Abstract
Introduction:
The literature is lacking information on the anatomy and the osseous dimensions of the thoracic intervertebral foramen (IVF). We describe the anatomy of the broader area, and we proceed with morphometric data of the vertebrae and the foramina. Depiction of these features is provided with imaging and illustrations. The purpose of this paper is to survey and present the anatomy of the foramen as a whole and provide baseline statistical data.
Materials and Methods:
We review relevant literature, and we present data obtained from skeletal samples of known population and sex. One hundred and nineteen thoracic vertebrae of ten cadaveric spines from the prefecture of Eastern Macedonia and Thrace, Greece, were selected. Statistical analysis measuring the vertical height and the foraminal width of each vertebra was made in accordance with sex.
Results:
No statistically important differences referring to the descriptive data of both sexes were found. However, statistically, important positive correlation between the vertebral height and the foraminal width was observed, especially for men. The components of the foramen including arteries and veins passing through or neighboring it, and the spinal nerves and roots are described and depicted.
Conclusions:
The osseous thoracic IVF reveals a glimpse of the in vivo structure and alterations of its width may be present in back pain and other degenerative diseases. Although it is crucial for surgeries and other interventional procedures of the thoracic spine, little is known about the precise anatomy and dimensions of this anatomical landmark.
Keywords: Cadaveric study, clinical anatomy, intervertebral foramen, review, thoracic
Introduction
The thoracic spine is the second segment of the vertebral column, located between the cervical and the lumbar vertebral segments, and it is the part of the vertebral column where protection and function of the thoracic viscera take precedence over segmental spinal mobility. The anteroposterior diameter of the vertebral bodies gradually increases from T1 to T12, whereas the transverse width decreases from T1 to T3 and then increases progressively down to T12. Normally, the vertical height of the thoracic vertebral bodies is about 2–3 mm less anteriorly than posteriorly, which partially contributes to thoracic kyphosis [Figure 1].[1] The thoracic spine is unique in regard to the complexity of its formation [Figure 2].[2]
Figure 1.
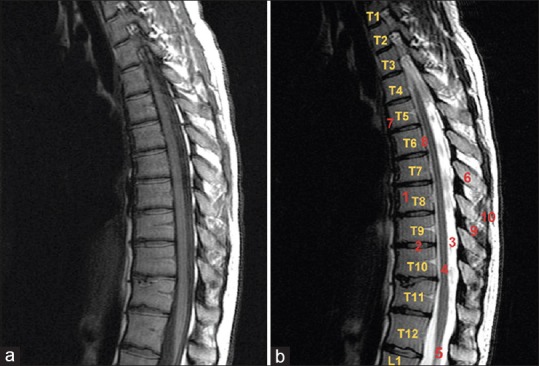
(a) Normal appearance of the thoracic spinal canal. Midline sagittal T1-weighted image; (b) midline sagittal T2-weighted fast spin echo image. 1: Vertebra, 2: normal intervertebral disk, 3: spinal canal, 5: spinal cord, 6: spinous process, 7: anterior longitudinal ligament, 8: posterior longitudinal ligament, 9: interspinous ligament, 10: supraspinous ligament
Figure 2.
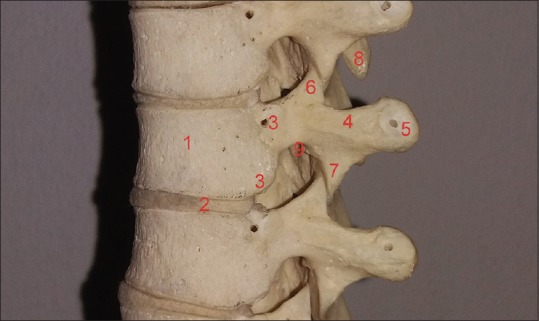
Cadaveric sample of the thoracic spine. 1: Vertebral body, 2: intervertebral disk, 3: superior and inferior costal facets, 4: transverse process, 5: transverse costal facet, 6: superior articular process, 7: inferior articular process, 8: spinous process, 9: inferior vertebral notch. (The small cavities appearing on the vertebrae are used to restraint them in the formation of the thoracic spine)
A significant entity of the vertebral column is the intervertebral foramen (IVF). This foramen is special due to its boundaries consisting of two movable joints: Ventral intervertebral joint and dorsal zygapophysial joint.[3] The proximity of these joints increases susceptibility of narrowing from arthritic structural alterations, thoracic disk herniations, and trauma of the thoracic spine.[1,4] This foramen has one anterosuperior part (formed by the inferior part of the pedicle and the posteroinferior part of the vertebral body) and another inferior and mobile part (formed by the posterior articular lamina covered by the yellow ligament, in the back, and by the posterolateral aspect of the intervertebral disk in front). This second articular part is the one that undergoes motion-related or degenerative changes, especially with regard to the lumbar spine.[5,6] In particular, the boundaries of the thoracic intervertebral foramina are formed anteriorly: From below upward, from a small posterolateral part of the body of the inferior vertebra, the posterolateral aspect of the adjacent vertebral bodies with the intervertebral disc included, and the posterolateral aspect of the body of the superior vertebra; superiorly: From the deep arch of the inferior vertebral notch of the superior vertebra; posteriorly: From below upward, from the superior articular process of the inferior vertebra, a part of the ventral aspect of the fibrous capsule of the facet synovial joint, and the inferior articular process of the superior vertebra; inferiorly: From the shallow arch of the superior vertebral notch of the inferior vertebra [Figure 2].[2,3]
Although thoracic spine anatomy is familiar to spinal surgeons, surprisingly little is known about the precise anatomy of the thoracic IVF as an anatomic entity. Furthermore, only a few studies have described the anatomy of the cervical and lumbar IVF including recent reviews of the literature.[7,8,9] We can also observe a lack of schematic depiction of the foraminal components and their anatomical relationships. As a result, to elucidate the clinical significance of the thoracic IVF, we study this foramen as a whole including spinal nerve roots, vessels, osseous, and ligamentous structures, and we further proceed to their schematic illustration. We also measure the vertical height of cadaveric vertebrae and the transverse length of the inferior vertebral notch of each vertebra referring to the size of the thoracic IVF.
Materials and Methods
We accompany our review of the literature with a statistical analysis of our cadaveric specimens referring to the correlation between the height of the vertebrae and the width of the thoracic IVFs in accordance with sex. The ethics of our study are in accordance with the Greek legislation about ethical standards. The vertical vertebral height and the width of the thoracic IVF of 120 well-preserved vertebrae were measured. Ten thoracic spines were studied, 6 men and 4 women. Specimens are from Northern Greece and especially from the prefecture of Eastern Macedonia and Thrace. Those vertebrae were collected from adult cadavers of different age and both sexes without known record of spine-related pain. No gross morphological alterations or malformations due to spinal disease were visible. Only one vertebra was excluded due to a defect in the posterior surface of the vertebral body.
As mentioned before, osteometric measurements of the vertical height of the vertebra and the foraminal width (transverse linear diameter of the inferior vertebral notch) were estimated. With regard to the first one, we measured the vertical height of each vertebra on either the right or left side and only a few millimeters anteriorly to the superior and inferior costal facets and not further because it is known that the vertical height of the thoracic vertebral bodies is about 2–3 mm less anteriorly than posteriorly and it is correlated with abnormal conditions of the spine.[1] To measure the foraminal width, we estimated the transverse linear diameter of the inferior vertebral notch. We used the distance between the middle of the red lines as seen in Figure 3. In particular, the dome of the inferior vertebral notch continues to two almost parallel osseous linear borders posteriorly and anteriorly. Those are superior to the inferior costal facets and the inferior articular facets. They are not easily affected by arthritic lesions, and this part of the foramen is not covered by the head of the rib in regard to the in vivo structure which forms a “false” foramen [Figure 4]. As a result, they tend to depict a more solid measurement. We decided not to take into consideration the vertebral levels since the adjacent ligaments were absent.
Figure 3.
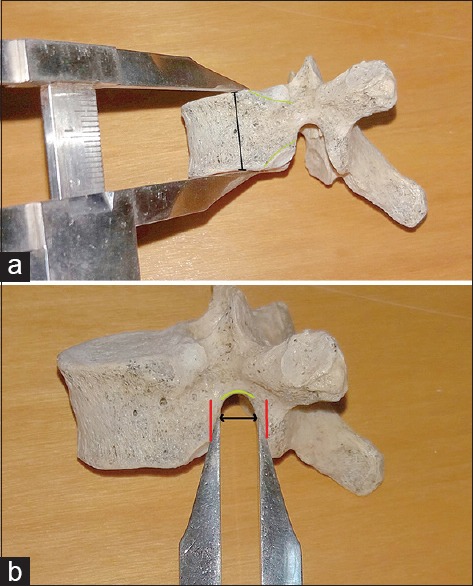
(a) Measurement of the height of the vertebral body and (b) of the foraminal width
Figure 4.
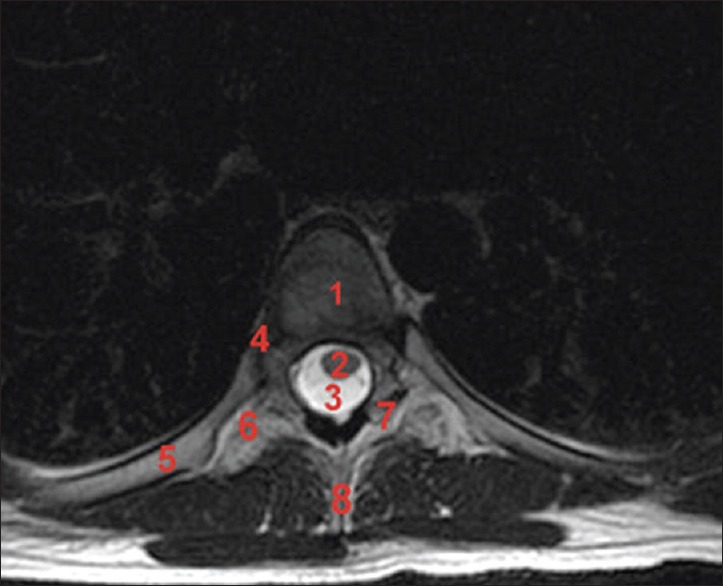
Axial T2-weighted fast spin echo image. 1: Vertebral body, 2: thoracic spinal cord, 3: cerebrospinal fluid, 4: head of the rib, 5: rib, 6: transverse process, 7: facet joints, 8: spinous process
The measurements were performed twice with a sliding caliper to the nearest 0.1 cm. If the two measurements showed a difference of more than 1 mm, a third measurement was taken and the average of all was used. The statistical analysis of this study was performed with the statistical package SPSS, version 17.00 (SPSS Inc., Chicago, IL, USA). Values of continuous variables are presented using number of participants (N), mean value, standard deviation (SD), median, and interquartile range. The correlation between the variables was studied with the Pearson and Spearman correlation coefficient.
Results
Data analysis
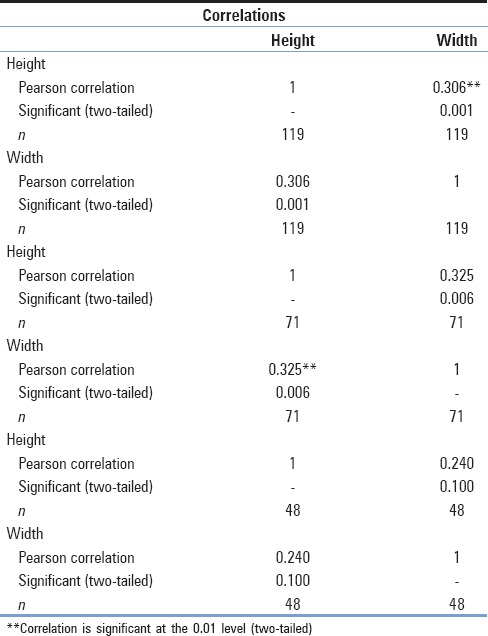
Overall, 119 vertebrae were measured. Of this sample, 71 were collected from male cadavers and 48 from female cadavers. The mean vertebral height and the foraminal width, respectively, were 1.94 and 0.84 cm along with SD of 0.21 cm for the first and 0.13 cm for the latter [Table 1]. No differences existed between the two sexes. Using the t-test, we concluded that for the parameter of vertebral height, the P value was estimated at 0.373 and that for the other foraminal width, the P value was estimated at 0.213 [Table 2]. However, statistically, important positive correlation between the vertebral height and the foraminal width was observed (r = 0.306, P = 0.001), which was stronger in men (r = 0.325, P = 0.006) than in women (r = 0.240, P = 0.100) [Table 3 and Graph 1].
Table 1.
Descriptive data of the sample
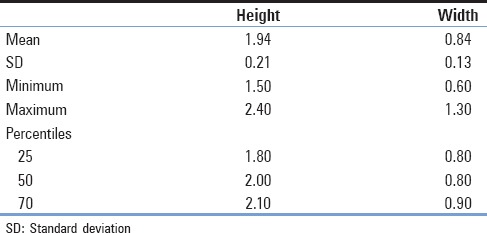
Table 2.
Sample statistics in accordance with sex and t-test for the two parameters
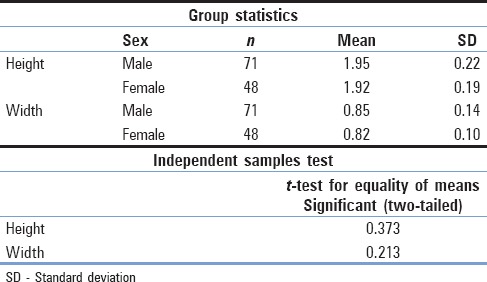
Table 3.
Correlation between the vertebral height and the foraminal width
Graph 1.
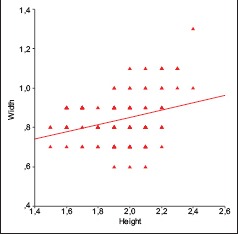
Graphical depiction of the correlation between vertebral height and foraminal width
Components of the thoracic intervertebral foramen
A thoracic IVF contains the spinal nerve root with its sheaths, two to six sinuvertebral nerves, spinal arteries (variable numbers), plexiform connections between the internal and external venous plexuses, numerous small lymphatic vessels, and fatty areolar network which fills the foramen [Figure 5].[10,11]
Figure 5.
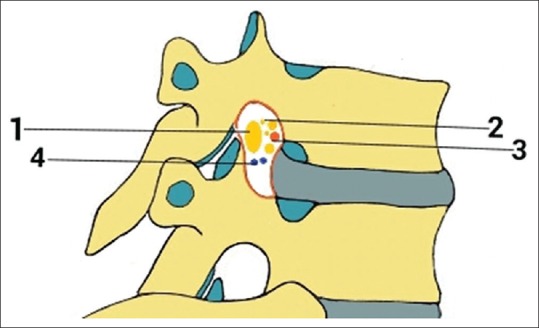
A view illustrating some of the components of the thoracic IVF including - 1: segmental spinal nerve/dorsal root ganglion, 2: sinuvertebral nerves and rami communicantes, 3: spinal branch of segmental arteries, 4: intervertebral veins
Spinal nerves and roots
They form by fusion of a posterior sensory spinal root with an anterior motor root. These join at each IVF. Typically, the nerve then divides into a posterior and an anterior primary ramus. The former supplies the vertebral muscles and dorsal skin. The anterior primary ramus in the thoracic region bears a white ramus communicans to the sympathetic ganglion. The nerves T2–T12 supply the skin and muscles of the trunk sequentially.[12]
The diameter of thoracic spinal nerve roots is much smaller than their respective foramina. As a result, they incompletely fill the foramina. The spinal roots course through the superior portion of the neural foramina, immediately inferior to the upper pedicle.[10,13,14] The trajectory of the thoracic nerve roots at the intervertebral foramina varies according to the thoracic level, with upper thoracic roots projecting upward, middle thoracic roots positioned in a horizontal plane, and lower thoracic roots projecting downward.[10]
The number of dorsal rootlets that emerge to give rise to a dorsal root varies at each spinal segment.[6,15,16] Kirazli et al. found that the average number of dorsal thoracic rootlets is 6.6 ± 0.8, which is higher than what Sindou found.[17,18] They counted 4–5 dorsal thoracic rootlets easily separated and identifiable. The interval between the thoracic nerve rootlets at each level is increased compared to the cervical and lumbosacral levels.[19] Bozkurt et al. found an average of 6.8 T1 segment rootlets in their study which corresponded with the results of three other separate studies.[15,18,19,20] In addition, they found that T1 segment contained the largest number of thoracic nerve rootlets, in contrast to the T6, T7, and T10 segments which contained the fewest.[19] Kirazli et al. found that the T1, T3, T4, T11, and T12 levels contained the largest number of dorsal thoracic rootlets while their results about the fewest correlate well with the observations of Bozkurt et al.[18,19] As the T1 spinal nerve contributes to form the brachial plexus, it can be explained why it has a high number of rootlets. Similarly, the high number of dorsal thoracic rootlets of the T11 and T12 levels is justified because of their contribution to the lumbar plexus. However, these results about T3 and T4 segments cannot be explained.[18]
A segmental spinal nerve is formed as ventral and dorsal rootlets within the subarachnoid space converge at the area of the IVF. The rootlets are surrounded by two layers of pia mater and the pia ends medial to the arachnoid while the arachnoid ends medial to the dura mater. As they advance toward the IVF, their pial and arachnoidal layers fuse with the dura mater of the thecal sac. The dorsal and ventral roots mostly exit through separate ostia in the dura. Each one of them has a separate dural sheath outside of the thecal sac which is surrounded loosely by a thin connective tissue sheath. This connective tissue sheath also surrounds the dorsal root ganglia.[21]
Sinuvertebral nerve
The sinuvertebral nerve, also known as the nerve of von Luschka, meningeal or recurrent meningeal nerve, originates from the anterior aspect of the spinal nerve distal to the dorsal root ganglion and receives some sympathetic branches from one or two rami communicantes close to its origin. An IVF can contain two to six sinuvertebral nerves of varying size. The sinuvertebral nerve courses through the IVF and reenters the spinal canal. As it enters the canal, the nerve has a variable course that it anastomoses with the sinuvertebral nerves of the other side and nearby segments. It forms a dense plexus over the posterior longitudinal ligament and a more irregular plexus over the anterior longitudinal ligament supplying both ligaments. It also supplies dura mater, blood vessels, annulus fibrosus, and periosteum of the vertebral body. In the thoracic level, it gives a branch that crosses the upper border of the neck of the nearby rib supplying the periosteum of the neck.[22,23]
Spinal arteries
The segmental arteries provide blood supply to the corresponding metamere. Two sequential segmental arteries supply each vertebra on each side. The spinal branch of the segmental artery, at the IVF, traverses medially and inserts into the vertebral canal via the IVF. It divides into three branches: An anterior and a posterior artery of the vertebral canal that provides blood to the bony spinal column and a radicular artery that supplies the dura and nerve root at every segmental level. Some accompany the ventral nerve root to supply the anterior surface of the spinal cord while some others accompany the dorsal nerve root and supply the posterolateral aspect of the spinal cord.[14] It is important to mention the presence of the artery of Adamkiewicz, also known as arteria radicularis magna, which is the largest anterior segmental medullary artery and arises from a left posterior intercostal artery at the level of the 9th–12th intercostal artery supplying the lower two-thirds of the spinal cord via the anterior spinal artery.[2]
Intervertebral veins
The internal vertebral venous plexus (anterior and posterior) is a network of veins lying within the spinal canal outside of the dura mater. They travel through the epidural space and are mainly supplied by radicular veins.[24,25] The external vertebral venous plexuses (anterior and posterior) surround the vertebral column and communicate through the internal venous plexuses with the intervertebral veins.
The basivertebral veins are intravertebral veins run horizontally from the anterior aspect to the posterior part of the body of the vertebra, where they connect with anterior internal vertebral venous system. Anteriorly, they anastomose with the anterior external vertebral venous plexus. They also unite the inferior and superior vena cava through connections with the azygous venous system and the lumbar veins.[25]
Internal and external vertebral venous plexuses communicate through the intervertebral veins.[24,25,26,27] Intraosseous spinal venography has also confirmed it.[28,29] The anterior internal vertebral venous plexuses are the most developed among the internal vertebral venous plexuses, comprising on each side: A lateral and a medial longitudinal venous network.[24,26] Transverse plexuses connect the internal vertebral plexuses in front and behind the dural sheath. Therefore, an epidural venous ring is formed. Its part lying behind the body of the vertebra is more developed as it receives the anastomosis of the basivertebral veins.[24]
Transforaminal ligaments
Various foraminal ligaments may be present in the thoracic intervertebral foramina, closely related to the exiting nerve root. The superior corporopedicular ligament extends from the superior pedicle traversing obliquely, anteriorly, and inferiorly to the posterolateral vertebral body and related annulus fibrosus; the inferior corporopedicular ligament extends from the inferior pedicle traversing obliquely, anteriorly, and superiorly to the posterolateral vertebral body and related annulus fibrosus; the superior transforaminal ligament extends from the arches of the superior and inferior vertebral notches to the articular capsule of the superior pedicle; the mid-transforaminal ligament runs from the annulus fibrosus and superior and inferior corpopedicular ligaments to the articular capsule; the inferior transforaminal ligament extends from the junction of the annulus fibrosus and the posterior vertebral body to the superior articular facet [Figure 6].[4,10]
Figure 6.
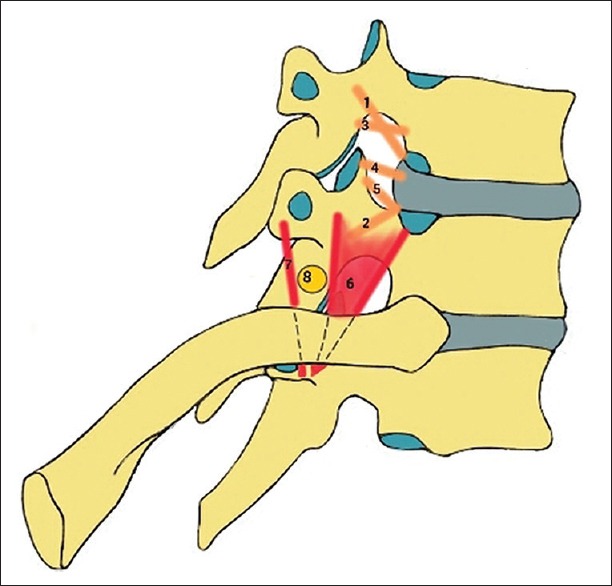
Schematic illustration depicting transforaminal and extraforaminal ligaments: corporopedicular 1: superior and 2: inferior ligament; 3: Superior transforaminal ligament; 4: mid-transforaminal ligament; 5: inferior transforaminal ligament; costotransverse 6: superior and 7: inferior ligament; 8: spinal nerve located in the extraforaminal space
Extraforaminal ligament attachments of the thoracic spinal nerves
A number of ligaments attach the thoracic spinal nerves to spinal structures at the extraforaminal region. They are considered to provide protection against traction and compression, as well as optimal positioning of the nerve. From the second to ninth thoracic levels, the extraforaminal ligament attachments consist of two parts: A superior and an inferior one. The superior part is identified as the superior costotransverse ligament and runs from the costovertebral joint and the superior transverse process to the inferior transverse process. This ligament is ventrally attached to the spinal nerves. On the other side, the inferior ligament extends from the superior to the inferior transverse process attaching the nerve dorsally. At the 10th thoracic level, the inferior part may be missing, and at the 11th level, the spinal nerve is dorsally attached to the internal intercostals membrane and caudally to the capsule of the costovertebral joint and the IVF.[30]
Shape of the thoracic intervertebral foramen
The thoracic IVFs face laterally with the transverse processes behind them. In addition, the articulations of the head of a rib with the costal demifacets and the capsules of double synovial joints contribute to forming the anteroinferior boundaries of the first to tenth thoracic foramina [Figure 4].[10,11] However, it is referred that the head of a rib can create a false foramen as it seems to form the caudal border of the foramen, obscuring the superior vertebral notch of the inferior vertebra. To provide visibility to the true IVF, the rib head and its facet must be removed.[10,31]
The shape of the foramina is variable: Oval (26.6%), auricular (58%), or teardrop (17.4%), and they face laterally. Laterally, they are covered by a fascial sheet, which is part of the anterior layer of the thoracolumbar fascia. Medially, there is the dural sleeve with its emerging nerve root. Usually, there are two separate oval perforations in the fascia, one for the nerve root and a smaller one for the intervertebral blood vessels.[10]
Discussion
Unlike previous studies, the results of our study indicate that there are no statistically important differences between men and women. In our study, statistically important positive correlation between the vertebral height and the foraminal width is observed, especially for men. The measures of central tendency show that our two parameters present strict measurements. A lack of statistic measurements referring to the thoracic IVF is being observed, and the majority of information about the osseous IVF size refers to the lumbar foramen. It is important to mention that there is no other Greek study known in the literature that has made osteometric assessments of vertebrae. As far as correlation between individual age and osseous IVF size is concerned, the data of clinical reports are ambiguous. For example, Rühli et al. showed that there is a lack of correlation between these parameters while Humphreys et al. showed that exists as age-related alterations.[9,32] In addition, it is considered that individual stature has a significant role in spinal morphology and length. Again, Rühli et al. found that there is no significant correlation between IVF width and vertebral body height or sagittal diameter, respectively, unlike our findings. No correlation was found in their study between IVF width and individual stature or femur robusticity. Since age-related alterations affecting the bony structures of the foramen have been reported, it could be assumed that they would influence the IVF width.[9] This is contrary to some reports on side-dependence of the spinal morphologic values.[33,34] However, others reports suggest that there is side-independence of the spinal dimensions.[16,35,36] In terms of sexual dimorphism, Rühli et al. found that IVF width is greater in females than males. It should be noted that females have considerably smaller femur measurements than males.[5] Previous reports on the sex-dependent size of spinal structures that enclose neural elements are in accordance with this.[37,38,39,40,41]
The existing anatomic reviews have not described the anatomy of the foramen thoroughly. The anatomy of the thoracic IVF is complex; nevertheless, it is a significant entity among spine surgeons. Symptomatic thoracic disc herniations are rare and account for less than 1% of all protruded discs. They occur most commonly between the third and fifth decades of life. The most common level is T11/T12, with 75% of all thoracic disc prolapses occurring below T8. A majority of conditions, including degenerative disk diseases, herniated discs, scoliosis, compression fractures, kyphosis, spondylolisthesis, bone spurs-tumors, or other diseases, can be treated either with mini-thoracotomy, fusion, laminectomy or by placing lateral mass screws. This surgical access can be done by partial excision of the facet joint or pedicle, an area adjacent to the thoracic IVF.[42] Recent advances and successful outcomes have made minimally invasive techniques such as the endoscopic transforaminal thoracic foraminotomy and discectomy a safe and effective treatment option.[43] Surgeons and interventional radiologists have to fully understand the three-dimensional anatomy of the thoracic IVF to conduct an optimal procedure.
Conclusion
The thoracic IVF continues to be a poorly defined and depicted region of the spinal canal and more osteometric measurements are needed to be made. The osteometric assessment of the thoracic IVF is just an approximate estimation of its in vivo size, which depends on soft tissue and dynamic components. These measurements provide just a glimpse of the age-related alterations of its neural content. However, the ratio of the two parameters could have a clinical role on computed tomography and magnetic resonance imaging as a quick assessment and may be a prognostic factor of spinal diseases relating to the thoracic IVF concerning spine surgeries and other related interventional procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.el-Khoury GY, Whitten CG. Trauma to the upper thoracic spine: Anatomy, biomechanics, and unique imaging features. AJR Am J Roentgenol. 1993;160:95–102. doi: 10.2214/ajr.160.1.8416656. [DOI] [PubMed] [Google Scholar]
- 2.Moore KL, Dalley AF. Clinically Oriented Anatomy. 5th ed. Baltimore: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 3.Drake R, Vogl W, Mitchell AV. Gray's Anatomy for Medical Students. 2nd ed. New York: Churchill Livingstone; 2009. [Google Scholar]
- 4.Gilchrist RV, Slipman CW, Bhagia SM. Anatomy of the intervertebral foramen. Pain Physician. 2002;5:372–8. [PubMed] [Google Scholar]
- 5.Rosse C, Gaddum Rosse P. The vertebral canal, spinal cord, spinal nerves, and segmental innervation. In: Rosse C, Gaddum-Rosse P, editors. Hollinshead's Textbook of Anatomy. 5th ed. Ch. 12-13. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 6.Wong DA, Transfeldt E. Musculoskeletal anatomy, neuroanatomy, and biomechanics of the lumbar spine. In: Wong DA, Transfeldt E, editors. Macnab's Backache. 4th ed. Ch. 1. Philadelphia: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 7.Gkasdaris G, Kapetanakis S. Clinical anatomy and significance of the lumbar intervertebral foramen: A review. J Anat Soc India. 2015;64:166–73. [Google Scholar]
- 8.Sioutas G, Kapetanakis S. Clinical anatomy and clinical significance of the cervical intervertebral foramen: A review. Folia Morphol (Warsz) 2016;75:143–8. doi: 10.5603/FM.a2015.0096. [Doi: 10.5603/FM.a2015.0096] [DOI] [PubMed] [Google Scholar]
- 9.Rühli FJ, Müntener M, Henneberg M. Human osseous intervertebral foramen width. Am J Phys Anthropol. 2006;129:177–88. doi: 10.1002/ajpa.20263. [DOI] [PubMed] [Google Scholar]
- 10.Dickman CA, Rosenthal DJ. Thoracoscopic Spine Surgery. New York: Thieme; 1999. [Google Scholar]
- 11.Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. 40th ed. London: Elsevier; 2008. [Google Scholar]
- 12.Harold E. Anatomy of the spinal nerves and dermatomes. Anaesth Intensive Care Med. 2009;10:536–7. [Google Scholar]
- 13.Ebraheim NA, Jabaly G, Xu R, Yeasting RA. Anatomic relations of the thoracic pedicle to the adjacent neural structures. Spine (Phila Pa 1976) 1997;22:1553–6. doi: 10.1097/00007632-199707150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Naidich TP, MR . Imaging of the Spine. Philadelphia: Elsevier; 2011. [Google Scholar]
- 15.Kubo Y, Waga S, Kojima T, Matsubara T, Kuga Y, Nakagawa Y. Microsurgical anatomy of the lower cervical spine and cord. Neurosurgery. 1994;34:895–90. doi: 10.1227/00006123-199405000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Zhou MW, Wang WT, Huang HS, Zhu GY, Chen YP, Zhou CM. Microsurgical anatomy of lumbosacral nerve rootlets for highly selective rhizotomy in chronic spinal cord injury. Anat Rec (Hoboken) 2010;293:2123–8. doi: 10.1002/ar.21213. [DOI] [PubMed] [Google Scholar]
- 17.Sindou M. Dorsal root entry lesions. In: Burchiel K, editor. Surgical Management of Pain. New York: Thieme Medical Publishers; 2002. pp. 701–3. [Google Scholar]
- 18.Kirazli O, Tatarli N, Güçlü B, Ceylan D, Ziyal I, Keles E, et al. Anatomy of the spinal dorsal root entry zone: Its clinical significance. Acta Neurochir (Wien) 2014;156:2351–8. doi: 10.1007/s00701-014-2252-0. [DOI] [PubMed] [Google Scholar]
- 19.Bozkurt M, Canbay S, Neves GF, Aktüre E, Fidan E, Salamat MS, et al. Microsurgical anatomy of the dorsal thoracic rootlets and dorsal root entry zones. Acta Neurochir (Wien) 2012;154:1235–9. doi: 10.1007/s00701-012-1395-0. [DOI] [PubMed] [Google Scholar]
- 20.Xiang JP, Liu XL, Xu YB, Wang JY, Hu J. Microsurgical anatomy of dorsal root entry zone of brachial plexus. Microsurgery. 2008;28:17–20. doi: 10.1002/micr.20438. [DOI] [PubMed] [Google Scholar]
- 21.Tubbs RS, Lobashevsky A, Oakes P, D’Antoni AV, Hattab E, Topp K, et al. Meningeal relationships to the spinal nerves and rootlets: A gross, histological, and radiological study with application to intradural extramedullary spinal tumors. Childs Nerv Syst. 2015;31:675–81. doi: 10.1007/s00381-015-2648-z. [DOI] [PubMed] [Google Scholar]
- 22.Middleditch A, Oliver J. Functional Anatomy of the Spine. Oxford: Elsevier; 2005. [Google Scholar]
- 23.Ombregt L. A System of Orthopaedic Medicine. Edinburgh: Elsevier; 2013. [Google Scholar]
- 24.Demondion X, Lefebvre G, Fisch O, Vandenbussche L, Cepparo J, Balbi V. Radiographic anatomy of the intervertebral cervical and lumbar foramina (vessels and variants) Diagn Interv Imaging. 2012;93:690–7. doi: 10.1016/j.diii.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Groen RJ, du Toit DF, Phillips FM, Hoogland PV, Kuizenga K, Coppes MH, et al. Anatomical and pathological considerations in percutaneous vertebroplasty and kyphoplasty: A reappraisal of the vertebral venous system. Spine (Phila Pa 1976) 2004;29:1465–71. doi: 10.1097/01.brs.0000128758.64381.75. [DOI] [PubMed] [Google Scholar]
- 26.Chaynes P, Verdié JC, Moscovici J, Zadeh J, Vaysse P, Becue J. Microsurgical anatomy of the internal vertebral venous plexuses. Surg Radiol Anat. 1998;20:47–51. doi: 10.1007/BF01628115. [DOI] [PubMed] [Google Scholar]
- 27.Pearce JM. The craniospinal venous system. Eur Neurol. 2006;56:136–8. doi: 10.1159/000095706. [DOI] [PubMed] [Google Scholar]
- 28.Clemens HJ. The nervous systems of the human spinal column; Morphology and functional significance. Berlin: De Gruyter; 1961. [Google Scholar]
- 29.Vogelsang H. Intraosseous Spinal Venography. Amsterdam: Excerpta Medica; 1970. [Google Scholar]
- 30.Kraan GA, Hoogland PV, Wuisman PI. Extraforaminal ligament attachments of the thoracic spinal nerves in humans. Eur Spine J. 2009;18:490–8. doi: 10.1007/s00586-009-0881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abuzayed B, Tuna Y, Gazioglu N. Thoracoscopic anatomy and approaches of the anterior thoracic spine: Cadaver study. Surg Radiol Anat. 2012;34:539–49. doi: 10.1007/s00276-012-0949-4. [DOI] [PubMed] [Google Scholar]
- 32.Humphreys SC, Hodges SD, Patwardhan A, Eck JC, Covington LA, Sartori M. The natural history of the cervical foramen in symptomatic and asymptomatic individuals aged 20 60 years as measured by magnetic resonance imaging. A descriptive approach. Spine (Phila Pa 1976) 1998;23:2180–4. doi: 10.1097/00007632-199810150-00007. [DOI] [PubMed] [Google Scholar]
- 33.Anderson RJ. Observations on the diameters of human vertebrae in different regions. J Anat Physiol. 1883;17(Pt 3):341–4. [PMC free article] [PubMed] [Google Scholar]
- 34.Marchesi D, Schneider E, Glauser P, Aebi M. Morphometric analysis of the thoracolumbar and lumbar pedicles, anatomo-radiologic study. Surg Radiol Anat. 1988;10:317–22. doi: 10.1007/BF02107905. [DOI] [PubMed] [Google Scholar]
- 35.Banta CJ, 2nd, King AG, Dabezies EJ, Liljeberg RL. Measurement of effective pedicle diameter in the human spine. Orthopedics. 1989;12:939–42. doi: 10.3928/0147-7447-19890701-06. [DOI] [PubMed] [Google Scholar]
- 36.Kothe R, O’Holleran JD, Liu W, Panjabi MM. Internal architecture of the thoracic pedicle. An anatomic study. Spine (Phila Pa 1976) 1996;21:264–70. doi: 10.1097/00007632-199602010-00002. [DOI] [PubMed] [Google Scholar]
- 37.Eisenstein S. The morphometry and pathological anatomy of the lumbar spine in South African negroes and caucasoids with specific reference to spinal stenosis. J Bone Joint Surg Br. 1977;59:173–80. doi: 10.1302/0301-620X.59B2.873978. [DOI] [PubMed] [Google Scholar]
- 38.Hasue M, Kikuchi S, Sakuyama Y, Ito T. Anatomic study of the interrelation between lumbosacral nerve roots and their surrounding tissues. Spine (Phila Pa 1976) 1983;8:50–8. doi: 10.1097/00007632-198301000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Porter RW, Hibbert C, Wellman P. Backache and the lumbar spinal canal. Spine. 1980;5:99–105. doi: 10.1097/00007632-198003000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Porter RW, Wicks M, Ottewell D. Measurement of the spinal canal by diagnostic ultrasound. J Bone Joint Surg Br. 1978;60(B):481–4. doi: 10.1302/0301-620X.60B4.711793. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi S, Hasue M, Nishiyama K, Ito T. Anatomic and clinical studies of radicular symptoms. Spine (Phila Pa 1976) 1984;9:23–30. doi: 10.1097/00007632-198401000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Patel N. Surgical disorders of the thoracic and lumbar spine: A guide for neurologists. J Neurol Neurosurg Psychiatry. 2002;73(Suppl 1):i42–8. doi: 10.1136/jnnp.73.suppl_1.i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nie HF, Liu KX. Endoscopic transforaminal thoracic foraminotomy and discectomy for the treatment of thoracic disc herniation. Minim Invasive Surg 2013. 2013 doi: 10.1155/2013/264105. 264105. [DOI] [PMC free article] [PubMed] [Google Scholar]


