Abstract
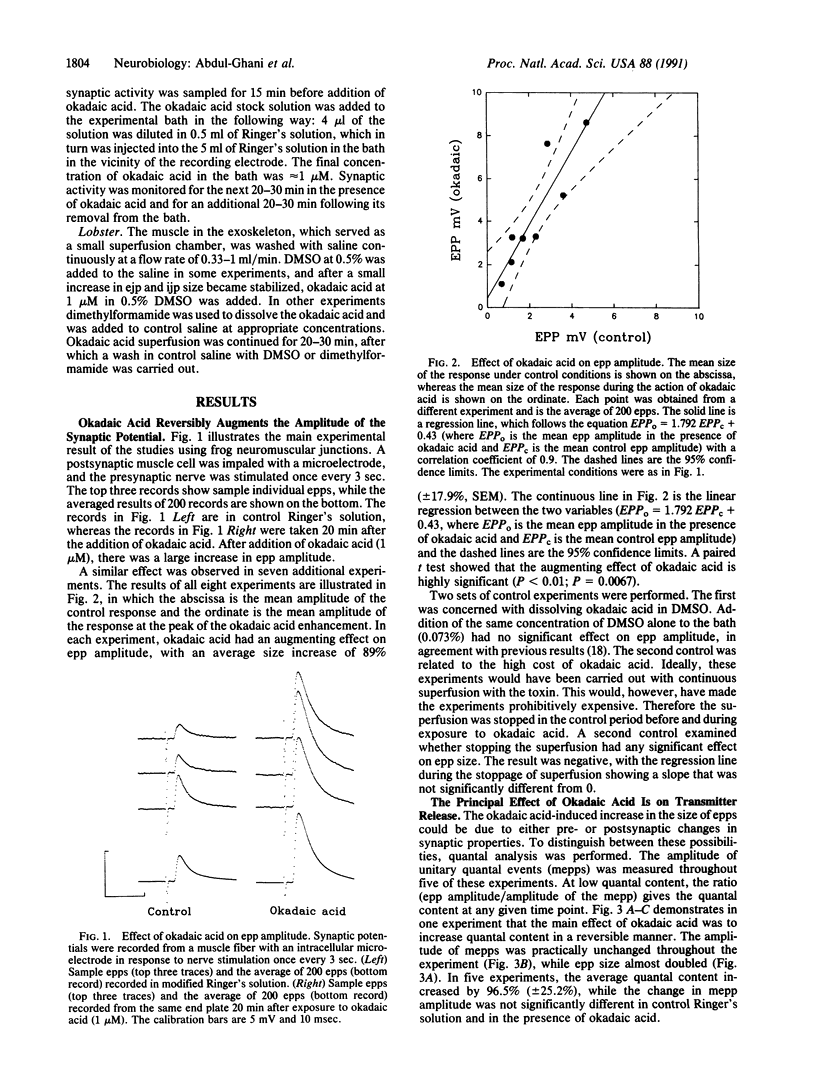
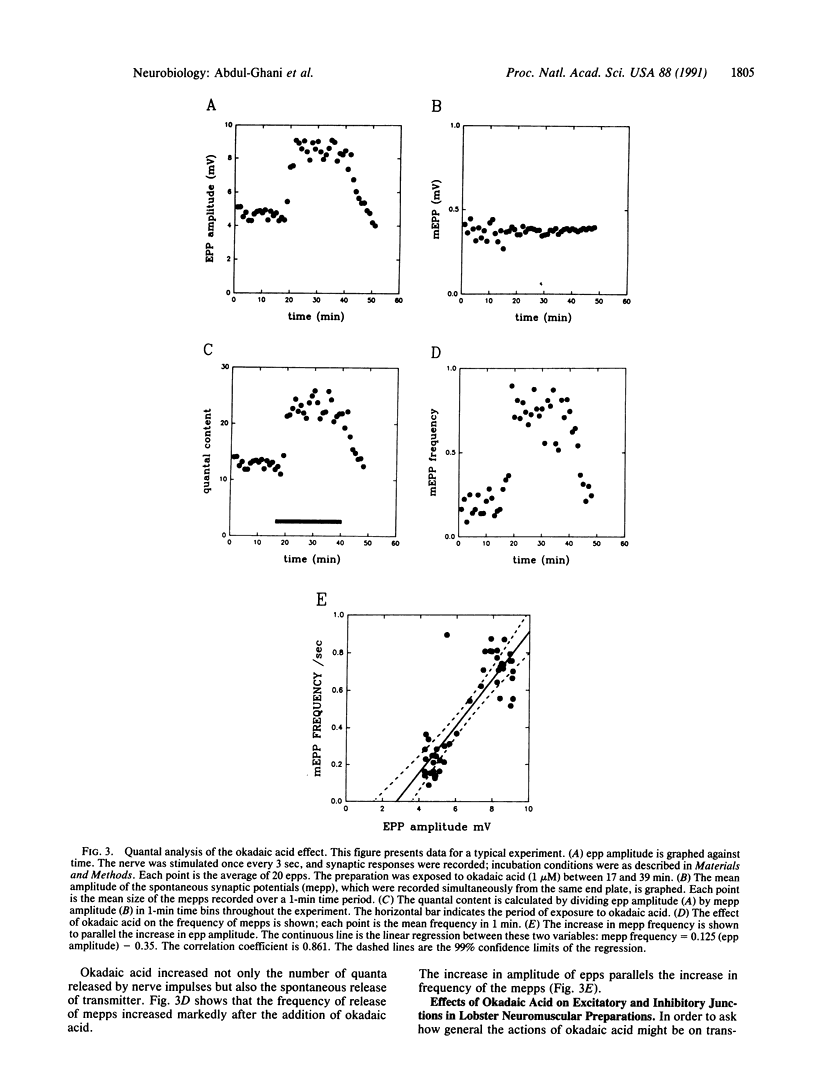
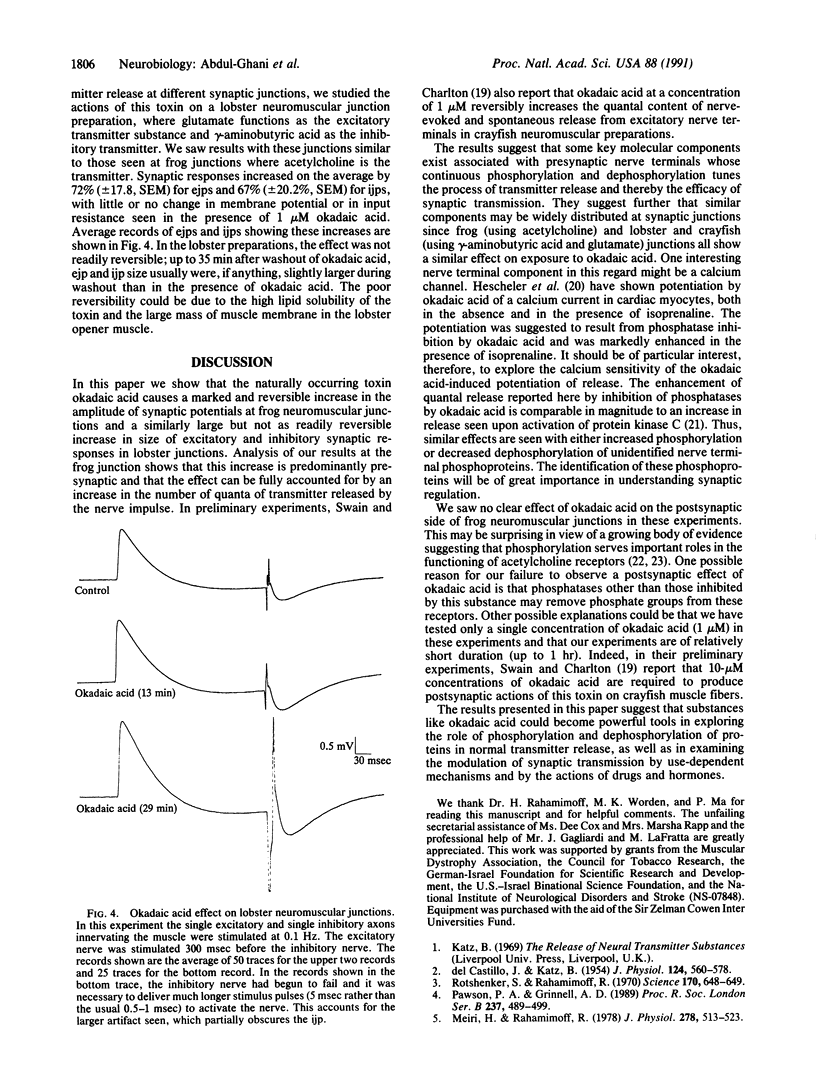
To test the hypothesis that continual phosphorylation and dephosphorylation of protein components of nerve terminals might be important determinants of synaptic efficacy, the effect of okadaic acid, a potent natural inhibitor of two serine threonine protein phosphatases (phosphatase 1 and phosphatase 2A), was examined on synaptic transmission at frog (cholinergic) and lobster (glutamatergic and GABAergic) neuromuscular junctions. At frog junctions, the addition of 1 microM okadaic acid to the extracellular fluid caused almost a doubling of the amplitude of the end-plate potential. The effect of okadaic acid was reversible. Quantal analysis showed that the augmenting effect was presynaptic, resulting from an increase in the number of quanta of transmitter released by a nerve impulse. Where was no significant change in the amplitude of spontaneously liberated miniature end-plate potentials, but their frequency of release increased in parallel with the increase in amplitude of the nerve-evoked synaptic potential. Similar studies with lobster neuromuscular junctions showed increases in the size of both excitatory and inhibitory synaptic responses that were similar in magnitude to the effects seen in the frog junctions. No significant changes in membrane potential or in input resistance accompanied the increased response size. These results suggest that transmitter release at a variety of junctions using different transmitters is constantly modulated by phosphorylation and dephosphorylation of important protein components within nerve terminals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Ghani M., Meiri H., Rahamimoff R. Vasopressin produces long-lasting increase in transmitter release. Brain Res. 1990 May 7;515(1-2):355–357. doi: 10.1016/0006-8993(90)90623-j. [DOI] [PubMed] [Google Scholar]
- Browning M. D., Huganir R., Greengard P. Protein phosphorylation and neuronal function. J Neurochem. 1985 Jul;45(1):11–23. doi: 10.1111/j.1471-4159.1985.tb05468.x. [DOI] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D. The modulation of neurotransmitter release at synaptic junctions. Rev Physiol Biochem Pharmacol. 1983;98:63–175. doi: 10.1007/BFb0033867. [DOI] [PubMed] [Google Scholar]
- Geron N., Meiri H. The fusogenic substance dimethyl sulfoxide enhances exocytosis in motor nerve endings. Biochim Biophys Acta. 1985 Oct 10;819(2):258–262. doi: 10.1016/0005-2736(85)90181-6. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., McGuinness T. L., Huganir R. L., Greengard P. Role of protein phosphorylation in neuronal signal transduction. FASEB J. 1989 Mar;3(5):1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Huganir R. L. Regulation of the nicotinic acetylcholine receptor by protein phosphorylation. J Recept Res. 1987;7(1-4):241–256. doi: 10.3109/10799898709054988. [DOI] [PubMed] [Google Scholar]
- Kravitz E. A. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science. 1988 Sep 30;241(4874):1775–1781. doi: 10.1126/science.2902685. [DOI] [PubMed] [Google Scholar]
- Meiri H., Rahamimoff R. Clumping and oscillations in evoked transmitter release at the frog neuromuscular junction. J Physiol. 1978 May;278:513–523. doi: 10.1113/jphysiol.1978.sp012321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Iversen L. L., Hall Z. W., Kravitz E. A. Release of gamma-aminobutyric acid from inhibitory nerves of lobster. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1110–1115. doi: 10.1073/pnas.56.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson P. A., Grinnell A. D. Oscillation period of MEPP frequency at frog neuromuscular junctions is inversely correlated with release efficacy and independent of acute Ca2+ loading. Proc R Soc Lond B Biol Sci. 1989 Sep 22;237(1289):489–499. doi: 10.1098/rspb.1989.0061. [DOI] [PubMed] [Google Scholar]
- Rotshenker S., Rahamimoff R. Neuromuscular synapse: stochastic properties of spontaneous release of transmitter. Science. 1970 Nov 6;170(3958):648–649. doi: 10.1126/science.170.3958.648. [DOI] [PubMed] [Google Scholar]
- Shapira R., Silberberg S. D., Ginsburg S., Rahamimoff R. Activation of protein kinase C augments evoked transmitter release. Nature. 1987 Jan 1;325(6099):58–60. doi: 10.1038/325058a0. [DOI] [PubMed] [Google Scholar]



