Abstract
Background:
About 10% of the patients had surveillance drug-related mutations for nonnucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs) in an Indian study. It was also reported that resistance was maximum for nucleoside reverse transcriptase inhibitors (NRTIs) and minimum for PIs.
Methods:
The present study was a cross-sectional assessment of 21 human immunodeficiency virus (HIV)-infected individuals attending a HIV care center in a tertiary care center in Mumbai, Maharashtra, India. All HIV-infected individuals included in the present analysis were tested for CD4 count, viral load, and resistance to antiretrovirals (ARVs).
Results:
A total of 13 male and 8 female were included in the present analysis. Of these, 18 were treatment naive and three were treatment experienced patients. In treatment-naive patients, the proportion of high-level resistance (HLR) was 2% for NRTIs, 5% for PIs, and 11% for NNRTIs. In treatment-naive patients, high susceptibility was observed for darunavir (89%) followed by lopinavir (72%) and fosamprenavir (67%) among PIs. Similarly, susceptibility was high for NRTIs lamivudine (94%), emtricitabine (94%), and tenofovir (89%). However, we found HLR for nevirapine (39%) even in treatment-naive patients.
Conclusions:
The proportion of HLR was relatively low for PIs and NRTIs, compared with NNRTIs in treatment-naive patients. We also reported a high correlation in resistance patterns among drugs belonging to the same group. Thus, it may be useful to conduct ARV resistance even in newly infected HIV patients and those receiving medications for the first time.
Key words: Human immunodeficiency virus drug resistance, India, treatment experienced, treatment-naive
INTRODUCTION
India currently has about 2.1 million people living with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) and an adult HIV prevalence of 0.27%.[1] The most common routes of transmission are sexual (89%), parent to child (5%), and injecting drug use.[2] Even though antiretroviral therapy (ART) was available to those who could afford it for about two decades in India and was regularly used by physicians, the National AIDS Control Organisation (NACO) Department of AIDS Control initiated free ART in 2004. From a modest start of 25 ART centers and 6845 patients on ART in 2005; currently, NACO has about 425 ART centers and 768,000 people on ART. Thus, there has been an increase the number of people on ART.[1]
One major concern while administering ART is the presence of resistance to these drugs.[3] Apart from clinical implications of ART resistance, the cost of management increases with the presence of resistance mutations.[4] Given, the elaborate laboratory set up required for antiretroviral (ARV) resistance testing, it is expensive and not easily available to a large proportion of HIV-infected individuals in resource-limited countries such as India.
However, with increased use of ART in the Indian population, it is important to document the resistance patterns and the clinical implications, if any, of these in the Indian population. Thus, this study was undertaken to assess the resistance to protease inhibitors (PIs), nucleoside reverse transcriptase inhibitors (NRTIs), and non-NRTIs (NNRTIs) in treatment experienced and naive HIV-infected patients in Mumbai, Maharashtra, India.
METHODS
This study was a cross-sectional assessment of consecutive consenting 21 HIV-infected individuals attending an HIV care center in a tertiary care center in Mumbai, Maharashtra, India.
The study was conducted a private tertiary care center in South Mumbai, Maharashtra, India. Data were collected from consecutive HIV-infected patients in whom resistance patterns could be assessed. All HIV-infected individuals included in the present analysis were tested for CD4 count, viral load, and resistance to ARVs. We tested them for the following drugs: (1) PIs: Atazanavir (ATV), darunavir (DRV), fosamprenavir (FPV), indinavir (IDV), lopinavir (LPV), nelfinavir (NFV), saquinavir (SQV), and tipranavir (TPV); (2) NRTIs: Lamivudine (3TC), abacavir (ABC), azidothymidine (AZT), stavudine (D4T), didanosine (DDI), emtricitabine (FTC), and tenofovir (TDF); and (3) NNRTIs: Efavirenz (EFV), etravirine (ETR), nevirapine (NVP), and rilpivirine (RPV). The HIV RNA PCR was done using QIAGEN OneStep RT-PCR Kit (QIAGEN, Hilden, Germany) and HIV RNA real-time PCR was done using RoboGene HIV-1 Quantification Kit (ROBOSCREEN, Germany). A detailed explanation of the molecular diagnostic methods has been published elsewhere.[5] HIV Stanford Database was used for genotypic drug resistance analysis.[6] The resistance patterns were classified as: Susceptible (resistance mutation score [RMS] 0 and 5), potential low-level resistance (RMS 10), low-level resistance (RMS 15–25), intermediate resistance (RMS 30–55), and high-level resistance (HLR) (RMS 60 and above). We also calculated the overall prevalence HLR in all the resistance tests conducted among PIs, NRTIs, and NNRTIs.
Demographic data (age, sex), date of HIV testing, and treatment (regimen and duration) were also collected. All the data were entered in MS Excel (© Microsoft Corp., USA) and converted to Stata Version 13 (© StataCorp, College Station, TX, USA). We calculated the means and standard deviations (SDs) for the linear variables, and proportions for categorical variables. We compared the means using t-test and the differences in proportions were compared using the Fisher's exact test for low cell counts. We also calculated the correlations for resistance patters in these drugs.
The study was approved by the Ethics Committee of the hospital in which the study was conducted.
RESULTS
A total of 13 male and 8 female were included in the present analysis. The mean (SD) age of the participants was 38.9 (8.1) years; there was no significant difference between the ages of males (38.5 [5.8]) and females (39.5 [11.3]) (P = 0.78). Only three participants were on treatment currently. The median duration from the time of diagnosis was 71 days (range 0 days to 2496 days). The median CD4 count before drug resistance testing was 146 (range 12–674) and the median CD4 counts after drug resistance 246 (range 10–659); this change was significantly significant (P = 0.003). The mean (SD) log viral load of the subjects before resistance testing was 5.06 (0.74); this was significantly higher in those on treatment compared with those who were not (5.20 [0.68] vs. 4.23 [0.56], P = 0.03).
Treatment-naive patients
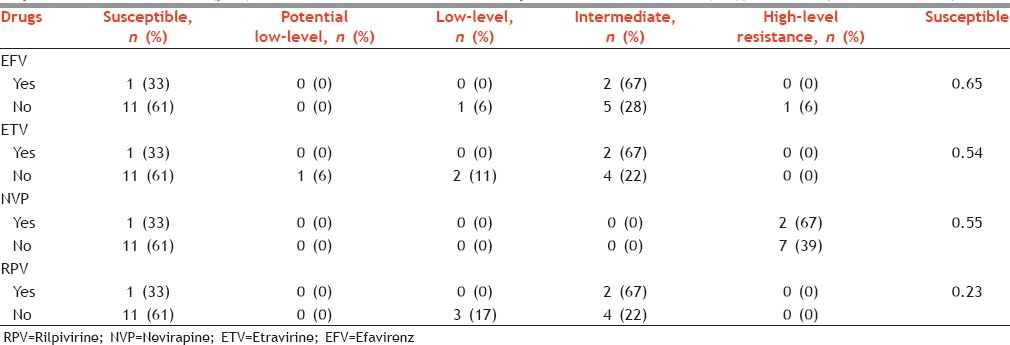
Among PIs, high susceptibility was observed for DRV (89%) followed by LPV (72%) and FPV (67%). HLR was observed only in NFV, ATV, and IDV. In general, susceptibility was high for most of the NRTIs: 3TC (94%), FTC (94%), and TDF (89%). HLR was also found only in FTC (6%) and 3TC (6%). The susceptibility was similar for all the NNRTI tested; it was 61% for EFV, ETV, NVP, and RPV. We recorded HLR for NVP.
A total of 144 resistance tests were conducted for PIs (we tested eight PIs for each of 18 treatment-naive patients); of these, we found seven cases of HLR (5%). Similarly, the prevalence of HLR among NRTIs was 2% and among NNRTIs was 11%; the proportion of HLR was significantly different across these three groups (P = 0.01).
Treatment experienced patients
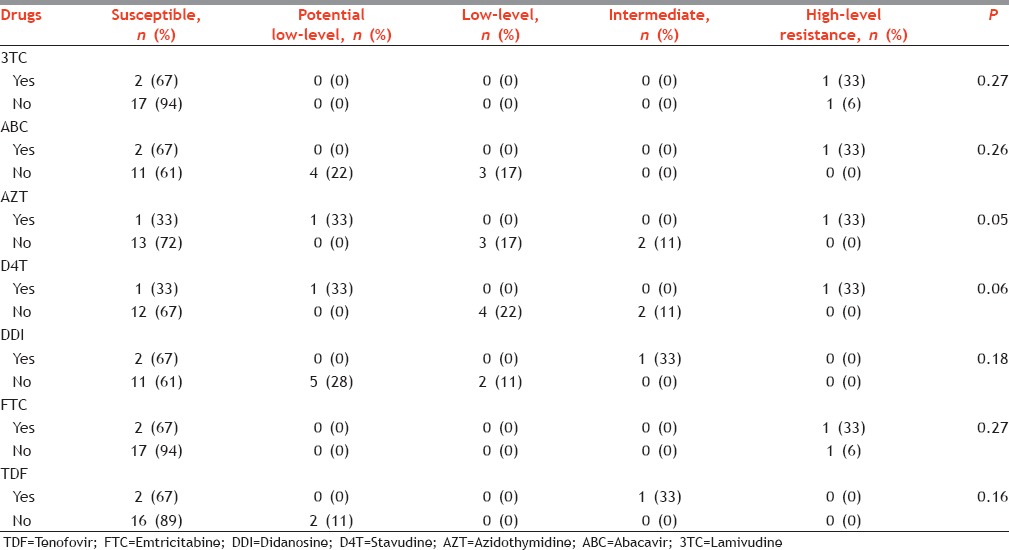
In treatment-experienced patients, HLR was found for IDV, NFV, TPV, ATV, FPV, and SQV. Similarly, it was also found for 3TC, ABC, AZT, D4T, and FTC. However, HLR was only found for NVP. Resistance patterns were significantly different in treatment naive and experienced patients for FPV, LPV, TPV, and AZT.
We have presented a detailed analysis of the resistance patterns for each drug in Tables 1–4.
Table 1.
Levels of resistance among protease inhibitor in those who were exposed to treatment (yes) and those who were not exposed to treatment (no), Mumbai, Maharashtra, India
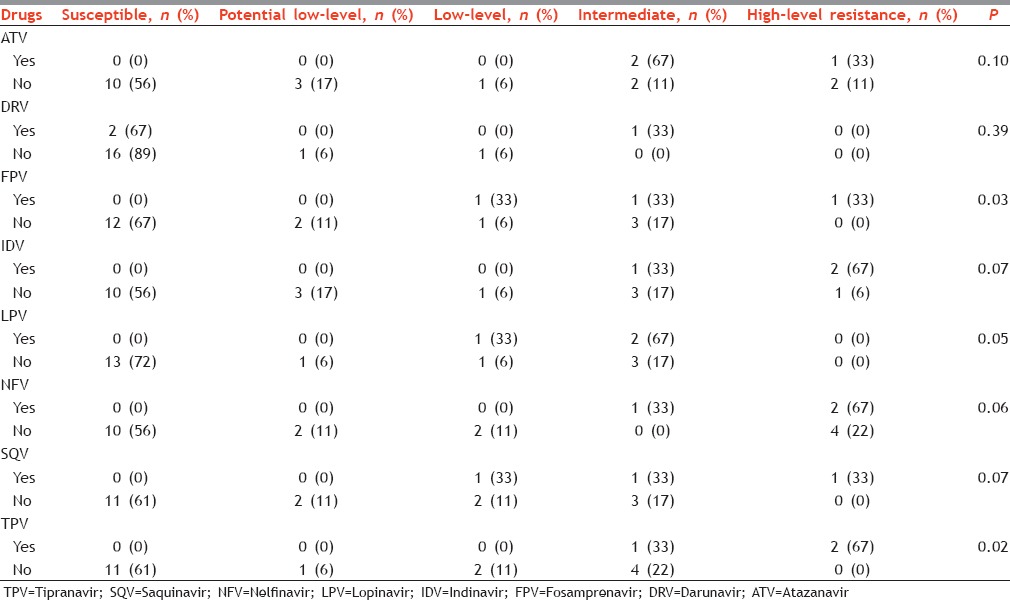
Table 4.
Levels of resistance and resistance mutation scores among protease inhibitors, nucleoside reverse transcriptase inhibitors, and nonnucleoside reverse transcriptase inhibitors in all tests conducted among 18 treatment-naive patients, Mumbai, Maharashtra, India*
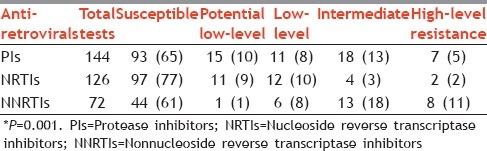
Table 3.
Levels of resistance among nonnucleoside reverse transcriptase inhibitors in those who were exposed to treatment (yes) and those who were not exposed to treatment (no), Mumbai, Maharashtra, India
As seen in Table 5, among the correlation of resistance patterns of PI, we found that ATV correlated highly with DRV, FPV, NFV, IDV, LPV, SQV, and TPV. We also found a high correlation in the resistance patterns of FPV and LPV (0.92), FPV and NFV (0.92), and NFV and IDV (0.99). In NRTIs, we found very high correlation in the resistance patterns of ABC and DDI, DDI and D4T, and D4T and AZT. Among NNRTIs, we found a high correlation in the resistance patterns of NVP and the other three NNRTIs (RPV [0.97], ETR [0.97], and EFV [0.98]). In general, the resistance patterns were similar within the same class of medications. Additional correlation values have been presented in Table 2.
Table 5.
Correlation matrix of resistance patters of drugs (protease inhibitors, nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors) among 18 treatment-naive patients, Mumbai, Maharashtra, India*
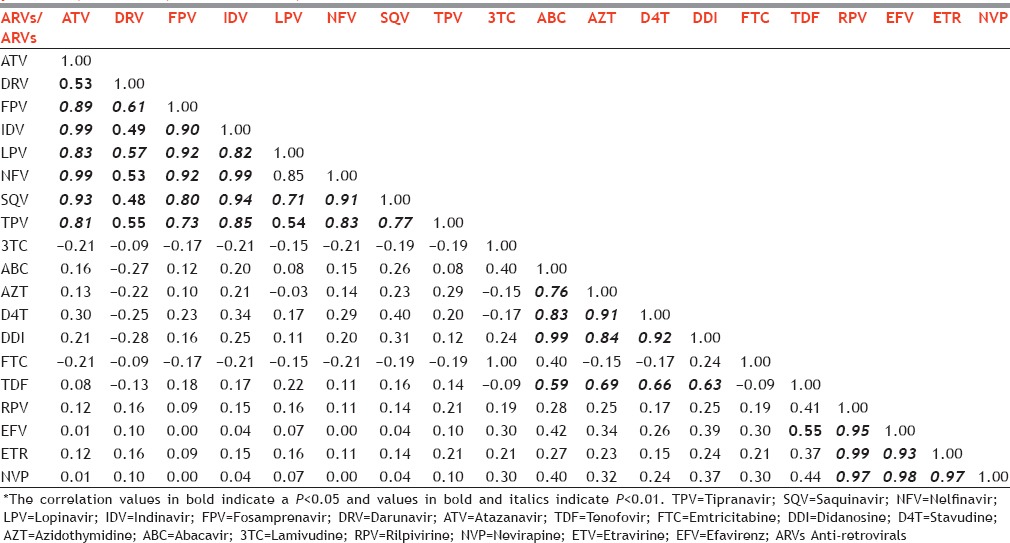
Table 2.
Levels of resistance among nucleoside reverse transcriptase inhibitors in those who were exposed to treatment (yes) and those who were not exposed to treatment (no), Mumbai, Maharashtra, India
DISCUSSION
HLR was relatively low for PIs and NRTIs. However, the highest proportion of HLR was found among NRTIs in treatment-naive patients. Furthermore, in these patients, susceptibility was highest for DRV among PIs, for 3TC and FTC among NRTIs, and similar for all NNRTIs tested. Interestingly, resistance patterns were correlated among the same groups of drugs, whereas the correlation was in general not significant across three groups of ARVs.
Although ARVs have been used in India for nearly two decades, there was limited literature on ARV resistance patterns.[7] However, there has been an increase in the number of reports highlighting the presence of ARV resistance in the Indian population. The pattern and proportion of resistance patterns vary in these studies. For instance, Deshpande et al. reported surveillance drug-related mutations for NNRTIs and PIs in about 10% of the patients.[8] However, Gupta et al. reported that about 96% had resistance to at least one ARV and the resistance was maximum for NRTIs and minimum for PIs.[9] Both these studies were from the same geographic location (Mumbai, Maharashtra, India). Interestingly, other authors have also reported high resistance patterns for NRTIs and NNRTIs and low to none for PIs.[10,11] We found resistance patterns to be high in treatment-experienced patients – particularly for PIs and NRTIs. Saravanan et al. also reported a relatively high proportion of resistance mutations for PIs, even though this was lower than that found for NRTIs.[12] A study by Richman et al. reported 76% resistance to any ARV drug, 71% resistance to NRTIs, 41% resistance to PIs, and 25% resistance to NNRTIs in the United States.[13] As reported elsewhere [5] some of the common mutations we found were M46 L, I47M, V82A/D/G, in addition to minor protease mutations or polymorphisms of L10I, V11F, L23H, K43I/R, A71D, G73A, T74A/P/S, and N83D.
The presence of resistance to various ARVs is important for clinical management of patients. Furthermore, the presence of resistance to ARVs even in treatment-naive patients is an important marker to suggest transmission of resistant viruses in the population. Interestingly, Sen et al., and Azam et al. did not find any mutations in treatment-naive patients.[14,15] The current NACO guidelines have suggested nevirapine-based regimens as first line of therapy in adults.[16] As per our findings, a high proportion of treatment experienced and a substantial proportion of treatment-naive patients have HLR to NVP. It has been well documented that single-dose NVP may result in a relatively high proportion of resistance mutations.[17,18,19] Lockman et al. found that LPV/ritonavir combination with TDF-FTC was superior compared with NVP-TDF-FTC combination in women who were exposed to single-dose NVP in pregnancy.[20] Since single-dose NVP was being used in the Prevention of Parent to Child Treatment Programme in India,[21] it is quite likely that there is the presence of resistance mutations to NVP in the population. Furthermore, as observed in our data resistance patterns in NVP and other NNRTIs may be highly correlated. Similar cross-resistance and class resistance in NNRTIs have also been reported by others.[19,22] The WHO also recommends the use of TDF + 3TC (or FTC) + EFV as the first line management for adult patients.[23] Given, the Cross-resistance among NNRTIs, it may be useful to assess the resistance before starting NNRTI-based therapies in patients.
The NACO guidelines suggest ATV-based regimens to be second-line treatment.[24] As observed in our data, susceptibility was relatively low to ATV even in treatment-naive patients. Furthermore, there was a high correlation of resistance patterns with other PIs. Thus, it may be worthwhile conducting a detailed ARV resistance testing before initiating second-line therapy. The PI which showed best susceptibility was DRV; thus, DRV may be a useful alternative for management of patients. Indeed, other authors have also reported high susceptibility to DRV and that it should be the preferred choice of drug for third-line management.[12,25] It may be worthwhile to consider DRV as a drug of choice for second-line treatment. In addition, LPV, TPV, and SQV may be useful alternatives for clinical management of patients.
We included data from 21 patients in our analysis; the sample was relatively small. Thus, the generalizability of these data may be limited nonetheless we have presented a detailed analysis of all three groups of ARVs. Furthermore, we have also presented correlation between resistance patterns of various ARVs. Although we have not included detailed information on resistance mutations in our manuscript, this has been presented elsewhere.[5]
In spite of the above limitations, this is an important contribution to the literature and has important treatment implications. We found a high proportion of HLR for many PIs in treatment-experienced patients; however, HLR was not observed in treatment-naive patients for most of the PIs. Similarly, HLR was not observed in for NRTIs in treatment-naive patients. HLR in treatment-naive patients was however relatively high for NNRTIs. Furthermore, there was a high correlation in general among drugs belonging to the same group. Thus, it may be useful to conduct ARV resistance even in newly infected HIV patients and those receiving medications for the first time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge Dr. P. D. Potdar and Department of Molecular Biology, Jaslok for help with microbiological assessment and We would like to acknowledge Dr. Prasita Kshirsagar, Associate Professor of Medicine (Rajiv Gandhi Medical College) for support. We would also like to acknowledge Dr. Maninder S. Setia, Consultant Epidemiologist for feedback on the manuscript.
REFERENCES
- 1.Annual Report 2013-14. New Delhi, India: Department of AIDS Control, Ministry of Health & Family Welfare, Government of India; 2014. National AIDS Control Organization. [Google Scholar]
- 2.Annual Report 2010-11. New Delhi, India: Department of AIDS Control, Ministry of Health & Family Welfare, Government of India; 2012. National AIDS Control Organization. [Google Scholar]
- 3.Frentz D, Van de Vijver DA, Abecasis AB, Albert J, Hamouda O, Jørgensen LB, et al. Increase in transmitted resistance to non-nucleoside reverse transcriptase inhibitors among newly diagnosed HIV-1 infections in Europe. BMC Infect Dis. 2014;14:407. doi: 10.1186/1471-2334-14-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krentz HB, Ko K, Beckthold B, Gill MJ. The cost of antiretroviral drug resistance in HIV-positive patients. Antivir Ther. 2014;19:341–8. doi: 10.3851/IMP2709. [DOI] [PubMed] [Google Scholar]
- 5.Potdar PD, Daswani BS, Rane NJ. Drug resistance mutations to protease and reverse transcriptase inhibitors in treatment naive HIV-1 clade C infected individuals from Mumbai, India. Int J Virol. 2011;7:13–23. [Google Scholar]
- 6.Stanford University HIV Drug Resistance Database. [Last accessed on 2016 Mar 15]. Available from: http://www.hivdb.stanford.edu/
- 7.Lakhashe S, Thakar M, Godbole S, Tripathy S, Paranjape R. HIV infection in India: Epidemiology, molecular epidemiology and pathogenesis. J Biosci. 2008;33:515–25. doi: 10.1007/s12038-008-0070-3. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande A, Karki S, Recordon-Pinson P, Fleury HJ. Drug resistance mutations in HIV type 1 isolates from naive patients eligible for first line antiretroviral therapy in JJ Hospital, Mumbai, India. AIDS Res Hum Retroviruses. 2011;27:1345–7. doi: 10.1089/AID.2011.0066. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Saple DG, Nadkarni G, Shah B, Vaidya S, Hingankar N, et al. One-, two-, and three-class resistance among HIV-infected patients on antiretroviral therapy in private care clinics: Mumbai, India. AIDS Res Hum Retroviruses. 2010;26:25–31. doi: 10.1089/aid.2009.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini S, Bhalla P, Gautam H, Baveja UK, Pasha ST, Dewan R. Resistance-associated mutations in HIV-1 among patients failing first-line antiretroviral therapy. J Int Assoc Physicians AIDS Care (Chic) 2012;11:203–9. doi: 10.1177/1545109711421217. [DOI] [PubMed] [Google Scholar]
- 11.Sinha S, Shekhar RC, Ahmad H, Kumar N, Samantaray JC, Sreenivas V, et al. Prevalence of HIV drug resistance mutation in the Northern Indian population after failure of the first line antiretroviral therapy. Curr HIV Res. 2012;10:532–8. doi: 10.2174/157016212802429785. [DOI] [PubMed] [Google Scholar]
- 12.Saravanan S, Vidya M, Balakrishnan P, Kantor R, Solomon SS, Katzenstein D, et al. Viremia and HIV-1 drug resistance mutations among patients receiving second-line highly active antiretroviral therapy in Chennai, Southern India. Clin Infect Dis. 2012;54:995–1000. doi: 10.1093/cid/cir967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richman DD, Morton SC, Wrin T, Hellmann N, Berry S, Shapiro MF, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393–401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]
- 14.Azam M, Malik A, Rizvi M, Rai A. Zero prevalence of primary drug resistance-associated mutations to protease inhibitors in HIV-1 drug-naive patients in and around Aligarh, India. J Infect Dev Ctries. 2014;8:79–85. doi: 10.3855/jidc.3480. [DOI] [PubMed] [Google Scholar]
- 15.Sen S, Tripathy SP, Patil AA, Chimanpure VM, Paranjape RS. High prevalence of human immunodeficiency virus type 1 drug resistance mutations in antiretroviral treatment-experienced patients from Pune, India. AIDS Res Hum Retroviruses. 2007;23:1303–8. doi: 10.1089/aid.2007.0090. [DOI] [PubMed] [Google Scholar]
- 16.Antiretroviral therapy guidelines for HIV-infected adults and adolescents – May 2013. New Delhi, India: Department of AIDS Control, Ministry of Health & Family Welfare, Government of India; 2013. National AIDS Control Organization. [Google Scholar]
- 17.Johnson JA, Li JF, Morris L, Martinson N, Gray G, McIntyre J, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 18.Palmer S, Boltz V, Martinson N, Maldarelli F, Gray G, McIntyre J, et al. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci U S A. 2006;103:7094–9. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajesh L, Ramesh K, Hanna LE, Narayanan PR, Swaminathan S. Emergence of drug resistant mutations after single dose nevirapine exposure in HIV-1 infected pregnant women in South India. Indian J Med Res. 2010;132:509–12. [PMC free article] [PubMed] [Google Scholar]
- 20.Lockman S, Hughes MD, McIntyre J, Zheng Y, Chipato T, Conradie F, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Updated guidelines for prevention of parent to child transmission (PPTCT) of HIV using multi drug anti-retroviral regimen in India. New Delhi, India: Department of AIDS Control Basic Services Division, Ministry of Health & Female Welfare, Government of India; 2013. National AIDS Control Organization. [Google Scholar]
- 22.Sluis-Cremer N. The emerging profile of cross-resistance among the nonnucleoside HIV-1 reverse transcriptase inhibitors. Viruses. 2014;6:2960–73. doi: 10.3390/v6082960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach June 2013. Switzerland, Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 24.National guidelines on second-line and alternative first-line ART for adults and adolescents – May 2013. New Delhi, India: Department of AIDS Control, Ministry of Health & Family Welfare, Government of India; 2013. National AIDS Control Organization. [Google Scholar]
- 25.Saravanan S, Madhavan V, Balakrishnan P, Smith DM, Solomon SS, Sivamalar S, et al. Darunavir is a good third-line antiretroviral agent for HIV type 1-infected patients failing second-line protease inhibitor-based regimens in South India. AIDS Res Hum Retroviruses. 2013;29:630–2. doi: 10.1089/aid.2011.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]


