Abstract
Context:
Low back pain (LBP) is a complex and disabling condition, and its treatment becomes a challenge.
Aims:
The aim of our study was to assess the clinical outcome of plasma rich in growth factors (PRGF-Endoret) infiltrations (one intradiscal, one intra-articular facet, and one transforaminal epidural injection) under fluoroscopic guidance-control in patients with chronic LBP. PRGF-Endoret which has been shown to be an efficient treatment to reduce joint pain.
Settings and Design:
The study was designed as an observational retrospective pilot study. Eighty-six patients with a history of chronic LBP and degenerative disease of the lumbar spine who met inclusion and exclusion criteria were recruited between December 2010 and January 2012.
Subjects and Methods:
One intradiscal, one intra-articular facet, and one transforaminal epidural injection of PRGF-Endoret under fluoroscopic guidance-control were carried out in 86 patients with chronic LBP in the operating theater setting.
Statistical Analysis Used:
Descriptive statistics were performed using absolute and relative frequency distributions for qualitative variables and mean values and standard deviations for quantitative variables. The nonparametric Friedman statistical test was used to determine the possible differences between baseline and different follow-up time points on pain reduction after treatment.
Results:
Pain assessment was determined using a visual analog scale (VAS) at the first visit before (baseline) and after the procedure at 1, 3, and 6 months. The pain reduction after the PRGF-Endoret injections showed a statistically significant drop from 8.4 ± 1.1 before the treatment to 4 ± 2.6, 1.7 ± 2.3, and 0.8 ± 1.7 at 1, 3, and 6 months after the treatment, respectively, with respect to all the time evaluations (P < 0.0001) except for the pain reduction between the 3rd and 6th month whose signification was lower (P < 0.05). The analysis of the VAS over time showed that at the end point of the study (6 months), 91% of patients showed an excellent score, 8.1% showed a moderate improvement, and 1.2% were in the inefficient score.
Conclusions:
Fluoroscopy-guided infiltrations of intervertebral discs and facet joints with PRGF in patients with chronic LBP resulted in significant pain reduction assessed by VAS.
Keywords: Chronic low back pain, fibrin matrix, plasma rich in growth factors, platelet-rich plasma, visual analog scale
Introduction
Low back pain (LBP) is a complex, personal experience encompassing multidimensional phenomena such as physiologic, sensory, affective, cognitive, behavioral, and sociocultural effects.[1] This endemic condition leads to disability with current estimates of 632 million people affected worldwide.[2] Although in most patients LBP is self-limiting, there is a subset of patients whose symptoms will reemerge labeling the condition as nonspecific, chronic LBP lasting longer than 3 months of evolution.[3] The pain is primarily related to mechanical factors which mediate in muscular strain, intervertebral disc degeneration (IVDD), facet-mediated pain, and radiculopathy though the precise mechanisms remain to be elucidated. Two fundamental spinal structures specialized in both shock-absorbing and load-bearing are facet joints and intervertebral discs (IVD) which in response to mechanical stress, inflammatory synovitis, and degenerative arthritis undergo chronic degenerative process which leads to chronic LBP.[4]
A remarkable clinical motivation for reducing pain arises from the need to return patients to their daily routines and improve their life quality. The appropriate treatment of chronic LBP remains a daunting challenge despite advances in the management of pain and inflammation by pharmacological and surgical procedures. Before a surgical procedure is performed, the patient should be offered, an incrementally progressive treatment plan, beginning with a minimally invasive interventional therapy[5] for symptomatic disc herniation and degeneration, and lumbar facet joint arthrosis.
An innovative biologically inspired approach to tissue repair is the application of plasma rich in growth factors (PRGF-Endoret),[6] which has been shown to be an efficient treatment to attenuate knee and hip pain of patients with osteoarthritis (OA) and to improve their clinical condition by reducing joint pain.[7,8]
In light of prior promising results in basic science[9,10] in preclinical, and in osteoarthritic patients whose chronic pain was significantly reduced,[7,11,12,13,14] we suggested four synergetic effects of PRGF-Endoret on synovial joint diseases, namely, a chondroprotective, anti-inflammatory, cell-phenotype modulation, and joint pain attenuation which make PRGF a potential candidate for treatment of vertebral facet joints and IVDD.[15]
The aim of our study was to assess the clinical outcome after one intradiscal, one intra-articular facet, and one transforaminal epidural injection of PRGF-Endoret under fluoroscopic guidance-control carried out in the operating theater setting.
Subjects and Methods
Study design
The study was designed as an observational retrospective pilot study. Eighty-six patients with a history of chronic LBP and degenerative disease of the lumbar spine who met inclusion and exclusion criteria were recruited between December 2010 and January 2012. Inclusion criteria were patients with LBP and symptoms of lumbago and/or sciatica ongoing for at least 3 months; nonresponse to initial pain management with oral nonsteroidal anti-inflammatory drugs (NSAIDs) and/or myorelaxant drugs; patients with failed back surgery syndrome; and age over 18 years. Patients were excluded from the study in case of acute illness, alcoholism, patients with ongoing labor conflict, or patients with mental illness or psychological conditions related to pain.
A preliminary clinical examination was carried out to characterize and diagnose the LBP, which was confirmed by magnetic resonance imaging or computerized tomography, and to assess the back pain as well. Before receiving the PRGF treatment and at the follow-up visits, patients back pain intensity was assessed using the visual analog scale (VAS).
This study was carried out in accordance with “World medical association Declaration of Helsinki” in its latest revised version (Seoul, 2008). The study protocol was reviewed and approved by the reference ethics committee. All patients provided written informed consent before entry into the study.
Plasma rich in growth factors preparation
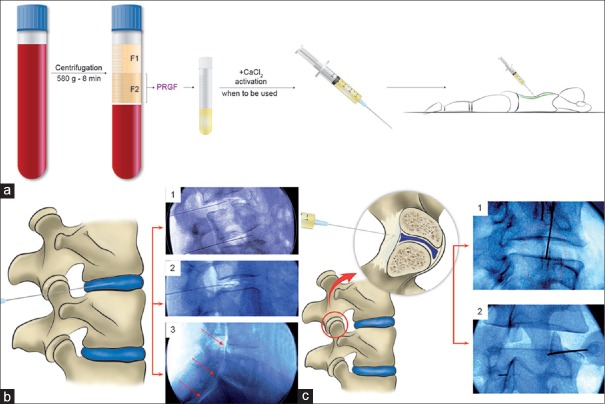
The patients were advised not to eat fatty food in the 6 h before the blood extraction. Twenty minutes before to the induction of sedation with 2.5 mg midazolam in addition to atropine intravenously, 20–36 mL of peripheral venous blood was withdrawn from the patient and collected in 9-mL tubes (TB9) containing sodium citrate (3.8% weight/vol). This blood then was centrifuged (PRGF system IV, BTI-Biotechnology Institute, Vitoria, Álava, Spain) at 580 g for 8 min at room temperature. The 2-mL plasma fraction (F2) located just above the sedimented red blood cells was collected (Endoret Traumatology Kit, BTI-Biotechnology Institute, Vitoria, Álava, Spain) in another tube but without aspirating the buffy coat [Figure 1a]. This plasma fraction (F2) presents a moderate enrichment in platelets (2-fold the platelet count of peripheral blood) with scarce leukocytes.[16] The upper volume of plasma containing a similar number of platelets as peripheral blood (F1) was collected in another tube. A few minutes before the percutaneous infiltration, the PRGF fraction to be applied was activated by adding 20 μL of PRGF activator (10% weight/vol calcium chloride) per milliliter of PRGF [Figure 1a].
Figure 1.
PRGF preparation and infiltration of disc and lumbar facet joint. (a) Illustrative representation of PRGF obtaining process. Before the percutaneous infiltration, the fraction 2 of PRGF was activated with PRGF activator. (b) Illustration and fluoroscopic-guided lumbar intervertebral disc (insert 1 and 2) images and peridural infiltration (insert 3). (c) Drawing and fluoroscopic-guided lumbar facet joint infiltrations of PRGF showing an intradiscal (insert 1) and intra-articular (insert 2) position of the needle tip
Plasma rich in growth factors injection procedure
All treatments were performed by the same experienced orthopedic surgeon. Patients were placed in either prone or lateral position depending on patient circumstances. Using sterile technique, disc, intra-articular facet, and peridural percutaneous PRGF infiltrations were performed under X-ray fluoroscopy with a horizontal C-arch (BV Libra, Philips, Eindhoven, The Netherlands). Briefly, the percutaneous disc infiltration approach was used at an oblique angle between 30° and 45° along the lateral margin of the inferior articular process of the vertebra [Figure 1b, inserts 1 and 2] and through the neuroforamen while preserving the nerve root. The position of the needle, which had a bent-shaped end (Quincke Chiba Needle, 22-gauge: 8 inch), was guided and confirmed under anteroposterior and lateral fluoroscopic view. Once the needle end was located at the degenerated or herniated disc and checked by infiltrating a little bit of activated PRGF under fluoroscopy, 4 mL of activated PRGF was injected into the nucleus pulposus and the surrounding dehydrated areas while paying careful attention not to allocate more than this PRGF quantity. When we removed the needle from the disc, using the same technical procedure, we performed the peridural infiltration injecting 2 mL of activated PRGF [Figure 1b, insert 3]. We carried out the spinal facet joints infiltration using the same procedure [Figure 1c, inserts 1 and 2]. While the patient lay prone, the joints to be injected were located and marked. After cleaning and draping, a 22-gauge spinal needle was inserted until it contacted the capsule of the joint, and then with careful and refined movements, the needle tip entered the joint. Once the needle position was confirmed under fluoroscopy, 0.5 mL of activated PRGF was injected into each of the facet joints.
The patient was observed for 1 h after the procedure to monitor for any undesirable reaction. All patients were asked to avoid strenuous activities in the ensuing 3 days. Patients receive antibiotic therapy during 1 week. Additional medical therapy or physiotherapy and the intake of any type of NSAID or painkilling medication were considered an exclusion criterion.
Evaluation and follow-up
Pain assessment was determined using a VAS, with a line whose ends are labeled as the extremes: Score of 0 denoting “no pain” and score of 10 denoting “pain as bad as it could be.”[1,17] The VAS score was evaluated at the first visit before (Baseline) and after the procedure at 1, 3, and 6 months. Reduction in VAS score was considered the outcome of the interventional therapy with PRGF.
Pain relief over time was categorized as excellent (0–3 score in VAS scale), moderate (VAS 3.1–6.5), and ineffective (VAS 6.6–10).[5]
The safety data were also collected. All adverse major and minor events were assessed and documented at each visit.
Statistical analysis
Descriptive statistics were performed using absolute and relative frequency distributions for qualitative variables and mean values and standard deviations for quantitative variables. Different normality tests (Kolmogorov–Smirnov and Shapiro–Wilk) were performed on each variable sample. The nonparametric Friedman statistical test was used to determine the possible differences between baseline and different follow-up time points on pain reduction after treatment. Statistical significance level was set on P < 0.05. SPSS version 15.0 for Windows statistical software package (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis.
Results
Study subjects
A total of 86 patients, 47 females (median age 58; age range, 22–81 years) and 39 males (median age 55; age range, 29–79 years) were treated and evaluated.
Clinical outcome after plasma rich in growth factors infiltration
The postprocedure pain reduction assessed by VAS showed a statistically significant drop from 8.4 ± 1.1 before the treatment to 4.0 ± 2.6, 1.7 ± 2.3, and 0.8 ± 1.7 at 1, 3, and 6 months after the treatment, respectively, with respect to all the time evaluations (P < 0.0001) except for the pain reduction between the 3rd and 6th months whose significance was lower (P < 0.05) [Figure 2]. Over time, the percentage of pain reduction was gradual until reaching 90% at the end of follow-up (6th month).
Figure 2.
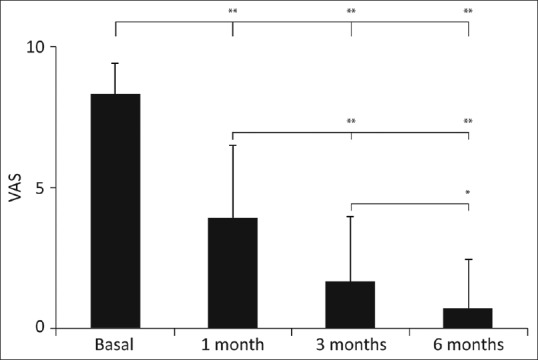
Graphic representation of the postprocedural pain reduction assessed by visual analog scale showing a statistically significant pain reduction from basal visual analog scale to first, third, and sixth months after the treatment, respectively, with respect to all the time evaluation (**P < 0.0001) except for the visual analog scale between the 3rd and 6th month whose signification was lower (*P < 0.05)
The analysis of the VAS over time showed that after the 1st month, 38.4% of patients had excellent (VAS 0–3) pain relief, 44.2% responded moderately (VAS 3.1–6.5), whereas the treatment had been ineffective in 17.4% (VAS 6.6–10). At 3rd month, 79.1% of patients had excellent pain relief, 14% had moderate relief, and for 7% had the pain relief was ineffective. At the end point of the study (6 months), 90.7% of patients showed an excellent score, 8.1% showed a moderate VAS score, and 1.2% of patients were included in the ineffective score [Figure 3].
Figure 3.
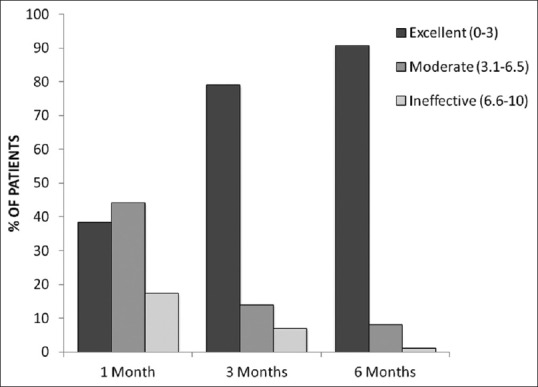
Analysis of the visual analog scale. At the end point of study (after 6 months), 90.7% of patients revealed an excellent score (visual analog scale 0–3), 8.1% had a moderate score (visual analog scale 3.1–6.5), and 1.2% of patients showed an ineffective score (visual analog scale 6.6–10)
Although the analysis of VAS throughout the follow-up revealed a significant and sustained improvement of clinical condition, the largest pain reduction was attained in the 1st month after the procedure (53% over a total of 90%).
Safety data
There were no major adverse events. None of the patients showed serious adverse effects after the treatment or during the ensuing days, apart from headache (five patients), soreness, or local skin bruising (seven patients).
Discussion
The results of the present study showed that a minimally invasive therapy consisting of PRGF-Endoret infiltrations of IVD and facet joints in patients with chronic LBP resulted in a significant pain reduction assessed by VAS. At the end of follow-up (6th month), 90.7% of patients showed a pain value of 0–3 in the VAS score, which is considered “excellent” in the literature.[5] The pain reduction of 90% at the end of 6th month is consistent with the values published by Sánchez et al.[7] for the treatment of OA of the hip with PRGF infiltration, and comparable to the values reported by Becker et al.[18] using epidural perineural injections of autologous conditioned serum on patients with lumbar radicular compression, and substantially better than the pain relief shown by infiltrating methylprednisolone in the lumbar facet joints,[19] triamcinolone in epidural perineural injections,[18] or betamethasone in periganglionar infiltration[5] in patients with LBP. Nevertheless, the course of treatment reported by Chatuverdi el al.[19] reached a pick of 93% of responders at 4th week of treatment and then declined to 62.5% at 3rd month of treatment, an outcome which is no consistent with our results.
Although the relative contribution of various structures in chronic LBP is varied, the pain source commonly is attributed to inflammatory response of facet joints and/or disc degeneration and herniation cells due to mechanical stressful stimuli.[19] Facet joints and IVD undergo chronic degenerative process in response to nonphysiological mechanical stresses, bringing about collagens and aggrecans cleavage, a decrease of collagen and proteoglycan synthesis, an increase of proteases and cell death which parallels to articular cartilage degeneration in OA and whose clinical hallmark is joint pain.[20]
There are several potential mechanisms by which PRGF-Endoret infiltrations might either suppress or slow down the progress of IVD and facet degeneration. PRGF-Endoret is an autologous product that conveys a mimetic biomaterial, namely, fibrin, which is embedded with a pool of growth factors (FGF, platelet-derived growth factor [PDGF], insulin-like growth factor-1 [IGF-1], vascular endothelial growth factor, transforming growth factor-β [TGF-β], hepatocyte growth factor [HGF], connective tissue growth factor [CTGF], and nerve growth factor) stemmed from activated platelets and plasma and acting as a biological scaffold from which a sustained and a gradual delivery of growth factors is released at the dysfunctional and degenerate sites instead of as a bolus delivery modality. Furthermore, a proteomic characterization study of PRGF fibrin matrix[21] reported a significant representation of acute-phase proteins, a strong network of interconnected proteins linked to the NF-kB pathway. This pathway is in part responsible for the inflammatory response of stressed cells such as chondrocytes, tenocytes, fibroblasts, and macrophages. In addition, both IVD and facet joints share many developmental, functional, and biological features with articular cartilage and synovial joints.[22]
In vitro, several growth factors present in platelet-rich plasma (PRP) such as TGF-β, IGF-1, and CTGF have been shown to exert a powerful effect on extracellular matrix (ECM) synthesis and proliferation in IVD.[23,24] Furthermore, PDGF and IGF1 have been shown to exert a cell survival action on IVD cells.[25] In addition, PRP through TGF-β or as a PRP whole product has been shown to promote the synthesis of ECM components such as proteoglycans and collagen in human nucleus pulposus cell cultures.[22,26] More importantly, PRP administration using biodegradable gelatin hydrogel microspheres into degenerated IVD animal model resulted in a preservation of water and disc height, suppression of the IVD degeneration progression, and a synthesis of proteoclycans 8 weeks after the treatment, highlighting the importance of both delivering growth factors in a sustained and gradual manner and the effectiveness for early IVD degeneration intervention.[27,28] Similar regenerative effects have been reported applying PRP on degenerative disc disease in animal models.[29]
Inflammation is a term that encompasses clinical, physiological, cellular, and molecular phenomena, with pain being the hallmark or the tip of iceberg underlying pro-inflammatory cytokine release, ECM catabolism, and cell death. Thus, pain and inflammation are flip sides of the same coin, namely, tissue damage. Data coming from animal studies strongly suggest that pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 are pivotal for the onset and maintenance of pain mainly stemmed from the damaged peripheral tissues.[30] In contrast, anti-inflammatory cytokines such as IL-4 and IL-10 have analgesic properties.[30] Some components present in PRGF-Endoret (HGF, LXA4, PF4, IGF-1, PDGF, and TGFβ)[10,31,32,33,34] inhibit the NF-kB signaling pathway in several cell lineages including macrophages, chondrocytes, and fibroblasts. NF-kB plays an important role in mediating the gene expression of pro-inflammatory cytokines such as TNFα, IL-1β, PGE2, and COX-1 and COX-2.[35]
As a consequence, it is reasonable to consider that PRGF-Endoret could exert an antiapoptotic, ECM-protective, anti-inflammatory, and pain reduction effect in a similar manner as in knee and hip synovial joints.[13,14,36] In macrophages, the inhibition of the NF-kB might contribute to the polarization from M1 to M2 phenotype, thereby favoring the resolution of inflammation and generating a switch in the ECM from a pro-inflammatory and algesic milieu to an anti-inflammatory and analgesic context. Furthermore, the in situ generate fibrin matrix would be gradually removed as a result of local activation of the tissue plasminogen activator/plasminogen system. This fibrinolytic remodeling process overlaps with the homing of survival fibrochondrocytes and migratory mesenchymal stem cell, which might had been attracted by chemoattractants such as SDF-1, HGF, IGF-1, TGFβ, or bFGF sequestered within the fibrin matrix and gradually released during the fibrinolytic remodeling process.[37]
This study has several drawbacks. The absence of a control (placebo) group in this study is certainly a limitation. There are other weaknesses in our study as to we did not perform a previous diagnostic block for patients’ selection, and therefore our diagnosis and selection of patients relied on a careful clinical examination. Another drawback to this study is the lack of measurement of physical activity levels before and after the treatment. Last but not the least, to limit the bias of a single assessment, the self-reported VAS pain scale should have been associated with other health survey questionnaires which encompass pain and functional evaluation. In the light of several limitations of our research, a randomized controlled clinical trial is considered imperative.
Conclusions
Taking into account these limitations and in the light of our preliminary data, we suggest that fluoroscopy-guided infiltrations of IVD and facet joints with plasma rich in growth factors could be an alternative therapy in patients with chronic low back. To the best of our knowledge, the present article is the first description of a PRP facet joint infiltration as a complementary approach to IVD infiltration in the LBP treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
EA is the scientific director at BTI - Biotechnology Institute, the company that has developed the PRGF-Endoret Technology. EA is the president of the Eduardo Anitua Foundation for Biomedical Research, Vitoria, Spain.
References
- 1.Mannion AF, Balagué F, Pellisé F, Cedraschi C. Pain measurement in patients with low back pain. Nat Clin Pract Rheumatol. 2007;3:610–8. doi: 10.1038/ncprheum0646. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Tulder M, Koes B. In: Chronic Low Back Pain, in Evidence-Based Chronic Pain Management. Stannard CF, Kalso E, Ballantyne J, editors. West Sussex, UK: John Wiley & Sons, Ltd; 2010. [Google Scholar]
- 4.Hwang SY, Lee JW, Lee GY, Kang HS. Lumbar facet joint injection: Feasibility as an alternative method in high-risk patients. Eur Radiol. 2013;23:3153–60. doi: 10.1007/s00330-013-2921-z. [DOI] [PubMed] [Google Scholar]
- 5.Oder B, Loewe M, Reisegger M, Lang W, Ilias W, Thurnher SA. CT-guided ozone/steroid therapy for the treatment of degenerative spinal disease – Effect of age, gender, disc pathology and multi-segmental changes. Neuroradiology. 2008;50:777–85. doi: 10.1007/s00234-008-0398-2. [DOI] [PubMed] [Google Scholar]
- 6.Anitua E, Sánchez M, Orive G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev. 2010;62:741–52. doi: 10.1016/j.addr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez M, Guadilla J, Fiz N, Andia I. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford) 2012;51:144–50. doi: 10.1093/rheumatology/ker303. [DOI] [PubMed] [Google Scholar]
- 8.Anitua E, Sánchez M, Aguirre JJ, Prado R, Padilla S, Orive G. Efficacy and safety of plasma rich in growth factors intra-articular infiltrations in the treatment of knee osteoarthritis. Arthroscopy. 2014;30:1006–17. doi: 10.1016/j.arthro.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Anitua E, Sanchez M, De la Fuente M, Zalduendo MM, Orive G. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg Sports Traumatol Arthrosc. 2012;20:1657–65. doi: 10.1007/s00167-011-1697-4. [DOI] [PubMed] [Google Scholar]
- 10.Bendinelli P, Matteucci E, Dogliotti G, Corsi MM, Banfi G, Maroni P, et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: Mechanisms of NF-κB inhibition via HGF? J Cell Physiol. 2010;225:757–66. doi: 10.1002/jcp.22274. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: A retrospective cohort study. Clin Exp Rheumatol. 2008;26:910–3. [PubMed] [Google Scholar]
- 12.Wang-Saegusa A, Cugat R, Ares O, Seijas R, Cuscó X, Garcia-Balletbó M. Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch Orthop Trauma Surg. 2011;131:311–7. doi: 10.1007/s00402-010-1167-3. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez M, Fiz N, Azofra J, Usabiaga J, Aduriz Recalde E, Garcia Gutierrez A, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28:1070–8. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Vaquerizo V, Plasencia MÁ, Arribas I, Seijas R, Padilla S, Orive G, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: A randomized controlled trial. Arthroscopy. 2013;29:1635–43. doi: 10.1016/j.arthro.2013.07.264. [DOI] [PubMed] [Google Scholar]
- 15.Wang SZ, Rui YF, Tan Q, Wang C. Enhancing intervertebral disc repair and regeneration through biology: Platelet-rich plasma as an alternative strategy. Arthritis Res Ther. 2013;15:220. doi: 10.1186/ar4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anitua E, Prado R, Sánchez M, Orive G. Platelet-rich plasma: Preparation and formulation. Oper Tech Orthop. 2012;22:25–32. [Google Scholar]
- 17.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: Intrasubject variability and correlation with a numeric scale. Anesth Analg. 1998;86:102–6. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Becker C, Heidersdorf S, Drewlo S, de Rodriguez SZ, Krämer J, Willburger RE. Efficacy of epidural perineural injections with autologous conditioned serum for lumbar radicular compression: An investigator-initiated, prospective, double-blind, reference-controlled study. Spine (Phila Pa 1976) 2007;32:1803–8. doi: 10.1097/BRS.0b013e3181076514. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi A, Chaturvedi S, Sivasankar R. Image-guided lumbar facet joint infiltration in nonradicular low back pain. Indian J Radiol Imaging. 2009;19:29–34. doi: 10.4103/0971-3026.44522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: The facet joints. Nat Rev Rheumatol. 2013;9:216–24. doi: 10.1038/nrrheum.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anitua E, Prado R, Azkargorta M, Rodriguez-Suárez E, Iloro I, Casado-Vela J, et al. High-throughput proteomic characterization of plasma rich in growth factors (PRGF-Endoret)-derived fibrin clot interactome. J Tissue Eng Regen Med. 2015;9:E1–12. doi: 10.1002/term.1721. [DOI] [PubMed] [Google Scholar]
- 22.Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet rich plasma. J Cell Physiol. 2006;209:744–54. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 23.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: Three dimensional culture in agarose or alginate and responsiveness to TGF beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 24.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007;16:1858–66. doi: 10.1007/s00586-007-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruber HE, Norton HJ, Hanley EN., Jr Anti apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2000;25:2153–7. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Akeda K, An HS, Pichika R, Attawia M, Thonar EJ, Lenz ME, et al. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine (Phila Pa 1976) 2006;31:959–66. doi: 10.1097/01.brs.0000214942.78119.24. [DOI] [PubMed] [Google Scholar]
- 27.Nagae M, Ikeda T, Mikami Y, Hase H, Ozawa H, Matsuda K, et al. Intervertebral disc regeneration using platelet rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13:147–58. doi: 10.1089/ten.2006.0042. [DOI] [PubMed] [Google Scholar]
- 28.Sawamura K, Ikeda T, Nagae M, Okamoto S, Mikami Y, Hase H, et al. Characterization of in vivo effects of platelet rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng Part A. 2009;15:3719–27. doi: 10.1089/ten.TEA.2008.0697. [DOI] [PubMed] [Google Scholar]
- 29.Obata S, Akeda K, Imanishi T, Masuda K, Bae W, Morimoto R, et al. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: A preclinical study. Arthritis Res Ther. 2012;14:R241. doi: 10.1186/ar4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uçeyler N, Schäfers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67–78. doi: 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- 31.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–9. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 32.Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, et al. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: Involvement of Src/PI 3K/AKT pathway? PLoS One. 2011;6:e28663. doi: 10.1371/journal.pone.0028663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coudriet GM, He J, Trucco M, Mars WM, Piganelli JD. Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: Implications for inflammatory mediated diseases. PLoS One. 2010;5:e15384. doi: 10.1371/journal.pone.0015384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Buul GM, Koevoet WL, Kops N, Bos PK, Verhaar JA, Weinans H, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39:2362–70. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 35.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Anitua E, Sánchez M, Orive G, Padilla S. A biological therapy to osteoarthritis treatment using platelet rich plasma. Expert Opin Biol Ther. 2013;13:1161–72. doi: 10.1517/14712598.2013.801450. [DOI] [PubMed] [Google Scholar]
- 37.Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–71. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]