Abstract
Background
The storage of platelets affects platelet integrity and functionality, a process named platelet storage lesion (PSL). Reduced adenosine diphosphate (ADP)-induced platelet aggregation is a typical manifestation of PSL. However, the role of ADP receptors in this context has not been evaluated yet. The aim of this study was, therefore, to investigate surface expression and function of the purinergic receptors P2Y1, P2Y12 and P2X1 in stored platelet concentrates.
Material and methods
Platelets were obtained from venous whole blood and from apheresis-derived platelet concentrates stored for 0, 2 and 5 days. Purinergic receptor expression was measured by flow cytometry and western blot analysis. Receptor function was determined by calcium-induced fluorescence (P2Y1 and P2X1) or by flow cytometric measurement of the platelet reactivity index (P2Y12).
Results
The basal surface expression and total content of purinergic receptors remained unchanged throughout storage. After an initial reduction during apheresis, P2X1-mediated calcium flux was maintained, whereas the P2Y1-mediated increase of calcium flux gradually decreased during the course of storage. In contrast, the platelet reactivity index was comparable in freshly obtained and stored platelets.
Discussion
The function of the P2Y12 receptor is maintained during storage of apheresis-derived platelet concentrates. However, the impairment of P2X1 and especially of P2Y1 receptor function indicated by decreased receptor-mediated calcium flux is an important mechanism contributing to reduced ADP responsiveness of stored platelets.
Keywords: platelet concentrate, storage lesion, purinergic receptors, adenosine diphosphate, aggregation
Introduction
Adenosine diphosphate (ADP) is a physiological platelet activator. It is stored at high concentrations in platelet dense granules and released from stimulated platelets to promote platelet activation1,2. ADP mediates its effects on platelets via purinergic receptors. There are three different purinergic receptors in human platelets: P2Y1, P2Y12 and P2X1. P2Y1 is a Gq-coupled ADP receptor, activating phospholipase C and stimulating calcium release from intracellular stores3,4. P2Y12 is coupled with the Gαi protein. The activation of P2Y12 results in adenylyl cyclase suppression and switching-off of the cAMP-dependent inhibitory pathway in platelets3,4. Simultaneous stimulation of both ADP receptors is required for the initiation of platelet aggregation3,4. P2X1, in contrast to P2Y1 and P2Y12, is an ATP-gated, non-selective cation channel. It causes a rapid calcium influx in platelets, synergises P2Y1 signalling5 and induces platelet shape change, but is not able to induce platelet aggregation6.
Platelets from stored apheresis-derived platelet concentrates (APC) undergo a process called storage lesion with changes in their signalling cascades leading to decreased platelet response to physiological agonists and impaired integrin activation, secretion or aggregation7. Various studies of stored APC revealed accumulation of soluble P-selectin, PF48 CD62-P-positive platelets9,10 and increased intracellular calcium levels11. Flow cytometric analysis of platelets in APC show decreased expression of surface glycoproteins (GP) IIb/IIIa and GPIb12,13 accompanied by the loss of high-affinity thrombin receptors on the platelet surface14,15.
As known phenomena of storage lesion, ADP signalling and ADP-induced aggregation are affected in stored platelets16,17. However, to date, there is a lack of detailed data on changes of platelet purinergic receptors associated with these alterations. The aim of this study was, therefore, to investigate the expression and function of purinergic receptors in stored APC in order to evaluate underlying mechanisms of impaired ADP responsiveness.
Materials and methods
Materials
ADP was from Haemochrom Diagnostica GmbH (Essen, Germany), thrombin receptor activating peptide-6 (TRAP-6) from Bachem (Bubendorf, Switzerland). Fluorescein isothiocyanate-conjugated goat anti-rabbit polyclonal antibody, prostaglandin E1 (PGE1), acetylsalicylic acid, probenecid, Pluronic F-127, 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid (HEPES) and apyrase were from Sigma-Aldrich Chemie GmbH (Muenchen, Germany). APC-conjugated mouse monoclonal anti-CD41a antibody was from BD Biosciences (Heidelberg, Germany). Rabbit polyclonal anti-P2Y1, anti-P2Y12 and anti-P2X1 antibodies were from Alomone Labs (Jerusalem, Israel). The selective P2Y1 receptor agonist [(1R,2R,3S,4R,5S)-4-[6-Amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxy- bicycle [3.1.0] hex-1-yl]methyl] diphosphoric acid mono ester trisodium salt (MRS2365), the selective antagonist of P2Y1(1R*,2S*)-4-[2-iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphornooxy)bicyclo-[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt (MRS2500), the agonist of P2X1 receptor α,β-methyleneadenosine 5′-triphosphate trisodium salt (α,β-MeATP), and the potent P2X1 antagonist 4,4′,4″,4‴-[carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino))] tetrakis-1,3-benzenedisulfonic acid, octasodium salt (NF449) were from R&D Systems GmbH (Wiesbaden-Nordenstadt Germany). Fluo-4A M Cell permeant was from Life Technologies GmbH (Darmstadt, Germany). The flow cytometric PLT VASP/P2Y12 Kit for the measurement of P2Y12 receptor function was from Stago GmbH (Düsseldorf, Germany).
Blood collection and apheresis-derived platelet concentrates
Venous whole blood samples (WB) and APC were obtained from seven informed healthy volunteer donors (4 male, 3 female, aged 20 to 46 years). Peripheral blood was collected into polystyrene tubes containing 3.2% citrate buffer (106 mM trisodium citrate, Sarstedt, Nümbrecht, Germany) before APC donation.
APC (2.5×1011 platelets in 250 mL of plasma) were collected using Trima Accel devices with version 9 software and the Trima Accel LRS Platelet, Plasma Set (Terumo BCT, Lakewood, CO, USA) obeying current guidelines and with the approval of authorities. The ratio of inlet blood volume to anticoagulant (ACD-A) was 10:1. On days 0 (2–3 hours after the finalised apheresis), two and five samples of APC were taken, under sterile conditions, for analysis. Analysis of WB samples on day 0 was started 30 minutes after blood collection.
Our studies with human platelets were approved by the local ethics committee of the University of Würzburg. The study was performed in accordance with our institutional guidelines and the Declaration of Helsinki.
Preparation of washed platelets
Washed platelets were prepared as described elsewhere18. Briefly, 3 mM EGTA was added to WB or to samples from APC to prevent platelet activation. Platelet-rich plasma (PRP) was obtained by centrifugation at 280 g for 5 minutes. Subsequently, samples of PRP and APC were centrifuged at 430 g for 10 minutes. The pelleted platelets were washed once in CGS buffer (120 mM sodium chloride, 12.9 mM trisodium citrate, 30 mM D-glucose, pH 6.5) and resuspended in HEPES buffer (150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM D-glucose, 10 mM HEPES, pH 7.4) to a final concentration of 3×108 platelets/mL.
Platelet aggregation
Platelet aggregation was measured using an APACT 4004 aggregometer (LabiTec, Ahrensburg, Germany). PRP was obtained by centrifugation of WB at 280 g for 5 minutes. PRP or material from stored APC, diluted with plasma to match the PRP platelet concentration, was stimulated with 10 μM ADP or 5 μM TRAP-6. Aggregation was measured for 5 minutes under continuous stirring at 1,000 rpm and 37 °C.
Flow cytometric analysis
Eleven microlitres of WB were diluted with 11 μL Dulbecco’s phosphate-buffered saline and stained with 3 μL anti-CD41a-APC and 5 μL of rabbit anti-purinergic receptor antibodies. In the case of APC, 25 μL of APC diluted with plasma to 1.5×108 platelets/mL were pre-incubated with 5 μL of anti-purinergic receptor antibodies (0.8–1.0 mg/mL) for 15 minutes at room temperature followed by incubation for 15 minutes at 37 °C. Samples were stopped with 0.1% formaldehyde, fixed for 10 minutes at room temperature and centrifuged for 2 minutes at 20,000 g. The pellet was resuspended in 100 μL Dulbecco’s phosphate-buffered saline/5 mM glucose/0.5% bovine serum albumin (BSA) and stained for 25 minutes with 1 μL fluorescein isothiocyanate-conjugated goat anti-rabbit antibody. Finally, the samples were diluted with 500 μL Dulbecco’s phosphate-buffered saline/5 mM glucose/0.5% BSA and analysed on a FACSCalibur flow cytometer from Becton Dickinson (Franklin Lakes, NJ, USA) using CELLQuest software, version 6.0. The platelet population was identified by its forward and side scatter distribution and 10,000 events were analysed for mean fluorescence.
Western blot analysis
Cell lysates of washed platelets containing 2 μg protein were loaded onto the gel, separated by sodium dodecylsulphate polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes. The membranes were incubated with appropriate primary antibodies overnight at 4 °C. For visualisation of the signal, goat anti-rabbit or anti-mouse IgG conjugated with horseradish peroxidase were used as secondary antibodies, followed by detection with an ECL detection kit (GE Healthcare, Piscataway, NJ, USA). Blots were analysed densitometrically using NIH Image J software (National Institutes of Health, Bethesda, MD, USA) for uncalibrated optical density.
Platelet preparation for the measurement of P2Y1 activity
To prepare platelets for the measurement of P2Y1 activity, 500 nM PGE1 was added to PRP (as described for the preparation of washed platelets) or to material from stored APC and then centrifuged at 430 g for 10 minutes. The pellet was washed with 5 mL of modified Tyrode buffer (10 mM HEPES, 150 mM NaCl, 3 mM KCl, 1 mM MgCl2, 5 mM glucose and 0.1% BSA, pH 6.5) containing 500 nM PGE1. Platelets were resuspended in modified Tyrode buffer without PGE1 and platelet concentration was adjusted to 0.6×108 platelets/mL19.
Platelet preparation for the measurement of P2X1 activity
To prepare platelets for the measurement of P2X1 activity, 1 mM acetylsalicylic acid and 0.3 U/mL apyrase were added to the PRP (as described for the preparation of washed platelets) or to material from stored APC and then centrifuged at 430 g for 10 minutes. The pellet was washed with 5 mL of modified Tyrode buffer containing 1 mM acetylsalicylic acid and 0.3 U/mL apyrase. Platelets were resuspended in modified Tyrode buffer containing 0.3 U/mL apyrase and platelet concentration was adjusted to 0.6×108 platelets/mL19.
Measurement of P2Y1 and P2X1 activity
The activity of platelet purinergic P2Y1 and P2X1 receptors was measured by calcium flux-induced fluorescence in Fluo-4AM loaded platelets after selective stimulation19. Briefly, in each well of a 96-well black plate, 100 μL of washed platelets were mixed with an equal volume of Hank’s buffered saline solution (HBSS) containing 10 mM HEPES, 0.1% BSA, 2.5 mM probenecid, 1 mM EGTA, 0.01% pluronic acid and 2 μM Fluo-4AM at pH 7.4. For P2X1 measurements, EGTA was substituted by 2.5 mM calcium and apyrase was added to the final concentration of 0.3 U/mL. The plate was incubated for 20 minutes at room temperature in the dark, followed by 20 minutes of incubation at 37 °C. During the last 10 minutes of incubation, 2 μL of 100 μM MRS2500, a P2Y1 antagonist, or 2 μL of 100 μM NF449, a P2X1 antagonist, were added. After measurement of the basal fluorescence (Ex 488 - Em 538; 20 measurements at 1 second), platelets were stimulated with 2 μL of 100 μM MRS2365, a P2Y1 agonist, or 2 μL of 100 μM α,β-MeATP, a P2X1 agonist. After stimulation, fluorescence values were measured every second for the next 3 minutes. Fluorescence signals were measured and analysed by a Fluoroscan Ascent Microplate Fluorometer from Fisher Scientific GmbH (Schwerte, Germany).
Measurement of P2Y12 activity
The activity of platelet P2Y12 receptor was measured by the flow cytometric PLT VASP/P2Y12 Kit. Briefly, aliquots of WB or APC diluted with plasma to 3×108 platelets/mL were stimulated with PGE1 alone or with a combination of PGE1 and ADP at room temperature. After stimulation, samples were fixed and stained as described in the manufacturer’s instructions, followed by flow cytometric measurement of fluorescence. The platelet reactivity index (PRI) was calculated using corrected mean fluorescence intensities (MFIc) as
Statistical analysis
Data are presented as mean±standard error of the mean (SEM). The n-values refer to the number of experiments, each made with different blood donors. Differences between groups were analysed by paired and unpaired Student’s t-test as appropriate using the MedCalc statistic programme (MedCalc Software bvba, Mariakerke, Belgium). P-values <0.05 were considered statistically significant.
Results
Platelet purinergic receptor expression is stable during storage of apheresis-derived platelet concentrates
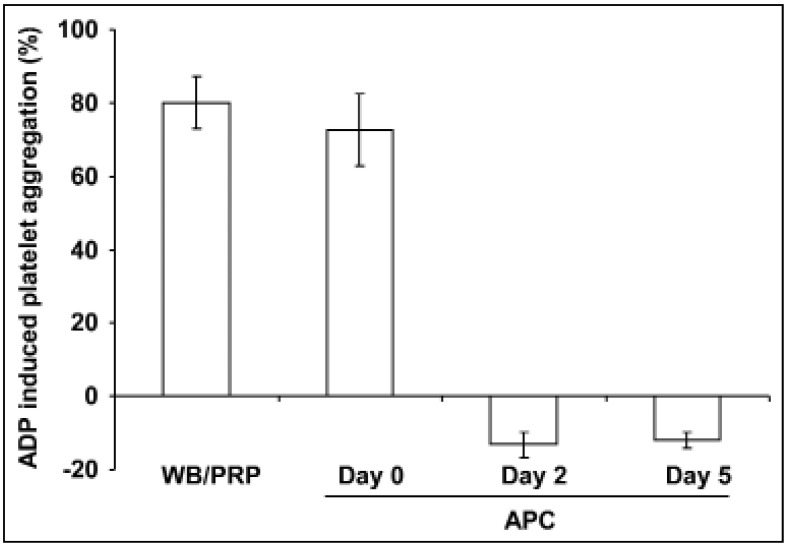
Stimulation with 10 μM ADP resulted in rapid, strong (80.1±7.28%) platelet aggregation in PRP from freshly obtained WB (Figure 1). Aggregation of platelets from APC shortly after apheresis showed comparable values (72.7±9.9%). Platelets from APC stored for 2 or 5 days had the capacity for change shape, as indicated by negative light transmission values after activation (Figure 1), but they were not able to aggregate even with ADP concentrations up to 100 μM (data not shown). In contrast, 5 μM TRAP-6-induced aggregation was only partially reduced by approximately 25% on day 2 and by approximately 50% on day 5 (data not shown).
Figure 1.
ADP-induced platelet aggregation in PRP from fresh WB and from stored APC.
PRP from fresh WB (black line), from freshly prepared APCs (day 0, black dashed line) and from APC stored for 2 days (grey line) or for 5 days (grey dashed line), diluted with platelet-poor plasma to adjust to the same platelet concentration as in PRP, were stimulated with 10 μM ADP. Results are presented as mean %±SEM of maximal aggregation; n=7.
ADP: adenosine diphosphate; APC: apheresis-derived platelet concentrates; PRP: Platelet-rich plasma; WB: whole blood; SEM: standard error of the mean.
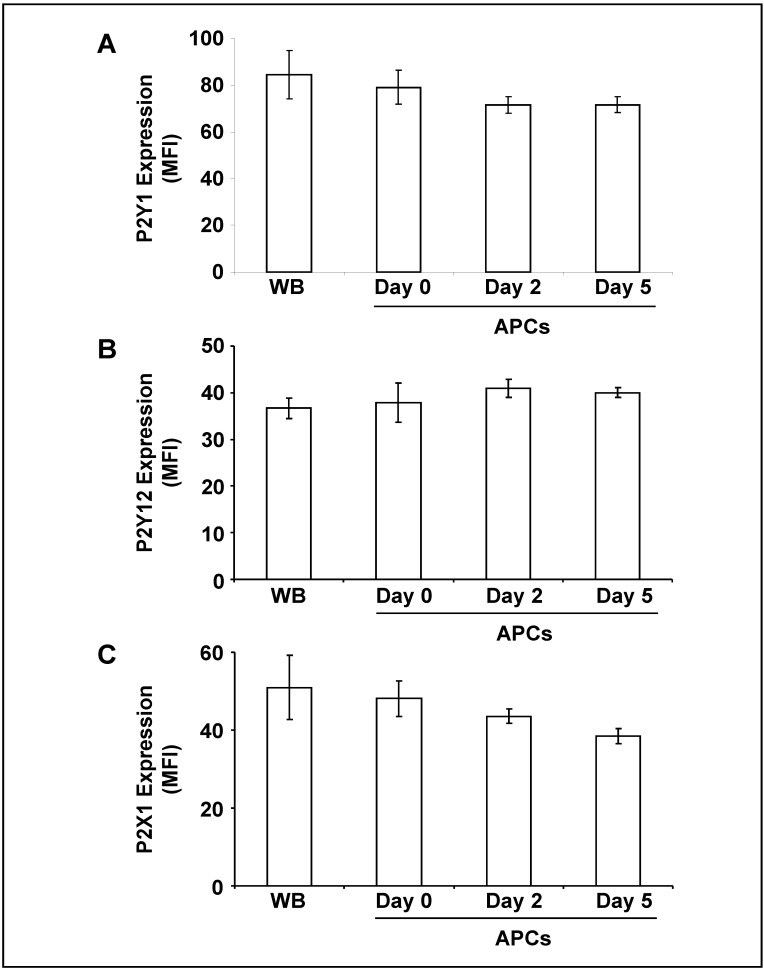
The analysis of purinergic receptor surface expression revealed that basal levels of P2Y1 and P2Y12 did not change significantly during storage of APC (Figure 2A,B). The level of P2X1 showed a weak, but not significant tendency to decrease (Figure 2C).
Figure 2.
Surface expression of purinergic receptors on platelets from fresh WB and from stored APC.
The histograms show the mean fluorescence intensity (MFI) of (A) P2Y1, (B) P2Y12 and (C) P2X1 surface expression on platelets as indicated. Results are presented in absolute arbitrary units as mean±SEM; n=7.
WB: whole blood; APC: apheresis-derived platelet concentrates; SEM: standard error of the mean.
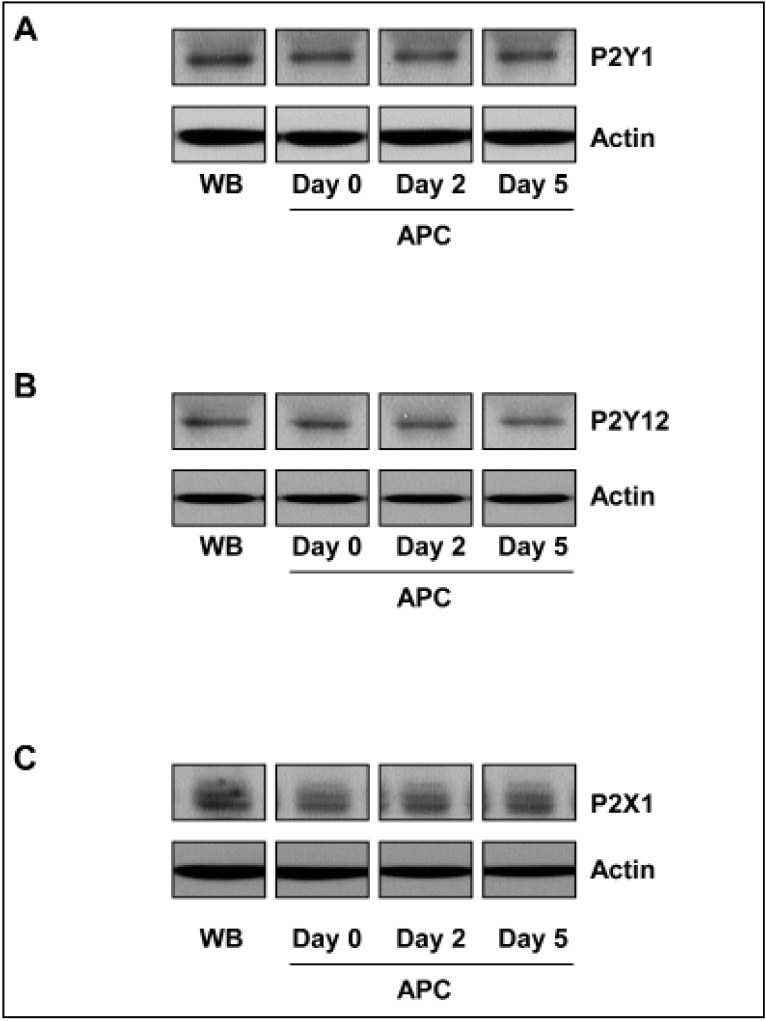
In addition, western blot analysis was performed to determine the total amount of purinergic receptors in stored platelets. For all three receptor types, the content remained unchanged during the course of storage (Figure 3).
Figure 3.
Total contents of purinergic receptors in platelets during storage.
The lysates of washed platelets (1×108 per mL) from WB and from APC on days 0, 2, and 5 were analysed by western blot for (A) P2Y1, (B) P2Y12, and (C) P2X1 receptors. The blots are representative of six independent experiments. Actin was used as a loading control.
WB: whole blood; APC: apheresis-derived platelet concentrates.
The functional activity of platelet P2Y1 and P2X1 receptors, but not of P2Y12, is reduced during storage of apheresis-derived platelet concentrates
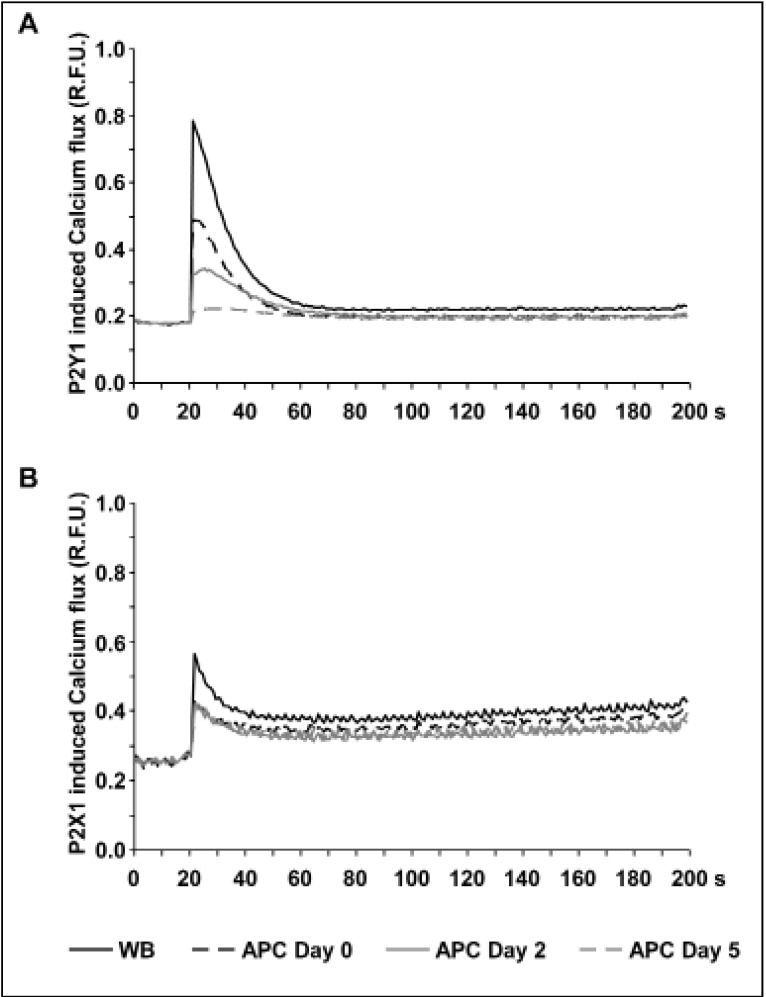
In freshly obtained WB, 1 μM MRS2365 generated a rapid 4.4±0.5-fold increase of maximal calcium-induced fluorescence compared to basal levels (Figure 4A). In freshly prepared APC, stimulation with MRS2365 only resulted in 2.7±0.2-fold elevation of calcium-induced fluorescence levels indicating that the apheresis procedure alone has the potential to affect P2Y1 function. During the course of storage, calcium-induced fluorescence levels after P2Y1 stimulation were further decreased with an increment of 1.9±0.2-fold on day 2 and only 1.2±0.1-fold on day 5 (Figure 4A).
Figure 4.
Impaired calcium flux mediated by P2Y1 and P2X1 receptors in platelets from stored APC.
The figures show the calcium-induced fluorescence curves in Fluo-4AM-loaded platelets from WB (black line), from freshly prepared APC (day 0, black dashed line) and from APC stored for 2 days (grey line) or 5 days (grey dashed line) after stimulation with (A) the P2Y1 agonist MRS2365 (A) or (B) the P2X1 agonist α,β-MeATP. Representative fluorescence curves of at least six independent experiments are shown.
R.F.U.: relative fluorescence units; WB: whole blood; APC: apheresis-derived platelet concentrates.
The functional activity of P2X1 receptors was assessed by calcium-induced fluorescence in Fluo-4AM loaded platelets stimulated with α,β-MeATP, a selective agonist of the P2X1 receptor. One micromole per litre of α,β-MeATP induced a rapid and more than 2-fold (2.2±0.2-fold) increase of maximal fluorescence in platelets from freshly donated WB (Figure 4B). In APC on day 0, calcium-induced fluorescence reached only 1.6±0.2-fold higher levels after P2X1 stimulation, pointing to a degradation of P2X1 receptor function after apheresis, similar to that of the P2Y1 receptor. However, in contrast to P2Y1, there was no progressive impairment of P2X1 function during continued storage (Figure 4B).
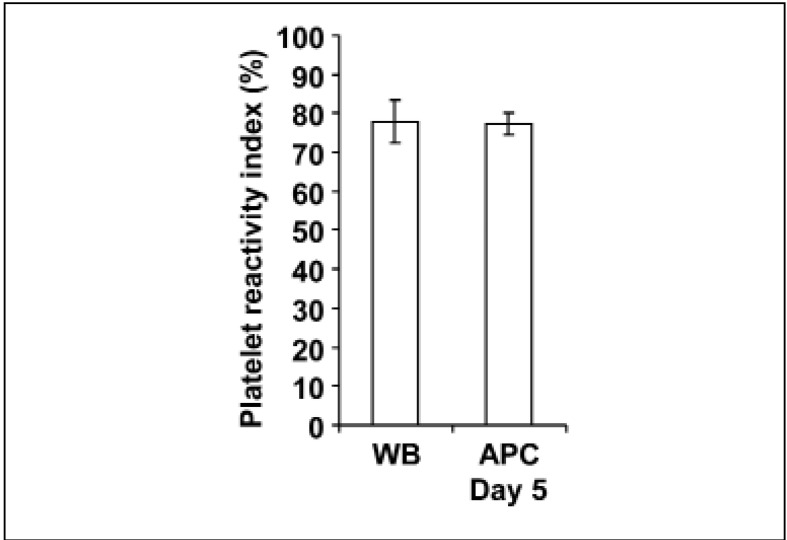
The activity of the P2Y12 receptor was determined by the PRI. Platelets from fresh WB had a mean PRI value of 77.49±5 .41% (Figure 5). The PRI values in PRP generated from the same WB were similar with 74.59±3.31% (data not shown). The PRI value of platelets in APC stored for 5 days was 77.17±2.82% and not different compared to that of fresh WB (Figure 5).
Figure 5.
Unchanged P2Y12 receptor function during storage of APC.
The histogram shows the mean PRI values in platelets from freshly obtained WB and from APC stored for 5 days. Results are presented as mean %±SEM; n=6.
WB: whole blood; APC: apheresis-derived platelet concentrates; SEM: standard error of the mean.
Discussion
Degradation of ADP-induced platelet aggregation is a common finding in stored platelet concentrates17,20–23. In this study, performed with APC, induced platelet aggregation using 10 μM ADP was tampered after apheresis and then diminished in APC on days 2 and 5 of storage (Figure 1). However, the capacity for shape change was maintained and aggregation was still inducible by the agonist TRAP-6, as in other recent studies24,25, indicating that stored platelets had preserved residual in vitro function after 5 days of storage.
Since PSL is associated with alterations on the platelet surface, e.g. with increased CD62P expression and decreased GPIIb/IIIa and GPIb/IX complex expression14, we examined to what extent impaired ADP responsiveness in stored APC is conditioned by changes of platelet purinergic receptor expression and function.
ADP exerts its platelet activation via purinergic receptors. There are three different known purinergic receptors in human platelets: P2Y1, P2Y12 and P2X13,4,6. Flow cytometric analysis of platelet surface expression did not reveal any significant differences in the basal levels of these receptors, either on platelets from freshly obtained WB or on platelets from APC stored for 5 days (Figure 2A–C).
The purinergic receptors P2Y1 and P2Y12 are not only expressed on the platelet surface, but also abundantly present inside platelets, in association with the membrane of α-granules and the open canalicular system26,27. Since the availability of receptors from different compartments may play an important role in platelet activation, the total content of these receptors was analysed in platelet lysates by western blot, which showed that the values were unchanged during storage (Figure 3). Reduced ADP responsiveness is obviously not the effect of enhanced removal of purinergic receptors. However, tampered redistribution or mobilisation of the receptors may contribute to the phenomenon, an issue which needs to be addressed in further studies.
The functional integrity of purinergic receptors is another important prerequisite for the initiation of ADP-induced platelet aggregation. The P2Y1 and the P2X1 receptors are involved in calcium regulation of platelets so that specific agonists (MRS2365 and α,β-MeATP) and Fluo-4 loading of platelets were used to measure their functional activity.
The apheresis procedure alone induced a distinct decrease of P2Y1-mediated calcium release, an effect that became permanently emphasised during storage of APC (Figure 4A). Unlike P2Y1, once reduced after apheresis by approximately 30% (Figure 4B), P2X1-mediated calcium flux remained constant in the course of APC storage confirming different underlying mechanisms of calcium regulation by these two purinergic receptors6.
The flow cytometric platelet vasodilator-stimulated phosphoprotein (VASP)/P2Y12 assay based on ADP-induced inhibition of PGE1-induced, cAMP-mediated VASP phosphorylation and presented as PRI is a common method to measure the function of the P2Y12 receptor18,28. The PRI values in samples from WB were within the reference range of healthy individuals29. In comparison to levels in WB, the PRI levels remained unchanged after apheresis and during storage of APC indicating undamaged functional activity of the P2Y12 receptors.
The P2Y12 receptor is the pharmacological target of ticagrelor and thienopyridines30–32, which are used for the treatment of patients with cardiovascular disease and implanted coronary stents33,34. The inhibition of this receptor is well known to be associated with an anti-aggregatory effect and bleeding diathesis35. Preserved activity of the P2Y12 receptor in stored platelets may, therefore, be an important requirement for the physiological functionality of transfused platelets in vivo.
The turnover and the degradation of significant proteins involved in different signal cascades may be responsible for the observed phenomena. For example, in a recent study, it was found that cGMP levels in platelets from stored APC increased due to the degradation of phosphodiesterase 5A activity, whereas the cAMP-dependent inhibitory signalling was not affected25. In this context, it is remarkable that the functional activity of the P2Y12 receptor - also signalling via the cAMP-dependent pathway - was not affected during the course of storage.
In general, it should be kept in mind that storage is accompanied by apoptosis and necrosis affecting the expression and function of platelet receptors. Shortening storage time would, therefore, improve the quality of platelet concentrates, in addition to reducing potential hazards caused by bacterial contaminations.
Conclusions
The impairment of the P2X1 and especially of the P2Y1 receptor function indicated by decreased receptor-mediated calcium flux is an important mechanism contributing to reduced ADP responsiveness of platelets from stored APC. Further studies should address the molecular basis of affected purinergic receptor function, because such knowledge is essential for the development and evaluation of novel strategies to minimise PSL.
Acknowledgements
The Authors thank their colleagues of the technical staff of the Institute of Transfusion Medicine and Haemotherapy for donation management and preparation of the platelet concentrates.
Footnotes
Authorship contributions
JK, MB, and AKob designed the research; KW, AKoe, PY, AKob acquired the data; JK, KW, AKob analysed and interpreted the data; JK, MB, and AKob drafted and revised the paper.
The Authors declare no conflict of interest.
References
- 1.White JG. Anatomy and Structural Organization of the Platelet. Philadephia: JB Lippincott; 1994. [Google Scholar]
- 2.Holmsen H. Platelet Secretion and Energy Metabolism. Philadephia: JB Lippincott; 1994. [Google Scholar]
- 3.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA. 1998;95:8070–4. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunapuli SP, Dorsam RT, Kim S, Quinton TM. Platelet purinergic receptors. Curr Opin Pharmacol. 2003;3:175–80. doi: 10.1016/s1471-4892(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 5.Vial C, Rolf MG, Mahaut-Smith MP, Evans RJ. A study of P2X1 receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors. Br J Pharmacol. 2002;135:363–72. doi: 10.1038/sj.bjp.0704486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunapuli SP. P2 receptors and platelet activation. Scientific World J. 2002;2:424–33. doi: 10.1100/tsw.2002.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauwenberghs S, van Pampus E, Curvers J, et al. Hemostatic and signaling functions of transfused platelets. Transfus Med Rev. 2007;21:287–94. doi: 10.1016/j.tmrv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Bock M, Rahrig S, Kunz D, et al. Platelet concentrates derived from buffy coat and apheresis: biochemical and functional differences. Transfus Med. 2002;12:317–24. doi: 10.1046/j.1365-3148.2002.00392.x. [DOI] [PubMed] [Google Scholar]
- 9.Holme S, Sweeney JD, Sawyer S, Elfath MD. The expression of P-selectin during collection, processing, and storage of platelet concentrates: relationship to loss of in vivo viability. Transfusion. 1997;37:12–7. doi: 10.1046/j.1537-2995.1997.37197176945.x. [DOI] [PubMed] [Google Scholar]
- 10.Thiele T, Iuga C, Janetzky S, et al. Early storage lesions in apheresis platelets are induced by the activation of the integrin alphaIIbbeta(3) and focal adhesion signaling pathways. J Proteomics. 2012;76:297–315. doi: 10.1016/j.jprot.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 11.Feinberg H, Sarin MM, Batka EA, et al. Platelet storage: changes in cytosolic Ca2+ actin polymerization and shape. Blood. 1988;72:766–9. [PubMed] [Google Scholar]
- 12.Bock M, Gawaz MP, Dietzler A, et al. Single-donor platelet concentrates: changes of surface glycoproteins during storage. Haemostasis. 1994;24:230–5. doi: 10.1159/000217106. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Mody M, Herst R, et al. Flow cytometric analysis of platelet function in stored platelet concentrates. Transfus Sci. 1999;20:129–39. doi: 10.1016/s0955-3886(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 14.Lozano ML, Rivera J, Gonzalez-Conejero R, et al. Loss of high-affinity thrombin receptors during platelet concentrate storage impairs the reactivity of platelets to thrombin. Transfusion. 1997;37:368–75. doi: 10.1046/j.1537-2995.1997.37497265336.x. [DOI] [PubMed] [Google Scholar]
- 15.Schlagenhauf A, Kozma N, Leschnik B, et al. Thrombin receptor levels in platelet concentrates during storage and their impact on platelet functionality. Transfusion. 2012;52:1253–9. doi: 10.1111/j.1537-2995.2011.03475.x. [DOI] [PubMed] [Google Scholar]
- 16.McShine RL, Weggemans M, Das PC, et al. The use of fresh plasma and a plasma-free medium to enhance the aggregation response of stored platelets. Platelets. 1993;4:338–40. doi: 10.3109/09537109309013237. [DOI] [PubMed] [Google Scholar]
- 17.Shapira S, Friedman Z, Shapiro H, et al. The effect of storage on the expression of platelet membrane phosphatidylserine and the subsequent impact on the coagulant function of stored platelets. Transfusion. 2000;40:1257–63. doi: 10.1046/j.1537-2995.2000.40101257.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz UR, Geiger J, Walter U, Eigenthaler M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets--definition and detection of ticlopidine/clopidogrel effects. Thromb Haemost. 1999;82:1145–52. [PubMed] [Google Scholar]
- 19.Liu EC, Abell LM. Development and validation of a platelet calcium flux assay using a fluorescent imaging plate reader. Anal Biochem. 2006;357:216–24. doi: 10.1016/j.ab.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 20.Murphy S, Gardner FH. Platelet storage at 22 degrees C; metabolic, morphologic, and functional studies. J Clin Invest. 1971;50:370–7. doi: 10.1172/JCI106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Minno G, Silver MJ, Murphy S. Stored human platelets retain full aggregation potential in response to pairs of aggregating agents. Blood. 1982;59:563–8. [PubMed] [Google Scholar]
- 22.Curvers J, van Pampus EC, Feijge MA, et al. Decreased responsiveness and development of activation markers of PLTs stored in plasma. Transfusion. 2004;44:49–58. doi: 10.1111/j.0041-1132.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 23.Osman A, Hitzler WE, Meyer CU, et al. Effects of pathogen reduction systems on platelet microRNAs, mRNAs, activation, and function. Platelets. 2015;26:154–63. doi: 10.3109/09537104.2014.898178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobsar A, Klinker E, Kuhn S, et al. Increasing susceptibility of nitric oxide-mediated inhibitory platelet signaling during storage of apheresis-derived platelet concentrates. Transfusion. 2014;54:1782–9. doi: 10.1111/trf.12584. [DOI] [PubMed] [Google Scholar]
- 25.Kobsar A, Putz E, Yilmaz P, et al. Decreasing phosphodiesterase 5A activity contributes to platelet cGMP accumulation during storage of apheresis-derived platelet concentrates. Transfusion. 2014;54:1008–14. doi: 10.1111/trf.12360. [DOI] [PubMed] [Google Scholar]
- 26.Nurden P, Poujol C, Winckler J, et al. Immunolocalization of P2Y1 and TPalpha receptors in platelets showed a major pool associated with the membranes of alpha-granules and the open canalicular system. Blood. 2003;101:1400–8. doi: 10.1182/blood-2002-02-0642. [DOI] [PubMed] [Google Scholar]
- 27.Haberstock-Debic H, Andre P, Mills S, et al. A clopidogrel-insensitive inducible pool of P2Y12 receptors contributes to thrombus formation: inhibition by elinogrel, a direct-acting, reversible P2Y12 antagonist. J Pharmacol Exp Ther. 2011;339:54–61. doi: 10.1124/jpet.111.184143. [DOI] [PubMed] [Google Scholar]
- 28.Geiger J, Teichmann L, Grossmann R, et al. Monitoring of clopidogrel action: comparison of methods. Clin Chem. 2005;51:957–65. doi: 10.1373/clinchem.2004.047050. [DOI] [PubMed] [Google Scholar]
- 29.Pampuch A, Cerletti C, de Gaetano G. Comparison of VASP-phosphorylation assay to light-transmission aggregometry in assessing inhibition of the platelet ADP P2Y12 receptor. Thromb Haemost. 2006;96:767–73. [PubMed] [Google Scholar]
- 30.Ahmad S, Storey RF. Development and clinical use of prasugrel and ticagrelor. Curr Pharm Des. 2012;18:5240–60. doi: 10.2174/138161212803251989. [DOI] [PubMed] [Google Scholar]
- 31.Barn K, Steinhubl SR. A brief review of the past and future of platelet P2Y12 antagonist. Coron Artery Dis. 2012;23:368–74. doi: 10.1097/MCA.0b013e3283564930. [DOI] [PubMed] [Google Scholar]
- 32.Secco GG, Parisi R, Mirabella F, et al. P2Y12 inhibitors: pharmacologic mechanism and clinical relevance. Cardiovasc Hematol Agents Med Chem. 2013;11:101–5. doi: 10.2174/1871525711311020005. [DOI] [PubMed] [Google Scholar]
- 33.Metharom P, Berndt MC, Baker RI, Andrews RK. Current state and novel approaches of antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2015;35:1327–38. doi: 10.1161/ATVBAHA.114.303413. [DOI] [PubMed] [Google Scholar]
- 34.Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–73. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 35.Cattaneo M. The platelet P2Y(1)(2) receptor for adenosine diphosphate: congenital and drug-induced defects. Blood. 2011;117:2102–12. doi: 10.1182/blood-2010-08-263111. [DOI] [PubMed] [Google Scholar]