ABSTRACT
Siderophores are small molecular iron chelators that are produced by microbes and whose most notable function is to sequester iron from the host and provide this essential metal nutrient to microbes. Recent studies have proposed additional, noncanonical roles for siderophores, including the acquisition of noniron metals and modulation of host functions. Recently, Holden et al. (V. I. Holden, P. Breen, S. Houle, C. M. Dozois, and M. A. Bachman, mBio 7:e01397-16, 2016, http://dx.doi.org/10.1128/mBio.01397-16) showed that siderophores secreted by Klebsiella pneumoniae during lung infection induce stabilization of the transcription factor HIF-1α, increase the expression of proinflammatory cytokines in the lung, and promote dissemination of K. pneumoniae to the spleen. Thus, their study demonstrated novel roles for siderophores in vivo, beyond iron sequestration. The interaction of siderophores with host cells further promotes the pathogenicity of K. pneumoniae and is likely relevant for other pathogens that also secrete siderophores in the host.
COMMENTARY
Iron is an essential nutrient for the host as well as for most microbes. In the host, free iron levels are extremely low (e.g., <10−24 M for Fe3+ in serum), as the metal is largely bound to proteins, and iron is further limited during infection through a process known as nutritional immunity (1). To overcome nutritional immunity, some bacteria and fungi produce siderophores, which are small molecules that chelate iron (2). Enterobactin (also known as enterochelin) is a catecholate siderophore produced by both commensal and pathogenic Enterobacteriaceae and has greater affinity for iron than host molecules, such as transferrin and lactoferrin (3). To combat this, the host has evolved to produce lipocalin-2 (also known as neutrophil gelatinase-associated lipocalin, siderocalin, and 24p3), an antimicrobial protein that binds to iron-laden enterobactin, thereby preventing its reuptake by bacteria (4). Nevertheless, in the “tug of war” for iron, many enteric pathogens have acquired additional mechanisms to evade lipocalin-2 activity, in particular by producing and acquiring “stealth siderophores” that are not bound by lipocalin-2 (5). Examples of stealth siderophores include salmochelin (a C-glucosylated derivative of enterobactin) and yersiniabactin (a mixed-type siderophore). These molecules allow pathogens to evade lipocalin-2-mediated iron starvation and thereby confer an advantage to pathogens during infection and inflammation, when lipocalin-2 is highly expressed (6, 7).
The repertoire of siderophores varies among different microbial species and even among different strains. Indeed, various combinations of siderophores (e.g., enterobactin, salmochelin, yersiniabactin) are found among clinical isolates of Klebsiella pneumoniae (8), a member of the Enterobacteriaceae that causes pneumonia, urinary tract infection, and septicemia, largely in hospitalized patients. This diversity in siderophores impacts the replicative niche of K. pneumoniae in the host (8, 9) and suggests that siderophores contribute to pathogenesis via different mechanisms (10).
Long known to scavenge iron during infection, recent studies have highlighted additional siderophore functions. For example, enterobactin, but not yersiniabactin, appears to protect bacteria from oxidative stress (11). On the other hand, yersiniabactin has been shown to act as a chelator of additional metals, including copper and zinc. Strains such as uropathogenic Escherichia coli appear to use yersiniabactin’s copper-binding properties as a mechanism to resist copper toxicity (12), whereas yersiniabactin’s zinc-binding ability allows Yersinia pestis to resist zinc limitation in a septicemic plague mouse model (13).
In addition to promoting microbial growth by binding metals, there is emerging evidence that siderophores can modulate the host response. HIF-1α is a transcription factor that plays pivotal roles during infection (14). It was previously shown that siderophores secreted by enteric pathogens cause hypoxia-dependent activation of HIF-1α in the Peyer’s patches and in human epithelial and endothelial cells (15). Previously, Holden et al. demonstrated that enterobactin stabilizes HIF-1α in respiratory cells in vitro, thereby inducing expression of proinflammatory cytokines and enhancing lipocalin-2-mediated inflammation (16). In a more recently published mBio article by Holden et al. (17), the authors hypothesized that siderophores secreted by K. pneumoniae during lung infection can also have proinflammatory effects by interacting with host cells, thereby promoting pathogenicity during pneumonia.
To determine whether K. pneumoniae siderophores also have proinflammatory effects in vivo, Holden and colleagues employed a mutant that can secrete, but not take up, siderophores (a tonB mutant). When the K. pneumoniae tonB mutant was administered to mice, mass spectrometry of lung homogenates confirmed the presence of salmochelin and yersiniabactin. Moreover, these authors showed that the tonB mutant induced expression of wild-type proinflammatory cytokine levels at early time points postinfection. In comparison to an isogenic strain unable to secrete siderophores (an entB ybtS tonB mutant), the tonB mutant exhibited greater dissemination from the lung to the spleen. Interestingly, siderophores appeared to only induce a subset of proinflammatory proteins, including interleukin-6 and the neutrophil chemoattractants CXCL1 and CXCL2, whereas induction of other proinflammatory molecules (interleukin-1β [IL-1β], macrophage inhibitory protein-3α) was independent of siderophore secretion. Analysis of strains with mutations in different combinations of siderophores indicated that all siderophores contributed to the induction of host inflammation and promoted the dissemination of K. pneumoniae to the spleen. Both phenotypes were independent of lipocalin-2 expression, as similar levels of pathogen dissemination and host expression of proinflammatory cytokines were observed in both wild-type and lipocalin-2-deficient mice. This is in contrast to prior in vitro observations, where lipocalin-2 enhanced production of cytokines in airway epithelial cells (16), a difference that Holden et al. suggested might be the result of redundant signals compensating for lipocalin-2 deficiency in vivo.
As their prior study indicated that siderophores stabilize HIF-1α in respiratory cells (16), Holden and colleagues sought to investigate whether this occurs in vivo. To this end, they employed a transgenic mouse model that expresses a luciferase fusion to the oxygen-dependent domain (ODD) of HIF-1α, which becomes stabilized under low-oxygen or low-iron conditions. By performing these elegant experiments, Holden et al. demonstrated that siderophores secreted in the lung by K. pneumoniae lead to greater HIF-1α stabilization and that greater HIF-1α stabilization correlates with increased K. pneumoniae dissemination to the spleen. These authors then carried out a key experiment to show a functional link between siderophore-mediated HIF-1α stabilization and bacterial dissemination: infection of transgenic mice in which Hif1a deletion was induced postnatally only in lung epithelial cells. In Hif1a-deficient mice, K. pneumoniae dissemination to the spleen was significantly reduced, demonstrating a role for siderophore-dependent HIF-1α stabilization in promoting dissemination of K. pneumoniae.
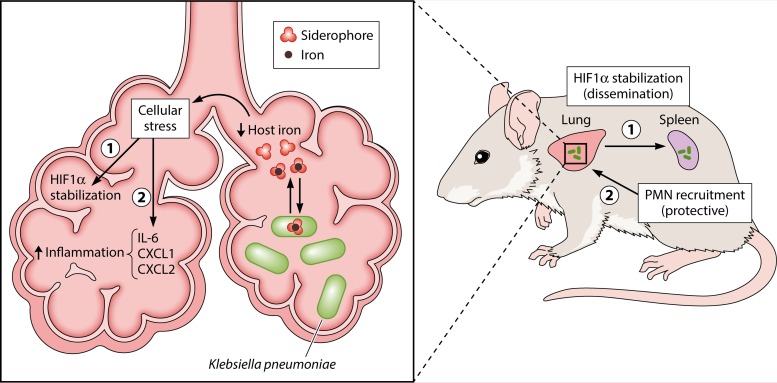
The primary significance of their study is its demonstration of a noncanonical role for siderophores in vivo during K. pneumoniae infection. In addition to providing iron to the pathogen, siderophores also promote K. pneumoniae dissemination to the spleen by inducing stabilization of HIF-1α in lung epithelial cells (Fig. 1). The study also demonstrated that HIF-1α enhances susceptibility to K. pneumoniae infection. Future investigations will need to address the mechanism by which epithelial HIF-1α promotes K. pneumoniae dissemination. An additional response induced by siderophores is the expression of proinflammatory cytokines, such as IL-6 and CXC chemokines, which was independent of HIF-1α in lung epithelial cells. These chemokines promote the recruitment of neutrophils, which in turn confer protection during K. pneumoniae infection but also contribute to pathology. Thus, therapeutic interventions aiming to inactivate siderophores may be beneficial to the host not only by inhibiting pathogens from acquiring iron but also by preventing pathogen dissemination while limiting pathology through modulating the immune system.
FIG 1 .
Working model. When Klebsiella pneumoniae enters the lung, the host responds to the infection by restricting iron availability. In order to obtain the iron necessary for its growth, K. pneumoniae secretes siderophores, which can bind iron with greater affinity than host proteins. The resulting lower levels of host iron induce stress in host cells. This stress response has two consequences for the progression of the infection: (i) HIF-1α stabilization, which ultimately leads to bacterial dissemination to peripheral organs like the spleen; (ii) the secretion of proinflammatory cytokines IL-6, CXCL1, and CXCL2. The chemokines CXCL1 and CXCL2 recruit neutrophils (polymorphonuclear lymphocytes [PMN]) to the site of infection, a response that is crucial for host protection from K. pneumoniae infection.
The views expressed in this Commentary do not necessarily reflect the views of this journal or of ASM.
Funding Statement
No applicable funding.
Footnotes
For the article discussed, see http://dx.doi.org/10.1128/mBio.01397-16.
Citation Behnsen J, Raffatellu M. 2016. Siderophores: more than stealing iron. mBio 7(6):e01906-16. doi:10.1128/mBio.01906-16.
REFERENCES
- 1.Cassat JE, Skaar EP. 2013. Iron in infection and immunity. Cell Host Microbe 13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crosa JH, Walsh CT. 2002. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66:223–249. doi: 10.1128/MMBR.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raymond KN, Dertz EA, Kim SS. 2003. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A 100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 5.Fischbach MA, Lin H, Liu DR, Walsh CT. 2006. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol 2:132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 6.Bachman MA, Miller VL, Weiser JN. 2009. Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog 5:e1000622. doi: 10.1371/journal.ppat.1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, Mak TW, Tsolis RM, Bevins CL, Solnick JV, Dandekar S, Bäumler AJ. 2009. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachman MA, Oyler JE, Burns SH, Caza M, Lépine F, Dozois CM, Weiser JN. 2011. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun 79:3309–3316. doi: 10.1128/IAI.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachman MA, Lenio S, Schmidt L, Oyler JE, Weiser JN. 2012. Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia. mBio 3:e00224-11. doi: 10.1128/mBio.00224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holden VI, Bachman MA. 2015. Diverging roles of bacterial siderophores during infection. Metallomics 7:986–995. doi: 10.1039/c4mt00333k. [DOI] [PubMed] [Google Scholar]
- 11.Achard ME, Chen KW, Sweet MJ, Watts RE, Schroder K, Schembri MA, McEwan AG. 2013. An antioxidant role for catecholate siderophores in Salmonella. Biochem J 454:543–549. doi: 10.1042/BJ20121771. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. 2012. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol 8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobrov AG, Kirillina O, Fetherston JD, Miller MC, Burlison JA, Perry RD. 2014. The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice. Mol Microbiol 93:759–775. doi: 10.1111/mmi.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nizet V, Johnson RS. 2009. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS, Matteoli G, Bohn E, Autenrieth IB, Karhausen J, Neumann D, Colgan SP, Kempf VA. 2008. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 134:756–767. doi: 10.1053/j.gastro.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Holden VI, Lenio S, Kuick R, Ramakrishnan SK, Shah YM, Bachman MA. 2014. Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1α and proinflammatory cytokine secretion in cultured respiratory epithelial cells. Infect Immun 82:3826–3836. doi: 10.1128/IAI.01849-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holden VI, Breen P, Houle S, Dozois CM, Bachman MA. 2016. Klebsiella pneumoniae siderophores induce inflammation, bacterial dissemination, and HIF-1α stabilization during pneumonia. mBio 7(5):e01397-16. doi: 10.1128/mBio.01397-16. [DOI] [PMC free article] [PubMed] [Google Scholar]