Abstract
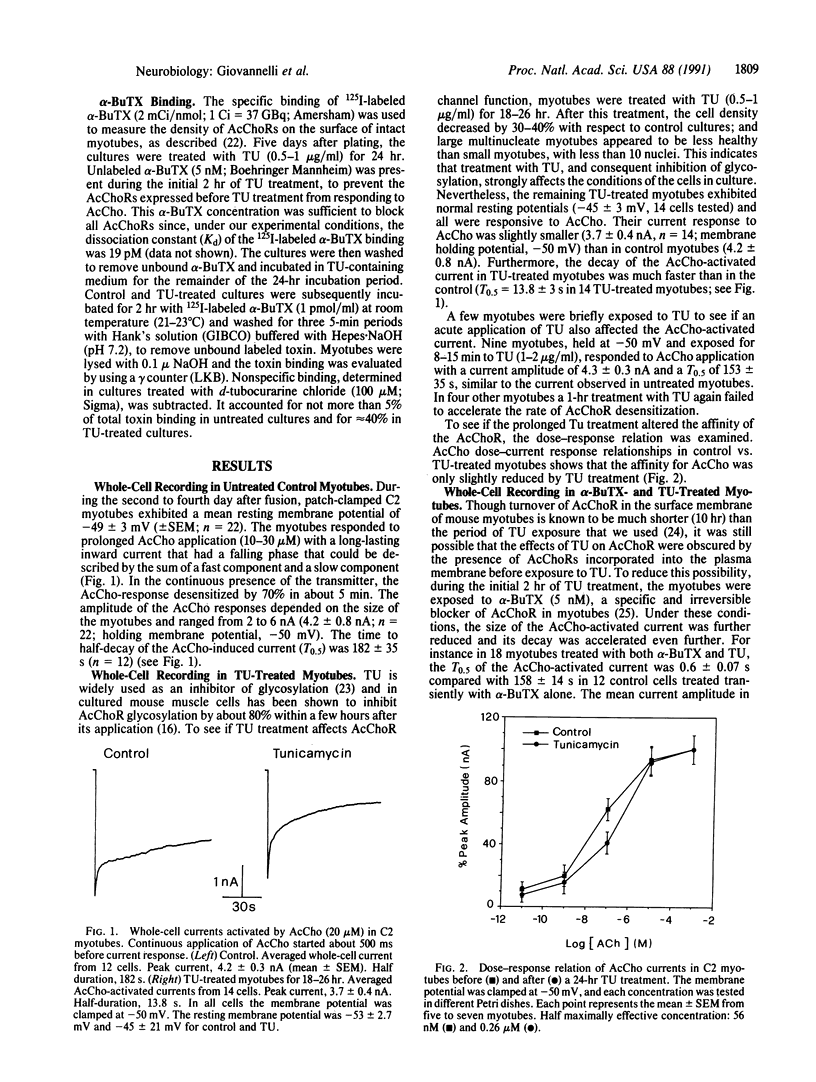
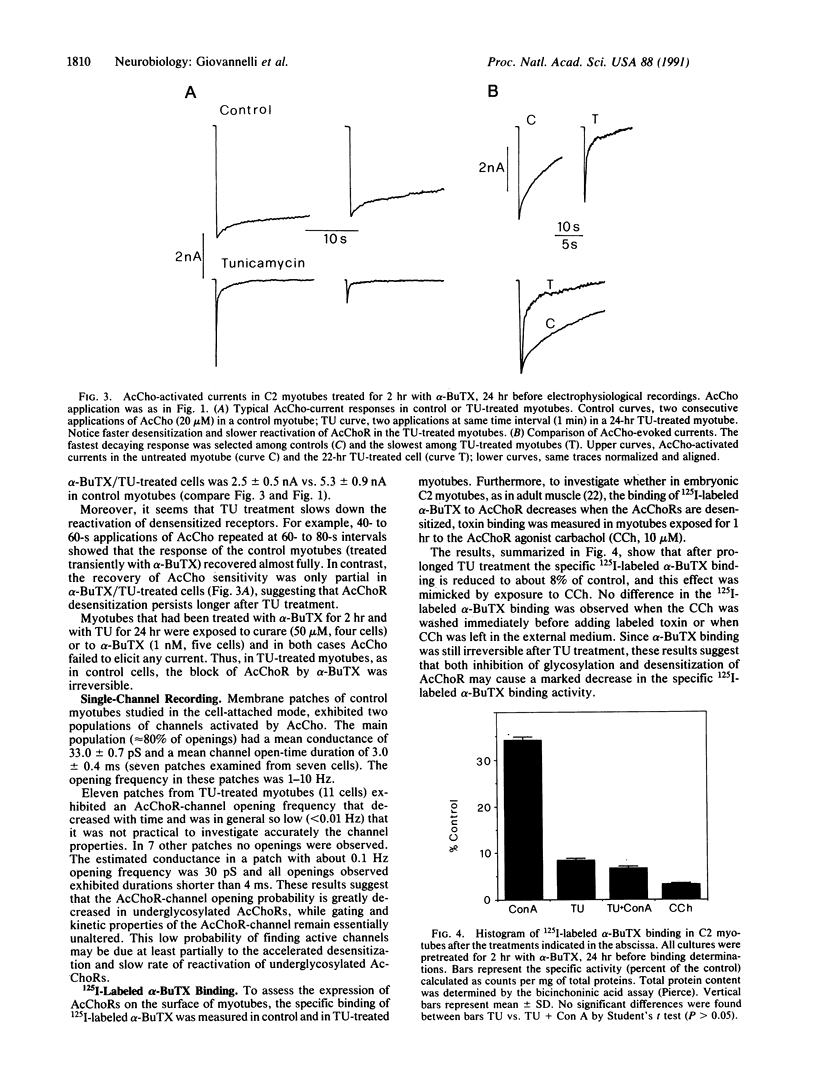
Whole-cell currents activated by acetylcholine (AcCho) were recorded in C2 mouse myotubes before and after prolonged treatment with tunicamycin, an inhibitor of glycosylation. In control cells the AcCho-induced currents decayed slowly even in the continuous presence of AcCho. After 24 hr of treatment with tunicamycin AcCho still elicited currents, but their size was significantly reduced and their decay was greatly accelerated. The binding of 125I-labeled alpha-bungarotoxin, a specific and irreversible antagonist of muscle AcCho receptors, was greatly reduced after tunicamycin treatment, and an equivalent reduction was observed after a long-lasting application of the AcCho agonist carbachol. We suggest that, after inhibition of glycosylation by tunicamycin, AcCho receptors are expressed correctly on the plasma membrane but these receptors desensitize more rapidly and are less efficient in binding alpha-bungarotoxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry. 1990 Apr 17;29(15):3591–3599. doi: 10.1021/bi00467a001. [DOI] [PubMed] [Google Scholar]
- Cossu G., Eusebi F., Senni M. I., Molinaro M. Increased endocytosis of acetylcholine receptors by dystrophic mouse myotubes in vitro. Dev Biol. 1985 Aug;110(2):362–368. doi: 10.1016/0012-1606(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi F., Grassi F., Molinaro M., Zani B. M. Acetylcholine regulation of nicotinic receptor channels through a putative G protein in chick myotubes. J Physiol. 1987 Dec;393:635–645. doi: 10.1113/jphysiol.1987.sp016845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M., Zani B. M. Agents that activate protein kinase C reduce acetylcholine sensitivity in cultured myotubes. J Cell Biol. 1985 Apr;100(4):1339–1342. doi: 10.1083/jcb.100.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hopfield J. F., Tank D. W., Greengard P., Huganir R. L. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988 Dec 15;336(6200):677–680. doi: 10.1038/336677a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1130–1134. doi: 10.1073/pnas.80.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R., Tzartos S., Lindstrom J. Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle cells. J Biol Chem. 1982 Mar 10;257(5):2694–2701. [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- Mishina M., Tobimatsu T., Imoto K., Tanaka K., Fujita Y., Fukuda K., Kurasaki M., Takahashi H., Morimoto Y., Hirose T. Location of functional regions of acetylcholine receptor alpha-subunit by site-directed mutagenesis. 1985 Jan 31-Feb 6Nature. 313(6001):364–369. doi: 10.1038/313364a0. [DOI] [PubMed] [Google Scholar]
- Mulle C., Benoit P., Pinset C., Roa M., Changeux J. P. Calcitonin gene-related peptide enhances the rate of desensitization of the nicotinic acetylcholine receptor in cultured mouse muscle cells. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5728–5732. doi: 10.1073/pnas.85.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., McMillan J., Wolfson H., O'Brien J. C. Acetylcholine receptor metabolism in a nonfusing muscle cell line. J Biol Chem. 1977 Mar 25;252(6):2143–2153. [PubMed] [Google Scholar]
- Prives J. M., Olden K. Carbohydrate requirement for expression and stability of acetylcholine receptor on the surface of embryonic muscle cells in culture. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5263–5267. doi: 10.1073/pnas.77.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives J., Bar-Sagi D. Effect of tunicamycin, an inhibitor of protein glycosylation, on the biological properties of acetylcholine receptor in cultured muscle cells. J Biol Chem. 1983 Feb 10;258(3):1775–1780. [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Assembly and N-glycosylation of all ACh receptor subunits are required for their efficient insertion into plasma membranes. Brain Res Mol Brain Res. 1989 May;5(3):183–192. doi: 10.1016/0169-328x(89)90034-x. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Change in desensitization of cat muscle acetylcholine receptor caused by coexpression of Torpedo acetylcholine receptor subunits in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):367–371. doi: 10.1073/pnas.86.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Effect of tunicamycin on the expression of functional brain neurotransmitter receptors and voltage-operated channels in Xenopus oocytes. Brain Res. 1988 Nov;464(3):191–199. doi: 10.1016/0169-328x(88)90025-3. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989 Dec;3(6):665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Zona C., Eusebi F., Miledi R. Glycosylation is required for maintenance of functional voltage-activated channels in growing neocortical neurons of the rat. Proc R Soc Lond B Biol Sci. 1990 Mar 22;239(1295):119–127. doi: 10.1098/rspb.1990.0011. [DOI] [PubMed] [Google Scholar]



