Abstract
Purpose
Compared with young White women, young Black women are more likely to present with aggressive breast cancer (BC) subtypes that are potentially linked to worse health-related quality of life (HRQOL); however, there is limited consensus regarding HRQOL needs among young Black BC survivors. Employing Ferrell's framework on QOL in BC (i.e., physical, psychological, social, and spiritual well-being), we conducted a systematic review on HRQOL among Black BC survivors aged <50 years and proposed recommendations for advancing HRQOL research and care for this population.
Methods
Literature searches were conducted in MED-LINE/PubMed, EMBASE, CINAHL, and PsycINFO to identify relevant articles published from 1995 to 2015. Abstracts and full-text articles were screened using predetermined inclusion/exclusion criteria and evaluated for quality.
Results
A total of 2533 articles were identified, but six met eligibility criteria. Most studies examined multiple HRQOL domains, with the psychological domain most represented. Compared with their older, White, and BC-free counterparts, young Black BC survivors reported greater fear of dying, unmet supportive care needs, financial distress, and lower physical/functional well-being. However, spiritual well-being appeared favorable for young Black survivors. Research gaps include the absence of longitudinal studies and under-representation of studies examining physical, social, and particularly, spiritual HRQOL in young Black BC survivors.
Conclusions
Young Black BC survivors generally experience suboptimal HRQOL after BC diagnosis. As few studies have reported on HRQOL among this group, future research and oncology care should prioritize young Black women in ways that recognize their unique concerns, in order to ensure better HRQOL outcomes both during and after treatment.
Keywords: Breast cancer, Black African-American, Premenopausal, Quality of life
Introduction
Breast cancer (BC) is the most common cancer among women worldwide [1]. Yet, studies indicate that BC risk and burden are not evenly shared between White and Black women in the U.S. For example, although BC incidence rates are similar in White and Black women, mortality rates are higher in Blacks [2–8]. These findings may be partly explained by the fact that Black women tend to be diagnosed at younger ages (i.e., premenopausal) [9, 10] and with more aggressive BC subtypes (i.e., hormone receptor negative, lymph node positive) [11, 12] that require more aggressive treatments (e.g., chemotherapy and radiation) and are associated with worse prognosis [11, 12]. Aggressive treatments and greater risk of death negatively impact both physical and psychosocial health-related quality of life (HRQOL) [13–16]. Given that Black women with BC are at increased risk for aggressive, premenopausal BC [17], understanding HRQOL needs specific to this population is imperative.
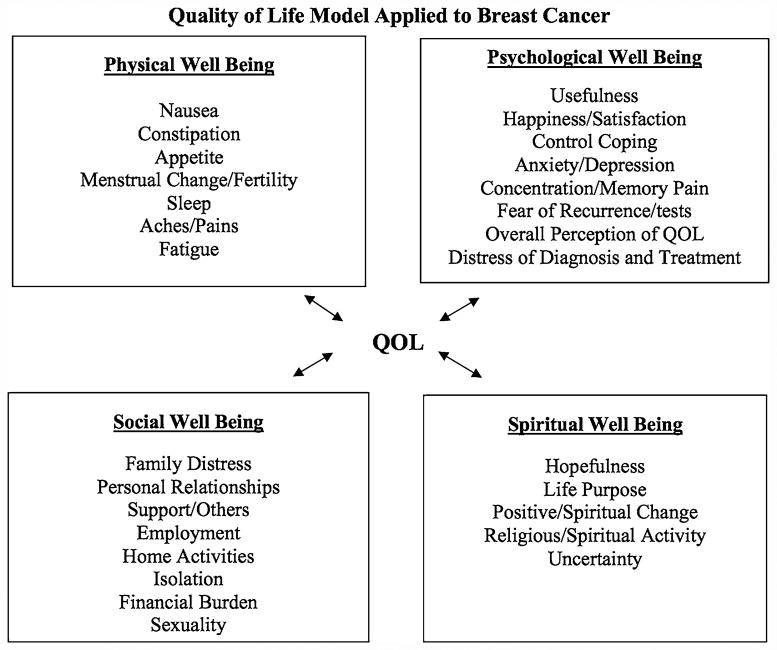
Four distinct HRQOL domains have been identified in BC survivors and vary by race: physical (e.g., fatigue, nausea, lymphedema), social (e.g., distress, sexuality, personal relationships), psychological (e.g., fear of recurrence, anxiety, depression), and spiritual (e.g., hopefulness, uncertainty about the future, self-discovery) well-being (Fig. 1) [17–19]. Overall, BC survivors report deterioration in physical and mental health during diagnosis, treatment, and survivorship [20]; however, Black BC survivors report poorer HRQOL than Whites [21–24]. Specifically, Blacks report worse physical and functional well-being [24, 25]. Conversely, Black BC survivors also report positive emotional growth throughout the BC care continuum, which may explain reports of fewer psychological and spiritual well-being decrements compared with White BC survivors [26, 27].
Fig. 1. Ferrell's Conceptual Framework on quality of life in Breast Cancer.
Overall, after adjusting for demographic and clinical characteristics, BC survivors younger than 50 years report worse HRQOL compared to their 50 and older counterparts [28, 29]. A systematic review on HRQOL in young BC survivors documented psychosocial HRQOL decrements such as depression and anxiety, as well as menopausal symptoms including weight gain, hot flashes, and vaginal dryness in this survivor group [28]. One potential explanation for these differences is that younger women are more likely to be active in the workforce and have childcare responsibilities than older women [30, 31]. Issues surrounding fertility and treatment-induced menopause may also be more concerning for younger BC survivors [30, 32, 33], who are more likely to be premenopausal at diagnosis [17]. Thus, compared with older women, younger women may experience more difficulty adjusting to a BC diagnosis [17, 30].
Independent of their BC diagnosis, young Black women may experience high levels of stress due to cultural and contextual factors [34–36]. Research on the nuanced experiences of life stressors among Black women suggest that Superwoman Schema, or perceived obligations to present an image of strength, suppress emotions, resist support from others, achieve success with inadequate resources, and prioritize caregiving over self-care can adversely affect health-promoting behaviors and HRQOL [35, 37]. Moreover, Network Stress, or the stress experienced by Black women as a result of life challenges experienced by family members and friends [36], might magnify ways in which stress negatively influences aspects of HRQOL in this population. Such role perceptions and stress-related processes may be more pronounced among young Black BC survivors who are faced with the unique intersectional experience and challenges of being young, Black, and diagnosed with BC.
Although previous systematic reviews investigated HRQOL in Black women, these reviews failed to stratify outcomes by both race and age group, there by masking HRQOL needs specific to young Black BC survivors [21, 38–40]. Furthermore, previous studies limited definitions of “cancer survivor” to the period following active treatment or the 5-year survival landmark, overlooking HRQOL concerns immediately following diagnosis [21, 40]. As young Black BC survivors present with more aggressive disease, experience more burdensome treatments, and potentially worse HRQOL beginning from diagnosis, there is a need to better understand HRQOL concerns specific to this group. As such, we conducted a systematic review examining existing evidence on HRQOL patterns among young Black BC survivors.
Employing Ferrell's conceptual framework on QOL in Breast Cancer and a lifetime definition of BC survivor (diagnosis to death) [38, 41], we evaluated literature on HRQOL among Black BC survivors under age 50 [21, 39]. The objective of this study was to synthesize evidence on contemporary HRQOL concerns and needs among young Black BC survivors in the U.S., identify gaps in research and clinical practice, and propose recommendations for advancing HRQOL research and improving support services for this patient population.
Methods
Literature search strategy
A comprehensive literature search was developed and conducted in MEDLINE/PubMed, EMBASE, CINAHL, and PsycINFO to identify relevant articles published from 1995 (publication year of Ferrell's original article on Measurement of QOL in Cancer Survivors, which informed Ferrell's 1996 conceptual framework on QOL in Breast Cancer) [18, 19] through July 24, 2015. The literature search included Medical Subject Headings and Emtree headings and related text and keyword searches when appropriate, focusing on terms used to describe HRQOL in young Black BC survivors. An experienced librarian conducted the searches, with input from clinician and non-clinician research team members (see Appendix 1 for search strategy).
Inclusion and exclusion criteria
Eligibility criteria were developed with respect to the population, outcomes of interest, study design, and publication type. Studies were eligible for inclusion if they presented stratified data on HRQOL [18, 19] among Black female BC survivors under age 50. Fifty years was selected as the young women age cutoff because this is the mean age of menopause in Black women [42] and a commonly used cutoff in other studies examining BC-related outcomes in young women [28, 43, 44]. Consistent with current definitions of a “cancer survivor,” we employed a lifetime definition capturing time from diagnosis until death [41, 45]. Conference abstracts and non-peer-reviewed publications were excluded, as were non-empirical studies, non-English studies, non-U.S.-based studies, and studies not separately reporting HRQOL for our target population (i.e., Black BC survivors under age 50).
Study selection
Two trained research team members independently screened titles and abstracts for inclusion using the eligibility criteria. Studies with titles and abstracts that met the inclusion criteria or lacked adequate information to determine inclusion/exclusion underwent full-text review. A senior member of the review team resolved conflicts.
During the full-text review, two trained members of the team independently reviewed full-text articles for inclusion/exclusion. If both reviewers agreed that a study did not meet eligibility criteria, the study was excluded. If reviewers disagreed, conflicts were resolved by consulting a senior member of the team.
Literature search results
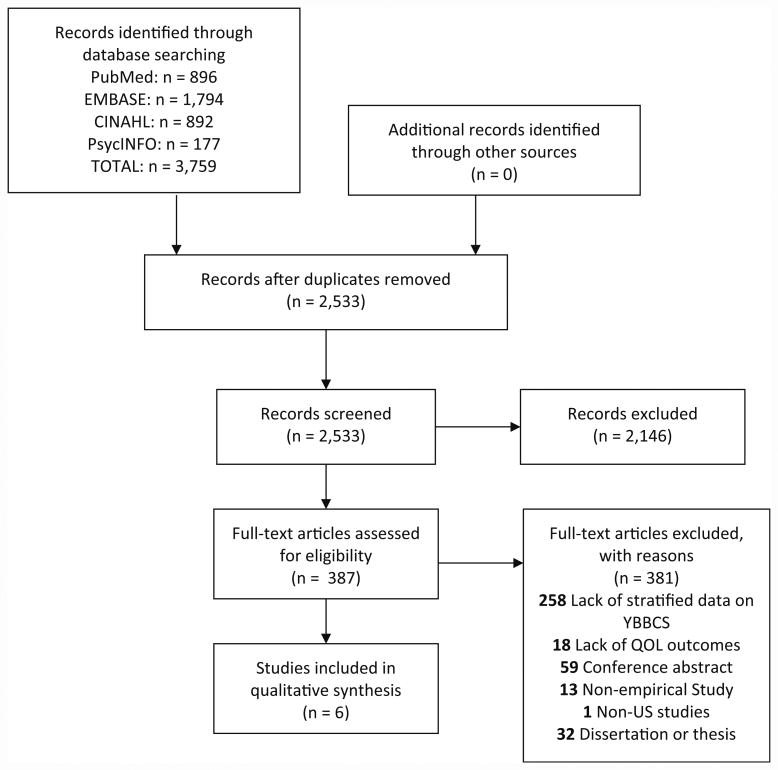
A total of 3759 articles were identified through database searching, of which 2533 were non-duplicates. A total of 2146 articles were excluded during the abstract screening phase, 381 articles were excluded during full-text review, and six articles met all eligibility criteria for inclusion (see Fig. 2 for article disposition).
Fig. 2. Literature flow diagram.
Data extraction and quality appraisal
Four independent reviewers abstracted data from the six eligible articles. Abstracted data were organized into a standardized table that included study purpose, conceptual framework, setting and participant eligibility criteria, sample characteristics, study design, HRQOL measures and instruments, and key HRQOL domain findings (based on Ferrell et al. [18, 19]) specific to young Black BC survivors (Table 1).
Table 1. Summary of included studies.
| First author (year) |
Study objectives (pg no.) |
Conceptual framework (pg no.) |
Setting and eligibility criteria (pg no.) |
Sample and key participant characteristics (pg no.) |
Study design and data collection methods (pg no.) |
HRQOL measures [and instrument, if applicable] (pg no.) |
HRQOL dimension(s) [physical, psychological, social, spiritual] (pg no.) |
Key findings for YBBCS (pg no.) |
Appraisal tool and number of items met on checklist out of total (STROBE or CASP) |
|---|---|---|---|---|---|---|---|---|---|
| Beder (1995) | Identify the unique social support challenges faced by socioeconomically disadvantaged Black women who had mastectomies and use these findings to instruct social workers and service providers on how to better serve this patient population—pg 57 | N/A | Setting: recruited from 6 New York City area hospitals Eligibility criteria: Black women, 65 years or younger with a first-time diagnosis of stage I, II, or III breast cancer, and who underwent a mastectomy—pg 58 |
Sample: 50 socioeconomically disadvantaged Black women who were 3 months post-mastectomy and 50 socioeconomically disadvantaged Black women who were 12 months post-mastectomy (N = 100)—pg 57 Participant characteristics: Age range: 25–65 85 % US-born 78 % not married 71 % had 12 years or less of education 97 % had children 69 % were working at the time of their surgery 33 % lived alone—pg 61 |
Study design: cross-sectional, quantitative—Pg 57 Data collection Method: survey interview—pg 58 |
HRQOL measures included psychological adjustment (Psychological Adjustment to Illness Scale - PAIS, Derogatis and Derogatis, 1990) Social support (Interpersonal Support Evaluation List - ISEL, Cohen 1985)—pg 59 |
Social well-being—pg 55 psychological well-being—Pg 57 | Black breast cancer survivors aged 25–45 years reported significantly worse adjustment (i.e., psychological well-being) compared to Black breast cancer survivors aged 45-65 years—pg 66 Black breast cancer survivors aged 25–45 years reported greater levels of perceived social support (i.e., social well-being) compared with Black breast cancer survivors aged 45-65 years—pg 67 |
13/22 (STROBE) |
| Gibson (2014) | Describe breast cancer fear according to phase of survivorship, determine whether breast cancer fear levels differed across survivorship phases, and determine the relationship between fear and age in Black breast cancer survivors—pg135 | Psychological well-being construct defined in the QOL Model by Ferrell & Grant (1996)—pg 137 | Setting: recruited from outpatient oncology facilities in the southeastern US, and Black/African-American cancer organizations, church groups, sorority groups, and other organizations Eligibility criteria: Black breast cancer survivors—pg 137 |
Sample: 46 women from outpatient facilities and 116 women from Black women's cancer organizations (TV — 162) Participant characteristics: Age range: 31-85 87 % were not receiving any active treatment Mean income range between $30,000 and 34,999, with 40 % making more than $40,000 66 % were college educated—pg 138 |
Study design: cross-sectional, quantitative Data collection Method: secondary data analysis of previously collected data—pg 137-138 |
HRQOL measures included (Quality of Life/Breast Cancer Psychological Weil-Being (PWB) Subscale, Ferrell and Grant, 1996))—pg. 137-138<br>Breast cancer fear (Adapted from the Quality of Life/Breast Cancer Psychological Weil-Being (PWB) Subscale, Ferrell and Grant, 1996)—pg. 137-138 | Psychological well-being—pg 138 | Black breast cancer survivors aged 31–49 years reported lower levels of fear (psychological well-being) compared with Black breast cancer survivors aged 50-64 and 64-85 years—pg 140 | 17/22 (STROBE) |
| Janz (2009) | Determine whether racial/ethnic differences in quality of life exist among White, Black, and Latina women in the early phase of breast cancer survivorship—pg 212 | N/A | Setting: recruited from Los Angeles and Detroit Surveillance Epidemiology and End Results (SEER) program registries Eligibility criteria: women aged 20-79 years diagnosed with primary ductal carcinoma in situ (DCIS) or invasive stages I, II, or III breast cancer—pg 213 |
Sample: 2268 Black, White, and Latina women (mean time since diagnosis — 9 months)—pg 212 Participant characteristics: Age range: 20–79 Black and Latina women were more likely than White women to be under age 50 at time of diagnosis—pg 215 |
Study design: cross-sectional, quantitative Data collection Method: survey interview—pg 213 |
HRQOL measures included physical well-being; emotional well-being; functional well-being; and social/family well-being (The Functional Assessment of Cancer Therapy-Breast (FACT-B))–pg 214 | Physical well-being and psychological well-being—pg 214 | Black breast cancer survivors under age 50 reported lower levels of physical and psychological well-being compared with Black breast cancer survivors aged 50-70 and >70 years—pg 220 | 21/22 (STROBE) |
| Lewis (2012) | Examine how Black women cope with breast cancer and whether societal pressures may add to coping difficulties—pg 2 | N/A | Setting: recruited through a national survey Eligibility criteria: Black women younger than 45 years that were at least 1 year post-diagnosis of breast cancer, off active treatment other than hormonal therapy, and demonstrated adequate English skills—pg 2–3 |
Sample: 33 women Participant characteristics: Age range: 25–4-5–pg 1 |
Study design: cross-sectional, mixed methods Data collection method: survey interview—Pg 3 |
HRQOL measures included the impact of cancer on women's living situations, employment, relationships, fertility, and sexuality (Instrument: N/A—internally developed by study research team)—pg 3 | Psychological and social well-being—pg 1; physical well-being—pg 8); spiritual well-being—Pg. 8 | Black breast cancer survivors under age 50 (entire cohort) reported problems with relationships and isolation (social well-being), emotional and functional support (social well-being), and infertility and sexual dysfunction (physical well-being)—pg 6 61 % of Black breast cancer survivors reported increased anxiety (psychological well-being) related to fear of dying—pg 6 Black breast cancer survivors reported strong spiritual well-being—pg 8 |
7/10 (CASP) 20/22 (STROBE) |
| Morrow (2014) | Demonstrate the differential effects of age, race, cancer diagnosis, and treatment on quality of life in young breast cancer survivors—Pg21 | N/A | Setting: Recruited from University of Texas MD Anderson cancer center Eligibility criteria: Women aged 18 or older who were younger than 45 at the time of breast cancer diagnosis, were more than 12 months past their breast cancer diagnosis, and have received or were currently undergoing surgery, chemotherapy, radiation therapy, or adjuvant endocrine therapy for breast cancer—pg 21–22 |
Sample: 1090 Black, Asian, Hispanic, Native American, and White women Participant characteristics: Age range: 18–45 78 % married More than half underwent a mastectomy —pg 23 |
Study design: cross-sectional, quantitative Data collection method: self-administered survey—pg 22 |
HRQOL measures included the appearance concerns, financial problems, distress over recurrence, family-related distress, and benefits of cancer (Quality of Life in Adult Cancer Survivors (QLACS))—pg 21–22 | Social well-being—pg 22 | Black breast cancer survivors under age 45 reported greater levels of financial distress (social well-being) compared with White breast cancer survivors under age 45—pg 28 | 21/22 (STROBE) |
| Sheppard (2013) | Assess levels of depressive symptomatology in Black women with breast cancer compared to women without breast cancer, and examine demographic, psychosocial, and clinical factors correlated with depression—pg 292 | N/A | Setting: recruited from Washington, DC and surrounding suburbs Eligibility criteria: Entire Cohort - Black women between 40 and 50 years, not currently being treated for depression, and not currently engaged in abuse of illicit drugs. Breast Cancer Cohort -Women with breast cancer were eligible if diagnosed with breast cancer within 12 months of data collection—pg 292–293 |
Sample: 152 (n = 76 breast cancer cases; n = 76 non-breast cancer controls) women Participant characteristics: Age range: 40–50 Over 25 % with incomes over $40,000 Over 65 % were college educated—pg 294 |
Study design: cross-sectional, quantitative Data collection method: self-administered survey—pg 294 |
HRQOL measures included depressive symptomatology-(Beck Depression Inventory-Short Form) effective functioning, adaptability, and personal resourcefulness (Barron's Ego Strength Scale (MMPI-2))—pg 294 |
Psychological and physical well-being—pg 294 | Black breast cancer survivors aged 40-50 reported higher levels of depression (psychological well-being) compared with Black women aged 40-50 without breast cancer—pg 294 Black breast cancer survivors aged 40-50 reported worse functioning (physical well-being) compared with Black women aged 40-50 without breast cancer—pg 294 |
20/22 (STROBE) |
HRQOL Health-related quality of life, CASP Critical appraisal skills program, STROBE Strengthening the reporting of observational studies, YBBCS Young Black breast cancer urvivors
Two independent reviewers assessed the quality of each article using the 22-item Strengthening the Reporting of Observational Studies (STROBE) tool [46]. One study, which employed qualitative and quantitative methods, was also assessed using the ten-item Critical Appraisal Skills Program (CASP) qualitative tool [47]. Table 1 includes an appraisal tool ratio, calculated as the number of STROBE/ CASP criteria met by the study divided by the total number of tool criteria [21]. No studies were excluded based on quality appraisal outcomes.
Results
Summary of existing literature
Among the six articles included, five were cross-sectional quantitative studies and one was a cross-sectional mixed-methods study (Table 1). Eligibility criteria related to time since diagnosis varied, with one study sampling only BC survivors within 12 months of diagnosis, two studies sampling BC survivors at least 12 months post-diagnosis, and three studies sampling BC survivors regardless of time since diagnosis. Three studies were limited to Black BC survivors, two studies focused on young women, and one study recruited only young Black BC survivors. One study employed a conceptual framework, specifically Ferrell and Grant's Quality of Life Model [18, 19]. All studies employed a range of validated HRQOL measurement tools, including the Psychological Adjustment to Illness Scale [48], Interpersonal Support Evaluation List [49], Quality of Life/Breast Cancer Psychological Well-Being Subscale [18, 19], Functional Assessment of Cancer Therapy-Breast [50], Quality of Life in Adult Cancer Survivors [51], Beck Depression Inventory-Short Form [52], and Barron's Ego Strength Scale [53]. All studies met 50 % of the critical appraisal criteria, and five studies met over 70 % of appraisal criteria. A summary of key findings by HRQOL domain is described in the following sub-sections.
Psychological well-being
Five studies reported on at least one aspect of psychological well-being in young Black BC survivors. In one study, younger Black BC survivors reported significantly worse psychological adjustment post-surgery than older Black BC survivors [54]. Two studies examined fear in Black BC survivors. Findings from one study suggested that compared with older survivors, younger Black BC survivors were less likely to report high levels of fear of recurrence (67 vs. 37 %) [55]. However, another study indicated that 61 % of young Black BC survivors reported anxiety related to fear of dying [56]. Moreover, one study documented lower psychological well-being among younger Black BC survivors relative to older Black survivors [57]. Similarly, another study found that young Black BC survivors were more likely to report depressive symptoms than young Black women with no BC history [58].
Social well-being
Issues related to social support, relationship strain, and financial distress were described in three studies. One study reported greater levels of social support among younger Black BC survivors compared with older Black survivors [54]. Findings from another study revealed substantial emotional (70 %) and functional support (56 %) needs among young Black BC survivors [56]. Most young Black BC survivors in that study indicated that they received emotional (58 %) and functional support (67 %) from families and community; however, 30 % reported unmet emotional support needs around time of diagnosis. In the same study, romantic relationship strain and feeling isolated emerged as key concerns. Finally, a third study found that young Black BC survivors reported higher levels of financial distress than their White counterparts [59].
Physical well-being
Findings from the three studies reporting on physical functioning suggest that young Black BC survivors experience substantial decrements in physical well-being. Compared with older Black BC survivors and young Black women without BC, young Black BC survivors reported lower physical well-being and functioning [57, 58]. Issues related to infertility and sexual dysfunction (e.g., loss of sexual desire, vaginal dryness, pain during intercourse) were reported among young Black participants in one study [56]. Approximately half of young Black BC survivors in that study reported wanting to have a child near time of BC diagnosis; however, 48 % of women did not recall discussing infertility with providers and fewer than 15 % became pregnant since completing BC treatment. This study, however, did not report on pregnancy attempts or infertility.
Spiritual well-being
One study reported on spiritual well-being in young Black BC survivors. Fifty percent of women reported that their BC diagnosis positively changed their outlook for the future and helped them develop a new appreciation for life. Additionally, 18 % indicated that their illness strengthened their spirituality and religious faith [56].
Gaps in existing literature
Several gaps in the existing literature are worth noting. Overall, few studies were grounded in HRQOL theory and none were guided by theories that accounted for race- or age-related differences in HRQOL (e.g., Superwoman Schema, Network Stress, Weathering Effect [35, 36, 60–62]). Such theories can help inform our understanding of modifiable pathways leading to differential HRQOL outcomes in young Black BC survivors. Additionally, no study quantified potential mediating factors (i.e., targets for intervention, such as patient–provider communication, supportive care access) impacting HRQOL outcomes. Similarly, the absence of longitudinal studies examining HRQOL changes over time and intervention studies targeting HRQOL outcomes in young Black BC survivors represents a significant research gap that hinders progress in identifying critical time points for intervention and effective strategies for optimizing HRQOL in this survivor group.
Moreover, compared with the psychological domain, evidence on physical, social, and spiritual HRQOL in this patient population is scarce. Previous studies indicate that Black cancer survivors typically experience worse physical functioning and side effects relative to Whites [25, 26]. Therefore, exploring this aspect of HRQOL in young Black BC survivors is especially important, as their physical HRQOL needs may differ from their older counterparts. Furthermore, despite high levels of spirituality commonly reported among Black women [63–66], only one study examined spirituality in young Black BC survivors.
Discussion
This systematic review summarized and critiqued the limited available literature describing HRQOL among young Black BC survivors across the BC continuum, from diagnosis until death, and highlights the need for more research on HRQOL in this important patient population. The review revealed that young Black women generally experience worse overall HRQOL after BC diagnosis, compared with their older Black, younger White, and older White counterparts, as well as compared with young Black women without BC. Although comparison groups varied across studies, regardless of the comparison, young Black women with BC fared worse in most HRQOL domains, except in spiritual well-being (where there was no between group comparisons). With respect to psychological well-being, young Black women with BC reported greater fear of dying, but not fear of recurrence, compared with older Black women [55, 56]. They also reported greater depressive symptoms compared with young Black women without cancer [58]. Regarding social well-being, despite reporting greater social support than older Black women with BC, 30 % of young Black women expressed unmet social needs [58]. Compared with young White women with BC, young Black women experienced greater financial distress [59]. Moreover, relative to older Black women with BC and young Black women without BC, young Black BC survivors reported lower physical and functional HRQOL [57, 58].
These findings suggest a need for routine HRQOL assessments, especially distress screening, among young Black BC survivors. Distress screening identifies psychosocial (cognitive, behavioral, emotional), social, and spiritual signs that may indicate depression, anxiety, financial distress, social isolation and lack of social support, or other threats to individual well-being [67]. Ideally, screening for distress in oncology patients should occur during an initial medical visit, employ a standardized and validated instrument, and, when warranted, lead to timely referrals to psychosocial services, financial counseling, or other support services [68]. Given the 2012 American College of Surgeons Commission On Cancer (CoC) recommendation that providers should screen patients for psychosocial distress, findings from our review suggest that young, Black women, in particular, may benefit more from systematic distress screening than other subgroups since their burden of distress is greater across most HRQOL domains. However, as shown in this review, for young Black women, it is essential that distress-screening tools target areas such as depression and financial distress. The National Comprehensive Cancer Network Distress Thermometer and Problem List is one screening instrument that can be used quickly and efficiently to identify cancer patients in distress [69]. Using this tool, 30 % of BC patients have reported high levels of distress (scores [5 on a ten-point scale), but more research is needed in large, racially diverse populations to understand whether or not young Black women report greater distress using such instruments, and if so, whether referral and intervention mechanisms need to be tailored in ways to mitigate distress in this population [70].
Moreover, our findings have important implications for clinical practice and supportive care. First, because HRQOL varies by race and age, with young Black women more often experiencing worse HRQOL, routinized distress screening [67–70] can potentially help providers identify and address unique psychosocial issues that disproportionately affect this population. Specifically, routine screening can enhance providers' surveillance and follow-up on these issues (e.g., social isolation) and may lead to referral (e.g., social worker and mental health services) and care delivery that is more timely, appropriate, patient centered, and culturally sensitive than has historically been provided.
Second, patient and survivor resources must target specific needs of young Black women; for example, by offering culturally relevant interventions [71], including peer support counseling that emphasizes unique experiences and concerns of young Black women—many of whom are employed, with young children or fertility intentions that may be less relevant to older women. Moreover, because experiences of Superwoman Schema and Network Stress are well documented in Black women [35, 36], approaches that simultaneously promote stress management, effective coping with multiple demands, and the importance of self-compassion and self-care may be especially beneficial to improving HRQOL in young Black BC survivors [72].
Third, young Black women often present with advanced BC disease, resulting in aggressive treatments [11]. Providers and support staff should be transparent in informing young Black women regarding treatment expectations and outcomes and addressing specific treatment-related concerns unique to this group (e.g., treatment-induced infertility).
Finally, considering the greater financial vulnerability of young Black BC survivors, evidenced by higher financial distress reported in this review [59], and greater representation of young Black women among uninsured and Medicaid populations [73], systematic referrals to financial counselors, social workers, and other support services to address financial concerns may be particularly important to mitigating financial-related stress and anxiety that young Black women with cancer differentially experience.
These findings suggest a number of research implications. First, despite initially identifying 2533 potentially relevant articles, after applying eligibility criteria, only six articles were included in this review. Still, these articles met most STROBE/CASP quality appraisal criteria [46, 47]. The paucity of HRQOL data, however, points to a critical need for additional HRQOL studies and culturally tailored psychosocial interventions targeting young Black BC survivors and the providers serving them. Furthermore, given that all six studies were cross-sectional, future work should assess HRQOL changes over time across the BC continuum, as cross-sectional HRQOL assessments may limit our understanding of experiences and needs of this group. This review also highlights how studies providing only overall estimates of HRQOL (i.e., non-domain-specific) may fail to recognize spirituality as a protective coping mechanism among young Black women [56]. Therefore, exploring more fully (and designing interventions to enhance) spiritual well-being may be especially impactful in this group.
Several limitations warrant mention. In this review, studies which categorized “young” BC survivors as those less than 55 years of age were identified, but excluded studies based on the predetermined eligibility criteria. As described, 50 years is the age threshold commonly reported in the literature [28, 43, 44] to dichotomize young and old women and reflects the mean age at menopause in Black women. Therefore, studies using alternative definitions of “young” were excluded from this review. However, one would not expect findings among Black BC survivors ages 50–55 to vary substantially from results reported in this study. Finally, findings should be interpreted with a focus on understanding HRQOL experiences of Black women with BC who are less than 50 years of age, and not experiences of older Black women, young Black women without cancer, women with cancers other than BC, or other minority groups, all of which were outside the scope of this review.
Based on our findings, the following recommendations for advancing HRQOL research and improving supportive care services for this patient population are proposed: (1) Expanding HRQOL research among young Black BC survivors is critical. Moreover, because young Black women are not often the focus of targeted resources and social support and less often enrolled in clinical trials [74], clinicians and researchers should broaden the aperture to design and test interventions that encompass the needs of this group (e.g., whole-person, family, and culturally oriented care), with specific recruitment strategies to promote inclusion of young Black BC survivors. (2) HRQOL assessments (e.g., distress screening) should be routinized in patient and survivorship care, as recommended by current CoC guidelines [68], with attention to providing opportunities to elicit patient-reported outcomes, in non-academic and lower resourced oncology environments, where many Black women with BC are being treated [75]. (3) Providers should recognize that young Black women's BC experiences may differ significantly from older White and older Black women's experiences (e.g., young Black women may experience and witness more aggressive BC and BC-related deaths in their communities, which may influence risk perception and fatalistic attitudes). In light of these differences, providers and supportive care staff should be aware of this lived experience and seek opportunities to understand and address these perceptions, socially embedded norms/expectations, and behaviors. (4) Considering that young Black women are potentially facing competing demands within their family structures and stress within social and economic networks (e.g., juggling unpaid time off from work with the need to complete expensive BC treatments), providers should be cognizant of and responsive to the possible existence of unique social roles and compounded stressors among some Black women, as well as culturally nuanced strategies for stress-coping, managing daily obligations, and caring for others [34, 72]. As such, programs and services that focus on optimizing resilience (including self-care and stress management) and provide resources to help ease BC burden in the context of other competing demands may be most impactful in this population. In summary, young Black women must be prioritized in the oncology care environment in ways that recognize their unique concerns, competing demands, and HRQOL needs to ensure optimal HRQOL outcomes both during and after BC treatment.
Supplementary Material
Acknowledgments
CAS and SBW have received a Research Grant from Pfizer for another unrelated study. This article is dedicated in memory of Meleshia Daye, whose courage and strength as a Young Black Woman with Breast Cancer inspired this contribution to research and clinical practice.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-016-3963-0) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of Interest All other authors have no conflicts of interest to disclose.
References
- 1.WHO. Breast cancer: prevention and control. World Health Organization; 2013. [Accessed 24 Sept 2013]. http://www.who.int/cancer/detection/breastcancer/en/index1.html. [Google Scholar]
- 2.ACS. Breast cancer facts and figures 2015–2016. Am Cancer Soc. 2015:2016. Access 02(03/2016) [Google Scholar]
- 3.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005) Cancer Epidemiol Biomarkers Prev. 2009;18(1):121–131. doi: 10.1158/1055-9965.epi-08-0679. [DOI] [PubMed] [Google Scholar]
- 4.Reeder-Hayes KE, Wheeler SB, Mayer DK. Health disparities across the breast cancer continuum. Semin Oncol Nurs. 2015;31(2):170–177. doi: 10.1016/j.soncn.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL, Prentice R. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 6.Newman LA, Mason J, Cote D, Vin Y, Carolin K, Bouwman D, Colditz GA. African–American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African–American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94(11):2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 7.Ghafoor A, Jemal A, Ward E, Cokkinides V, Smith R, Thun M. Trends in breast cancer by race and ethnicity. CA Cancer J Clin. 2003;53(6):342–355. doi: 10.3322/canjclin.53.6.342. [DOI] [PubMed] [Google Scholar]
- 8.Vastag B. Breast cancer racial gap examined: no easy answers to explain disparities in survival. J Am Med Assoc. 2003;290(14):1838–1842. doi: 10.1001/jama.290.14.1838. [DOI] [PubMed] [Google Scholar]
- 9.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. Trends in breast cancer by race and ethnicity: update 2006. CA. 2006;56(3):168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 11.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson WF, Jatoi I, Devesa SS. Distinct breast cancer incidence and prognostic patterns in the NCI's SEER program: suggesting a possible link between etiology and outcome. Breast Cancer Res Treat. 2005;90(2):127–137. doi: 10.1007/s10549-004-3777-3. [DOI] [PubMed] [Google Scholar]
- 13.Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Titus-Ernstoff L, Skalla K, Bakitas M, Silberfarb PM. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J Clin Oncol. 2005;23(19):4399–4405. doi: 10.1200/jco.2005.03.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carver CS, Smith RG, Petronis VM, Antoni MH. Quality of life among long-term survivors of breast cancer: different types of antecedents predict different classes of outcomes. Psycho-oncology. 2006;15(9):749–758. doi: 10.1002/pon.1006. [DOI] [PubMed] [Google Scholar]
- 15.Ganz PA, Rowland JH, Meyerowitz BE, Desmond KA. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 1998;152:396–411. doi: 10.1007/978-3-642-45769-2_38. [DOI] [PubMed] [Google Scholar]
- 16.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 17.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)–negative, progesterone receptor (PR)–negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 18.Ferrell BR, Grant M, Funk B, Garcia N, Otis-Green S, Schaffner ML. Quality of life in breast cancer. Cancer Pract. 1996;4(6):331–340. [PubMed] [Google Scholar]
- 19.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4(6):523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 20.Smith AW, Alfano CM, Reeve BB, Irwin ML, Bernstein L, Baumgartner K, Bowen D, McTiernan A, Ballard-Barbash R. Race/ethnicity, physical activity, and quality of life in breast cancer survivors. Cancer Epidemiol Biomark Prev. 2009;18(2):656–663. doi: 10.1158/1055-9965.epi-08-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mollica M, Nemeth L, Newman SD, Mueller M. Quality of life in african american breast cancer survivors: an integrative literature review. Cancer Nurs. 2015;38(3):194–204. doi: 10.1097/ncc.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 22.Pinheiro LC, Wheeler SB, Chen RC, Mayer DK, Lyons JC, Reeve BB. The effects of cancer and racial disparities in health-related quality of life among older Americans: a case-control, population-based study. Cancer. 2015;121(8):1312–1320. doi: 10.1002/cncr.29205. [DOI] [PubMed] [Google Scholar]
- 23.Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Hellemann G. Examining predictive models of HRQOL in a population-based, multiethnic sample of women with breast carcinoma. Qual Life Res. 2007;16(3):413–428. doi: 10.1007/s11136-006-9138-4. [DOI] [PubMed] [Google Scholar]
- 24.Ashing-Giwa K. Quality of life and psychosocial outcomes in long-term survivors of breast cancer. J Psychosoc Oncol. 2000;17(3–4):47–62. doi: 10.1300/J077v17n03_03. [DOI] [Google Scholar]
- 25.Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African-American and White long term breast carcinoma survivors. Cancer. 1999;85(2):418–426. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Von Ah DM, Russell KM, Carpenter J, Monahan PO, Qianqian Z, Tallman E, Ziner KW, Storniolo AM, Miller KD, Giesler RB, Haase J, Otte J, Champion VL. Health-related quality of life of african american breast cancer survivors compared with healthy African American women. Cancer Nurs. 2012;35(5):337–346. doi: 10.1097/NCC.0b013e3182393de3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton JB, Powe BD, Pollard AB, 3rd, Lee KJ, Felton AM. Spirituality among African American cancer survivors: having a personal relationship with God. Cancer Nurs. 2007;30(4):309–316. doi: 10.1097/01.NCC.0000281730.17985.f5. [DOI] [PubMed] [Google Scholar]
- 28.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 29.Arndt V, Merx H, Sturmer T, Stegmaier C, Ziegler H, Brenner H. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. Eur J Cancer. 2004;40(5):673–680. doi: 10.1016/j.ejca.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J clin Oncol. 2003;21(22):4184–4193. doi: 10.1200/jco.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 31.Mor V, Malin M, Allen S. Age differences in the psy-chosocial problems encountered by breast cancer patients. J Natil Cancer Inst Monogr. 1994;16:191–197. [PubMed] [Google Scholar]
- 32.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17(8):2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 34.Woods-Giscombé CL, Lobel M. Race and gender matter: a multidimensional approach to conceptualizing and measuring stress in African American women. Cult Divers Ethn Minor Psychol. 2008;14(3):173–182. doi: 10.1037/1099-9809.14.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods-Giscombe CL. Superwoman schema: African American women's views on stress, strength, and health. Qual Health Res. 2010;20(5):668–683. doi: 10.1177/1049732310361892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods-Giscombe CL, Lobel M, Zimmer C, Wiley Cene C, Corbie-Smith G. Whose stress is making me sick? Network-stress and emotional distress in African-American women. Issues Ment Health Nurs. 2015;36(9):710–717. doi: 10.3109/01612840.2015.1011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black AR, Woods-Giscombé C. Applying the stress and ‘strength’ hypothesis to black women's breast cancer screening delays. Stress Health. 2012;28(5):389–396. doi: 10.1002/smi.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powe BD, Hamilton J, Hancock N, Johnson N, Finnie R, Ko J, Brooks P, Boggan M., Jr Quality of life of African American cancer survivors—a review of the literature. Cancer. 2007;109(2 Suppl):435–445. doi: 10.1002/cncr.22358. [DOI] [PubMed] [Google Scholar]
- 39.Chopra I, Kamal KM. A systematic review of quality of life instruments in long-term breast cancer survivors. Health Qual life Outcomes. 2012;10:14. doi: 10.1186/1477-7525-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41(17):2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Cho J, Jung SY, Lee JE, Shim EJ, Kim NH, Kim Z, Sohn G, Youn HJ, Kim KS, Kim H, Lee JW, Lee MH. A review of breast cancer survivorship issues from survivors' perspectives. J Breast Cancer. 2014;17(3):189–199. doi: 10.4048/jbc.2014.17.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL. Onset of natural menopause in African American women. Am J Public Health. 2003;93(2):299–306. doi: 10.2105/ajph.93.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy AM, Yang J, Armstrong K. Increasing disparities in breast cancer mortality from 1979 to 2010 for US black women aged 20 to 49 years. Am J Public Health. 2015;105(Suppl 3):S446–S448. doi: 10.2105/ajph.2014.302297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda: 2016. [Google Scholar]
- 45.ACS. Life after cancer: survivorship by the numbers. American Cancer Society; 2014. [Google Scholar]
- 46.Elm V. The strengthening the reporting of observational studies in epidemiology (STROBE)statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 47.CASP. Critical appraisal skills programme (CASP) CASP Checklists 2014 [Google Scholar]
- 48.Derogatis LR. The psychosocial adjustment to illness scale (PAIS) J Psychosom Res. 1986;30(1):77–91. doi: 10.1016/0022-3999(86)90069-3. [DOI] [PubMed] [Google Scholar]
- 49.Cohen SH, Hoberman HM. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;13:99–125. [Google Scholar]
- 50.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 51.Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing quality of life in adult cancer survivors (QLACS) Qual Life Res. 2005;14(4):1007–1023. doi: 10.1007/s11136-004-2147-2. [DOI] [PubMed] [Google Scholar]
- 52.Furlanetto LM, Mendlowicz MV, Romildo Bueno J. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87–91. doi: 10.1016/j.jad.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Schuldberg D. Ego-strength revised: a comparison of the MMPI-2 and MMPI-1 versions of the barron ego-strength scale. J Clin Psychol. 1992;48(4):500–505. doi: 10.1002/1097-4679(199207)48:4<500::aid-jclp2270480410>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 54.Beder J. Perceived social support and adjustment to mastectomy in socioeconomically disadvantaged black women. Soc Work Health Care. 1995;22(2):55–71. doi: 10.1300/j010v22n02_04. [DOI] [PubMed] [Google Scholar]
- 55.Gibson LM, Thomas S, Parker V, Mayo R, Wetsel MA. Breast cancer fear in African American breast cancer survivors. J Cult Divers. 2014;21(4):135–144. [PubMed] [Google Scholar]
- 56.Lewis PE, Sheng M, Rhodes MM, Jackson KE, Schover LR. Psychosocial concerns of young African American breast cancer survivors. J Psychosoc Oncol. 2012;30(2):168–184. doi: 10.1080/07347332.2011.651259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Alderman A, Hamilton AS, Graff J, Katz SJ. Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv. 2009;3(4):212–222. doi: 10.1007/s11764-009-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheppard VB, Llanos AA, Hurtado-de-Mendoza A, Taylor TR, Adams-Campbell LL. Correlates of depressive symptomatology in African-American breast cancer patients. J Cancer Surviv. 2013;7(3):292–299. doi: 10.1007/s11764-013-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrow PK, Broxson AC, Munsell MF, Basen-Enquist K, Rosenblum CK, Schover LR, Nguyen LH, Hsu L, Castillo L, Hahn KM, Litton JK, Kwiatkowski DN, Hortobagyi GN. Effect of age and race on quality of life in young breast cancer survivors. Clin Breast Cancer. 2014;14(2):e21–e31. doi: 10.1016/j.clbc.2013.10.003. doi:10.1016/j.clbc. 2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. doi: 10.2105/ajph.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ware JE., Jr Methodology in behavioral and psychosocial cancer research. conceptualizing disease impact and treatment outcomes. Cancer. 1984;53(10 Suppl):2316–2326. doi: 10.1002/cncr.1984.53.s10.2316. [DOI] [PubMed] [Google Scholar]
- 62.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 63.Bourjolly JN. Differences in religiousness among black and white women with breast cancer. Soc Work Health Care. 1998;28(1):21–39. doi: 10.1300/J010v28n01_02. [DOI] [PubMed] [Google Scholar]
- 64.Gibson LM, Hendricks CS. Integrative review of spirituality in African American breast cancer survivors. ABNF J. 2006;17(2):67–72. [PubMed] [Google Scholar]
- 65.Holt CL, Schulz E, Caplan L, Blake V, Southward VL, Buckner AV. Assessing the role of spirituality in coping among African Americans diagnosed with cancer. J Relig Health. 2012;51(2):507–521. doi: 10.1007/s10943-011-9453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres E, Dixon C, Richman AR. Understanding the breast cancer experience of survivors: a qualitative study of African American women in rural eastern North Carolina. J Cancer Educ. 2016;31(1):198–206. doi: 10.1007/s13187-015-0833-0. [DOI] [PubMed] [Google Scholar]
- 67.MCC. Psychological distress screening in cancer patients. Michigan Cancer Consortium; Lansing: 2013. [Google Scholar]
- 68.CoC. Cancer program standards 2012: ensuring patient-centered care. American College of Surgeons; Chicago: 2012. [Google Scholar]
- 69.NCCN. NCCN Distress thermometer and problem list for patients. 1st. National Comprehensive Cancer Network; Washington: 2016. [Google Scholar]
- 70.Dabrowski M, Boucher K, Ward JH, Lovell MM, Sandre A, Bloch J, Carlquist L, Porter M, Norman L, Buys SS. Clinical experience with the NCCN distress thermometer in breast cancer patients. J Natl Compr Cancer Netw. 2007;5(1):104–111. doi: 10.6004/jnccn.2007.0011. [DOI] [PubMed] [Google Scholar]
- 71.Whitehead NE, Hearn LE. Psychosocial interventions addressing the needs of Black women diagnosed with breast cancer: a review of the current landscape. Psycho-Oncology. 2015;24(5):497–507. doi: 10.1002/pon.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woods-Giscombé CL, Black AR. Mind-Body interventions to reduce risk for health disparities related to stress and strength among African American women: the potential of mindfulness-based stress reduction, loving-kindness, and the NTU therapeutic framework. Complement Health Pract Rev. 2010;15(3):115–131. doi: 10.1177/1533210110386776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duckett PAS. Health coverage for the black population today and under the affordable care Ac. The Henry J Kaiser Family Foundation; Menlo Park: 2013. [Google Scholar]
- 74.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 75.Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Med Care. 2009;47(7):765–773. doi: 10.1097/MLR.0b013e31819e1fe7. doi:10.1097/MLR.0b013e31 819e1fe7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.