Summary
While single nucleotide somatic mutations in the proximal promoter of the human telomerase reverse transcriptase (TERT) gene create novel consensus sequences for transcription factors that enhance TERT expression, the precise mechanism of how telomerase is re-activated in cancer cells remains poorly understood. In this issue, Ak1nc1lar and colleagues (1) identify a potential mechanism of TERT reactivation that is mediated by a novel long-range chromatin interactions between the TERT promoter on chromosome 5p and a 300 kb upstream region. This permits recruitment of the transcription factor GABPA in mutant TERT promoters but not in wild-type promoters.
Telomeres in mammals are chromosome ends that are composed of thousands of the canonical repetitive sequence TTAGGG (2). Telomeres shorten with each cell division due to the end replication problem (incomplete lagging strand DNA synthesis at the end) and post-replication end processing (telomeric overhang generation processes). In proliferating stem and germ-line cells, telomeres can be partially maintained by the cellular reverse transcriptase, termed telomerase. Telomerase adds new TTAGGG repeats onto the telomeric overhangs, but due to lack or insufficient telomerase activity, human somatic cells can only divide until at least some telomere ends become critically shortened (uncapped and recognized as a DNA double strand break). Most premalignant human lesions harbor very short telomeres and it is generally thought that the repression of telomerase and progressive telomeres shortening may have evolved in large long-lived species as an initial anti-tumor protective mechanism (2). To continue to divide, pre-malignant cancer cells need to acquire a telomere maintenance mechanism during neoplastic transformation. The vast majority of human cancers (>85%) maintain their telomere length via telomerase reactivation (2). All human tissues express the functional telomerase templating RNA component (TERC), whereas most human adult tissues do not express telomerase reverse transcriptase (TERT). During early human fetal development telomerase is expressed and, in a tissue specific manner, the TERT gene becomes silenced. While the regulation of TERT is complex and involves transcriptional, post-transcriptional and epigenetic modifications, the precise mechanism of activation of telomerase in cancer has not been resolved.
The TERT gene has a GC rich core promoter containing CpG islands that are mostly methylated in normal cells (1). The hypermethylation of the TERT core promoter is believed to be one of the major mechanisms for TERT repression but what changes the methylation status during fetal development is not known. In addition, the TERT promoter does not have typical transcriptional elements such as TATA boxes or CCAAT boxes; instead it contains GC-boxes, which are the consensus binding sites for the transcription factor Sp1. Masking of the Sp1 binding site by G-quadruplex formation in GC-boxes may be another mechanism for TERT promoter repression. Thus, transcriptional up-regulation or reactivation of the TERT gene is a critical step in tumorigenesis and multiple mechanisms have been proposed for reactivating TERT gene in cancer. These include mutation or deletion in the TERT promoter, TERT gene amplification, epigenetic alterations, and TERT gene alternative splicing factors (2). How cancer cells activate the silenced TERT gene still remains largely unknown.
From genetic linkage analysis and whole-genome sequencing, it has been recently reported that TERT gene can be reactivated by proximal promoter mutation (3,4). These are single nucleotide mutations in the proximal promoter of the TERT gene; cytosine to thymidine transition at −124 bp and −146 bp upstream of the translation start site. These TERT promoter mutations are very close to the transcription start site of the TERT gene (−46 bp and −68 bp upstream of transcription start site). Importantly, these are also located in the GC-boxes in the TERT core promoter (−90 bp to −22 bp upstream of transcription start site). These two TERT promoter mutations (−124C>T, −146C>T) are now considered among the most common noncoding mutations in cancer. For example, monoallelic TERT promoter mutations are common in melanomas (~85%), glioblastomas (~84%), hepatocellular carcinomas (~44%), liposarcomas (~79%) and urothelial cancers (~47%) (3-5). However, TERT promoter mutations are much less common (<10%) in lung, colon, esophageal, pancreatic, breast, and prostate cancers (6). It is believed that these mutations generate a de novo consensus binding motif (GGAA) for the E-twenty-six (Ets) transcription factor. Ets family transcription factors, GABPA/Ets1 have been identified as the binding factor in most cancer cells with TERT promoter mutations (7,8). A better understanding what facilitates specific proximal promoter mutations in the TERT gene could provide clues for developing therapeutic strategies to block telomerase activation in most human cancers. In this issue of Cancer discovery, Ak1nc1lar and colleagues (1) demonstrate a novel mechanism of mutant TERT promoter reactivation via long-range chromatin interactions.
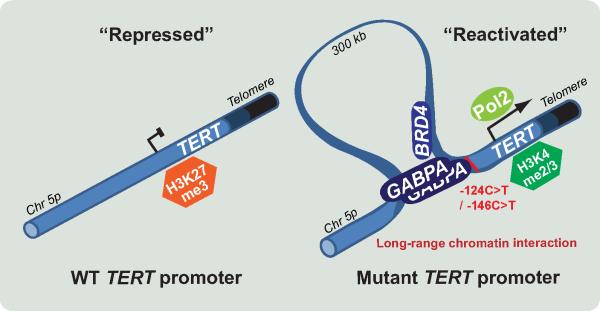
Taking advantage of CRISPR/Cas9 genome editing techniques, Ak1nc1lar et al (1) generated a series of isogenic cancer cell lines with wild-type and mutant TERT promoters. They showed that reversal of the mutant to wild-type TERT promoter led to heterochromatin changes in TERT promoter region as well as reduction in telomerase activity. This observation is consistent with the previous report that the mutant TERT promoter allele exhibits H3K4me2/3 (active chromatin histone mark) and recruits the GABPA transcription factor, whereas wild-type TERT allele retain H3K27me3 (repressed chromatin histone mark) and does not recruit the GABPA transcription factor (9,10). Since it has been recently proposed that GABPA may have a potential role in engaging long-range chromatin interactions, Ak1nc1lar investigated whether GABPA bound to mutant TERT promoter can engage long-range chromatin interactions. They performed circular chromosome capture assays to test whether the three-dimensional genome organization changed in the vicinity of mutant TERT promoter locus compared to wild-type TERT. Importantly, they found that mutant TERT promoters display long-range chromatin interactions with a region 300kb upstream of the TERT promoter (chr5: 1,556,087-1,558,758), and that these interactions are mediated by GABPA.
By performing additional genome editing experiments, they also showed that introducing a mutant sequence in wild-type TERT promoter could change long-range chromatin interactions. Moreover, the acute depletion of GABPA by using GABPA siRNA induced significant reduction of long-range chromatin interaction. These experiments indicate that long-range chromatin interactions are reversible changes and dependent on a single nucleotide mutation sequence via GABPA binding. The most striking part of this study is that Ak1nc1lar (1) generated knockout cell lines targeting the interacting region (chr5: 1,556,087-1,558,758) far from the TERT locus. The removal of the interacting region reduced TERT expression and altered the epigenetic status in the proximal TERT promoter region without any change in GABPA expression. These experiments strongly support the importance of the interaction between the proximal TERT promoter and chr5: 1,556,087-1,558,758 intergenic region in driving mutant TERT promoter activity (Figure 1).
Figure 1.
TERT promoter mutation reactivates the silenced TERT promoter via generating long-range chromatin interactions. Ak1nc1lar and colleagues (1) show that TERT reactivation by the proximal promoter mutation is directly mediated by a novel long-range chromatin interaction between the TERT promoter and a 300 kb upstream region (chr5: 1,556,087-1,558,758). GABPA proteins might generate long-range chromatin interactions by linking the mutant TERT promoter and the specific interacting region.
In summary, the report by Ak1nc1lar and colleagues (1) identifies a novel mechanism of TERT reactivation by cancer specific TERT promoter mutations that are mediated by a long-range chromatin interactions between the TERT promoter and a region 300kb from the TERT promoter. Targeting this long-range chromatin interaction exclusively harbored in cancers with TERT promoter mutation may be a potential therapeutic strategy for inhibiting telomerase activity and the indefinite cell proliferation that is one of the hallmarks of cancer. However, there are additional fundamental questions that remain unsolved regarding the potential roles of TERT promoter mutations in tumorigenesis. For example, we still do not know whether TERT promoter mutations are sufficient for re-activating completely silenced TERT genes in normal human cells (e.g., normal fibroblasts). In addition, we do not know why many common cancer types do not demonstrate TERT promoter mutations? Perhaps in these tumor types genomic amplifications, rearrangements, and TERT pre-mRNA alternative splicing factors may predominate. Finally, a potential mechanism to connect TERT promoter mutations, to the epigenetic changes observed by Ak1nc1lar et al (1), to the well-established fact that most cancers occur in the older segment of the population, may be the location of the TERT gene in humans and other large long-lived mammals. The human TERT gene is located within a megabase to the telomere on chromosome 5p, and one possibility is that TERT expression auto-regulates itself. While it is not known why telomerase is silenced at very specific times during human fetal development or why human telomeres are maintained within a small range of sizes, there is mounting evidence that when telomeres reach a specific initial length, three dimensional chromatin structures involving telomere position effects over long distances (TPE-OLD) may silence many genes including the TERT gene (2). This would serve as a mechanism to silence telomerase to prevent the early onset of cancer. Thus, as we age and telomeres progressively shorten, it is reasonable to suggest that in a monoallelic specific manner the chromatin silencing effects at one TERT promoter may change, facilitating TERT promoter mutations. This is an example of antagonist pleiotropy when one gene controls more than one trait and one of these traits is beneficial to the organism fitness early in life (silencing telomerase, reducing the early onset of cancer) and one is detrimental to the organism fitness later in life (telomerase reactivation in cancer).
Acknowledgments
Grant Support
We acknowledge support from the National Cancer Institute (Lung SPORE P50CA70907); R01 AG001228, and a distinguished chair from the Southland Financial Foundation in Geriatrics Research. This work was performed in laboratories constructed with support from NIH grant C06 RR30414.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors disclose no potential conflicts of interest.
References
- 1.Akıncılar SC, Khattar E, Boon PLS, Unal B, Fullwood MJ, Tergaonkar V. Long-range chromatin interactions drive mutant Tert promoter activation. Cancer Discov. 2016 doi: 10.1158/2159-8290.CD-16-0177. this issue. [DOI] [PubMed] [Google Scholar]
- 2.Shay JW. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016;6(6):584–93. doi: 10.1158/2159-8290.CD-16-0062. doi 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–61. doi: 10.1126/science.1230062. doi 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 4.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–9. doi: 10.1126/science.1229259. doi 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borah S, Xi L, Zaug AJ, Powell NM, Dancik GM, Cohen SB, et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347(6225):1006–10. doi: 10.1126/science.1260200. doi 10.1126/science.1260200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. doi 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 7.Bell RJ, Rube HT, Kreig A, Mancini A, Fouse SD, Nagarajan RP, et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015;348(6238):1036–9. doi: 10.1126/science.aab0015. doi 10.1126/science.aab0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Zhou QL, Sun W, Chandrasekharan P, Cheng HS, Ying Z, et al. Non-canonical NF-kappaB signalling and ETS1/2 cooperatively drive C250T mutant TERT promoter activation. Nat Cell Biol. 2015;17(10):1327–38. doi: 10.1038/ncb3240. doi 10.1038/ncb3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern JL, Theodorescu D, Vogelstein B, Papadopoulos N, Cech TR. Mutation of the TERT promoter, switch to active chromatin, and monoallelic TERT expression in multiple cancers. Genes Dev. 2015;29(21):2219–24. doi: 10.1101/gad.269498.115. doi 10.1101/gad.269498.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi L, Schmidt JC, Zaug AJ, Ascarrunz DR, Cech TR. A novel two-step genome editing strategy with CRISPR-Cas9 provides new insights into telomerase action and TERT gene expression. Genome Biol. 2015;16:231. doi: 10.1186/s13059-015-0791-1. doi 10.1186/s13059-015-0791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]