Summary
Super-enhancers are tissue specific cis-regulatory elements that drive expression of genes associated with cell identity and malignancy. A cardinal feature of super-enhancers is that they are transcribed to produce long non-coding RNAs (eRNAs). It remains unclear whether epigenetically indistinguishable super-enhancers robustly activate genes in situ and if these functions are attributable to eRNAs or the DNA element. CRISPR/Cas9 was used to systematically delete three discrete super-enhancers at the Nanog locus in embryonic stem cells, revealing functional differences in Nanog transcriptional regulation. One distal super-enhancer 45 kb upstream of Nanog (−45 enhancer) regulates both nearest neighbor genes Nanog and Dppa3. Interestingly, eRNAs produced at the −45 enhancer specifically regulate Dppa3 expression by stabilizing looping of the −45 enhancer and Dppa3. Our work illustrates that genomic editing is required to determine enhancer function and points to a method to selectively target a subset of super-enhancer regulated genes by depleting eRNAs.
Keywords: embryonic stem cells, transcriptional regulation, super-enhancers, eRNAs, long non-coding RNAs
Introduction
Embryonic stem cells (ESCs) are distinguished by two unique properties: the ability to undergo unlimited self-renewal while maintaining pluripotency, the capacity to differentiate into all embryonic germ layers. Transcription factors (TFs) form the core machinery that maintain pluripotency by binding ESC specific cis-regulatory elements (CREs) that regulate transcription independent of distance and orientation (Chen et al., 2008). While there have been great advances in identifying the genomic location of ESC specific enhancers based on epigenetic signatures, much remains unknown about their exact mechanism and contribution to cell identity. Murine ESCs are an ideal model to study epigenetic regulation of cell identity as the TF and chromatin interaction networks (e.g. ChIP-Seq and Hi-C) controlling pluripotency have been well characterized resulting in identification of highly active cell-type specific CREs, termed super-enhancers or stretch enhancers, which associate with genes critical to cell identity (Whyte et al., 2013). In addition, super-enhancers play a critical role in driving disease processes such as cancer and targeting them with small molecule inhibitors is an active area of research (Lovén et al., 2013). Murine ESCs have 231 super-enhancers, which are large (~10 kb) CREs occupied by an extremely high density of pluripotency critical TFs Nanog, Oct4, and Sox2 (NOS) and coactivators such as Mediator (Whyte et al., 2013). Further, super-enhancers are defined by very high levels of the active enhancer epigenetic mark Histone 3 Lysine 27 Acetylation (H3K27Ac), are bound by the initiating form of RNA polymerase II (Serine 5 phosphorylated, Ser5P RNAPII), and bidirectionally transcribed to produce unspliced long non-coding RNAs (lncRNAs) termed enhancer RNAs (eRNAs; Pulakanti et al., 2013; Hnisz et al., 2013). eRNAs are a mark of highly active enhancers (Ørom et al., 2010; Kim et al., 2010; De Santa et al., 2010; Pulakanti et al., 2013) and have diverse roles in regulating transcription including stabilizing enhancer-promoter looping by tethering cohesin and Mediator complexes at interacting CREs (Li et al., 2013; Lai et al., 2013; Hsieh et al., 2014; Feng et al., 2006; Figure S1A). How discrete super-enhancers in close proximity work together to regulate genes and the relative contribution of the DNA element and its transcribed product (eRNA) to gene expression is currently unknown.
The 160 kb extended Nanog locus on murine chromosome 6 contains four genes (Apobec1, Gdf3, Dppa3, Nanog) that have critical roles early in embryonic development and are NOS regulated in ESCs. Nanog is a homeobox transcription factor and is required for pluripotency and self-renewal in ESCs (Chambers et al., 2003; Mitsui et al., 2003). Enhancer-promoter interactions at the extended Nanog locus have been studied revealing a complex network of putative CREs that interact and may co-regulate genes (Levasseur et al., 2008; Apostolou et al., 2013; de Wit et al., 2013). Interestingly, the Nanog locus contains three super-enhancers, but the relative contribution of each CRE to Nanog expression has not been studied in situ. We have previously demonstrated one distal super-enhancer 45 kb upstream of Nanog is transcribed and produces ESC specific eRNAs (Pulakanti et al., 2013). Thus, the extended Nanog locus is an ideal genomic region to study the function of multiple super-enhancers and the regulatory capacity of the eRNAs they produce.
Here we demonstrate striking differences in super-enhancer transcriptional regulation of Nanog. In addition, we show that a distal super-enhancer differentially regulates multiple neighboring genes and that eRNAs produced at this super-enhancer selectively activate expression of one neighboring gene. Thus, eRNAs are critical to enhancer function. This study provides new insight into the mechanism of transcribed enhancers in a cluster of co-regulated genes, specifically parsing out genes that cluster with a highly active transcribed enhancer versus a subset of genes the enhancer regulates.
Results
Super-Enhancers Cluster with Nanog
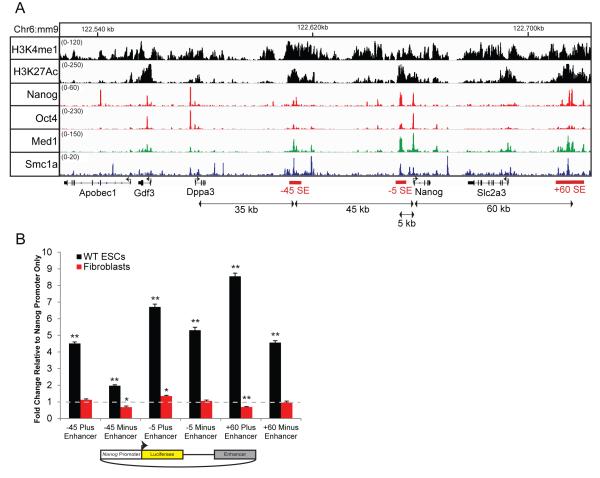
Whyte et al., (2013) identified 231 tissue specific super-enhancers in murine ESCs, which represent less than 5% of all enhancers. Subsequent studies have examined chromatin regions that interact with these highly active elements by genome wide chromosome conformation capture approaches such as Hi-C or 4C, suggesting a functional role of long-range chromatin interactions in the maintenance of pluripotency (Schoenfelder et al., 2015; Apostolou et al., 2013; de Wit et al., 2013). Nanog is unique from other pluripotent loci as it lies within 100 kb of three super-enhancers: 45 kb upstream (−45 enhancer), 17 kb region immediately upstream, and 60 kb downstream (+60 enhancer) of Nanog (Figure 1A). In this manuscript we focus on a RNAPII occupied enhancer 5 kb upstream of Nanog (−5 enhancer) as it is the most active element within the large proximal super-enhancer region (Figure 1A). Luciferase reporter assays show that all three super-enhancers increase transcriptional activity from the Nanog promoter in both orientations in ESCs but not in fibroblasts, indicating tissue-specific enhancer activity of the Nanog promoter (Pulakanti et al., 2013; Das et al., 2011; Figure 1B).
Figure 1.
Nanog neighbors three putative super-enhancers. (A) Integrated Genome Viewer (IGV) image of genome wide chromatin immunoprecipitation (ChIP-Seq) data sets in wild-type (WT) ESCs at the extended Nanog locus. Super-enhancers are indicated below in red. (B) Luciferase reporter plasmids containing the Nanog promoter and a fragment of either the −45, −5, or +60 enhancer in both orientations were transfected into WT ESCs or a fibroblast cell line (NIH/3T3). All samples had a fold change (Y-axis) and statistical analysis calculated relative to a plasmid containing Nanog promoter only which was set to one (dashed line). n = 3. Error bars indicate the standard error of the mean between experimental replicates. * p < 0.05, ** p < 0.01 using a one-sample Student’s t-test.
A common feature of all enhancers is that they interact with promoters by looping out the intervening chromatin segment (Guo et al., 2011; Nora et al., 2013; Sanyal et al., 2013; Rao et al., 2014; Figure S1A). To detect interaction of each super-enhancer with Nanog we used chromosome conformation capture (3C). Previous work has shown that the −5 and −45 enhancers interact with Nanog (Levasseur et al., 2008; Apostolou et al., 2013), which we confirmed (Figures 2A and 2B). In addition, we identified an unappreciated interaction between the +60 enhancer and Nanog in ESCs (Figures 2C). Interactions were increased above a control fibroblast cell line where Nanog is not expressed. Further, published Hi-C data shows clustering of numerous CREs at the extended Nanog locus, similar to our 3C data (Figure S1B). To verify locus wide clustering of other CREs at this locus in ESCs, we demonstrate an interaction between Dppa3 and +60 enhancer (Figure S1C) and an enhancer:enhancer interaction between the −45 and −5 enhancers (Figure S1D). In sum, we confirm locus wide clustering of co-regulated (NOS occupied) CREs with Nanog that function as putative enhancers in reporter assays.
Figure 2.
A looping event is detected between Nanog and three super-enhancers by chromosome conformation capture (3C). (A-C) X-axis indicates genomic distance (kb) from the Nanog promoter (anchor) and the Y-axis indicates normalized interaction ratio. The peak interaction was normalized to one for each WT ESC experimental replicate. IGV image showing HaeIII cut sites, 3C primers, and ChIP-Seq binding profiles in WT ESCs are shown below each graph. The anchor primer is not shown in Figure 2C. Error bars indicate the standard error of the mean between experimental replicates. * p < 0.05 and # p < 0.05, ## p < 0.01 using a one-sample and two-sample Student’s t-test respectively.
Functional Differences of Nanog Super-Enhancers
Super-enhancers have been proposed to control genes critical to cell identity such as Nanog. Given that all three super-enhancers are putative Nanog enhancers we asked whether each CRE is required for robust Nanog expression. To determine in situ enhancer function we systematically deleted each CRE using CRISPR/Cas9 genomic editing (Figure 3A). To delete the −5 and +60 enhancer we designed sequence specific guide RNAs (gRNAs) flanking each enhancer (Figures S2A and S2B). We obtained multiple independent clones containing large biallelic deletions (10.5 kb) of the +60 enhancer. Using the same approach we efficiently recovered clones containing only monoallelic deletions (2.5 kb) of the proximal −5 enhancer (Figure S2C). Finally, using an alternative CRISPR-mediated HDR approach, we obtained several clones containing biallelic deletions (2.85 kb) of the −45 enhancer (Figure S2D). All super-enhancer deleted clones recovered retain colony morphology and stain positive for alkaline phosphatase, a surface marker of pluripotency (Figure S2E).
Figure 3.
Genomic editing reveals functional differences in super-enhancer regulation of Nanog. (A) Schematic of CRISPR-mediated super-enhancer deletion at the Nanog locus. (B) Expression of genes within the extended Nanog locus in WT ESCs. Two representative clones are shown for −45 (Neo present), −5, and +60 enhancer deleted clones. Error bars indicate the standard error of the mean between experimental replicates. n = 3. All samples had a fold change (Y-axis) and statistical analysis (one-sample Student’s t-test) calculated relative to WT ESCs that were set to one (dashed line). A two-sample Student’s t-test was used to compare clones. * p < 0.05, ** p < 0.01 and ## p < 0.01 using a one-sample and two-sample Student’s t-test respectively. (C) Chart depicting changes in gene expression of Nanog and Dppa3 following deletion of each enhancer.
Surprisingly, knockout of individual super-enhancers resulted in different effects on gene expression at the extended Nanog locus. Specifically, biallelic deletion of the −45 enhancer results in decreased expression of both nearest neighbor genes Nanog and Dppa3, while biallelic deletion of the +60 enhancer results in minimal changes in expression of any gene at the Nanog locus (Figures 3B and 3C). Presence of a Neomycin resistance (Neo) cassette in place of the −45 enhancer had modest effects on Dppa3 expression but did not alter Nanog expression. Differences in expression of Apobec1 and Oct4 between −45 enhancer deleted clones with and without Neo is likely clone to clone variation (Figure S3C). Interestingly, monoallelic −5 enhancer deleted ESCs show an approximately 50% decrease in Nanog expression. This suggests that the −5 enhancer is required for robust Nanog expression and biallelic deleted clones were not recovered likely due to intolerance for enhancer loss secondary to loss of Nanog expression, in line with work demonstrating Nanog+/− but not Nanog−/− mice are viable (Mitsui et al., 2003). No significant change in expression was observed at other nearby NOS regulated genes (Gdf3, Apobec1) following deletion of any enhancer (Figure 3B). While −5 and −45 enhancer deleted clones showed a 40-50% decrease in Nanog expression, ESCs retain wild-type expression of other pluripotency-associated TFs indicating the pluripotency network is intact (Figure S3A). Accordingly, there was no biologically significant increase (ten to hundreds fold) in a range of lineage-specific markers as we have observed previously when ESCs lose pluripotency and differentiate (Stelloh et al., 2016; Figure S3B). These findings are in contrast to genome editing studies at the Sox2 locus where biallelic deletion of a distal super-enhancer in ESCs resulted in an 80-90% decrease in Sox2 expression and elevated expression of germ layer lineage markers (Zhou et al., 2014; Li et al., 2014). Taken together, despite identical epigenetic signatures, reporter activity, and chromatin interaction, genomic editing reveals important functional variation of super-enhancers in Nanog regulation, where only the most proximal CRE (−5 enhancer) appears critical to Nanog expression. These results provide insights into the complexity of enhancer function during development and demonstrate that isolated super-enhancers have differential function on cell critical gene expression which can only be uncovered by deleting the CREs (Figure 3C). Moreover, this parallels work showing differential function of constituent enhancers within one large super-enhancer (Huang et al., 2016).
−45 Enhancer Regulates Nanog and Dppa3
Surprisingly, deletion of the −45 enhancer resulted in an approximately 60% decrease in Dppa3 expression (Figures 3B). Dppa3 lies 35 kb upstream of the −45 enhancer and has roles in DNA imprinting in very early embryogenesis but is dispensable for pluripotency (Nakamura et al., 2007; Figures 1A and 3B). To confirm that reduction in Dppa3 expression is not a result of decreased Nanog (or vice versa) we depleted either Dppa3 or Nanog mRNA by RNAi. Nanog depletion resulted in a greater than 10-fold increase in Dppa3 expression, consistent with a previous report that Nanog represses Dppa3 (Sharov et al., 2008; Figure S4A). Dppa3 depletion led to minimal changes in Nanog expression and other pluripotency genes such as Sall4 (Figure S4B), demonstrating that Dppa3 does not regulate pluripotency. We identified a novel interaction of the −45 enhancer with the Dppa3 promoter in ESCs using 3C, which was not present in fibroblasts (Figure 4A). Finally, we show that the −45 enhancer activates Dppa3 in reporter assays in ESCs but not in fibroblasts (Figure 4B). Thus, we determined that the −45 enhancer regulates both nearest neighbor genes within a highly active region of co-regulated genes in ESCs. Further, the modest increase in Dppa3 expression in −5 enhancer monoallelic ESCs demonstrates that the −5 enhancer regulates Nanog but not Dppa3 (Figures 3B and 3C). Interestingly, despite studies showing clustering of Apobec1 and Gdf3 with Nanog (Levasseur et al., 2008; Apostolou et al., 2013), our genome editing results reveal that none of the super-enhancers regulate these genes (Figure 3B). This suggests linear DNA proximity of the super-enhancer is of functional importance within this compact cluster of co-regulated CREs.
Figure 4.
−45 Enhancer regulates Dppa3. (A) A looping event is detected between the −45 enhancer and Dppa3 by 3C in WT ESCs. X-axis indicates genomic distance (kb) from Dppa3 promoter (anchor) and the Y-axis indicates normalized interaction ratio. The peak interaction was normalized to one for each WT ESC experimental replicate. IGV image showing HaeIII cut sites, 3C primers, and ChIP-Seq binding profiles in WT ESCs is shown below the graph. (B) A luciferase reporter plasmid containing the Dppa3 promoter and a fragment of the −45 enhancer in both orientations was transfected into WT ESCs or a fibroblast cell line (NIH/3T3). All samples had a fold change (Y-axis) and statistical analysis calculated relative to a plasmid containing Dppa3 promoter only which was set to one (dashed line). n = 3. For (A) and (B) error bars indicate the standard error of the mean between experimental replicates. * p < 0.05 and # p < 0.05 using a one-sample and two-sample Student’s t-test respectively.
eRNAs Produced at the −45 Enhancer Specifically Regulate Dppa3
In just a few years since their discovery, eRNAs have been shown to have diverse functions in transcriptional regulation, including roles in enhancer-promoter looping (Lai et al., 2013; Li et al., 2013; Hsieh et al., 2014). We previously identified ESC specific eRNAs produced at the −45 enhancer (Pulakanti et al., 2013). The −45 enhancer serves as an interesting opportunity to study eRNA function as this CRE regulates two neighboring genes: Nanog and Dppa3 (Figures 1-4). To identify activating properties of −45 eRNAs at these two genes, we targeted eRNAs for depletion with modified antisense oligonucleotides (ASOs). eRNAs are restricted to the nucleus and therefore ASOs, which act by a nuclear specific RNAse H mechanism, have been used to efficiently deplete them. ASOs were designed to target peak levels of eRNAs as determined by Global Run-on Sequencing (GRO-Seq; Kaikkonen et al., 2013; Li et al., 2013; Figure S2D), which detects nascent RNA transcription and thus is a sensitive technique to detect eRNAs. Our level of eRNA depletion is comparable to other studies as ASOs reproducibly depleted eRNAs by more than 60% relative to a non-targeting ASO (Kaikkonen et al., 2013; Li et al., 2013; Figure 5A). Maintaining greater than 50% depletion is challenging as eRNAs are a short-lived molecule and restricted to focal region(s) of the nucleus. Interestingly, 24 hour depletion of eRNAs at the −45 enhancer showed greater than a 40% decrease in Dppa3 expression, with no change in Nanog expression (Figure 5A). This is in contrast to changes following −45 enhancer deletion, which result in decreased expression of both genes (Figure 3B). This demonstrates specificity of eRNAs to regulate only one of the nearest neighbor genes and that eRNAs are partially required for the cis-activating properties of this super-enhancer.
Figure 5.
−45 eRNAs regulate Dppa3 expression by stabilizing enhancer-promoter interaction(s). (A) Twenty-four hour depletion of −45 eRNAs using antisense oligonucleotides (ASOs) results in a reduction in Dppa3 expression. All samples had a fold change (Y-axis) and statistical analysis calculated relative to non-targeting ASO which was set to one (dashed line). n = 4. (B) Chromatin interactions (3C) between the −45 enhancer and neighboring cis-regulatory elements following eRNA depletion using eRNA ASO 1. Statistical analysis was calculated relative to the non-targeting ASO which was set to one (dashed line). n = 3. (C) Chromatin interactions (3C) between Nanog and the −5 and +60 enhancers in a −45 enhancer deleted ESC line (Neo removed). Statistical analysis was calculated relative to WT ESCs which was set to one (dashed line). n = 3. For (A-C) error bars indicate standard error of the mean between experimental replicates. * p < 0.05, ** p < 0.01 using a one-sample Student’s t-test. (D) Two-dimensional schematic of the extended Nanog locus before and after −45 eRNA depletion. −45 eRNAs maintain interaction of Dppa3 and the −45 enhancer and high levels of Dppa3 expression.
Previous studies have shown that lncRNAs interact with epigenetic regulators involved in DNA methylation (Berghoff et al., 2013), catalyzation of enhancer histone marks H3K27Ac (Wang et al., 2008), and Histone 3 lysine 4 methylation (H3K4me; Yang et al., 2014). To examine the mechanism by which eRNAs regulate Dppa3, we first tested for changes in active epigenetics marks of the enhancer. Following eRNA depletion we observed no change in H3K4me1 or H3K27Ac levels at the −45 enhancer and a putative transcribed enhancer upstream of Oct4 (control genomic region on a different chromosome) by chromatin immunoprecipitation (Figures S5A and S5B). Highly active enhancers also exhibit DNA hypomethylation compared to typical enhancers. Following eRNA depletion, we observed no change in DNA methylation at two CpG clusters within the −45 enhancer (Pulakanti et al., 2013; Figure S5C). In sum, transient depletion of eRNAs does not result in alteration of epigenetics marks of an active enhancer, implying eRNAs are not required for their maintenance.
Recent work highlights a role of eRNAs in stabilizing enhancer-promoter looping through interactions with the cohesin and Mediator complexes (Li et al., 2013; Lai et al., 2013). Cohesin is enriched at super-enhancers which are RNAPII bound and transcribed (Hnisz et al., 2013). Using published enhancer data sets we show that Smc1a (a component of the cohesin complex) is enriched at all ESC transcribed enhancers relative to eRNA negative enhancers (Pulakanti et al., 2013; Figure S5D). This suggests that eRNAs may have a generalized role to tether looping factors such as cohesin to stabilize chromatin interactions in ESCs. To determine the mechanism underlying altered Dppa3 expression, we tested whether eRNA depletion disrupts enhancer-promoter looping by 3C. Using eRNA ASO 1, which results in the most efficient depletion of eRNAs, we observed a ~ 50% decrease in looping of the −45 enhancer at the Dppa3 promoter (Figures 5B). There was no change in interaction of the −45 enhancer with Nanog or the −5 enhancer (Figure 5B). Other interactions at the Nanog locus were also unchanged following eRNA depletion (Figure S5E). Thus, −45 eRNAs stabilize specific enhancer-promoter interactions within a cluster of pluripotency-associated genes to drive robust Dppa3 expression. Our results are in agreement with studies showing no change in active enhancer marks following disruption of enhancer-promoter looping by depleting cohesin (Seitan et al., 2013). Finally, we confirmed that deletion of the −45 enhancer does not alter other super-enhancer interactions with Nanog (Figure 5C); further substantiating that −45 eRNAs specifically regulate a single gene within the cluster of CREs and that the −5 enhancer is sufficient to maintain pluripotency (Figures 3B and 5C).
Discussion
In summary, our data demonstrates in situ genomic editing is required to unravel the functional role of enhancers, particularly when numerous co-regulated CREs cluster within a compacted chromatin space. Expression changes following CRE ablation suggests there is a super-enhancer functional hierarchy at the Nanog locus where the −5 enhancer is the only CRE required for robust Nanog expression and pluripotency. Further, by combining genome editing with eRNA depletion we demonstrate that eRNAs have a functional role by specifically regulating one of the enhancer associated genes (Figure 5D). This is in contrast to recent work by Paralkar et al., (2016) where depletion of an enhancer transcribed long intergenic non-coding RNA (Lockd), which are a distinct class of lncRNA from eRNA and capable of acting in trans, did not phenocopy gene expression changes following enhancer deletion. Rather, our work supports a model whereby eRNAs have critical roles in organizing three-dimensional nuclear interactions, thereby activating a select subset of interacting genes regulated by a super-enhancer (Li et al., 2013; Lai et al., 2013; Hsieh et al., 2014).
Under commonly used in vitro serum/LIF culture conditions Nanog and Dppa3 are two of the most heterogeneously expressed genes on a cell to cell basis (Galonska et al., 2015). ESCs maintained in serum free defined conditions containing two kinase inhibitors to suppress differentiation cues (2i; Mek and Gsk3 inhibitors) have higher and more homogenous levels of Nanog as well as Dppa3 (Silva et al., 2009; Galonska et al., 2015). These ESCs are in an epigenetically and transcriptionally distinct “naïve” pluripotent state, likely as a result of dynamic NOS binding at enhancer elements (Galonska et al., 2015). Interestingly, gene expression differences in Nanog and Dppa3 in these two in vitro conditions parallels expression changes observed in WT and −45 enhancer deleted ESCs. This suggests that dynamic epigenetic changes in the −45 enhancer landscape in the two ESC states results in differential regulation of Nanog and Dppa3.
In addition to driving expression of lineage critical factors during development, super-enhancers play a critical role in pathologic processes, including malignancies (Hnisz et al., 2013). Inhibition of transcriptional co-activators and chromatin regulators that densely occupy super-enhancers has been shown to decrease aberrant expression of oncogenes in tumor cells including Myc (Lovén et al., 2013). However, current approaches using small-molecules to target coactivators such as Brd4 lack specificity, and disrupt most genes regulated by super-enhancers. Our work points to a method to selectively target a subset of genes regulated by super-enhancers in pathologic states. Antisense oligonucleotides, which are used as RNA-targeting therapies for a variety of diseases (Arun et al., 2016), offer an intriguing alternative to small molecules as they allow direct pharmacological targeting of activating eRNAs at oncogenic loci.
Experimental Procedures
Cell Culture, RNA Isolation, and Quantitative Real Time RT-PCR
ESCs were cultured under previously described conditions (Rao et al., 2010). Total RNA isolation, cDNA conversion, and quantitative PCR was performed as previously described (Pulakanti et al., 2013; Stelloh et al., 2016). Primers used for RT-qPCR, ChIP-qPCR, and 3C are listed in Table S1 (all primers in this study were designed using mm9).
Reporter Plasmid Generation
Luciferase reporter plasmids were generated and analyzed similar to Pulakanti et al., (2013). Each enhancer was cloned into the SalI site (in both orientations) and each promoter into the KpnI/XhoI site of the Firefly luciferase plasmid (pGL2, Promega). Reporter constructs were transiently transfected into cells using Lipofectamine 2000 (Invitrogen) along with a control plasmid (Renilla Luciferase, pRL-EF) for thirty hours.
Chromosome Conformation Capture (3C)
3C libraries for ESC and NIH/3T3 crosslinked chromatin were generated as described in Stelloh et al., (2016) using a modified protocol from Hagège et al., (2007). Interaction between the anchoring point and distal fragments was determined by SYBR Green qPCR and normalized to BAC templates. Fold changes were divided by relative interaction at the Ercc3 locus (a housekeeping gene known to loop in all cell types) to account for differences in library generation (Table S1). At least two independent libraries were used for each cell line and statistical analysis was calculated relative to WT ESCs. For each genomic region tested the peak normalized interaction for each ESC library was set to one.
Genome Editing
To generate −45 Nanog super-enhancer deleted ESC clones, a single guide RNA (gRNA) designed using the CRISPR design tool (http://crispr.mit.edu/) was cloned into the Cas9 expressing vector px459 (Cong et al., 2013). A homology directed repair (HDR) vector (pL452) containing a floxed Neomycin resistance (Neo) cassette flanked by homology regions was co-transfected along with the gRNA in WT ESCs. Individual clones that were resistant to both puromycin and G418 were isolated and expanded for genotyping (Table S1). To generate −5 and +60 Nanog super-enhancer deleted ESC clones we used two gRNA (designed same as above) as described in Zhou et al., (2014) and Huang et al., (2016) (Table S1).
Antisense Oligonucleotides
Antisense Oligonucleotides (ASOs) targeting eRNAs produced at the −45 Nanog super-enhancer were designed by Integrated DNA Technologies (Table S1). ASOs were designed with phosphorothioate bonds and a 10 bp gapmer flanked by 5 bp blocks of 2′-O-methyl modified ribonucleotides that protect the ASO from nuclease degradation. ASOs were transiently transfected at 100 nM with Lipofectamine 2000.
Supplementary Material
Acknowledgements
We thank Christopher Glass and Minna Kaikkonen for the suggestion of using ASOs for eRNA depletion, Kim Lennox (Integrated DNA Technologies) for designing ASOs, and Sergey Tarima for assistance with statistical analysis. This work was supported in part by funding from NIDDK (DK10350) to SB and the MCW MSTP T32 (NIGMS, GM080202). The majority of support came from Midwest Athletes against Childhood Cancer, American Society of Hematology, and Hyundai Hope on Wheels, all to SR.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, and one table.
Author Contributions
SB performed all experiments, while MR provided assistance with specific experiments. KP assisted with computational analysis. SB and SR conceived the study and designed experiments.
References
- Apostolou E, Ferrari F, Walsh RM, Bar-Nur O, Stadtfeld M, Cheloufi S, Stuart HT, Polo JM, Ohsumi TK, Borowsky ML, et al. Genome-wide chromatin interactions of the Nanog locus in pluripotency, differentiation, and reprogramming. Cell Stem Cell. 2013;12:699–712. doi: 10.1016/j.stem.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun G, Diermeier S, Akerman M, Chang KC, Wilkinson JE, Hearn S, Kim Y, MacLeod AR, Krainer AR, Norton L, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff EG, Clark MF, Chen S, Cajigas I, Leib DE, Kohtz JD. Evf2 (Dlx6as) lncRNA regulates ultraconserved enhancer methylation and the differential transcriptional control of adjacent genes. Development. 2013;140:4407–4416. doi: 10.1242/dev.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Jena S, Levasseur DN. Alternative splicing produces Nanog protein variants with different capacities for self-renewal and pluripotency in embryonic stem cells. J Biol Chem. 2011;286:42690–42703. doi: 10.1074/jbc.M111.290189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, Muller H, Ragoussis J, Wei CL, Natoli G. A large fraction of extragenic RNA Pol II transcription sites overlap enhancers. Plos Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Bouwman BA, Zhu Y, Klous P, Splinter E, Verstegen MJ, Krijger PH, Festuccia N, Nora EP, Welling M, et al. The pluripotent genome in three dimensions is shaped around pluripotency factors. Nature. 2013;501:227–231. doi: 10.1038/nature12420. [DOI] [PubMed] [Google Scholar]
- Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galonska C, Ziller MJ, Karnik R, Meissner A. Ground State Conditions Induce Rapid Reorganization of Core Pluripotency Factor Binding before Global Epigenetic Reprogramming. Cell Stem Cell. 2015;17:462–470. doi: 10.1016/j.stem.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Gerasimova T, Hao H, Ivanova I, Chakraborty T, Selimyan R, Oltz EM, Sen R. Two forms of loops generate the chromatin conformation of the immunoglobulin heavy-chain gene locus. Cell. 2011;147:332–343. doi: 10.1016/j.cell.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagège H, Klous P, Braem C, Splinter E, Dekker J, Cathala G, De Laat W, Forné T. Quantitative analysis of chromosome conformation capture assays (3C-qPCR) Nature Protocols. 2007;2:1722–1733. doi: 10.1038/nprot.2007.243. [DOI] [PubMed] [Google Scholar]
- Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Fei T, Chen Y, Li T, Gao Y, Wang X, Sun T, Sweeney CJ, Lee GS, Chen S, et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc Natl Acad Sci. 2014;111:7319–7324. doi: 10.1073/pnas.1324151111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Liu X, Li D, Shao Z, Cao H, Zhang Y, Trompouki E, Bowman TV, Zon LI, Yuan GC, et al. Dynamic Control of Enhancer Repertoires Drives Lineage and Stage-Specific Transcription during Hematopoiesis. Dev Cell. 2016;36:9–23. doi: 10.1016/j.devcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikkonen MU, Spann NJ, Heinz S, Romanoski CE, Allison KA, Stender JD, Chun HB, Tough DF, Prinjha RK, Benner C, Glass CK. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51:310–325. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Ørom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, Shiekhattar R. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur DN, Wang J, Dorschner MO, Stamatoyannopoulos JA, Orkin SH. Oct4 dependence of chromatin structure within the extended Nanog locus in ES cells. Genes Dev. 2008;22:575–580. doi: 10.1101/gad.1606308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rivera CM, Ishii H, Jin F, Selvaraj S, Lee AY, Dixon JR, Ren B. CRISPR reveals a distal super-enhancer required for Sox2 expression in mouse embryonic stem cells. PLoS One. 2014;9:e114485. doi: 10.1371/journal.pone.0114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Arai Y, Umehara H, Masuhara M, Kimura T, Taniguchi H, Sekimoto T, Ikawa M, Yoneda Y, Okabe M, et al. PGC7/Stella protects against DNA demethylation in early embryogenesis. Nat Cell Biol. 2007;9:64–71. doi: 10.1038/ncb1519. [DOI] [PubMed] [Google Scholar]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar VR, Taborda CC, Huang P, Yao Y, Kossenkov AV, Prasad R, Luan J, Davies JO, Hughes JR, Hardison RC, et al. Unlinking an lncRNA from Its Associated cis Element. Mol Cell. 2016;62:104–110. doi: 10.1016/j.molcel.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulakanti K, Pienello L, Stelloh C, Blinka S, Allred J, Milanovich S, Peterson J, Wang A, Yuan GC, Rao S. Enhancer Transcribed RNAs are produced from hypomethylated genomic regions in a Tet-dependent manner. Epigenetics. 2013;8:1303–1320. doi: 10.4161/epi.26597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Zhen S, Roumiantsev S, McDonald LT, Yuan GC, Orkin SH. Differential roles of Sall4 isoforms in embryonic stem cell pluripotency. Mol Cell Biol. 2010;22:5364–5380. doi: 10.1128/MCB.00419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2013;489:109–113. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder S, Furlan-Magaril M, Mifsud B, Tavares-Cadete F, Sugar R, Javierre BM, Nagano T, Katsman Y, Sakthidevi M, Wingett SW, et al. The pluripotent regulatory circuitry connecting promoters to their long-range interacting elements. Genome Res. 2015;25:582–597. doi: 10.1101/gr.185272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitan VC, Faure AJ, Zhan Y, McCord RP, Lajoie BR, Ing-Simmons E, Lenhard B, Giorgetti L, Heard E, Fisher AG, et al. Cohesin-based chromatin interactions enable regulated gene expression within preexisting architectural compartments. Genome Res. 2013;23:2066–2077. doi: 10.1101/gr.161620.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Masui S, Sharova LV, Piao Y, Aiba K, Matoba R, Xin L, Niwa H, Ko MS. Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genomics. 2008;9:269. doi: 10.1186/1471-2164-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelloh C, Reimer MH, Pulakanti K, Blinka S, Peterson J, Pinello L, Jia S, Roumiantsev S, Hessner MJ, Milanovich S, et al. The cohesin-associated protein Wapal is required for proper Polycomb-mediated gene silencing. Epigenetics Chromatin. 2016;9:14. doi: 10.1186/s13072-016-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YW, Flynn RA, Chen Y, Qu K, Wan B, Wang KC, Lei M, Chang HY. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. eLife. 2014;3:e02046. doi: 10.7554/eLife.02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Katsman Y, Dhaliwal NK, Davidson S, Macpherson NN, Sakthidevi M, Collura F, Mitchell JA. A Sox2 distal enhancer cluster regulates embryonic stem cell differentiation potential. Genes Dev. 2014;28:2699–2711. doi: 10.1101/gad.248526.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.