Abstract
Background
Heart Failure (HF) is an important and growing public health problem in women. Risk factors for incident hospitalized HF with preserved (HFpEF) compared to reduced ejection fraction (HFrEF) in women, and differences by race/ethnicity, are not well characterized.
Methods and Results
We prospectively evaluated the risk factors for incident hospitalized HFpEF and HFrEF in a multi-racial cohort of 42,170 post-menopausal women followed for a mean of 13.2 years. Cox regression models with time dependent covariate adjustment were used to define risk factors for HFpEF and HFrEF. Differences by race/ethnicity regarding incidence rates, baseline risk factors and their population attributable risk percentage (PAR%) were analyzed. Risk factors for both HFpEF and HFrEF were as follows: older age, Caucasian race, diabetes, cigarette smoking, and hypertension. Obesity, history of coronary heart disease (other than myocardial infarction (MI)), anemia, atrial fibrillation and more than one co-morbidity, were associated with HFpEF but not HFrEF. History of MI was associated with HFrEF but not HFpEF. Obesity was found to be a more potent risk factor for African American women compared with Caucasian women for HFpEF (p for interaction= 0.007). For HFpEF, the PAR% was greatest for hypertension (40.9%) followed by obesity (25.8%), with the highest PAR% found in African Americans for these risk factors.
Conclusions
In this multi-racial cohort of postmenopausal women, obesity stands out as a significant risk factor for HFpEF, with the strongest association in African American women.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00000611.
Keywords: post-menopausal woman, preserved ejection fraction heart failure, reduced ejection heart failure, race, ethnicity
Heart failure (HF) is a major and growing public health problem in the United States (U.S.) that accounts for over one million hospital admissions per year and affects close to 6 million Americans.1 Of patients with incident HF in epidemiological studies, 40% to 71% have HF with preserved, rather than reduced, ejection fraction (HFpEF versus HFrEF) and HFpEF is more common in women.1–7 HFpEF is increasing in prevalence1 and as opposed to HFrEF, limited effective therapy are presently available. In order to guide future therapeutic considerations, there is a need to better understand the risk factors and natural history of HFpEF.
The epidemiology of HFpEF has largely been studied in Caucasian cohorts.2, 4–7 Some existing studies have examined incident HF in multi-ethnic cohorts but without ejection fraction data or have studied survival of those with prevalent HFpEF.8–11 As such, there is an important need to evaluate risk factors for incident HFpEF and HFrEF, especially in women who are understudied.
The Women’s Health Initiative (WHI) recently re-adjudicated HF in a sub-cohort of women that differentiates acute from chronic HF and allows for the evaluation of incident hospitalized HFpEF and HFrEF.12,13 We therefore sought to identify risk factors for HFpEF and HFrEF in women in this sub-cohort and to better understand the role of race/ethnicity in explaining any differences in HFpEF and its risk factors. For those HFpEF and HFrEF risk factors that were amenable to prevention, we assessed their population attributable risk percentage (PAR%) to estimate the amount of HFpEF and HFrEF that could be theoretically reduced if these risk factors were eliminated.
Methods
Study Population
The WHI recruited women nationwide in 40 clinical centers between 1993 and 1998. Details of the recruitment, baseline questionnaires and examinations performed have been published previously. 14–16 Briefly, study participants were women 50 to 79 years of age at baseline who had no terminal illness and were eligible for either the clinical trials or observational arm, completed baseline assessments, including several self-administered questionnaires of socio-demographic characteristics, medical history, reproductive and menstrual history, health behavior and family history of selected diseases. Of the 161,808, postmenopausal women in the original cohort, a sub-cohort of 44,174 women, were evaluated for hospitalized HF from baseline through January 13, 2015. To allow for evaluation of racial and ethnic differences with adequate statistical power, we excluded those whose self-reported race was Asian/Pacific islander, Native American or unknown race/ethnicity (n=1042). To evaluate a disease free cohort, we excluded 505 women with self-reported HF at baseline and 444 who had chronic HF upon their first adjudication. The final analytic cohort was 42,170 women. This study received Institutional Review Board approval and all participants signed informed consents at all 40 clinical centers.
Outcomes
Hospitalized HF was adjudicated based upon self-report of a hospitalization related to HF or coronary heart disease (CHD) by trained adjudicators.12,13 Details of the adjudication process for acute HF are given in appendix 1. Acute HF with an ejection fraction < 45% was considered HFrEF. Acute HF with an ejection fraction ≥ 45% was considered HFpEF. If no ejection fraction was available it was classified as HFuEF. We performed an additional sensitivity analysis defining HFpEF as an ejection fraction of ≥ 50% and HFrEF as < 50%.
The acute HF classification system utilized in this analysis has been shown to have good agreement with other HF epidemiologic algorithms including: Framingham (69.5%), modified Boston (63.7%), NHANES (60.9%), Gothenburg (59.5%), ICD-9-CM (62.9%) and demonstrate modest kappa coefficients (0.32–0.10).12
Clinical covariates
Race/ethnicity was self-reported as black or African American, Hispanic/Latino, white or Caucasian (not of Hispanic origin), or other.14,15 Clinical covariates include: age, education, income, cigarette smoking, current hormone use, hypertension, diabetes mellitus, atrial fibrillation, coronary heart disease, chronic lung disease, physical activity, medication use, alcohol use, co-morbid conditions, and anemia. Details can be found in appendix 2.
Statistical Methods
Baseline characteristics are reported separately by race/ethnicity, and due to differences in age in the race/ethnicity groups, percentages are adjusted to the five-year age distribution of the HT participants. Annualized event rates were also age-adjusted as above; with 95% confidence intervals computed using a bootstrap method with 5,000 repetitions.
Primary analyses used time-to-event methods based on the Cox regression model. Time is defined as days from randomization in the HT, or from WHI enrollment for non-HT participants to the event or censoring. Censoring is the earliest of HF of a different type, death, end-of-follow-up, or January 13, 2015. All models were stratified by study component (clinical trial or observational study), and age strata used for clinical trial randomization. Cox regression models use the Wald chi-square test to evaluate the effect of each individual variable while simultaneously adjusting for all variables in the model. Tests for interactions are based on likelihood ratio tests. PAR% were calculated using the standard definitions.17
Results
Of the sub-cohort of 42,170 women evaluated for incident hospitalized HF, 51.2% were Caucasian, 33.6% were African American and 15.2%, were Hispanic. At baseline, African American and Hispanic women were younger than Caucasian women; therefore age-adjusted baseline comparisons have been made. (Table 1) African American women had lower incomes, were more likely to be obese, less physically active, and had higher prevalence of hypertension, diabetes, CHD, myocardial infarction (MI), stroke than their Caucasian counterparts. In addition, African American women were more likely to have had a hysterectomy, have anemia, and less likely to be on aspirin. Hispanic women were less educated, had lower incomes, had less health insurance, more likely to have diabetes and have had a hysterectomy and were less likely to be current smokers and to take aspirin than Caucasian women.
Table 1.
Baseline characteristics by Race/Ethnicity in 42,170 participants
| Caucasian (N=21603) | African American (N=14159) | Hispanic (N=6408) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | %1 | N | %1 | N | %1 | |
| Age at Screening (y) | ||||||
| 50–59 | 6388 | 32.5 | 5939 | 32.5 | 3235 | 32.5 |
| 60–69 | 9998 | 45.3 | 6043 | 45.3 | 2491 | 45.3 |
| 70–79 | 5217 | 22.2 | 2177 | 22.2 | 682 | 22.2 |
|
| ||||||
| Education | ||||||
| Less than college degree | 14532 | 67.5 | 9031 | 65.1 | 4977 | 79.1 |
| College degree or higher | 6954 | 32.5 | 4945 | 34.9 | 1316 | 20.9 |
|
| ||||||
| Family Income | ||||||
| <$35,000/year | 10013 | 48.1 | 7083 | 57.3 | 3540 | 65.8 |
| $35,000–$49,999/year | 4425 | 21.6 | 2369 | 17.6 | 926 | 15.6 |
| $50,000–$74,999/year | 3535 | 17.5 | 2211 | 15.8 | 726 | 11.4 |
| ≥ $75,000/year | 2543 | 12.8 | 1392 | 9.3 | 486 | 7.2 |
|
| ||||||
| Body Mass Index (kg/m2) | ||||||
| <25 | 6125 | 28.4 | 2271 | 16.6 | 1577 | 25.6 |
| 25 – <30 | 7634 | 35.4 | 4600 | 33.2 | 2418 | 38.7 |
| 30 – <35 | 4722 | 22.0 | 3771 | 27.0 | 1480 | 23.0 |
| ≥ 35 | 3008 | 14.2 | 3388 | 23.2 | 863 | 12.6 |
|
| ||||||
| History of MI | 429 | 1.9 | 437 | 3.4 | 72 | 1.3 |
|
| ||||||
| History of CHD2 | 827 | 3.7 | 826 | 6.7 | 185 | 3.6 |
|
| ||||||
| Stroke ever | 196 | 0.9 | 347 | 2.7 | 100 | 1.9 |
|
| ||||||
| History of Hypertension (taking meds or BP ≥ 140/90) | 7944 | 38.6 | 8238 | 62.2 | 2118 | 39.2 |
|
| ||||||
| Treated Diabetes (pills or shots) | 924 | 4.2 | 1626 | 12.1 | 445 | 7.5 |
|
| ||||||
| History of Cancer (except NMSC) | 687 | 3.2 | 1112 | 8.3 | 416 | 7.3 |
|
| ||||||
| Current Smoker | 2168 | 10.4 | 1584 | 10.7 | 452 | 6.4 |
|
| ||||||
| Dyslipidemia | 2495 | 12.4 | 2083 | 16.7 | 887 | 16.7 |
|
| ||||||
| Hysterectomy | 7872 | 36.3 | 7839 | 55.1 | 2866 | 45.2 |
|
| ||||||
| Oophorectomy | ||||||
| None | 16312 | 75.9 | 8451 | 60.1 | 4575 | 71.6 |
| Unilateral/partial/unknown number | 2140 | 9.9 | 2478 | 18.2 | 647 | 11.0 |
| Bilateral | 3083 | 14.2 | 3069 | 21.8 | 1094 | 17.4 |
|
| ||||||
| History of Atrial Fibrillation | 718 | 3.3 | 675 | 5.2 | 177 | 3.2 |
|
| ||||||
| History of Chronic Lung Disease | 704 | 3.6 | 517 | 4.1 | 163 | 3.0 |
|
| ||||||
| Anemia (Hgb < 11 gm/dL) | 66 | 0.3 | 315 | 2.4 | 46 | 0.8 |
|
| ||||||
| Co-morbidity (Charlson Index) | ||||||
| 0 | 14012 | 66.9 | 7870 | 56.9 | 3970 | 62.9 |
| 1 | 4103 | 19.4 | 3373 | 25.4 | 1308 | 22.3 |
| 2 | 2192 | 10.3 | 1502 | 11.6 | 578 | 10.5 |
| ≥ 3 | 741 | 3.5 | 773 | 6.1 | 227 | 4.3 |
|
| ||||||
| Diuretics use | 2314 | 10.5 | 3268 | 24.1 | 385 | 6.9 |
|
| ||||||
| Beta Blocker use | 1485 | 6.7 | 1068 | 7.9 | 318 | 5.6 |
|
| ||||||
| Aspirin use (≥80 mg) | 4673 | 21.2 | 1833 | 13.9 | 667 | 11.7 |
|
| ||||||
| Current Hormone Therapy use3 | ||||||
| E-alone | 3908 | 18.0 | 3184 | 21.4 | 1528 | 23.2 |
| E-alone placebo/non-user | 3964 | 18.3 | 4642 | 33.7 | 1332 | 21.9 |
| E+P | 7028 | 32.6 | 1266 | 8.3 | 1188 | 16.7 |
| E+P placebo/non-user | 6703 | 31.1 | 5047 | 36.6 | 2347 | 38.2 |
|
| ||||||
| Any prior Hormone Therapy use | 5716 | 26.3 | 2127 | 15.5 | 881 | 14.3 |
|
| ||||||
| Any Insurance | 19894 | 92.2 | 12646 | 92.7 | 4858 | 83.3 |
|
| ||||||
| Alcohol Intake | ||||||
| Non/past drinker | 5953 | 27.6 | 7029 | 51.6 | 2724 | 45.0 |
| <1 drink/wk | 7268 | 34.1 | 4384 | 30.7 | 2147 | 33.5 |
| 1 – <7 drinks/wk | 5428 | 25.4 | 1935 | 13.5 | 1123 | 16.8 |
| 7+ drinks/wk | 2781 | 12.9 | 604 | 4.2 | 291 | 4.7 |
|
| ||||||
| Total Energy Expenditure/wk from Physical Activity (METhr/wk) | ||||||
| <1.25 | 4383 | 22.5 | 3752 | 27.2 | 1597 | 25.1 |
| 1.25 – <6.25 | 4837 | 24.6 | 3560 | 26.2 | 1532 | 25.5 |
| 6.25 – <15.3 | 5140 | 26.0 | 3286 | 24.2 | 1447 | 24.5 |
| ≥15.35 | 5312 | 26.9 | 3054 | 22.4 | 1468 | 24.9 |
|
| ||||||
| White (N=21603) | Black (N=14159) | Hispanic (N=6408) | ||||
|
| ||||||
| N | Mean (SD)4 | N | Mean (SD)4 | N | Mean (SD)4 | |
|
| ||||||
| Age at Screening | 21603 | 63.4 (6.7) | 14159 | 63.3 (6.7) | 6408 | 63.3 (6.7) |
|
| ||||||
| Heart Rate (bpm) | 21586 | 70.1 (11.8) | 14134 | 70.7 (13.3) | 6397 | 69.0 (11.5) |
Percentages are age adjusted to the 5 year age distribution of the Hormone Trial participants.
CHD includes MI, CABG/PCI and angina requiring medication.
Current use is randomization arm for the HT Trial participants, or current E-alone use reported at baseline for non-HT participants with a hysterectomy, or current E+P use reported at baseline for non-HT participants without a hysterectomy.
Mean (SD) are age adjusted to the 5 year age distribution of the Hormone Trial participants.
Of the 42,170 women, followed for a mean of 13.2 years, there were 1952 cases of acute incident hospitalized HF. Of these 1952 cases, 70.7% had troponin measures, 38.9% had BNP and 6.1% had NT Pro-BNP assessed. Ejection fraction was determined at the time of HF hospitalization in 1419 cases (73%). Of these, 85% were determined by transthoracic echocardiogram, 1% by radionucleotide ventriculogram, 11% by angiography, 1% by stress echo, and 2% by transesophageal echocardiogram.
Of the 1952 cases of acute incident hospitalized HF, 902 (46.2%) met the definition of HFpEF, 508 (26.0%) were HFrEF, 533 (27.3%) were of unknown ejection fraction and 9 cases initially had a reduced ejection fraction which improved to normal. Annualized incidence rates were 0.35% for incident hospitalized HF, 0.16% for HFpEF and 0.09% for HFrEF with higher incident rates for HFpEF, compared to HFrEF for all race/ethnicity groups. Caucasian women were more likely to develop both HFpEF and HFrEF compared to African American and Hispanic women, with age-adjusted annualized incidence (%) and 95% CI of 0.20 (0.18, 0.21) for HFpEF and 0.10 (0.09,0.11) for HFrEF for Caucasian women, 0.0.15 (0.13,0.17) for HFpEF, 0.10(0.08,0.12) for HFrEF for African American women and 0.08 (0.06,0.010) for HFpEF, and 0.05 (0.03,0.07) for HFrEF for Hispanic women.
Risk factors for HFpEF compared to HFrEF
We examined risk factors for hospitalized incident HFpEF and HFrEF (Table 2). Compared to Caucasian women Hispanic women had a lower risk for both HFpEF and HFrEF in fully adjusted models, while African American women had a lower risk of HFpEF. Risk factors for both incident hospitalized HFpEF and HFrEF were older age, hypertension, diabetes at baseline and interim diabetes, current smoking, and interim MI, CHD and cancer. Anemia had a similar increased magnitude of risk for HFpEF and HFrEF but didn’t reach statistical significance for HFrEF. Risk factors of incident hospitalized HFpEF, but not HFrEF, were obesity, history of CHD other than MI, more than one co-morbidity, and hysterectomy with partial oophorectomy but not bilateral oophorectomy and atrial fibrillation. Risk factors for HFrEF and not HFpEF were history of MI and elevated heart rate was of borderline significance.
TABLE 2.
Risk Factors for HFpEF and HFrEF Overall and Stratified by Race/Ethnicity
| Risk Factor | HFpEF HR (95% CI)1 Total |
HFrEF HR (95% CI) Total |
HFpEF HR (95% CI) Caucasian |
HFrEF HR (95% CI) Caucasian |
HFpEF HR (95% CI) African American |
HFrEF HR (95% CI) African American |
HFpEF HR (95% CI) Hispanic |
HFrEF HR (95% CI) Hispanic |
|---|---|---|---|---|---|---|---|---|
| Age (ref=50–59) | **** | *** | **** | *** | ** | |||
| 60–69 | 2.46 (1.95, 3.10) | 1.48 (1.11, 1.97) | 2.82 (2.05, 3.87) | 1.97 (1.30, 2.97) | 2.03 (1.40, 2.94) | 1.16 (0.75, 1.81) | 2.74 (1.25, 6.04) | 0.93 (0.34, 2.57) |
| 70–69 | 5.22 (4.05, 6.73) | 2.76 (2.01, 3.79) | 6.24 (4.49, 8.67) | 3.80 (2.48, 5.84) | 4.03 (2.61, 6.21) | 1.74 (1.01, 3.01) | 2.46 (0.75, 8.02) | 2.08 (0.62, 6.98) |
| Race (ref=White) | **** | * | ||||||
| Black | 0.59 (0.47, 0.75) | 0.77 (0.57, 1.04 | ||||||
| Hispanic | 0.47 (0.32, 0.69) | 0.54 (0.33, 0.90) | ||||||
| Income (ref=$50–$75K) | * | * | * | * | ||||
| < $35K/year | 1.26 (0.99, 1.60) | 1.79 (1.23, 2.61) | 1.10 (0.84, 1.45) | 1.94 (1.20, 3.14) * | 1.88 (1.08, 3.28)* | 1.53 (0.80, 2.90) | 1.74 (0.47, 6.48) | 2.73 (0.26, 29.10) |
| $35–<$50K/year | 0.96 (0.73, 1.27) | 1.59 (1.05, 2.39) | 0.91 (0.67, 1.25) | 2.01 (1.20, 3.34) | 1.26 (0.66, 2.41) | 0.88 (0.40, 1.93) | 0.87 (0.18, 4.11) | 1.64 (0.11, 24.37) |
| ≥$75K/year | 0.77 (0.53, 1.13) | 1.53 (.94, 2.51) | 0.61 (0.38, 0.96) | 1.73 (0.93, 3.23) | 1.77 (0.86, 3.66) | 1.23 (0.52, 2.92) | NA | 2.07 (0.09, 49.09) |
| College education (ref=<college degree) | 0.93 (0.78, 1.12) | 0.92 (0.72, 1.18) | 1.01 (0.82, 1.25) | 0.90 (0.67, 1.22) | 0.74 (0.50, 1.08) | 1.09 (0.69, 1.72) | 1.22 (0.49, 3.00) | 0.88 (0.16, 4.77) |
| Hypertension (ref=No) | 1.57 (1.33, 1.86)**** | 1.99 (1.59, 2.51)**** | 1.57 (1.30, 1.90)**** | 2.07 (1.58, 2.71)**** | 1.80 (1.22, 2.67)** | 1.60 (1.01, 2.54)* | 1.22 (0.57, 2.60) | 4.24 (1.25, 14.32)* |
| Heart Rate per 5 bpm | 1.00 (0.97, 1.03) | 1.04 (1.00, 1.08)* | 0.99 (0.95, 1.03) | 1.05 (1.00, 1.09)* | 1.02 (0.97, 1.08) | 1.05 (0.99, 1.11) | 1.09 (0.97, 1.29) | 0.81 (0.58, 1.13) |
| Hx MI (ref=No) | 1.08 (0.74, 1.57) | 2.50 (1.60, 3.90)**** | 1.05 (0.66, 1.67) | 3.37 (1.91, 5.94)**** | 0.97 (0.49, 1.93) | 1.95 (0.90, 4.24) | 4.51 (0.40, 50.41) | 4.12 (0.14, 125.57) |
| Hx CHD other than MI (ref=No) | 1.60 (1.17, 2.19)** | 1.22 (0.79, 1.87) | 1.57 (1.07, 2.30)* | 0.93 (0.52, 1.65) | 1.95 (1.11, 3.41)* | 1.63 (0.80, 3.34) | 0.50 (0.04, 5.66) | 3.19 (0.44, 23.02) |
| Hx Stroke (ref=No) | 1.35 (0.87, 2.10) | 1.25 (0.68, 2.29) | 1.55 (0.86, 2.77) | 1.02 (0.41, 2.57) | 1.31 (0.64, 2.69) | 1.44 (0.61, 3.43) | NA | NA |
| DM (ref=No) | 1.84 (1.41, 2.39)**** | 2.16 (1.49, 3.14)**** | 1.76 (1.26, 2.47)** | 1.74 (1.06, 2.86)* | 2.30 (1.44, 3.68)*** | 2.44 (1.28, 4.64)** | 0.12 (0.01, 1.26) | 25.43 (2.54, 254.18)** |
| Dyslipidemia (ref=No) | 0.91 (0.74, 1.11) | 1.09 (0.84, 1.42) | 0.91 (0.71, 1.17) | 1.11 (0.80, 1.54) | 0.89 (0.59, 1.34) | 0.97 (0.58, 1.62) | 1.81 (0.76, 4.28) | 0.87 (0.24, 3.18) |
| Oophorectomy (ref=None) | * | * | ||||||
| Unilateral/partial/ unknown number | 1.40 (1.11, 1.77 | 0.80 (0.57, 1.13) | 1.43 (1.08, 1.91) | 0.85 (0.55, 1.32) | 1.26 (0.82, 1.95) | 0.79 (0.45, 1.39) | 1.86 (0.64, 5.38) | NA |
| Bilateral | 1.15 (0.91, 1.46) | 0.85 (0.62, 1.16) | 1.23 (0.92, 1.65) | 1.03 (0.69, 1.53) | 0.99 (0.63, 1.55) | 0.62 (0.35, 1.12) | 0.91 (0.31, 2.66) | 0.63 (0.14, 2.80) |
| Hx Cancer (ref=No) | 1.20 (0.82, 1.74) | 1.45 (0.87, 2.42) | 1.48 (0.93, 2.36) | 1.75 (0.88, 3.47) | 0.84 (0.40, 1.74) | 0.82 (0.33, 2.06) | 0.48 (0.08, 3.02) | 31.18 (1.64, 593.30)* |
| Co-morbidity (ref=None) | ||||||||
| 1 or more | 1.34 (1.10, 1.63)** | 1.20 (0.92, 1.58) | 1.30 (1.02, 1.65) | 1.23 (0.89, 1.72) | 1.67 (1.11, 2.52)* | 1.21 (0.70, 2.07) | 0.45 (0.13, 1.57) | 0.24 (0.02, 2.44) |
| BMI (ref=BMI<25) | **** | **** | **** | * | ||||
| 25–<30 | 1.11 (0.88, 1.40) | 0.91 (0.68, 1.21) | 1.00 (0.78, 1.29) | 0.87 (0.62, 1.21) | 3.57 (1.40, 9.08) | 1.10 (0.60, 2.03) | 1.39 (0.43, 4.43) | 1.10 (0.21, 5.65) |
| 30–<35 | 1.35 (1.06, 1.72) | 1.00 (0.74, 1.36) | 1.08 (0.81 1.43) | 1.12 (0.79, 1.60) | 6.27 (2.49, 15.77) | 0.81 (0.41, 1.59) | 0.90 (0.23, 3.44) | 1.75 (0.32, 9.66) |
| ≥ 35 | 2.36 (1.84, 3.03) | 0.87 (0.61, 1.24) | 2.10 (1.57, 2.80) | 0.69 (0.43, 1.11) | 7.50 (2.96, 18.98) | 1.09 (0.56, 2.13) | 4.29 (1.24, 14.90) | 3.09 (0.48, 19.80) |
| Current smoking (ref=never/past) | 2.17 (1.72, 2.74)**** | 2.14 (1.59, 2.86)**** | 2.75 (2.09, 3.61)**** | 2.40 (1.64, 3.52)**** | 1.44 (0.89, 2.33) | 1.74 (1.04, 2.91)* | 0.71 (0.09, 5.53) | 2.52 (0.52, 12.19) |
| Physical Activity (ref=<1.25 METhr/wk) | ||||||||
| 1.25–<6.25 | 0.91 (0.75, 1.11) | 0.92 (0.70, 1.20) | 0.94 (0.74, 1.20) | 0.95 (0.68, 1.34) | 0.77 (0.52, 1.13) | 0.81 (0.49, 1.33) | 1.62 (0.53, 4.12) | 1.08 (0.30, 3.88) |
| 6.25–<15.3 | 0.81 (0.66, 1.00) | 0.72 (0.54, 0.96) | 0.83 (0.65, 1.07) | 0.72 (0.50, 1.03) | 0.73 (0.48, 1.11) | 0.75 (0.44, 1.27) | 0.87 (0.29, 2.60) | 0.45 (0.08, 2.47) |
| ≥15.3 | 0.74 (0.59, 0.93) | 0.74 (0.54, 1.00) | 0.75 (0.57, 0.98) | 0.77 (0.53, 1.12) | 0.65 (0.41, 1.03) | 0.59 (0.32, 1.09) | 1.32 (0.46, 3.82) | 1.34 (0.30, 6.08) |
| Chronic Lung Disease (ref=No) | 1.27 (0.90, 1.79) | 1.54 (0.98, 2.41) | 1.50 (1.02, 2.22)* | 1.22 (0.67, 2.23) | 0.73 (0.31, 1.72) | 2.05 (0.96, 4.38) | 0.44 (0.05, 4.06) | 0.82 (0.03, 21.58) |
| Anemia (ref=No) | 1.91 (1.07, 3.40)* | 1.83 (0.85, 3.90) | 0.73 (0.18, 2.98) | NA | 3.04 (1.58, 5.85)*** | 2.62 (1.05, 6.57)* | NA | 35.19 (4.54, 272.68)*** |
| Atrial fibrillation (ref=No) | 1.39 (1.02, 1.90)* | 1.08 (0.69, 1.70) | 1.41 (0.97, 2.07) | 0.94 (0.51, 1.71) | 1.38 (0.78, 2.45) | 1.38 (0.67, 2.84) | 0.55 (0.07, 4.69) | NA |
| Beta blocker use (ref=No) | 1.21 (0.95, 1.54) | 0.77 (0.53, 1.11) | 1.19 (0.89, 1.59)** | 0.75 (0.48, 1.17) | 1.16 (0.72, 1.87) | 0.67 (0.30, 1.50) | 2.11 (0.58, 7.69) | 2.82 (0.54, 14.83) |
| Aspirin use (ref=No) | 1.08 (0.90, 1.29) | 1.28 (1.00, 1.62)* | 1.12 (0.92, 1.37) | 1.32 (1.00, 1.75)* | 0.87 (0.57, 1.33) | 1.05 (0.62, 1.79) | 0.95 (0.32, 2.86) | 0.77 (0.17, 3.52) |
| Current HT use (ref=E-alone placebo/non-use) | ||||||||
| E-alone | 1.20 (0.97, 1.48) | 0.76 (0.57, 1.02) | 1.29 (0.99, 1.69) | 0.75 (0.52, 1.10) | 1.17 (0.78, 1.73) | 0.88 (0.52, 1.50) | 0.86 (0.33, 2.16) | 0.34 (0.07, 1.73) |
| E+P | 0.95 (0.73, 1.24) | 0.67 (0.47, 0.93) | 1.09 (0.80, 1.48) | 0.77 (0.51, 1.15) | 0.60 (0.26, 1.37) | 0.52 (0.21, 1.29) | 0.43 (0.11, 1.64) | 0.11 (0.01, 1.40) |
| E+P placebo/non-use | 1.05 (0.83, 1.34) | 0.72 (0.53, 0.98) | 1.20 (0.88, 1.63) | 0.78 (0.52, 1.17) | 0.86 (0.55, 1.34) | 0.71 (0.42, 1.22) | 0.69 (0.24, 2.03) | 0.50 (0.13, 1.96) |
| Any prior HT use (ref=None) | 0.97 (0.81, 1.17) | 1.21 (0.95, 1.53) | 0.90 (0.73, 1.11) | 1.23 (0.93, 1.61) | 1.37 (0.92, 2.05) | 1.22 (0.72, 2.08) | 0.50 (0.15, 1.71) | 0.93 (0.22, 3.96) |
| Alcohol (ref=1–<7 drinks/wk) | ||||||||
| Non/past | 0.94 (0.75, 1.17) | 1.01 (0.75, 1.37) | 0.92 (0.71, 1.19) | 1.20 (0.84, 1.71) | 0.74 (0.45, 1.20) | 0.68 (0.38, 1.23) | 4.47 (1.00, 20.03) | 0.99 (0.17, 5.90) |
| <1 drinks/wk | 0.95 (0.76, 1.18) | 0.93 (0.69, 1.26) | 0.95 (0.74, 1.21) | 0.97 (0.67, 1.38) | 0.86 (0.52, 1.42) | 0.81 (0.45, 1.47) | 2.38 (0.51, 11.08) | 0.67 (0.11, 4.13) |
| ≥7 drinks/wk | 1.07 (0.79, 1.44) | 1.08 (0.73, 1.61) | 1.04 (0.76, 1.44) | 1.10 (0.70, 1.71) | 0.91 (0.36, 2.25) | 0.90 (0.33, 2.48) | NA | 1.61 (0.10, 26.31) |
| Any insurance (ref=None) | 0.89 (0.64, 1.24) | 1.10 (0.69, 1.75) | 0.77 (0.51, 1.16) | 1.36 (0.68, 2.72) | 1.33 (0.66, 2.66) | 0.89 (0.43, 1.83) | 1.26 (0.45, 3.54) | 0.97 (0.21, 4.42) |
| Interim MI (ref=No) | 1.83 (1.28, 2.62)** | 2.21 (1.40, 3.50)*** | 1.64 (1.09, 2.47)* | 1.92 (1.08, 3.40)* | 3.10 (1.38, 6.96)** | 3.32 (1.37, 8.04)** | 1.98 (0.16, 23.81) | 6.18 (0.74, 51.90) |
| Interim CHD-not MI (ref=No) | 1.39 (1.05, 1.84)* | 1.85 (1.27, 2.69)** | 1.60 (1.16, 2.19)** | 1.69 (1.07, 2.69)* | 0.79 (0.41, 1.52) | 2.20 (1.07, 4.54)* | 1.40 (0.32, 6.21) | 4.09 (0.74, 22.65) |
| Interim DM (ref=No) | 1.61 (1.31, 1.98)**** | 1.29 (0.95, 1.75) | 1.84 (1.43, 2.36)**** | 1.60 (1.10, 2.33)* | 1.24 (0.83, 1.84) | 0.81 (0.47, 1.41) | 1.80 (0.76, 4.28) | 2.09 (0.46, 9.52) |
| Interim Cancer (ref=No) | 1.56 (1.24, 1.94)*** | 1.61 (1.17, 2.23)** | 1.59 (1.22, 2.06)**** | 1.44 (0.97, 2.15) | 1.49 (0.91, 2.44) | 2.71 (1.52, 4.84)*** | 2.70 (0.89, 8.18) | NA |
CHD: MI, CABG, PCI or angina requiring medication
DM: diabetes mellitus treated with pills or shots
Hypertension: hypertension treated with medication or BP ≥ 140/90
NA: insufficient number of cases to estimate HR
Statistical significance indicated as follows:
p-value < 0.05,
p-value <0.01,
p-value < 0.001,
p-value < 0.0001.
For risk factors with more than 2 levels, the statistical significance applies to the inclusion of the entire term in the model.
All HR and 95% CI are estimated from multivariable Cox proportional hazard models stratified by study component (clinical trial or observational study) and age strata (50–54, 55–59, 60–69, 70–79), and adjusted for all listed risk factors simultaneously.
Differences in HFpEF between African American, Hispanic American and Caucasian women
We evaluated risk factors for incident hospitalized HFpEF and HFrEF stratified by race/ethnicity. (Table 2) Most risk factors were similar between the three race groups except for a significant interaction with obesity (p=0.007). Compared to BMI <25 kg/m2, BMI categories 30–34.9, and ≥35kg/m2 placed African American women at greater risk for HFpEF (HR= 6.27, 95% CI 2.49, 15.77; HR=7.50, 95% CI 2.96, 18.98) compared to Caucasian women (HR= 1.08, 95% CI 0.81, 1.43; HR=2.10, 95%CI 1.57, 2.80). Hispanic women with BMI >35 kg/m2also were at a significantly increased risk for HFpEF, HR=4.29,95%CI 1.24, 14.90).
Population Attributable Risk% for HFpEF and HFrEF
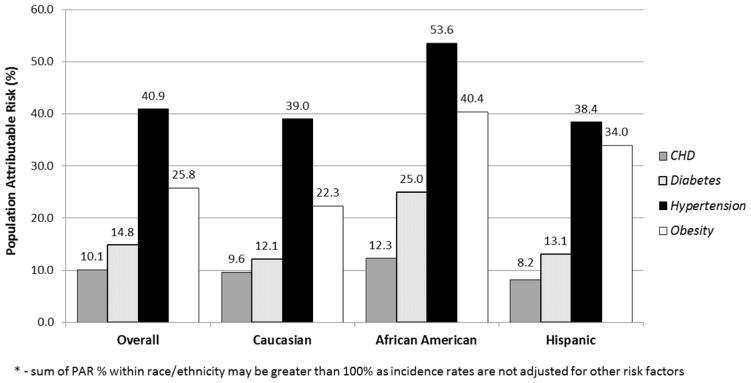
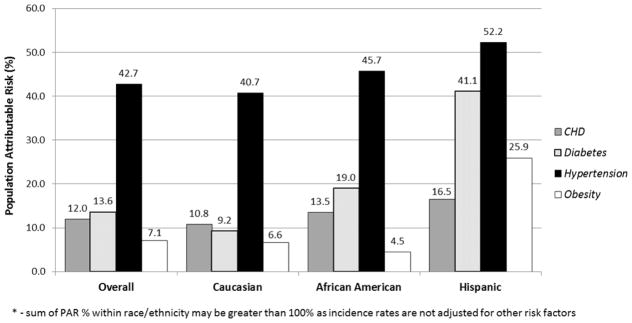
In order to assess the impact of potential preventive strategies for HFpEF and HFrEF, the PAR% for risk factors that are amenable to change and prevalent in the population (hypertension, obesity, diabetes, and CHD) were calculated. (Figure 1)
Figure 1.
Figure 1A. Population attributable risk* by race and ethnicity for heart failure with preserved ejection fraction
* - sum of PAR % within race/ethnicity may be greater than 100% as incidence rates are not adjusted for other risk factors
Figure 1B. Population attributable risk* by race and ethnicity for heart failure with reduced ejection fraction
* - sum of PAR % within race/ethnicity may be greater than 100% as incidence rates are not adjusted for other risk factors
For HFpEF, approximately 2/3rds of the PAR% is associated with hypertension and obesity while diabetes and CHD make up approximately 1/4th of the PAR%. For African American women, hypertension and obesity were associated with over 90% of the PAR% and for Hispanic women the same risk factors were associated with approximately 72% of the PAR%. For HFrEF, hypertension showed the strongest PAR% in all three race/ethnicity groups.
Discussion
This study of incident hospitalized HFpEF and HFrEF in a multi-ethnic cohort of women confirms previous findings that HFpEF is of greater incidence than HFrEF in post-menopausal women and that risk factors for both HFpEF and HFrEF include age, CHD, diabetes, smoking and hypertension. Robust associations for HFpEF, but not HFrEF, include obesity, number of co-morbid conditions, anemia and atrial fibrillation. As expected, MI is a risk factor for HFrEF. This study is unique in describing the importance of obesity as a risk factor for HFpEF and its PAR%, with special significance for African American women. While the important role of hypertension as a risk factor for both HFpEF and HFrEF is well documented, the important role of obesity as a risk factor in women for HFpEF is less well known.
Ho et al18 found a similar result for obesity as a risk factor for incident HFpEF in both sexes in the Framingham Heart study, as did Gupta et al11 in the Atherosclerosis Risk in Communities study for prevalent HFpEF in African Americans. Lam et al found stronger association of obesity in women compared to men in the baseline assessment of participants in the I-PRESERVE trial.19 Browers et al in the Dutch Prevend study found obesity to be a risk factor for both HFpEF and HFrEF.20 The pathophysiologic mechanisms by which higher BMI levels are associated with higher rates of incident HFpEF may well be related to adverse effects on obesity on skeletal muscle, oxidative stress, inflammation, and insulin resistance, all contributors to HFpEF.21 Recently Paulus and Tschope22 have proposed that obesity through the above mechanisms may induce changes in the coronary microvascular endothelium while Mohammed et al 23 has shown associations with coronary microvascular rarefaction with HFpEF as another potential mechanism.
While overweight and obesity are risk factors for incident HF and for HFpEF in most studies, both Haas et al and Kao have demonstrated in the I-PRESERVE and CHARM preserved trials that HFpEF participants with either lower BMIs and higher BMIs predicted more cardiovascular events and decreased survival.24, 25 This apparent paradox might be explained by cardiac cachexia and nutritional deficiencies associated with lower BMI26 while a BMI > 35kg/m2 is associated with higher rates of glucose intolerance, metabolic syndrome, chronic inflammation, all of which contribute to worse cardiovascular outcomes and higher levels of mortality.21
The importance of overweight and obesity in the potential prevention of HFpEF in women, especially in African American women, is noteworthy given its high PAR%. Why overweight and obesity places African American women at higher risk for HFpEF compared to Caucasian women even when adjusting for diabetes and hypertension is unknown but differences in inflammatory obesity, insulin sensitivity, and visceral fat distribution might play a role in these findings. The potential synergy between weight loss and exercise in obese, sedentary women and their impact on the prevention of HFpEF is worthy of future trials. Indeed in those with HFpEF, a recent trial showed an improvement in peak oxygen consumption with additive effects for weight loss and exercise. 27
Our study is the largest study in post-menopausal women to evaluate clinical risk factors for incident hospitalized HF with preserved and reduced ejection fractions and allows for race/ethnicity comparisons. An additional strength of our study was that it utilized a well validated classification system in defining new onset incident hospitalized HF and its subtypes.
Our study has several important caveats to consider when evaluating its conclusions. First it relied upon hospitalized HF and therefore outpatient diagnosed HF was not captured. However outpatient diagnosed HF is less than 25% of HF, is equal distributed between HFpEF and HFrEF and leads to subsequent hospitalization within a relatively short period of time. 28 In addition, ejection fraction information while captured in the majority of HF outcomes was missing in 27%, leading to potential misclassification bias. We may have overestimated the frequency of HFrEF by using an EF of <45% compared to a more stringent EF of <40% in order to categorize as many participants as either HFpEF or HFrEF in our cohort. In addition, the differential association between some risk factors and type of heart failure could be due to dependent censoring, although unlikely. We performed a sensitivity analysis using ≥50% ejection fraction defining HFpEF and <50% defining HFrEF and found similar results. We have limited power in our comparison of risk factors for Hispanic American women due to small number of HF events in Hispanic women.
Conclusion
This study demonstrated the higher incidence rate for new onset hospitalized HFpEF compared to HFrEF. Differential risk factors for new onset HFpEF included obesity, number of comorbidities, anemia and atrial fibrillation, whereas cigarette smoking, diabetes mellitus, hypertension, CHD were risk factors for both types of HF. Obesity was found to be a more potent risk factor for African American women compared to Caucasian women for HFpEF and showed a trend in Hispanic women. Since HFpEF is growing in incidence and prevalence as the population ages, and limited effective treatment for HFpEF are presently available, preventive strategies focusing on hypertension and obesity given their high PAR% appear most promising.
Supplementary Material
Clinical Perspective.
Heart failure (HF) is a major and growing public health problem in the United States, especially in older women. HFpEF is increasing in prevalence and as opposed to HFrEF, limited effective therapy are presently available. In order to guide future therapeutic considerations, there is a need to better understand the risk factors and natural history of HFpEF compared to HFrEF. This study compared risk factors for incident HFpEF and HFrEF and explored differences by race/ethnicity in 42,170 post-menopausal women followed for 13 years. Obesity and hypertension were both highly prevalent and extreme obesity (BMI>35) was a potent risk factors for HFpEF (HR= 2.36) and not HFrEF (HR=1.00) with a stronger association in African American women (HR=7.50) compared with Caucasian women (HR=2.10). The association of obesity with HFpEF could be associated with the adverse effects of obesity on skeletal muscle, oxidative stress, inflammation and insulin resistance which may lead to changes in coronary microvascular endothelium or changes in coronary microvascular rarefaction. The reason for the differential association of obesity on the risk of HFpEF in African American women compared to Caucasian women needs further investigation.
Acknowledgments
The authors thank the WHI participants, clinical sites, investigators, and staff for their dedicated efforts.
Sources of Funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services (contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C). The opinions expressed are those of the authors and do not necessarily reflect the views of the National Institutes of Health/U.S. Department of Health and Human Services.
Footnotes
Disclosures: C. Eaton: none, M. Pettinger: none, J. Rossouw: none, L. Martin: none, R. Foraker: none, A. Quddus: none, S. Liu: none, Wampler N: none, W Wu: none, J. Manson: none, K. Margolis: none, M. Allison: none, G. Corbie-Smith: none, W. Rosamond: none, K. Breathett: none, L. Klein: none.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, Fabsitz RR, Howard BV. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–1096. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 4.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 6.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL Cardiovascular Health Study Research Group. Importance of heart failure with preserved systolic function in patients > or = 65 years of age: CHS Research Group--Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 8.Eaton CB, Abdulbaki AM, Margolis KL, Manson JE, Limacher M, Klein L, Allison MA, Robinson JG, Curb JD, Martin LA, Liu S, Howard BV. Racial and Ethnic Differences in Incident Hospitalized Heart Failure in Post-Menopausal Women: The Women’s Health Initiative. Circulation. 2012;126:688–96. doi: 10.1161/CIRCULATIONAHA.111.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lina JAC. Difference in the incidence of congestive heart failure by ethnicity: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta DK, Shah AM, Castagno D, Takeuchi M, Loehr LR, Fox ER, Butler KR, Mosley TH, Kitzman DW, Solomon SD. Heart Failure With Preserved Ejection Fraction in African Americans: The ARIC (Atherosclerosis Risk In Communities) Study. JACC Heart Fail. 2013;1:156–163. doi: 10.1016/j.jchf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: A comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang P, Chambless L, Shahar E, Bertoni A, Russell S, Ni H, He M, Mosley T, Wagenknecht L, Samdarshi T, Wruck L, Rosamond W. Incidence and Survival of Hospitalized Acute Decompensated Heart Failure in Four US Communities (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2014;113:504–510. doi: 10.1016/j.amjcard.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langer R, White E, Lewis C, Kotchen J, Hendrix S, Trevisan M. The Women’s Health Initiative observational study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 15.Design of the Women’s Health Initiative clinical trial and observational study. Women’s Health Initiative Study Group. Controlled Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 16.Hennekens CH, Buring JE. Epidemiology in Medicine. Little, Brown and Co; Boston Toronto: pp. 90–96. [Google Scholar]
- 17.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam CS, Carson PE, Anand IS, Rector TS, Kuskowski M, Komajda M, McKelvie RS, McMurray JJ, Zile MR, Massie BM, Kitzman DW. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction. The irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) Trial. Circ Heart Fail. 2012;5:571–578. doi: 10.1161/CIRCHEARTFAILURE.112.970061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–1431. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 21.Upadhya B, Haykowsky M, Eggebeen J, Kitzman D. Sarcopenic Obesity and the Pathogenesis of Exercise Intolerance in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2015;12:205–214. doi: 10.1007/s11897-015-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulus W, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–671. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 23.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in Heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haass M, Kitzman D, Anand I, Miller A, Zile M, Massie B, Carson P. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the I-PRESERVE Trial. Circ Heart Fail. 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17:925–935. doi: 10.1002/ejhf.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F0, Pitt B, O’Connor CM. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patient with heart failure with preserved ejection. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.