant effect on LVD. A, gs and Kplant were positively correlated with each other, and they were negatively correlated with LVD. A, gs and Kplant were positively correlated with the inter-vein distance and major vein thickness. Therefore, the decreased photosynthesis and hydraulic conductance in rice plants under water deficit conditions are related to the decrease in the major vein thickness.
Photosynthesis is an important physiological process that is very sensitive to abiotic stresses1,2. Diffusive (stomatal or mesophyll conductance) and biochemical impairments are considered two major responses that decrease photosynthesis under drought conditions3,4. Stomatal conductance (gs) is a fundamental process required for CO2 acquisition and is regulated by stomatal opening and closing5,6. A decreasing leaf turgor pressure and an increasing vapor pressure deficit (VPD) closes the stomata rapidly in response to water deficit condition7. Thus, stomatal limitation is a key cause of the decrease in A that occurs under water-limited conditions7,8.
The water transportation capacity of plants is known as the plant hydraulic conductance9,10, which is determined by the root, stem and leaf hydraulic conductance (Kleaf)11. The root contribution ranges from one-third to one-half of the internal plant resistances12,13. The transpiration rate (E) or stomatal conductance exhibit significant and linear correlations with Kplant in a number of higher plants and rice plant14,15,16,17,18. Therefore, the capacity of water transport system controls the plant growth as it maintains the hydraulic link between the roots and leaves19.
The leaf hydraulic architecture is the key location for gas exchange between the plant and its environment20,21, and extra-vascular resistance imposes one-quarter or higher resistance (≥30%) in Kleaf22,23. A decrease in Kleaf leads to stomatal closure, which reduces photosynthesis24,25. Therefore, a strong correlation has been observed between gs and Kleaf22,23,26. The leaf venation architecture is a perfect illustration of a highly efficient irrigation structure27,28. Veins are made up of phloem and xylem vessels implanted in parenchyma, rarely in sclerenchyma, that are wrapped in bundle sheath cells. Leaf veins in monocots of the Poaceae family are divided into three categories (major, minor and transverse veins) in addition to the leaf midrib, and are different in sizes and functions29,30,31,32,33. The major longitudinal veins run from the leaf lamina into the leaf sheath, while minor longitudinal veins mostly terminate at the junction of the leaf lamina and leaf sheath34,35,36,37.
The leaf venation architecture has many functions, including mechanical support38, sugars and hormone transportation39, and replacement of water lost through E during photosynthetic processes23. An enormous variation is found in the vein arrangement, size and density, and in the geometry of phloem and xylem vessels within the leaf vascular bundles. Thicker veins have a greater water transportation and sugar translocation capacity due to the greater number and/or size of the xylem and phloem vessels40.
During the last two decades, numerous studies have been carried out to explore the relationship between Kleaf and leaf vein structure. The leaf vein length per unit leaf area is called as the vein length per unit area (VLA) or leaf vein density (LVD). Positive, negative41,42 and no correlations43 have been found between LVD and Kleaf in these studies. In our previous study, a significant positive correlation between Kleaf, Kplant and LVD was observed in rice plants under well watered condition, but no relationship was observed between Kleaf and Kplant under drought stress, although Kleaf showed a positive correlation with LVD44.
It is still unknown which vein property in rice crops is more closely related to the leaf photosynthetic rate and Kplant under drought conditions. The current study had the following objectives: (i) to elaborate the effects of PEG-induced water deficit stress (PEG-IWDS) on gas exchange parameters; (ii) to elaborate weather the LVD or leaf vein thickness is related to Kplant; and (iii) to elaborate whether the LVD or leaf vein thickness is related to gas exchange parameters under PEG-IWDS.
Results
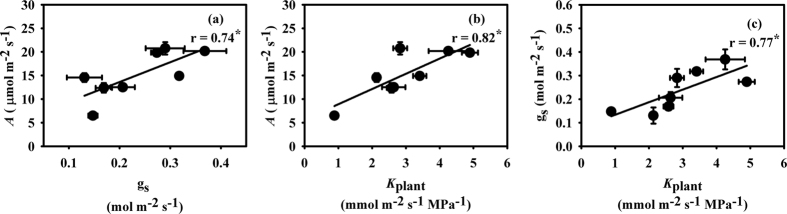
PEG-induced water deficit stress decreased the gas exchange parameters. More severe depression was observed in the IR-64 variety than in the Hanyou-3 variety (Table 1). IR-64 had a significant decrease in A under all PEG-IWDS conditions, while A was decreased non significantly under 5% PEG-IWDS in Hanyou-3. Under 15% PEG-IWDS, A was decreased by 68.7% in IR-64 compared with a smaller decrease of 27.8% in Hanyou-3. Hanyou-3 showed a significant decrease in gs under 15% PEG-IWDS, and IR-64 revealed a significant decrease in gs under both the 10% and 15% PEG-IWDS conditions. The intercellular CO2 concentration (Ci) increased under all stress levels in both varieties, but a significant increase was observed in IR-64 under 15% PEG-IWDS. Hanyou-3 and IR-64 both showed a significant decrease in E under 15% PEG-IWDS, but a more severe decrease (70.7%) was observed in IR-64 than Hanyou-3 (55.2%). The decrease in leaf water potential (Ψleaf) was only significant in IR-64 under 15% PEG-IWDS. There was a positive relationship between A and gs (Fig. 1a). Kplant showed a significant decrease in both varieties under 15% PEG-IWDS, although IR-64 showed a more severe decrease (68.8%) than Hanyou-3 (49.9%) (Table 1). A and gs showed positive correlations with Kplant (Fig. 1b,c).
Table 1. Effects of PEG-induced water deficit stress on photosynthesis (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (E) and leaf water potential (Ψleaf) of newly-developed leaves of two rice varieties at the vegetative stage.
| Varieties | Treatment | A (μmol m−2 s−1) | gs (mol m−2 s−1) | Ci (μmol mol−1) | E (mmol m−2 s−1) | Ψleaf (MPa) | Kplant (mmol m−2 s−1 MPa−1) |
|---|---|---|---|---|---|---|---|
| Hanyou-3 | WWC | 20.2 ± 0.3a | 0.37 ± 0.04a | 236 ± 4 | 6.29 ± 0.86a | −1.48 ± 0.03bc | 4.26 ± 0.58a |
| PEG-IWDS5% | 19.9 ± 0.3a | 0.27 ± 0.01ab | 258 ± 3 | 6.45 ± 0.31a | −1.37 ± 0.02a | 4.90 ± 0.24a | |
| PEG-IWDS10% | 14.9 ± 0.4b | 0.32 ± 0.00ab | 312 ± 4 | 4.07 ± 0.23ab | −1.37 ± 0.03ab | 3.41 ± 0.19ab | |
| PEG-IWDS15% | 14.6 ± 0.9b | 0.13 ± 0.03b | 277 ± 27 | 2.82 ± 0.04b | −1.70 ± 0.02c | 2.13 ± 0.03b | |
| IR-64 | WWC | 20.8 ± 1.3a | 0.29 ± 0.04a | 258 ± 10 | 4.44 ± 0.32a | −1.57 ± 0.04ab | 2.83 ± 0.21a |
| PEG-IWDS5% | 12.5 ± 0.4b | 0.21 ± 0.02ab | 263 ± 4 | 3.77 ± 0.49a | −1.48 ± 0.02a | 2.64 ± 0.35a | |
| PEG-IWDS10% | 12.4 ± 1.0b | 0.17 ± 0.02b | 260 ± 6 | 3.27 ± 0.46a | −1.44 ± 0.06ab | 2.98 ± 0.15a | |
| PEG-IWDS15% | 6.5 ± 0.6c | 0.15 ± 0.01b | 314 ± 3 | 1.30 ± 0.08b | −1.85 ± 0.02b | 0.88 ± 0.05b | |
| ANOVA | |||||||
| Treatment (T) | ** | ** | ns | ** | ** | * | |
| Variety (V) | ns | ns | ns | ns | ns | * | |
| T × V | ** | ns | ns | ns | ns | ns | |
Water deficit stress was simulated by adding 5, 10 or 15% (W/V) PEG6000 to the nutrient solution.
WWC, well-watered condition; PEG-IWDS, PEG-induced water deficit stress. The data are presented as the means ± SE with 3 replicates. ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001. The data followed by the different letters of each variety within a single column are significant at P < 0.05 level.
Figure 1.
Relationships between photosynthesis (A) and stomatal conductance (gs) (a) and plant hydraulic conductance (Kplant) (b) and relationship between gs and Kplant (c). The data are presented as the mean values of 3 replicates. *P < 0.05.
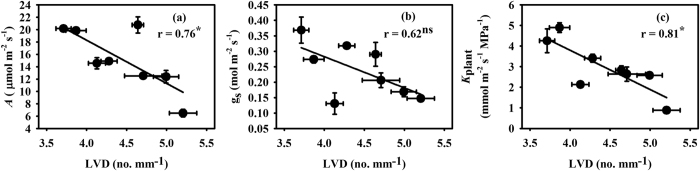
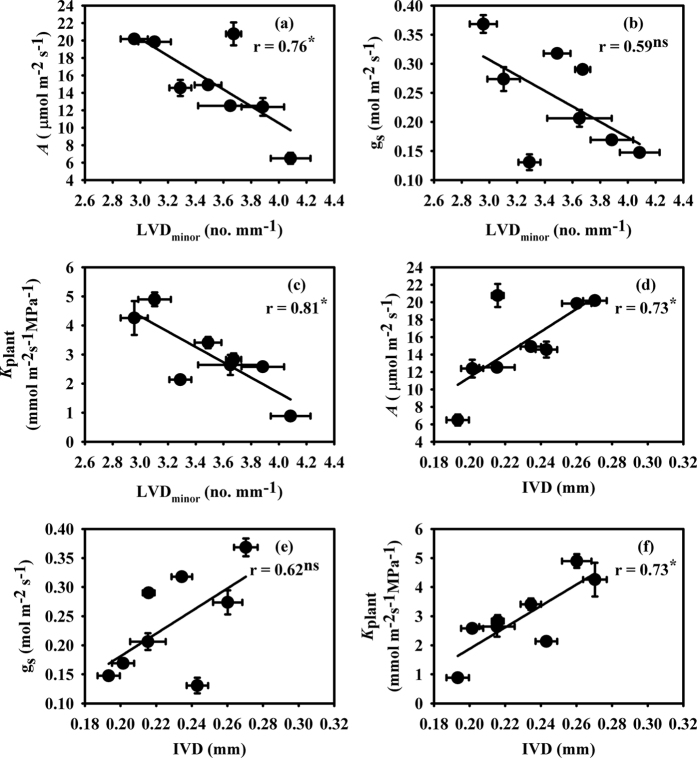
Leaf size was decreased under all PEG-IWADS conditions in Hanyou-3, but in IR-64, it was only significantly decreased under 15% PEG-IWDS (Table 2). Compared with IR-64, a more severe decrease in leaf size was observed in Hanyou-3 under all PEG-IWDS conditions. Leaf size showed positive correlation with the major, and minor vein thickness as well as with inter-vein distances (IVD), while it showed negative correlations with LVD and LVDminor (data not shown). LVD and LVDminor showed non-significant increases in both varieties under all PEG-IWADS conditions. Interestingly, IR-64 had a higher leaf vein density than Hanyou-3 under all treatment conditions. On the other hand, IVD decreased non-significantly under all treatment conditions in both varieties, and Hanyou-3 had a higher IVD than IR-64. LVD had a negative correlation with A and Kplant, but a non-significant relationship with gs (Fig. 2). Similarly, LVDminor had negative correlations with A and Kplant (Fig. 3a,c), but gs was not significantly related to LVDminor (Fig. 3b). IVD was positively correlated with A and Kplant (Fig. 3d,f) and was not related to gs (Fig. 3e).
Table 2. Effects of PEG-induced water deficit stress on the single leaf area, leaf vein density (LVD), minor leaf vein density (LVDminor), and inter-vein distance (IVD) of newly developed leaves of two rice varieties at the vegetative stage.
| Varieties | Treatment | Single leaf area (cm2) | LVD (no. mm−1) | LVDminor (no. mm) | IVD (mm) |
|---|---|---|---|---|---|
| Hanyou-3 | WWC | 72.3 ± 1.1a | 3.71 ± 0.09a | 2.96 ± 0.10a | 0.270 ± 0.007a |
| PEG-IWDS5% | 53.3 ± 2.8b | 3.87 ± 0.13a | 3.10 ± 0.12a | 0.260 ± 0.008a | |
| PEG-IWDS10% | 52.5 ± 1.6bc | 4.28 ± 0.10a | 3.49 ± 0.10a | 0.234 ± 0.006a | |
| PEG-IWDS15% | 40.0 ± 1.4c | 4.13 ± 0.11a | 3.29 ± 0.08a | 0.243 ± 0.006a | |
| IR-64 | WWC | 25.9 ± 0.4a | 4.64 ± 0.07a | 3.67 ± 0.05a | 0.216 ± 0.003a |
| PEG-IWDS5% | 21.7 ± 0.9ab | 4.71 ± 0.23a | 3.65 ± 0.23a | 0.215 ± ± 0.010a | |
| PEG-IWDS10% | 22.7 ± 1.2ab | 4.99 ± 0.16a | 3.88 ± 0.15a | 0.201 ± 0.006a | |
| PEG-IWDS15% | 19.4 ± 0.2b | 5.21 ± 0.17a | 4.08 ± 0.14a | 0.193 ± 0.006a | |
| ANOVA | |||||
| Treatment (T) | *** | ns | ns | ns | |
| Variety (V) | *** | ** | * | * | |
| T × V | * | ns | ns | ns | |
Water deficit stress was simulated by adding 5, 10 or 15% (W/V) PEG6000 to the nutrient solution.
WWC, well-watered condition; PEG-IWDS, PEG-induced water deficit stress. The data are presented as the means ± SE with 3 replicates. ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001. The data followed by the different letters of each variety within a single column are significant at P < 0.05 level.
Figure 2.
Relationship of photosynthesis (A) (a), stomatal conductance (gs) (b) and plant hydraulic conductance (Kplant) (c) with leaf vein density (LVD). The data are presented as the mean values of 3 replicates. ns, not significant; *P < 0.05.
Figure 3.
Relationships of photosynthesis (A) (a), stomatal conductance (gs) (b) and plant hydraulic conductance (Kplant) (c) with minor leaf vein density (LVDminor) and inter vein distance (IVD) (d–f). The data are presented as the mean values of 3 replicates. ns, not significant; *P < 0.05.
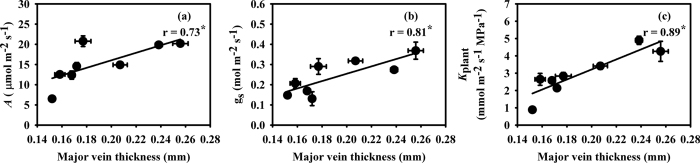
Major vein thickness decreased significantly in Hanyou-3 under 10 and 15% PEG-IWDS while a non-significant decrease was observed in IR-64 under all PEG-IWDS conditions (Table 3). Minor vein thickness decreased significantly in Hanyou-3 under 5% PEG-IWDS but non-significantly decreased under 10 and 15% PEG-IWDS. Moreover, the decrease in minor vein thickness was non-significant in IR-64 under all PEG-IWDS conditions. Major vein thickness showed a positive correlation with A, gs and Kplant (Fig. 4). However, leaf minor vein thickness did not show any significant relationship with gas exchange or Kplant (data not shown).
Table 3. Effects of PEG-induced water deficit stress on the leaf major and minor vein thickness of newly developed leaves of two rice varieties at the vegetative stage.
| Varieties | Treatment | Major vein thickness (mm) | Minor vein thickness (mm) |
|---|---|---|---|
| Hanyou-3 | WWC | 0.256 ± 0.006a | 0.120 ± 0.007a |
| PEG-IWDS5% | 0.238 ± 0.003a | 0.093 ± 0.002b | |
| PEG-IWDS10% | 0.207 ± 0.006b | 0.100 ± 0.001ab | |
| PEG-IWDS15% | 0.172 ± 0.002c | 0.101 ± 0.001ab | |
| IR-64 | WWC | 0.177 ± 0.006a | 0.098 ± 0.002a |
| PEG-IWDS5% | 0.158 ± 0.004a | 0.092 ± 0.001a | |
| PEG-IWDS10% | 0.168 ± 0.003a | 0.092 ± 0.001a | |
| PEG-IWDS15% | 0.152 ± 0.002a | 0.096 ± 0.001a | |
| ANOVA | |||
| Treatment (T) | *** | ns | |
| Varieties (V) | *** | ns | |
| T × V | * | ns | |
Water deficit stress was simulated by adding 5, 10 or15% (W/V) PEG6000 to the nutrient solution.
WWC, well-watered condition; PEG-IWDS, PEG-induced water deficit stress. The data are presented as the means ± SE with 3 replicates. ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001. The data followed by the different letters of each variety within a single column are significant at P < 0.05 level.
Figure 4.
Relationships of photosynthesis (A) (a), stomatal conductance (gs) (b) and plant hydraulic conductance (Kplant) (c) with leaf major vein thickness. The data are presented as the mean values of 4 replicates. *P < 0.05.
Discussion
Stomatal closure in response to water deficit stress will limit photosynthesis by restricting CO2 entry from the ambient environment into the intercellular air spaces of mesophyll cells45,46,47,48. Moreover, decreased gm and impaired biochemical processes are non-stomatal limitations to photosynthesis that occur under severe or long-term water deficit conditions49,50,51. It is therefore logical that photosynthesis exhibited positive correlations with gs or/and gm in previous studies52,53,54,55. In the current study, A was also positively correlated with gs (Fig. 1a). Chaves et al.7 reported that increased VPD and reduced turgor potential are major causes of stomatal closure under water-limited conditions. However, it is the boundary layers (leaf and canopy), as well as the driving force (VPD), that determine E, while Kplant determines the water potential at that E9,10. Thus, a high Kplant can maintain a high gs and the consequent A without leading to desiccation of the plant leaves14,56,57,58,59. Linear correlations between Kplant and E or gs were previously found in a number of higher plant species15,16,17,18. Kleaf is a major component of Kplant, the positive correlation between Kplant and gas exchange may be related to Kleaf. In the present study, the positive correlations between gs, A and Kplant suggest that Kplant is one of the key regulators of photosynthesis (Fig. 1).
The environmental signals present before and during leaf development determines the vein traits, like other leaf traits including leaf size and stomatal density60,61. Plasticity in vein traits was observed within the canopy and across environments for a given plant species. In this study, plasticity in leaf size, LVD, IVD and vein thickness were also observed under different PEG-IWDS conditions. Sack et al.62 suggested that LVD has a key influence on hydraulic conductance, gs and A, and LVD is positively correlated with A. In the present study, A was negatively correlated with LVD and LVDminor (Figs 2a and 3a) and positively correlated with IVD (Fig. 3d). This negative relationship between A and LVD is in accordance with negative relationships reported in angiosperms63,64,65,66,67, but different from the study by Xiong et al.68, who did not observe any relationship between gs, A and LVD during studies of the Oryza genus under well-watered condition.
Rice leaves are small and have more highly lobed mesophyll cells than C4 crop species69. They also have a lower LVD than C4 crops due to the higher number of mesophylls between veins. C4 plants, such as Setaria viridis and sorghum, have seven veins per millimeter, but rice has fewer than six veins per millimeter70. Although rice (C3) and maize (C4) both belong to the tropical-warm temperate grass family, rice has higher rates of photorespiration. This higher rate of photorespiration decreases the photosynthetic capacity by 30–35% at 30–35 °C ambient temperature71, and drought conditions make this more severe, so rice does not attain the full potential photosynthesis like C4 plants.
Kplant was negatively correlated with LVD (Fig. 2c), while it had a positive correlation with IVD (Fig. 3f). Mesophyll cells are more numerous in C3 than C4 plants, which increases IVD in C3 plants70,72 and reduces their Kleaf23,43. Smillie et al.73 reported that IVD of rice plants is more dependent on cell size than cell number, which suggests that the lower IVD under water deficit conditions is mostly a result of more tightly packed, small mesophyll cells. The tightly packed mesophyll cells in smaller leaves under water deficit (Table 2) would produce more resistance in the apoplastic pathway for water transport in leaves, which would decrease Kleaf and the subsequent Kplant.
Vein size also decreases under drought stress in addition to leaf vein differentiation. Martre and Durand74 reported that the vascular tissue is composed of xylem and phloem cells, and it carries out the transportation of different compounds. The flow rate of this transportation is determined by the size of the xylem and phloem cells. Decreases in the diameters of the xylem and phloem vessels were observed in Ctenanthe setosa, Vigna unguiculata and Triticum aestivum under water deficient conditions75,76,77, likely because the thin xylem vessels provide protection from cavitation under water-limited conditions78. In the present study, the major and minor vein thicknesses were also decreased under PEG-IWDS. The Hanyou-3 and IR-64 varieties showed more severe decreases in the major vein thickness (32.8% and 14.1%) than in the minor vein thickness (15.9% and 1.3%) under 15% PEG-IWDS (Table 3). The major, minor (longitudinal) and transverse veins have different sizes and functions29,30,31,33. The major leaf veins are the main supply lines for receiving water directly from the roots via the stem and leaf sheath, as they run from the leaf blade into the sheath while minor veins terminate at the junction of the leaf blade and sheath34,35,36,37. Water absorbed by the roots rises through the major veins from the leaf base to the leaf tips. After exiting the major veins, water reaches the minor veins via transverse veins and is finally distributed to mesophyll cells or is used for transpiration via stomata31,32,33. Ocheltree et al.79 suggested that gs is strongly correlated with extra vascular resistance (outside large veins) under normal water regimes, while large vein resistance has a strong correlation with gs under drought conditions. The current study suggests that the decreased major vein thickness that occurred under PEG-IWDS would increase the major vein resistance and restrict water uptake from the roots to leaves, and hence decreased Kplant and subsequently gs and A.
Based on the present findings, we conclude that PEG-IWDS deceases Kplant, photosynthesis, leaf vein thickness and IVD, while it increases LVD and LVDminor. LVD is negatively correlated with Kplant and photosynthesis, while major vein thickness is positively correlated with Kplant, gs and A under PEG-IWDS condition in rice crops.
Materials and Methods
Plant materials
Two rice cultivars, Hanyou-3 and IR-64, were selected because they had different drought tolerances with regard to photosynthesis in previous study. Hanyou-3 is considered drought-tolerant, while IR-64 is considered drought-sensitive. Seeds were surface-sterilized for 90 minutes using 10% H2O2, then washed with tap water to remove any residual H2O2. The seeds were germinated on moist filter paper until the radical emerged in the laboratory, then they were transferred to a seedling tray with tap water under natural environmental conditions. Seedlings were supplied with 1/8th-strength Hoagland solution on the fifth day of germination to avoid nutrient deficiency. Seedlings were transplanted after fifteen days of germination. Each bucket contained 10.5 L Hoagland solution. Seedlings were transplanted using a split block design such that each bucket had four seedlings of each variety. This experiment had six replicates and four treatments: the well-watered condition (WWC) and 5%, 10% and 15% (w/v) PEG-IWDS. Treatments were applied when seedlings reached 40 days of age. The composition of the full strength nutrient solution was as follows: macronutrients (mg l−1): 40 N as (NH4)2SO4 and Ca(NO3)2, 10 P as KH2PO4, 40 K as K2SO4 and KH2PO4, and 40 Mg as MgSO4; micronutrients (mg l−1): 2.0 Fe as Fe-EDTA, 0.5 Mn as MnCl2∙4H2O, 0.05 Mo as (NH4)6Mo7O24∙4H2O, 0.2 B as H3BO3, 0.01 Zn as ZnSO4∙7H2O, 0.01 Cu as CuSO4∙5H2O, 2.8 Si as Na2SiO3∙9H2O. Dicyandiamide was added to the nutrient solution as a nitrification inhibitor. Solutions were changed every fifth day, and the pH was maintained at 5.50 ± 0.05 every day by adding 0.1 molL−1HCl or NaOH. The experiment was conducted under natural environmental conditions in Huazhong Agricultural University (114.37E, 30.48N) Wuhan, Hubei, China.
Gas exchange measurements
The gas exchanges were measured inside a growth chamber to avoid the fluctuations of the outdoor environment. The photosynthetic photon flux density (PPFD) was controlled to 1,000 μmol m−2 s−1 using T5 fluorescent lamps and halogen incandescent lamps fixed on a down and upward moving panel. There were three fans built in the roof of the growth chamber to avoid over-heating of the growth chamber, and the air temperature was set to 30/25 °C day/night with 11 h photoperiod. The relative humidity in the growth chamber was controlled at 65%..
Leaf area measurement
Three newly expanded leaves for each variety and replicate were detached, followed by leaf area measurement using a leaf area meter (Li-Cor 3000 C, Li-Cor, NE, USA).
Leaf vein density measurement
Rice leaf veins were divided into three categories based on their size (i.e., midrib, major and minor veins) to calculate the leaf vein density73. One centimeter leaf sections were excised with a razor blade from the middle portion of newly-developed leaves after measuring the leaf width. These sections were immediately immersed in tap water and carried to a laboratory to observe all visible longitudinal leaf vein numbers. In the laboratory, all visible leaf veins (sum of the midrib, major and minor leaf veins) were counted under 40x magnification using a light microscope (SA3300, Beijing Tech Instrument Co., Ltd, Beijing, China). IVD was calculated by dividing the leaf width with the respective total longitudinal leaf vein numbers. LVD was calculated as total vein length per leaf area, and LVDminor was calculated as the total minor vein length per leaf area.
Measurement of plant hydraulic conductance
During the gas exchange measurements, newly and fully developed leaves were used to measure the day time leaf water potential using a WP4C Dewpoint Potential Meter (Decagon, Pullman, WA, USA). Kplant was calculated following the formula described by Brodribb and Holbrook81:
 |
where Ψsolution was 0 for WWC, and was −0.05, −0.18 and −0.38 MPa, respectively, for the 5%, 10% and 15% PEG-IWDS.
Leaf vein thickness measurement
Minor vein thickness was measured for each side of the leaf (avoiding midribs) using a leaf thickness measuring instrument (YI-20030A, China Jiliang University), while major vein thickness was measured using a DTG03 digital thickness gauge (Digital Micrometers Ltd, Sheffield, UK).
Statistical analysis
One and two-way analyses of variance (ANOVA) were applied to assess the differences between treatments with Statistics 8.1 analytical software. Linear regression and correlation analysis were performed to test the possible correlations between the studied parameters using Sigma Plot 12 (SPSS Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Tabassum, M. A. et al. Influence of leaf vein density and thickness on hydraulic conductance and photosynthesis in rice (Oryza sativa L.) during water stress. Sci. Rep. 6, 36894; doi: 10.1038/srep36894 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was financially supported by the national key research and development program of China (2016YFD0300102), the National Natural Science Foundation of China (31301840), a Foundation for the Author of National Excellent Doctoral Dissertation of PR China (201465), and Fundamental Research Funds for the Central Universities (2662015PY031).
Footnotes
Author Contributions Y.L. and M.A.T. conceived and designed the research. M.A.T. conducted the experiments and collected and analyzed data. M.A.T. wrote the manuscript. G.L.Z., A.H., M.A.W. and M.S. helped to collect data during the experiments and gave valuable comments. Y.L. revised the manuscript.
References
- Hsiao T. C. & Acevedo E. Plant responses to water deficits, water-use efficiency, and drought resistance. Agricultural meteorology 14, 59–84 (1974). [Google Scholar]
- Huang B. Recent advances in drought and heat stress physiology of turfgrass — A review. Acta Horticulturae 661, 185–192 (2004). [Google Scholar]
- Flexas J., Bota J., Loreto F., Cornic G. & Sharkey T. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology 6, 269–279 (2004). [DOI] [PubMed] [Google Scholar]
- Flexas J. et al. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell & Environment 30, 1284–1298 (2007). [DOI] [PubMed] [Google Scholar]
- Medici L. O., Azevedo R. A., Canellas L. P., Machado A. T. & Pimentel C. Stomatal conductance of maize under water and nitrogen deficits. Pesquisa Agropecuária Brasileira 42, 599–601 (2007). [Google Scholar]
- Dodd I. C. Hormonal interactions and stomatal responses. Journal of Plant Growth Regulation 22, 32–46 (2003). [Google Scholar]
- Chaves M., Flexas J. & Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany 103, 551–560 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida A. K. & Das A. B. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety 60, 324–349 (2005). [DOI] [PubMed] [Google Scholar]
- Tyree M. & Zimmermann M. Xylem Structure and the Ascent of Sap, Springer. Berlin, Germany (2002). [Google Scholar]
- Cowan I. An electrical analogue of evaporation from, and flow of water in plants. Planta 106, 221–226 (1972). [DOI] [PubMed] [Google Scholar]
- Martre P. et al. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology 130, 2101–2110 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau T. & Inra R. H. The use of tree root suckers to estimate root water potential. Plant, Cell & Environment 14, 585–591 (1991). [Google Scholar]
- Javot H. & Maurel C. The role of aquaporins in root water uptake. Annals of Botany 90, 301–313 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer F. & Grantz D. Stomatal and hydraulic conductance in growing sugarcane: stomatal adjustment to water transport capacity. Plant, Cell & Environment 13, 383–388 (1990). [Google Scholar]
- Sperry J. & Pockman W. Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis. Plant, Cell & Environment 16, 279–287 (1993). [Google Scholar]
- Saliendra N. Z., Sperry J. S. & Comstock J. P. Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196, 357–366 (1995). [Google Scholar]
- Meinzer F. et al. Environmental and physiological regulation of transpiration in tropical forest gap species: the influence of boundary layer and hydraulic properties. Oecologia 101, 514–522 (1995). [DOI] [PubMed] [Google Scholar]
- Hubbard R., Ryan M., Stiller V. & Sperry J. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant, Cell & Environment 24, 113–121 (2001). [Google Scholar]
- Tyree M. T. & Sperry J. S. Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Biology 40, 19–36 (1989). [Google Scholar]
- Whitehead D. Regulation of stomatal conductance and transpiration in forest canopies. Tree Physiology 18, 633–644 (1998). [DOI] [PubMed] [Google Scholar]
- Brodribb T. J., Feild T. S. & Jordan G. J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L., Cowan P., Jaikumar N. & Holbrook N. The ‘hydrology’ of leaves: co‐ordination of structure and function in temperate woody species. Plant, Cell & Environment 26, 1343–1356 (2003). [Google Scholar]
- Sack L. & Holbrook N. M. Leaf hydraulics. Annual Review in Plant Biology 57, 361–381 (2006). [DOI] [PubMed] [Google Scholar]
- Sperry J. S. Hydraulic constraints on plant gas exchange. Agricultural and Forest Meteorology 104, 13–23 (2000). [Google Scholar]
- Johnson D. M., Woodruff D. R., McCulloh K. A. & Meinzer F. C. Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiology 29, 879–887 (2009). [DOI] [PubMed] [Google Scholar]
- Brodribb T. J., Holbrook N. M., Zwieniecki M. A. & Palma B. Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytologist 165, 839–846 (2005). [DOI] [PubMed] [Google Scholar]
- Pelletier J. D. & Turcotte D. L. Shapes of river networks and leaves: are they statistically similar? Philosophical Transactions of the Royal Society of London B: Biological Sciences 355, 307–311 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Nebelsick A., Uhl D., Mosbrugger V. & Kerp H. Evolution and function of leaf venation architecture: a review. Annals of Botany 87, 553–566 (2001). [Google Scholar]
- Yamazaki K. Studies on the connecting strand of the vascular systemin rice leaves. Proceedings of the Crop Science Society of Japan 29, 400–403 (1960). [Google Scholar]
- Lush W. Leaf structure and translocation of dry matter in a C3 and a C4 grass. Planta 130, 235–244 (1976). [DOI] [PubMed] [Google Scholar]
- Altus D. & Canny M. Loading of assimilates in wheat leaves. I. The specialization of vein types for separate activities. Functional Plant Biology 9, 571–581 (1982). [Google Scholar]
- Altus D. & Canny M. Water pathways in wheat leaves. I. The division of fluxes between different vein types. Functional Plant Biology 12, 173–181 (1985). [Google Scholar]
- Altus D., Canny M. & Blackmann D. Water pathways in wheat leaves. II. Water-conducting capacities and vessel diameters of different vein types, and the behaviour of the integrated vein network. Functional Plant Biology 12, 183–199 (1985). [Google Scholar]
- Chonan N., Kawahara H. & Matsuda T. Morphology of vascular bundles of leaves in gramineous crops. I. Observations on vascular bundles of leaf blades, sheaths and internodes in rice plants. Proceedings of the Crop Science Society of Japan 42, 425–432 (1974). [Google Scholar]
- Colbert J. T. & Evert R. F. Leaf vasculature in sugarcane (Saccharum officinarum L.). Planta 156, 136–151 (1982). [DOI] [PubMed] [Google Scholar]
- Dannenhoffer J. M. & Evert R. F. Development of the vascular system in the leaf of barley (Hordeum vulgare L.). International Journal of Plant Sciences, 143–157 (1994). [Google Scholar]
- Russell S. & Evert R. Leaf vasculature in Zea mays L. Planta 164, 448–458 (1985). [DOI] [PubMed] [Google Scholar]
- Niklas K. J. Effects of vibration on mechanical properties and biomass allocation pattern of capsella bursa-pastoris (cruciferae). Annals of Botany 82, 147–156 (1998). [Google Scholar]
- Kehr J. & Buhtz A. Long distance transport and movement of RNA through the phloem. Journal of Experimental Botany 59, 85–92 (2008). [DOI] [PubMed] [Google Scholar]
- Sack L. & Scoffoni C. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytologist 198, 983–1000 (2013). [DOI] [PubMed] [Google Scholar]
- Sack L. & Scoffoni C. Measurement of leaf hydraulic conductance and stomatal conductance and their responses to irradiance and dehydration using the Evaporative Flux Method (EFM). Journal of Visualized Experiments 70, e4179 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A. & Salleo S. Limitation of stomatal conductance by hydraulic traits: sensing or preventing xylem cavitation? Trees 15, 14–24 (2000). [Google Scholar]
- Flexas J., Scoffoni C., Gago J. & Sack L. Leaf mesophyll conductance and leaf hydraulic conductance: an introduction to their measurement and coordination. Journal of Experimental Botany 64, 3965–3981 (2013). [DOI] [PubMed] [Google Scholar]
- Tabassum M. A. et al. Rice (Oryza sativa L.) hydraulic conductivity links to leaf venation architecture under well-watered condition rather than PEG-induced water deficit. Acta Physiologiae Plantarum 38, 1–11 (2016). [Google Scholar]
- Chaves M. Effects of water deficits on carbon assimilation. Journal of Experimental Botany 42, 1–16 (1991). [Google Scholar]
- Cornic G. Drought stress inhibits photosynthesis by decreasing stomatal aperture–not by affecting ATP synthesis. Trends in Plant Science 5, 187–188 (2000). [Google Scholar]
- Wilson K. B., Baldocchi D. D. & Hanson P. J. Quantifying stomatal and non-stomatal limitations to carbon assimilation resulting from leaf aging and drought in mature deciduous tree species. Tree Physiology 20, 787–797 (2000). [DOI] [PubMed] [Google Scholar]
- Chaves M. M. et al. How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany 89, 907–916 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroco J. P., Rodrigues M. L., Lopes C. & Chaves M. M. Limitations to leaf photosynthesis in field-grown grapevine under drought—metabolic and modelling approaches. Functional Plant Biology 29, 451–459 (2002). [DOI] [PubMed] [Google Scholar]
- Bernacchi C. J., Portis A. R., Nakano H., von Caemmerer S. & Long S. P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology 130, 1992–1998 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J., Bota J., Escalona J. M., Sampol B. & Medrano H. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology 29, 461–471 (2002). [DOI] [PubMed] [Google Scholar]
- Escalona J. M., Flexas J. & Medrano H. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Functional Plant Biology 27, 87–87 (2000). [Google Scholar]
- Bota J., Medrano H. & Flexas J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytologist 162, 671–681 (2004). [DOI] [PubMed] [Google Scholar]
- Grassi G. & Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell & Environment 28, 834–849 (2005). [Google Scholar]
- Galle A. et al. The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): acclimation or limitation? Journal of Experimental Botany 60, 2379–2390 (2009). [DOI] [PubMed] [Google Scholar]
- Küppers M. Carbon relations and competition between woody species in a Central European hedgerow. Oecologia 64, 344–354 (1984). [DOI] [PubMed] [Google Scholar]
- Nardini A., Tyree M. T. & Salleo S. Xylem cavitation in the leaf of Prunus laurocerasusand and its impact on leaf hydraulics. Plant Physiology 125, 1700–1709 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar R. et al. Influences of whole plant water transport and drought on gas exchange within a chaparral community. Ecological Society of America Annual Meeting Tucson, AZ (2002). [Google Scholar]
- Hirasawa T., Ozawa S., Taylaran R. D. & Ookawa T. Varietal differences in photosynthetic rates in rice plants, with special reference to the nitrogen content of leaves. Plant Production Science 13, 53–57 (2010). [Google Scholar]
- Zwieniecki M., Boyce C. & Holbrook N. Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant, Cell & Environment 27, 357–365 (2004). [Google Scholar]
- Pantin F., Simonneau T. & Muller B. Coming of leaf age: control of growth by hydraulics and metabolics during leaf ontogeny. New Phytologist 196, 349–366 (2012). [DOI] [PubMed] [Google Scholar]
- Sack L. et al. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. Journal of Experimental Botany 64, 4053–4080 (2013). [DOI] [PubMed] [Google Scholar]
- Brodribb T. J. & Holbrook N. M. Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant, Cell & Environment 29, 2205–2215 (2006). [DOI] [PubMed] [Google Scholar]
- Maherali H., Moura C. F., Caldeira M. C., Willson C. J. & Jackson R. B. Functional coordination between leaf gas exchange and vulnerability to xylem cavitation in temperate forest trees. Plant, Cell & Environment 29, 571–583 (2006). [DOI] [PubMed] [Google Scholar]
- Fichot R. et al. Hydraulic efficiency and coordination with xylem resistance to cavitation, leaf function, and growth performance among eight unrelated Populus deltoides× Populus nigra hybrids. Journal of Experimental Botany 62, 2093–2106 (2011). [DOI] [PubMed] [Google Scholar]
- Gleason S. M. et al. Weak coordination among petiole, leaf, vein, and gas‐exchange traits across Australian angiosperm species and its possible implications. Ecology and Evolution 6, 267–278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls R. L. Angiosperm leaf vein patterns are linked to leaf functions in a global-scale data set. American Journal of Botany 98, 244–253 (2011). [DOI] [PubMed] [Google Scholar]
- Xiong D. et al. Leaf hydraulic conductance is coordinated with leaf morpho-anatomical traits and nitrogen status in the genus Oryza. Journal of Experimental Botany 66, 741–748 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burundukova O., Zhuravlev Y. N., Solopov N. & P’yankov V. A method for calculating the volume and surface area in rice mesophyll cells. Russian Journal of Plant Physiology 50, 133–139 (2003). [Google Scholar]
- Karki S., Rizal G. & Quick W. P. Improvement of photosynthesis in rice (Oryza sativa L.) by inserting the C4 pathway. Rice 6, 28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. Environmental and evolutionary preconditionsfor the origin and diversification of the C4 photosynthetic syndrome. Plant Biology 3, 202–213 (2001). [Google Scholar]
- Ueno O., Kawano Y., Wakayama M. & Takeda T. Leaf vascular systems in C3 and C4 grasses: a two-dimensional analysis. Annals of Botany 97, 611–621 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie I., Pyke K. & Murchie E. Variation in vein density and mesophyll cell architecture in a rice deletion mutant population. Journal of Eexperimental Botany 63, 4563–4570 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P. & Durand J. L. Quantitative analysis of vasculature in the leaves of Festuca arundinacea (Poaceae): implications for axial water transport. International Journal of Plant Sciences 162, 755–766 (2001). [Google Scholar]
- Kutlu N. et al. Changes in anatomical structure and levels of endogenous phytohormones during leaf rolling in Ctenanthe setosa under drought stress. Turkish Journal of Biology 33, 115–122 (2009). [Google Scholar]
- El-Afry M. M., El-Nady M. F., Abdelmonteleb E. B. & Metwaly M. M. S. Anatomical studies on drought-stressed wheat plants (Triticum aestivum L.) treated with some bacterial strains. Acta Biologica Szegediensis 56, 165–174 (2012). [Google Scholar]
- Farouk S. & Amany A. R. Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egyptian Journal of Biology 14, 14–16 (2012). [Google Scholar]
- Jacobsen A. L., Ewers F. W., Pratt R. B., Paddock W. A. & Davis S. D. Do xylem fibers affect vessel cavitation resistance? Plant Physiology 139, 546–556 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocheltree T. W., Nippert J. B., Kirkham M. B. & Prasad P. V. V. Partitioning hydraulic resistance in Sorghum bicolor leaves reveals unique correlations with stomatal conductance during drought. Functional Plant Biology 41, 25–36 (2014). [DOI] [PubMed] [Google Scholar]
- von Cammerer S. & Farquhar G. D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387 (1981). [DOI] [PubMed] [Google Scholar]
- Brodribb T. & Holbrook N. M. Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest. New Phytologist 158, 295–303 (2003). [Google Scholar]