Abstract
Transcranial magnetic stimulation (TMS) is a type of noninvasive brain stimulation used to study corticomotor excitability of the intact and injured brain. Identification of muscle representations in the motor cortex is typically done using a procedure called ‘hotspotting’, which involves establishing the optimal location on the scalp that evokes a maximum TMS response with minimum stimulator intensity. The purpose of this study was to report the hotspot locations for the tibialis anterior (TA) muscle representation in the motor cortex of healthy and post stroke individuals. A retrospective data analyses from 42 stroke participants and 32 healthy participants was conducted for reporting TMS hotspot locations and their spatial patterns. Single pulse TMS, using a 110 mm double cone coil, was used to identify the motor representation of the TA. The hotspot locations were represented as x and y-distances from the vertex for each participant. The mediolateral extent of the loci from the vertex (x-coordinate) and anteroposterior extent of the loci from the vertex (y-coordinate) was reported for each hemisphere: non-lesioned (XNLes, YNLes), lesioned (XLes, YLes) and healthy (XH, YH). We found that the mean hotspot loci for TA muscle from the vertex were approximately: 1.29 cm lateral and 0.55 cm posterior in the non-lesioned hemisphere, 1.25 cm lateral and 0.5 cm posterior in the lesioned hemisphere and 1.6 cm lateral and 0.8 cm posterior in the healthy brain. There was no significant difference in the x- and y-coordinates between the lesioned and non-lesioned hemispheres. However, the locations of the XNLes (p = 0.01) and XLes (p = 0.004) were significantly different from XH. The YNLes and YLes showed no significant differences from YH loci. Analyses of spatial clustering patterns using the Moran’s I index showed a negative autocorrelation in stroke participants (NLes: Moran’s I = −0.09, p < 0.001; Les: Moran’s I = −0.14, p = 0.002), and a positive autocorrelation in healthy participants (Moran’s I = 0.16, p < 0.001), suggesting that individuals with stroke demonstrated a more dispersed pattern of hotspot locations than healthy individuals. Our results suggest that the hotspot loci show different spatial patterns in healthy and stroke individuals. The hotspot locations from this study has the potential to provide a guideline for optimal stimulation locations for the TA muscle in healthy and post stroke individuals for neuromodulation procedures such as transcranial direct current stimulation.
Keywords: Transcranial magnetic stimulation, Corticomotor excitability, Hotspot, Tibialis anterior, Transcranial direct current stimulation
1. Introduction
Over the last 30 years, single pulse transcranial magnetic stimulation (TMS) has been increasingly used to examine corticomotor excitability of muscle representations in the intact and injured human brain. The TMS-induced motor evoked potential (MEP) is a common parameter used to quantify changes in corticomotor excitability of the desired motor area. Identification of the muscle representation on the motor cortex necessitates the placement of the TMS coil over the location on the head, where maximal responses from the muscle can be elicited. This optimal site of stimulation is typically referred to as the “motor hotspot”, and is defined as the coil position that evokes a largest average MEP with a minimum stimulator output. The location of the hotspot is not only important for studies that quantify temporal changes in cortical excitability but also an important target for other neuromodulation procedures, such as transcranial direct current stimulation (tDCS).
tDCS is a non-invasive, safe, portable, and low cost modality that has been effectively used to prime the neuromotor system to modulate corticomotor excitability and optimize functional outcomes [9,15]. In laboratory studies that investigate the neurophysiological effects of tDCS, researchers typically use single pulse TMS to locate the hotspot of the desired motor area before placing the active tDCS electrode. Although we agree that TMS is the ideal technique to map the brain and study the efficacy of tDCS, this is a critical limitation when translating a low cost intervention such as tDCS into clinical practice. TMS is expensive, technically demanding, and time consuming. Transition of tDCS as a clinical tool can be expedited if the precise anatomical location of muscle representations is known without the need for sophisticated equipment such as TMS or other brain mapping techniques such as magnetic resonance imaging. This is important, especially when considering the development of tDCS as a home-based treatment modality[4,23].
An alternate method to brain mapping using TMS would be to rely on the manual 10–20 International System for electrode placement [11]. The 10–20 system is commonly used for placement of electroencephalography (EEG) electrodes and relates external skull landmarks with the underlying predefined cortical areas. This system is widely used to describe electrode placements especially in EEG studies and has been used as a reference for many TMS and tDCS studies to locate specific cortical areas without neuronavigation. Although the 10–20 system accounts for some variability in individual skull size, one cannot be certain that the areas identified with this system correspond to desired anatomical location. Few cognitive neuroscience studies have reported the relationship between the 10–20 system with anatomical CT and MRI scans of the frontal area [8,25,28]. Although these authors encountered inter-individual variability due to cranial asymmetry, they found that important locations in the cranio-cerebral topography, such as the central and lateral Sylvian fissures and Brodmann’s areas, related well to EEG placement of electrodes. They further acknowledge that differences in spatial orientation of deeper surfaces of the brain may contribute to the variability of external surfaces, which may be pertinent while obtaining neurophysiology based measures such as TMS.
However, to our knowledge, the anatomical locations of TMS identified muscle representations in the motor cortex have not been reported in detail. Few lower limb studies have mentioned the TA hotspot to be approximately 1–3 cm posterior or lateral to the vertex [2,3,6,7,12,13,20,21], but none have provided detailed measurements of the hotspot in a large cohort of stroke participants. Hence, in this study we aimed to report the optimal TMS stimulation site for the tibialis anterior (TA) muscle representation using external landmarks identified with the 10–20 system in a large cohort of stroke and healthy participants. Furthermore, we compared the spatial patterns of somatotopic localizations of the TA muscle between healthy and post stroke individuals.
2. Material and methods
2.1. Study design
This is a retrospective study design, where TMS measurements were collected as a part of a larger randomized control trial in the Brain Plasticity Laboratory at the University of Illinois at Chicago. We include baseline TMS hotspot measurements from healthy and post stroke individuals who had participated in the previous study.
2.2. Participants
Data from 42 chronic stroke participants (age range 42–74 years, 27 males and 15 females) and 32 healthy participants (age range 21–32 years, 18 males and 14 females) were included. 19 stroke participants had left-sided hemi-paresis and 23 had right-sided hemi-paresis. We assessed footedness in healthy participants by asking with which leg they preferred to kick a ball. The right side was reported dominant in 30 participants, and the left side in 2 participants. The demographic characteristics of our participants with stroke are presented in Table 1.
Table 1.
Demographic characteristics of participants with stroke.
| Type of stroke | Ischemic (n = 40) Hemorrhagic (n = 2) |
|---|---|
| Time since stroke (years) | 4.4 ± 3.2 |
| Age at the time of lesion (years) | 55.3 ± 7.3 |
| FMA-LE | 21.3 ± 4.1 |
| Gait speed (m/s) | 0.7 ± 0.2 |
All values are represented as mean ± standard deviation. FMA-LE = Fugl-Meyer Motor Assessment scores of the paretic lower extremity (maximum score = 34). Self-selected gait speeds were measured with the 10-m Walk Test.
All healthy participants reported no history of prior neurological, cardiovascular or orthopedic dysfunction. Inclusion criteria for stroke patients included those with a first-ever mono-hemispheric stroke at least 6 months prior to participation, and those who had detectable MEPs from the paretic tibialis anterior muscle. Patients with contraindications to TMS, including those with metal implants, a history of seizures and medications that alter central nervous system excitability, were excluded from the study. Other exclusion criteria included presence of cognitive impairments, brainstem or cerebellar lesions, and weakness or spasticity preventing visible dorsiflexion.
All participants were informed of the research procedures and risks, and a written informed consent was obtained from everyone. All research methods were approved by Institutional Review Board at University of Illinois at Chicago, and conformed to the Declaration of Helsinki.
2.3. Electromyography (EMG) setup
Participants were seated comfortably in a chair with knees flexed to 90°. EMG data were collected bilaterally from the TA muscles in stroke participants and from the non-dominant TA muscle in healthy participants. Surface Ag/AgCl electrodes were placed over the muscle belly of the TA. The reference electrode was placed over the spinous process of the seventh cervical vertebra. Before placing the EMG electrodes, the skin was shaved if needed, and rubbed with alcohol to reduce impedance. EMG data were sampled at 2000 Hz, amplified (1000X) and band pass filtered (10–500 Hz) with a Delsys EMG system (Bagnoli 8, MA USA). The EMG data collection was performed using Spike2software (Cambridge Electronic design, Cambridge, UK). An estimate of maximum voluntary isometric contraction (MVC) for the tibialis anterior was obtained prior to collecting TMS data.
2.4. TMS
A tight fitting linen cap was placed over the participant’s head. Then, the following landmarks were identified using the 10–20 EEG system – the nasion (Ns), inion (In), Left (PAL) and Right (PAR) preauricular points to establish the vertex or Cz [11]. The intersection of the longitudinal nasion-inion (Ns-In) and lateral pre-auricular points (PAL-PAR) was labeled as the vertex (Cz).
Single pulse TMS was delivered by means of a Magstim 200 stimulator (Magstim, Dyfed, Wales UK) with a double cone coil (diameter 110 mm) using a posterior-anterior cortical current orientation [16,17]. Spike2 software was used to trigger the stimulator at 0.25 Hz frequency and record the trigger pulses. During TMS, the participants were provided with visual feedback of their muscle activity and instructed to maintain a tonic contraction of the TA that represented approximately 10–20% MVC. For the participants with stroke, the non-lesioned (NLes) and lesioned hemispheres (Les) were tested. For the healthy participants, the hemisphere (H) contra-lateral to the non-dominant foot was tested.
During the experiment, the coil was initially placed over the vertex (Cz), and then the coil was moved in small increments to find the location for producing the maximum MEP response for the contralateral TA muscle at the lowest stimulator intensity. This was defined as the “hotspot” of the muscle representation. The location of the double cone coil was traced on the cap.
After the TMS session, the intersection of the two embedded coils within the double cone was extrapolated onto the cap on the participant’s head, and detailed recordings of the location of the hotspot from the vertex were noted for each participant.
2.5. Data and statistical analyses
The study data were represented as x- and y-distances from the vertex, in centimeters, for each participant. For data analyses, the vertex was considered as the origin (0, 0) in the Cartesian plane (x-y coordinate system), and the distances were recorded on the respective axes. XNLes, YNLes, XLes, YLes, XH and YH were used to denote the x and y coordinates for hotspot loci for the non-lesioned, lesioned and healthy hemispheres respectively. Positive y values represent an anterior direction from the vertex, and negative values represent a posterior direction from the vertex. Positive x-distances represent the lateral extent of the loci from the vertex within the motor cortex contralateral to the tested muscle, and negative x-distance represent the lateral extent of the loci from the vertex within the ipsilateral motor cortex.
A paired t-test was used to examine differences in the hotspot loci (x and y coordinates separately) between the non-lesioned and lesioned loci. An independent samples t-test was used to examine the differences in the hotspot loci (x and y coordinates separately) between the lesioned and healthy loci, and the non-lesioned and healthy loci. Since the data were used twice for the analyses, a Bonferroni correction was incorporated and the p-value was adjusted accordingly. A p-value < 0.025 was considered significant.
Spatial distribution patterns of the hotspot loci in each group were estimated using a previously developed and validated mathematical tool called Moran’s I [18,19]. Moran’s I is an index of spatial autocorrelation which measures the similarity of values in neighboring places from a mean value, and provides an estimate of how related the values within each group are. Moran’s I is known to be a spatially weighted form of the Pearson’s correlation coefficient [29] and ranges from −1 to +1 (Fig. 1). A Moran’s I value near +1 indicates 100% spatial clustering, while a value near −1 indicates 100% spatial dispersion. A value close to 0 indicates spatial randomness. We used the following approach to calculate Moran’s I [19]. Based on the minimum and maximum values of the x and y data, the maximum Euclidean distance between any two points within each dataset was calculated. Once we obtained the maximum distance between all two points, a matrix was generated based on these distances.
where:
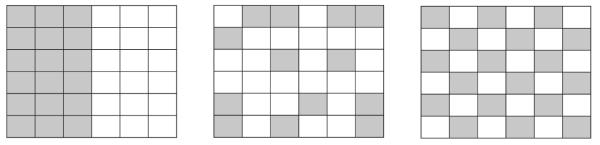
Fig. 1.
Moran’s I and autocorrelation.
The three figures schematically depict patterns in spatial data: positive autocorrelation, Moran’s I = 1 (left); no spatial autocorrelation, Moran’s I = 0 (center); and negative autocorrelation, Moran’s I = −1 (right). Moran’s I below 0 depicts a dispersed pattern of hotspot loci and Moran’s I above 0 depicts a clustered pattern of hotspot loci.
N = Number of observations, Wij = weighting factor = 1/d, d = the distance between loci in space, Xi − X̄ = deviation of the x-coordinate from mean, Xj − X̄ = deviation of the y-coordinate from mean.
For statistical hypothesis testing, the Moran’s I was transformed into a Z score using the following formula:
where:
where: E [Ii] = Expected Value of Moran’s I, V [Ii] = Variance of I
The Z scores were tested for assumptions of normality. When the p-value is small, and the absolute value of the Z score is large enough that it falls outside of the desired confidence level, the null hypothesis i.e., complete spatial randomness (no spatial patterns in the hotspot loci) can be rejected. This would indicate that the loci exhibit statistically significant clustering or dispersion, which is attributable to some underlying spatial process. Z values above 1.96 or below −1.96 (95% confidence interval) were considered to indicate statistical significance.
SPSS Statistics software (Version 22.0, SPSS Inc., Chicago, Illinois, USA) was used to perform the one way ANOVA, and Microsoft Excel was used to calculate the Moran’s I. A p-value of <0.05 was considered as significant for Moran’s I analyses.
3. Results
3.1. Location of the hotspot loci
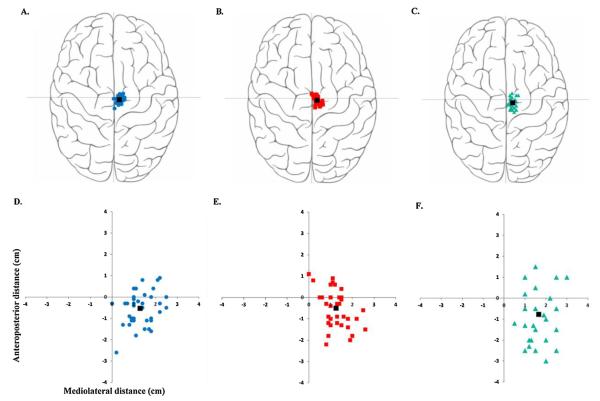
The distribution of the hotspot loci in the study participants is shown in Fig. 2. Average distance ± standard deviation for XNLes was 1.29 ± 0.57 cm, XLes was 1.25 ± 0.53 cm and XH was 1.65 ± 0.65 cm. The average y distance ± standard deviation for YNLes was −0.55 ± 0.77 cm, YLes was −0.50 ± 0.82 cm, and YH was −0.77 ± 1.36 cm. A majority of participants (30 stroke and 17 healthy) had their TA loci lateral and posterior to the vertex. 18 participants (7 stroke and 11 healthy) had their loci lateral and anterior to the vertex. 9 participants had their TA loci lateral to the vertex (y coordinate was 0). No participant revealed a negative x coordinate i.e., the hotspot location was always found to be on the contralateral motor cortex. Table 2 shows the descriptive data for the non-lesioned, lesioned and healthy hemispheres. Paired t-tests showed no differences between the XNLes and XLes loci (p = 0.71), and between the YNLes and YLes loci (p = 0.59). The independent samples t-test revealed significant differences between the XLes and XH loci [t(72) = −2.95, p = 0.004], and between the XNles and XH loci [t(72) = −2.53, p = 0.01] respectively. The independent samples t-test showed no differences between the YNLes and YH [t(72) = 0.85 p = 0.39] or between the YLes and YH [t(72) = 1.02, p = 0.3].
Fig. 2.
Hotspot loci in stroke and healthy participants. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The top panel (A–C) provides a visual depiction of the hotspot loci on a rendered brain image. The bottom panel (D–F) is an enlarged graphical representation of the anatomical measurements of the hotspots from the vertex (0, 0). Figure A represents the non-lesioned hemisphere (blue circles) of stroke patients. Figure B represents the lesioned hemisphere (red squares) of stroke patients. Figure C represents the non-dominant hemisphere (green triangles) of healthy participants. The black squares in each figure represent the mean loci of that hemisphere.
Table 2.
Descriptive data of the hotspot loci.
| Variable | Observations | Mean (cm) | SD (cm) | Min. (cm) | Max. (cm) |
|---|---|---|---|---|---|
| Non-lesioned hemisphere | |||||
| X NLes | 42 | 1.29 | 0.57 | 0 | 2.5 |
| Y NLes | 42 | −0.55 | 0.77 | −2.6 | 0.9 |
| Lesioned hemisphere | |||||
| X Les | 42 | 1.25 | 0.53 | 0 | 2.6 |
| Y Les | 42 | −0.50 | 0.82 | −2.2 | 1.1 |
| Healthy | |||||
| X H | 32 | 1.65 | 0.65 | 0.5 | 3 |
| Y H | 32 | −0.77 | 1.36 | −3 | 1.5 |
SD = standard deviation, Min. = minimum value, Max. = maximum value XNLes, YNLes, XLes, YLes, XH, YH represent the X and Y coordinates in the non-lesioned hemisphere, lesioned hemisphere of stroke participants, and non-dominant hemisphere in healthy participants respectively.
3.2. Moran’s I statistic
The lesioned and non-lesioned hemispheres showed negative values of Moran’s I, i.e. −0.14 (Z = −5.02, p = <0.001), and −0.09 (Z = −3.03, p = 0.002), suggesting a tendency towards spatial dis persion or negative autocorrelation within the stroke group. In the healthy hemisphere, a positive value of Moran’s I was noted (I = 0.23, Z = 3.87, p = <0.001), indicating that healthy participants showed a tendency towards spatial clustering or positive autocorrelation.
4. Discussion
In this study, we report spatial locations and patterns of hotspot loci for the TA muscle representation in the intact and injured motor cortex using standard TMS. Our results suggest that the somatotopic localization for the TA muscle representation, from the vertex, is found 1.29 cm lateral and 0.55 cm posterior in the non-lesioned hemisphere, 1.25 cm lateral and 0.50 cm posterior in the lesioned hemisphere of patients with stroke, and 1.60 cm lateral and 0.80 cm posterior in healthy individuals. The leg area of the human motor cortex is known to be embedded within the interhemispheric fissure at 3–4 cm depth from the scalp surface[1,10,26]. We believe that the optimal stimulation site reported here is lateral and posterior to the vertex as a result of the shape and angle of the stimulation-induced magnetic fields.
There was no difference in the location of the TA hotspot in the anterior-posterior direction between the healthy and post stroke motor cortices. However, hotspot loci in the mediolateral direction were significantly different between the healthy and stroke participants. The healthy participants showed the most lateral deviation from the vertex, and the hotspot of the paretic TA was closest to the vertex.
A more uniform pattern of clustering was noted in the healthy as compared to the stroke participants. There was a tendency for hotspot loci to be clustered in the healthy individuals, and dispersed in post stroke individuals. It is important to note that the distribution of the hotspot loci for the individuals with stroke ranged from 0 to 2.6 cm in the mediolateral direction and from −2.6 to 1.1 cm in the anteroposterior direction. As many tDCS studies use standard electrode configurations which are typically 8–35 cm2 in area, it is possible that one can use the reported hotspot locations in this study as a guideline to place large electrodes over the lower limb motor cortex. However, precise stimulation of one location over another (e.g. motor cortex vs. supplementary motor area, non-lesioned vs. lesioned) may not be possible with the reported data.
The anatomical locations of standard TMS placements at a group level for the tibialis anterior muscle in the intact and injured brains have not been reported before. A few studies have estimated the approximate locations of the TA hotspot to be within 1–3 cm lateral to the vertex [2,17]. In a previous study using image guided TMS, Niskanen et al. [22] reported that optimal stimulation site for the TA muscle was near the longitudinal fissure for healthy individuals, and that the observed variance in the location did not correlate with age or head circumference. In our study, we confirmed the optimal stimulation location using standard TMS, and furthermore, we reported the precise location in the anteroposterior and mediolateral coordinates on the skull. We expect these data to inform future clinical studies that utilize neuromodulation techniques, such as tDCS, to enhance neural plasticity to optimize motor learning. This may minimize the need for navigated brain stimulation techniques which are expensive, require technical expertise and not readily available in the clinic.
In this study, we used the Moran’s I to observe spatial patterns of hotspot loci distributions. It is not a surprising observation that the individuals with stroke demonstrated a more dispersed pattern than healthy individuals. Numerous brain imaging studies have indicated that reorganization of cortical representational maps contribute to stroke recovery [30]. This reorganization is thought to be a complex process induced by spontaneous recovery, as well as experience-dependent learning [14]. However, it is interesting to note that both the non-lesioned and lesioned hemispheres demonstrate the dispersed pattern, and this is in agreement with studies that have shown changes in cortical reorganization in the non-lesioned hemisphere as well [5,31,32].
The results of this study are limited by their generalizability to other lower limb muscle representations and to other neurological populations. Nonetheless, this study provides promising early evidence for spatial localizations of cortical motor representations, especially for the TA muscle. Our accuracy of hotspot localization may have been influenced by small movements of the hand-held coil, and the subsequent coil adjustment during the experiments, which may have caused changes in field strength and stimulus locations. Additionally, our reported measurements do not include variability in coil orientation. Current evidence suggests that adjustments in the coil orientation may induce variations in motor thresholds [24,27]. It is also important to note that we provide a rather simplified model of reporting our results in the XY plane and have not taken into consideration measurements in the Z plane. We also do not take into account the angle at which the stimulus is influencing the cortical neurons. Our results also do not account for inter-individual variations in skull shape and size, cytoarchitecture and neuroanatomy. All these factors minimize the accuracy of our findings. Given our observation that the optimal site is close to the vertex with minimal variance, we believe that this inaccuracy in location may be negligible when exploring a cheap and efficient way to apply tDCS in the clinical or home setting. Precise information can be obtained only with elegant techniques, such as magnetic resonance imaging (MRI) guided navigated coil positioning, which may be a prerequisite for accurate recordings. Future studies using neuro-navigational TMS or direct cortical stimulation, may establish more precise hotspot patterns for important lower limb muscles.
5. Conclusions
In summary, we report the x-y coordinates from the vertex for the optimal site of stimulation for the TA muscle as follows; non-lesioned hemisphere: 1.29 cm lateral and 0.55 cm posterior; lesioned hemisphere: 1.25 cm lateral and 0.5 cm posterior; and healthy brain: 1.6 cm lateral and 0.8 cm posterior. We observed different spatial patterns of their somatotopic localizations in the healthy and post stroke brain. Our study attempts to provide a guideline for optimal stimulation locations for the TA muscle in healthy and post stroke individuals.
HIGHLIGHTS.
We report the tibialis anterior hotspot loci obtained with TMS.
The hotspot loci were posterior and lateral to the vertex in our participants.
The loci showed more spatial clustering in healthy individuals compared to stroke.
Acknowledgements
This work was partly supported by a grant funded by the National Institute of Health (NIH) R01HD075777 (SM).
References
- [1].Allison T, McCarthy G, Luby M, Puce A, Spencer DD. Localization of functional regions of human mesial cortex by somatosensory evoked potential recording and by cortical stimulation. Electroencephalogr. Clin. Neurophysiol. 1996;100:126–140. doi: 10.1016/0013-4694(95)00226-x. [DOI] [PubMed] [Google Scholar]
- [2].Beaulieu LD, Massé-Alarie H, Brouwer B, Schneider C. Brain control of volitional ankle tasks in people with chronic stroke and in healthy individuals. J. Neurol. Sci. 2014;338:148–155. doi: 10.1016/j.jns.2013.12.038. [DOI] [PubMed] [Google Scholar]
- [3].Cacchio A, Paoloni M, Cimini N, Mangone M, Liris G, Aloisi P, Santilli V, Marrelli A. Reliability of TMS-related measures of tibialis anterior muscle in patients with chronic stroke and healthy subjects. J. Neurol. Sci. 2011;303:90–94. doi: 10.1016/j.jns.2011.01.004. [DOI] [PubMed] [Google Scholar]
- [4].Charvet LE, Kasschau M, Datta A, Knotkova H, Stevens MC, Alonzo A, Loo C, Krull KR, Bikson M. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front. Syst. Neurosci. 2015;9:26. doi: 10.3389/fnsys.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann. Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- [6].Christensen LO, Andersen JB, Sinkjaer T, Nielsen J. Transcranial magnetic stimulation and stretch reflexes in the tibialis anterior muscle during human walking. J. Physiol. 2001;531:545–557. doi: 10.1111/j.1469-7793.2001.0545i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp. Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- [8].Homan R, Herman J, Purdy P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiol. 1987;66:376–382. doi: 10.1016/0013-4694(87)90206-9. [DOI] [PubMed] [Google Scholar]
- [9].Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- [10].Iles JF, Cummings R. Electrical and magnetic stimulation of motor cortex in man. J. Physiol. 1992;446:223P. [Google Scholar]
- [11].Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroenceph. Clin. Neurophysiol. 1958;10:370–371. [Google Scholar]
- [12].Kamibayashi K, Nakajima T, Takahashi M, Akai M, Nakazawa K. Facilitation of corticospinal excitability in the tibialis anterior muscle during robot-assisted passive stepping in humans. Eur. J. Neurosci. 2009;30:100–109. doi: 10.1111/j.1460-9568.2009.06795.x. [DOI] [PubMed] [Google Scholar]
- [13].Khaslavskaia S, Ladouceur M, Sinkjaer T. Increase in tibialis anterior motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve. Exp. Brain Res. 2002;145:309–315. doi: 10.1007/s00221-002-1094-9. [DOI] [PubMed] [Google Scholar]
- [14].Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hearing Res. 2008;51:S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- [15].Madhavan S, Shah B. Enhancing motor skill learning with transcranial direct current stimulation – a concise review with applications to stroke. Front. Psychiatry. 2012;3:66. doi: 10.3389/fpsyt.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Madhavan S, Stinear JW. Focal and bi-directional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul. 2010;3:42. doi: 10.1016/j.brs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Madhavan S, Weber KA, 2nd, Stinear JW. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Exp. Brain Res. 2011;209:9–17. doi: 10.1007/s00221-010-2511-0. [DOI] [PubMed] [Google Scholar]
- [18].McGrew JC, Monroe CB. An Introduction to Statistical Problem Solving in Geography. 2nd ed Waveland Press; 2009. [Google Scholar]
- [19].Moran PA. Notes on continuous stochastic phenomena. Biometrika. 1950;37:17–23. [PubMed] [Google Scholar]
- [20].Morita H, Olivier E, Baumgarten J, Petersen NT, Christensen LO, Nielsen JB. Differential changes in corticospinal and Ia input to tibialis anterior and soleus motor neurones during voluntary contraction in man. Acta Physiol. Scand. 2000;170:65–76. doi: 10.1046/j.1365-201x.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- [21].Mrachacz-Kersting N, Fong M, Murphy BA, Sinkjaer T. Changes in excitability of the cortical projections to the human tibialis anterior after paired associative stimulation. J. Neurophysiol. 2007;97:1951–1958. doi: 10.1152/jn.01176.2006. [DOI] [PubMed] [Google Scholar]
- [22].Niskanen E, Julkunen P, Saisanen L, Vanninen R, Karjalainen P, Kononen M. Group-level variations in motor representation areas of thenar and anterior tibial muscles: navigated transcranial magnetic stimulation study. Hum. Brain Mapp. 2010;31:1272–1280. doi: 10.1002/hbm.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev. 2014;4 doi: 10.1002/14651858.CD008208.pub5. (CD008208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Richter L, Neumann G, Oung S, Schweikard A, Trillenberg P. Optimal coil orientation for transcranial magnetic stimulation. PLoS One. 2013;8:e60358. doi: 10.1371/journal.pone.0060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Steinmetz H, Furst G, Meyer BU. Craniocerebral topography within the international 10–20 system. Electroencephalogr. Clin. Neurophysiol. 1989;72:499–506. doi: 10.1016/0013-4694(89)90227-7. [DOI] [PubMed] [Google Scholar]
- [26].Terao Y, Ugawa Y, Hanajima R, Yumoto M, Kawahara Y, Yamamoto T, Shirouzu I, Kanazawa I. Motor cortical reflex myoclonus: a case study with MEG. Electroencephalogr. Clin. Neurophysiol. 1997;102:505–511. doi: 10.1016/s0013-4694(97)96122-1. [DOI] [PubMed] [Google Scholar]
- [27].Terao Y, Ugawa Y, Sakai K, Miyauchi S, Fukuda H, Sasaki Y, Takino R, Hanajima R, Furubayashi T, Pütz B, Kanazawa I. Localizing the site of magnetic brain stimulation by functional MRI. Exp. Brain Res. 1998;121:145–152. doi: 10.1007/s002210050446. [DOI] [PubMed] [Google Scholar]
- [28].Towle VL, Bolanos J, Suarez D, Tan K, Grzeszczuk R, Levin DN, Cakmur R, Frank SA, Spire JP. The spatial location of EEG electrodes: locating the best-fitting sphere relative to cortical anatomy. Electroencephalogr. Clin. Neurophysiol. 1993;86:1–6. doi: 10.1016/0013-4694(93)90061-y. [DOI] [PubMed] [Google Scholar]
- [29].Waller LA, Gotway CA. Applied Spatial Statistics for Public Health Data. Wiley; 2016. 2004. [Google Scholar]
- [30].Ward NS. Mechanisms underlying recovery of motor function after stroke. Postgrad. Med. J. 2005;81:510–514. doi: 10.1136/pgmj.2004.030809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann. Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]