Abstract
Background
Inflammatory myofibroblastic tumor (IMT) is a rare low-grade malignant neoplasm with a predilection for children and young adults, and typically arises in the lung, abdominopelvic region, and retroperitoneum. IMTs in the maxillofacial region are extreme rare. Approximately 50% of IMT harbor rearrangements of the anaplastic lymphoma kinase (ALK) gene at 2p23 with various fusion partners.
Case presentation
We herein report a case of intraosseous IMT of the mandible with a novel ATIC-ALK fusion. Tooth 43 did not erupt after the loss of tooth 83 in an 11-year-old girl with no previous history of trauma. Panoramic tomography showed a unilocular radiolucent lesion in the right anterior mandible resorbing the root of tooth 42 and the medial side of the root of tooth 44. Computed tomography revealed a well- circumscribed 3-cm osteolytic lesion of the right anterior mandible eroding the buccal cortical plate. The entire lesion was curetted out. A histopathological examination revealed the proliferation of plump spindle cells with a storiform architecture and lymphocytes scattered around spindle cells. The spindle cells showed diffuse cytoplasmic staining for ALK by immunohistochemistry. A fluorescence in situ hybridization analysis revealed the translocation of a part of the ALK gene locus at chromosome 2p23. A rapid amplification of cDNA ends analysis confirmed the rearrangement of ALK and identified ATIC as a partner of this ALK fusion mutant.
Conclusion
To the best of our knowledge, this is the first case of intraosseous IMT of the mandible with a novel ATIC-ALK fusion. We also herein reviewed similar tumors reported in the literature.
Keywords: Inflammatory myofibroblastic tumor, Mandible, Anaplastic lymphoma kinase gene, ATIC-ALK fusion, Fluorescence in situ hybridization, 5′ rapid amplification of cDNA ends, RT-PCR
Background
Inflammatory myofibroblastic tumors (IMTs) are more frequent in children and young adults, and typically arise in the lung, abdominopelvic region, and retroperitoneum [1, 2]. Unusual sites of involvement include the head and neck, genitourinary tract, heart, extremities, and central nervous system [2]. While these tumors are rare in the head and neck, they are even more uncommon in the mandible [3].
IMTs are mesenchymal neoplasms of intermediate malignant potential and histologically characterized by the proliferation of fibroblasts and myofibroblasts admixed with lymphocytes, plasma cells, eosinophils, and histiocytes [1]. The histological features of IMTs are often diagnostic, particularly through anaplastic lymphoma kinase (ALK) staining. The ALK gene has been implicated in the pathogenesis of IMTs, which supports the neoplastic origin of these tumors. Approximately 50% of IMTs harbor rearrangements in the ALK gene at 2p23 [4]. However, to the best of our knowledge, an ALK fusion mutation has not yet been demonstrated in IMTs in the jawbone.
We herein report a case of an intraosseous IMT in the mandible including a novel ATIC-ALK fusion mutation.
Case presentation
Clinical features
An 11-year-old girl presented with the delayed eruption of tooth 43 (two digit tooth notation system of the FDI) and mental nerve palsy. She had no history of trauma. An intraoral examination revealed that tooth 43 had not erupted after the loss of tooth 83 and the gingiva and alveolar mucosa of the right mandible were normal. Panoramic tomography showed a unilocular radiolucent lesion in the right anterior mandible resorbing the root of tooth 42 and the medial side of the root of tooth 44 (Fig. 1a). Tooth 43 was not impacted in the mandibular bone. Computed tomography showed a well-circumscribed non-expansile 3-cm osteolytic lesion in the right anterior mandible that eroded the buccal cortical plate and resorbed the root of tooth 42 and the medial side of the root of tooth 44 (Fig. 1b). Root resorption and cortical plate erosion suggested a locally aggressive tumor. The tumor lesion was entirely curetted out. No recurrence was detected in 18 months of follow-up.
Fig. 1.
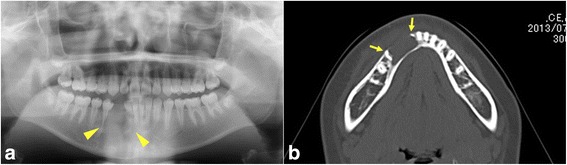
a Panoramic tomography showed the resorption of the root of tooth 42 and medial side of the root of tooth 44 (arrow heads). b Computed tomography revealed a well- circumscribed 3-cm osteolytic lesion in the right mandible that eroded the buccal cortical plate and resorbed the roots of tooth 42 and tooth 44 (arrows)
Histopathological features and differential diagnosis
A histopathological investigation revealed plump spindle cell proliferation with a storiform architecture and lymphocytes scattered around the spindle cells (Fig. 2a, b). The background stroma included a focal myxoid matrix. These spindle cells had ovoid nuclei with a pale eosinophilic cytoplasm, suggesting a myofibroblastic, myoepithelial, or fibroblastic lineage. Small islands of an odontogenic epithelium were detected. No mitosis or necrosis was observed. The histomorphological findings suggested that spindle cells were of a myofibroblastic, myoepithelial, or fibroblastic lineage. Small islands of an odontogenic epithelium suggested that this tumor was an odontogenic tumor. High-grade malignant tumors were excluded because neither mitosis nor necrosis was observed. Differential diagnoses included myofibroma, myoepithelioma, an odontogenic tumor, solitary fibrous tumor, giant cell granuloma, and IMT.
Fig. 2.
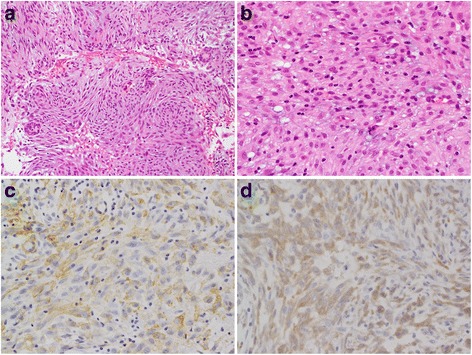
Hematoxylin and eosin staining (a, b) and immunohistochemical staining (c, d). a The proliferation of plump spindle cells with a storiform architecture with scattered lymphocytes. Epithelioid components with an appearance suggestive of odontogenic epithelioid cells were detected. b Spindle cells contained ovoid nuclei with a pale eosinophilic cytoplasm. c Spindle cells were positive for SMA. d Spindle cells were positive for ALK
Immunohistochemical findings
Immunohistochemical staining revealed that spindle cells were positive for α-smooth muscle actin (SMA) (Fig. 2c), ALK (Fig. 2d), CD10, and glial fibrillary acidic protein (GFAP), and negative for cytokeratin AE1/AE3, S100 proteins, and p63. The Ki67 labeling index was 3%.
Molecular pathological findings
FISH
A fluorescence in situ hybridization (FISH) (ALK) analysis was performed on a formalin-fixed paraffin-embedded (FFPE) tissue block. Rearrangements in the ALK gene at chromosome band 2p23 were detected by FISH utilizing a Vysis ALK break apart probe (Abott Molecular). The FISH analysis revealed the translocation of a part of the ALK gene locus (Fig. 3a).
Fig. 3.
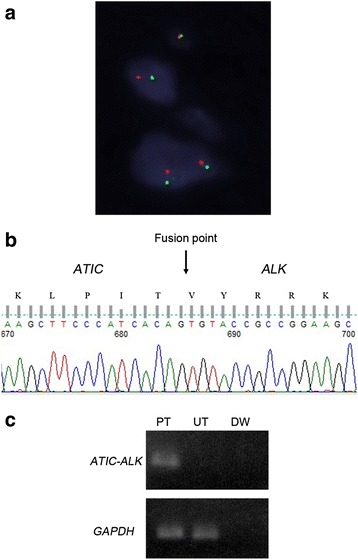
a FISH assay with a break apart probe for the ALK gene shows one intact yellow signal and one separated red and green signal per nucleus in tumor cells, indicating the presence of a rearrangement in the ALK gene. b The 5′ RACE analysis identified ATIC as a fusion partner. The sequence of the second round PCR product covering the fusion point (arrow) is shown. c The results of the RT-PCR analysis for ATIC-ALK are shown. PT: Present tumor, UT: Unrelated tumor (lung tumor), DW: Distilled Water, GAPDH: glyceraldehydes-3-phosphate dehydrogenase
RACE
Total RNA was extracted from frozen tissue using an RNeasy mini kit (Qiagen) according to the manufacturer’s instructions. In order to obtain cDNA fragments corresponding to a novel ALK fusion gene, a rapid amplification of cDNA ends (RACE) analysis was performed using the 5′-full RACE core set (TaKaRa Bio) according to the manufacturer’s instructions. First-strand cDNA synthesis was performed with a 5′ end-phosphorylated RT primer (5′-ATCTGGGCCTTGTATTTATCACTC). The primer set used for the first round of PCR was the A1 primer (5′-ACTTCCTGGTTGCTTTTGCTGGGGTAT) [5] and S1 primer (5′-AGAAGGAGCCACACGACAGGGGTAA). The primer set used for the second round of PCR was the S2 primer (5′-CTCCTTCACAAACCAGAGACCAA) and A2 primer (5′-TTCAGGCAGCGTCTTCACAG). The second round PCR product covering the break point was sub-cloned into a pT7Blue plasmid vector (TaKaRa Bio). The DNA sequence was analyzed using universal primers (M13M4 primer or T7 promoter primer) and the Big-dye terminator ver3.1 kit (Applied Biosystems). A RACE analysis confirmed a rearrangement in ALK and identified ATIC (NM_004044) as a partner of this ALK fusion mutant (Fig. 3b).
RT-PCR
First-strand cDNA was synthesized from total RNA using the SuperScript First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. RT-PCR was used to confirm the presence of the AITC-ALK fusion gene in this case. The primer set used was as follows: forward (5′-CCAAGCTTCCCATCACAGTGTACC) and reverse (5′-GAGGTCTTGCCAGCAAAGCAGTAG). RT-PCR was used to confirm the presence of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in this case. The primer set used was as follows: forward (5′-TGGAGTCCACTGGCGTCTTC) and reverse (5′-ATGACGAACATGGGGGCATCAG). A RT-PCR analysis confirmed the ATIC-ALK fusion in tumor cells (Fig. 3c).
Based on these results, the final diagnosis was IMT.
Discussion
We herein presented the first case of IMT with an ATIC-ALK fusion mutation primarily in the maxillofacial region. IMTs are rare mesenchymal tumors. Different terms have been applied to these lesions, namely, xanthogranuloma, plasma cell granuloma, inflammatory pseudotumor (IPT), and inflammatory myofibroblastic sarcoma [1, 6, 7]. The diverse nomenclature is descriptive and reflects the uncertainty regarding the true biological nature of these lesions. In 2013, the World Health Organization classification of soft tissue and bone defined IMT as a distinctive neoplasm composed of myofibroblastic and fibroblastic spindle cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes, and/or eosinophils [1]. The expression of ALK proteins is detected by anti-ALK immunohistochemistry in approximately 50% of IMT cases. ALK protein expression has been shown to reliably correlate with ALK gene rearrangements [8]. These lines of evidence support ALK-positive IMT being a distinct neoplastic entity. ALK immunostaining is an aid in the pathological diagnosis of IMTs, as well as in the differentiation of IMTs from other spindle cell neoplasms that fall within the broad category of IPTs. We reviewed 12 cases of intraosseous tumors of the mandible with similar histological features in the literature (Table 1) [7, 9–19]. Six cases were diagnosed as IPTs and 6 as IMTs in the mandible. ALK-positive IMT/IPT in the mandible was initially reported in 2005 [10] and only 4 cases including ours have been reported since then [10, 11, 19].
Table 1.
Clinicopathological features of IMTs in the mandible described in the present and previous reports
| No. | Authors (yr.) | Age (yr.) | Sex | Diagnosis | ALK-IHC | FISH | RT-PCR |
|---|---|---|---|---|---|---|---|
| 1 | Zegarelli (1974) [7] | 56 | F | Granuloma (?IPT) | NA | NA | NA |
| 2 | Inui (1993) [9] | 63 | M | IPT | NA | NA | NA |
| 3 | Brooks (2005) [10] | 82 | F | IMT | positive | NA | NA |
| 4 | Poh (2005) [11] | 42 | F | IMT | positive | NA | NA |
| 5 | Oh (2008) [12] | 20 | F | IPT | NA | NA | NA |
| 6 | Johann (2008) [13] | 33 | M | IPT | NA | NA | NA |
| 7 | Satomi (2010) [14] | 14 | F | IMT | negative | NA | NA |
| 8 | Date (2010) [15] | 70 | M | IPT | negative | NA | NA |
| 9 | Sasagawa (2011) [16] | 60 | M | IMT | NA | NA | NA |
| 10 | Gawande (2012) [17] | 20 | M | IPT | NA | NA | NA |
| 11 | Sah (2013) [18] | 30 | M | IMT | negative | NA | NA |
| 12 | Stringer (2014) [19] | 16 | M | IMT | positive | NA | NA |
| 13 | Present case | 11 | F | IMT | positive | positive | ATIC-ALK |
ALK anaplastic lymphoma kinase, IHC immunohistochemistry, FISH Fluorescene in situ hybridization, F female, M male, IPT Inflammatory pseudotumor, IMT Inflammatory myofibroblastic tumor, NA data not available
Thus, IMT may very rarely occur in the mandible. Otherwise, IMT may often be misdiagnosed because difficulties are associated with its histological differentiation from other spindle cell neoplasms and reactive lesions. Differential diagnoses include posttraumatic spindle cell nodules, giant cell granuloma, myofibroma, myoepithelioma, ameloblastic fibroma, odontogenic myxofibroma, IMT, a solitary fibrous tumor, low-grade myofibroblastic sarcoma, myofibrosarcoma, leiomyosarcoma, and spindle cell carcinoma. The immunohistochemical expression of the ALK protein is a very important hallmark for differentiating IMT from those described above. Therefore, if spindle cell lesions are detected in the jawbone, IMT must be included in the differential diagnosis and an immunohistochemical examination of ALK protein expression is needed.
The present tumor was confirmed to have the ATIC-ALK fusion mutation. Different ALK fusion partners, including TPM3, TPM4, CLTC, CARS, RANBP2, ATIC, SEC31L1, PPFIBP1, and DCTN1, in which TPM3 is the most common partner, have been identified in IMTs [5, 20–26]. ATIC-ALK fusion mutations are very rare, comprising approximately 1% of ALK-fusion mutations in anaplastic large cell lymphomas (ALCL) [27]. The hypothetical schema of the gene fusion between ATIC and ALK is shown in Fig. 4 [28]. To the best of our knowledge, one case of urinary bladder IMT with the ATIC-ALK fusion mutation and one case of IMT arising from the hyoid bone in a neonate with the ATIC-ALK fusion have been reported in the literature [23, 29]. Thus, the present case is the first IMT with the ATIC-ALK fusion mutation primarily in the mandible.
Fig. 4.
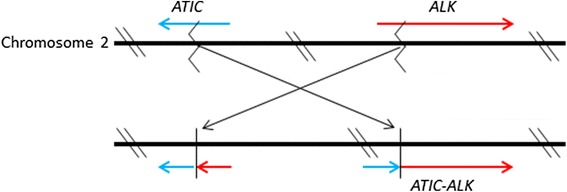
The hypothetical schema of the gene fusion between ALK and ATIC. ATIC-ALK fusion is generated by the pericentric inversion of chromosome 2 at the breakpoints of p23 and q35 [inv(2)(p23q35)], in which the ALK and ATIC genes are localized
The management of IMTs needs to entail complete surgical resection, including postoperative reassessments for at least 10 years [30]. Although the number of IMTs in the jawbone is limited, they appear to exhibit a favorable clinical course. However, it is important to emphasize that IMTs occasionally behave in an aggressive manner. Poh et al. reported the local recurrence of an intraosseous mandibular IMT 14 months after initial surgery [11]. Sasagawa et al. described a case of multiple IMTs involving the cranium, mandible, ischium, and calcaneus [16]. The ALK inhibitor, crizotinib, was recently demonstrated to be clinically beneficial for IMTs with ALK fusion mutations [26, 31–33].
Conclusion
IMTs are rare lesions in the mandible. The general consensus is that IMTs encompass a group of heterogeneous lesions with various biological behaviors. However, a proportion of cases are genuine neoplasms with the involvement of ALK fusion mutations. The recognition of this diagnostic entity is important for selecting appropriate treatments.
To the best of our knowledge, we are the first to describe IMT with the ATIC-ALK fusion mutation in the mandible.
Acknowledgments
We would like to sincerely thank Mr. Hideaki Mitsui for his technical support.
Funding
This paper was not financially supported.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Authors’ contributions
YT and KO carried out the molecular genetic studies and drafted the manuscript. SK provided histological and immunohistological evaluation. TS, SU and MM carried out the molecular genetic studies. MK attended the patient and arranged the clinical examination. KO conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient’s family for the publication of this case report and any accompanying images.
Ethics approval and consent to participate
Not applicable for Case Report.
Abbreviations
- IMT
Inflammatory myofibroblatic tumor
- ALK
Anaplastic lymphoma kinase
- FISH
Fluorescence in situ hybridization
- RACE
Rapid amplification of cDNA ends
- RT-PCR
Reverse transcription polymerase Chain reaction
- IPT
Inflammatory pseudotumor
Contributor Information
Yoko Tateishi, Phone: +81-45-787-2583, Email: ytateish@yokohama-cu.ac.jp.
Koji Okudela, Email: kojixok@yokohama-cu.ac.jp.
Shigeo Kawai, Email: skawai4836@yahoo.co.jp.
Takehisa Suzuki, Email: t380615@yokohama-cu.ac.jp.
Shigeaki Umeda, Email: umeda@yokohama-cu.ac.jp.
Mai Matsumura, Email: yoshima0112@yahoo.co.jp.
Mitomu Kioi, Email: kioi@yokohama-cu.ac.jp.
Kenichi Ohashi, Email: Ohashi@yokohama-cu.ac.jp.
References
- 1.Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumour. In Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F eds. World Health Organization classification of tumours soft tissue andbone. Lyon: IARC Press; 2013. p. 83–4.
- 2.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31(4):509–20. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 3.Park SB, Lee JH, Weon YC. Imaging findings of head and neck inflammatory pseudotumor. AJR Am J Roentgenol. 2009;193(4):1180–6. doi: 10.2214/AJR.09.2398. [DOI] [PubMed] [Google Scholar]
- 4.Cessna MH, Zhou H, Sanger WG, Perkins SL, Tripp S, Pickering D, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol. 2002;15(9):931–8. doi: 10.1097/01.MP.0000026615.04130.1F. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157(2):377–84. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudy HN, Scheingold SS. Solitary xanthogranuloma of the mandible; report of a case. Oral Surg Oral Med Oral Pathol. 1964;18:262–71. doi: 10.1016/0030-4220(64)90436-0. [DOI] [PubMed] [Google Scholar]
- 7.Zegarelli DJ, Rankow RM, Zegarelli EV. A large dental granuloma (? inflammatory pseudotumor) with unusual features: report of case. J Am Dent Assoc. 1974;89(4):891–4. doi: 10.14219/jada.archive.1974.0517. [DOI] [PubMed] [Google Scholar]
- 8.Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25(11):1364–71. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Inui M, Tagawa T, Mori A, Yoneda J, Nomura J, Fukumori T. Inflammatory pseudotumor in the submandibular region. Clinicopathologic study and review of the literature. Oral Surg Oral Med Oral Pathol. 1993;76(3):333–7. doi: 10.1016/0030-4220(93)90263-4. [DOI] [PubMed] [Google Scholar]
- 10.Brooks JK, Nikitakis NG, Frankel BF, Papadimitriou JC, Sauk JJ. Oral inflammatory myofibroblastic tumor demonstrating ALK, p53, MDM2, CDK4, pRb, and Ki-67 immunoreactivity in an elderly patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(6):716–26. doi: 10.1016/j.tripleo.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Poh CF, Priddy RW, Dahlman DM. Intramandibular inflammatory myofibroblastic tumor--a true neoplasm or reactive lesion? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(4):460–6. doi: 10.1016/j.tripleo.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Oh JH, Yim JH, Yoon BW, Choi BJ, Lee DW, Kwon YD. Inflammatory pseudotumor in the mandible. J Craniofac Surg. 2008;19(6):1552–3. doi: 10.1097/SCS.0b013e318188a2e9. [DOI] [PubMed] [Google Scholar]
- 13.Johann AC, Caldeira PC, Abdo EN, Sousa SO, Aguiar MC, Mesquita RA. Inflammatory myofibroblastic tumor of the alveolar mucosa of the mandible. Minerva Stomatol. 2008;57(1–2):59–63. [PubMed] [Google Scholar]
- 14.Satomi T, Watanabe M, Matsubayashi J, Nagao T, Chiba H. A successfully treated inflammatory myofibroblastic tumor of the mandible with long-term follow-up and review of the literature. Med Mol Morphol. 2010;43(3):185–91. doi: 10.1007/s00795-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 15.Date A, Yamagata K, Onizawa K, Yanagawa T, Karube R, Ishibashi N, et al. Inflammatory pseudotumor: report of a case in the mandible. Oral Maxillofac Surg. 2012;16(1):65–8. doi: 10.1007/s10006-010-0250-9. [DOI] [PubMed] [Google Scholar]
- 16.Sasagawa Y, Akai T, Itou S, Iizuka H. Multiple intraosseous inflammatory myofibroblastic tumors presenting with an aggressive clinical course: case report. Neurosurgery. 2011;69(4):E1010–5. doi: 10.1227/NEU.0b013e318223b651. [DOI] [PubMed] [Google Scholar]
- 17.Gawande PD, Sambhus M, Garde JB, Halli R, Deshmukh V, Kulkarni A, et al. Aggressive inflammatory pseudotumor of the mandible. J Craniofac Surg. 2012;23(4):1101–3. doi: 10.1097/SCS.0b013e318252da65. [DOI] [PubMed] [Google Scholar]
- 18.Sah P, Byatnal AA, Rao L, Narayanaswamy V, Radhakrishnan R. Inflammatory myofibroblastic tumor: a rapidly growing soft tissue mass in the posterior mandible. Head Neck Pathol. 2013;7(4):393–7. doi: 10.1007/s12105-013-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stringer DE, Allen CN, Nguyen K, Tandon R. Intraosseous inflammatory myofibroblastic tumor in the mandible: a rare pathologic case report. Case Rep Surg. 2014;2014:565478. doi: 10.1155/2014/565478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridge JA, Kanamori M, Ma Z, Pickering D, Hill DA, Lydiatt W, et al. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol. 2001;159(2):411–5. doi: 10.1016/S0002-9440(10)61711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cools J, Wlodarska I, Somers R, Mentens N, Pedeutour F, Maes B, et al. Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2002;34(4):354–62. doi: 10.1002/gcc.10033. [DOI] [PubMed] [Google Scholar]
- 22.Ma Z, Hill DA, Collins MH, Morris SW, Sumegi J, Zhou M, et al. Fusion of ALK to the ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37(1):98–105. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 23.Debiec-Rychter M, Marynen P, Hagemeijer A, Pauwels P. ALK-ATIC fusion in urinary bladder inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;38(2):187–90. doi: 10.1002/gcc.10267. [DOI] [PubMed] [Google Scholar]
- 24.Panagopoulos I, Nilsson T, Domanski HA, Isaksson M, Lindblom P, Mertens F, et al. Fusion of the SEC31L1 and ALK genes in an inflammatory myofibroblastic tumor. Int J Cancer. 2006;118(5):1181–6. doi: 10.1002/ijc.21490. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi K, Soda M, Togashi Y, Sugawara E, Hatano S, Asaka R, et al. Pulmonary inflammatory myofibroblastic tumor expressing a novel fusion, PPFIBP1-ALK: reappraisal of anti-ALK immunohistochemistry as a tool for novel ALK fusion identification. Clin Cancer Res. 2011;17(10):3341–8. doi: 10.1158/1078-0432.CCR-11-0063. [DOI] [PubMed] [Google Scholar]
- 26.Subbiah V, McMahon C, Patel S, Zinner R, Silva EG, Elvin JA, et al. STUMP un“stumped”: anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J Hematol Oncol. 2015;8:66. doi: 10.1186/s13045-015-0160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colleoni GW, Bridge JA, Garicochea B, Liu J, Filippa DA, Ladanyi M. ATIC-ALK: a novel variant ALK gene fusion in anaplastic large cell lymphoma resulting from the recurrent cryptic chromosomal inversion, inv(2)(p23q35) Am J Pathol. 2000;156(3):781–9. doi: 10.1016/S0002-9440(10)64945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wlodarska I, De Wolf-Peeters C, Falini B, Verhoef G, Morris SW, Hagemeijer A, et al. The cryptic inv(2)(p23q35) defines a new molecular genetic subtype of ALK-positive anaplastic large-cell lymphoma. Blood. 1998;92(8):2688–95. [PubMed] [Google Scholar]
- 29.Owusu-Brackett N, Johanson R, Schindel DT, Koduru P, Cope-Yokoyama S. A novel ALK rearrangement in an inflammatory myofibroblastic tumor in a neonate. Cancer Genet. 2013;206(9–10):353–6. doi: 10.1016/j.cancergen.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Meis-Kindblom JM, Kjellstrom C, Kindblom LG. Inflammatory fibrosarcoma: update, reappraisal, and perspective on its place in the spectrum of inflammatory myofibroblastic tumors. Semin Diagn Pathol. 1998;15(2):133–43. [PubMed] [Google Scholar]
- 31.Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Eng J Med. 2010;363(18):1727–33. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishio M, Murakami H, Horiike A, Takahashi T, Hirai F, Suenaga N, et al. Phase I study of ceritinib (LDK378) in Japanese patients with advanced, anaplastic lymphoma kinase-rearranged non-small-cell lung cancer or other tumors. J Thorac Oncol. 2015;10(7):1058–66. doi: 10.1097/JTO.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob SV, Reith JD, Kojima AY, Williams WD, Liu C, Vila DL. An unusual case of systemic inflammatory myofibroblastic tumor with successful treatment with ALK-inhibitor. Case Rep Pathol. 2014;2014:470340. doi: 10.1155/2014/470340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article.


