Abstract
Background
Infection with blood-borne viruses including hepatitis C (HCV) and hepatitis G (HGV) viruses is a substantial health problem. Varying prevalences of these infections in different studies reflect the role of predisposing risk factors in different countries or even different regions of a country.
Objectives
The objective of the present survey was to assess the prevalences of HCV and HGV virus infections among hemodialysis (HD) patients in Bandar Abbas, Hormozgan, Iran, 2015.
Methods
A total of 149 subjects with chronic renal failure undergoing HD at Shahid Mohammadi hospital in the Hormozgan province of southern Iran from January 1, 2015 to March 31, 2015 were evaluated for anti-HCV and antibodies against HGV E2 glycoprotein by census sampling method. Thereafter, all of the specimens were evaluated for molecular assays using polymerase chain reaction (PCR) and other techniques. Investigated data were recorded for each participant in a pre-designed data collection sheet. All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 19 for Windows by t-test and chi-square test (χ2).
Results
The mean age of patients was 56.23 ± 12.35 years (minimum age 18, maximum age 85). Both kinds of assays determined that five (3.36%) patients were HCV positive, whereas no HGV positives were diagnosed. The prevalence of HCV is associated with longer duration of HD (P value = 0.008), history of blood transfusion (P value = 0.037) and drug addiction (P value = 0.035).
Conclusions
History of drug addiction and/or blood transfusion and longer duration of HD treatment were the main risk factors determining the prevalence of HCV infection in the Hormozgan province of southern Iran in 2015. However, the values observed in the present investigation reflect the effective management techniques imposed by healthcare authorities and relevant organizations in recent years.
Keywords: Hepatitis C Virus, Hepatitis G Virus, Hemodialysis, Iran, Prevalence
1. Background
Hepatitis C (HCV) and G (HGV) viruses are significant causes of transmitted infections in hemodialysis (HD) patients (1). The infections are transmitted mainly by exposure to infected blood or blood products, infected medical equipment, intravenous drug abuse, HD, and organ transplantation (2). Therefore, the prevalence of HCV and HGV viruses in HD patients varies significantly across different geological locations, particularly due to differences in sanitation standards (3, 4).
Hepatitis C virus (HCV) is the most common cause of liver disease in dialysis units due to factors such as asymptomatic infection and lack of an effective vaccine (5). Chronic infection occurs in 50% - 80% of patients, which finally leads to hepatocellular carcinoma and cirrhosis (6). Chronic infection and its complications are facilitated by external factors, including viral co-infections such as hepatitis G virus (7). Although HCV and HGV are structurally similar and HGV is mostly concomitant with HCV, little data is available about HGV infection and pathogenesis (8).
HGV is a spherically shaped and enveloped virus measuring 40 - 60 nm in diameter. It has a 10 kb positive-sense single-stranded RNA genome that belongs to the family Flaviviridae. HGV can also develop drug resistance and lead to fulminant hepatitis (9). Therefore, HCV and HGV coinfection reduces the response of HCV and serum HGV-RNA to antiviral and interferon therapies (10).
The increasing use of HD for end stage renal disease (ESRD) predisposes patients to blood born viruses, including HCV and HGV (11). In recent decades, the healthcare authorities of various countries have established innovative strategies to reduce the incidence of these infections among dialysis patients. Therefore, such studies provide a valuable basis for comparisons among these different policies (12).
According to previous studies, the prevalence of hepatitis virus infections is higher in developing countries than in developed countries (9, 13-19). Few studies have been performed in southern Iran, especially in the Hormozgan province, to evaluate the prevalence of HCV and HGV virus infections among HD patients. In a survey carried out by Hassanshahi et al. (2011) in southeastern Iran, it was found that 44.7% of thalassemia and 31.5% of HD patients were infected with HCV. In addition, there was a significant association between HCV positivity and the frequency of blood transfusion and the duration of HD (20). Another study conducted by Mousavi et al. (2013) in the Hormozgan province indicated that the overall prevalence of HCV in Bandar Abbas, Hormozgan is relatively high, especially in HD patients. Based on their findings, the main risk factors for transmission in this geographical region were intravenous drug abuse, blood transfusion, and sexual contact (21). Additional data in regard to HCV transmission risk behaviors have been provided by Makiani et al. (2014). They revealed that having tattoos, undergoing phlebotomy, prison history, and unsafe sexual contact were more common among 15 - 45-year-old individuals in Bandar Abbas, Hormozgan (22).
2. Objectives
Despite the fact that there have been several studies on the prevalence of hepatitis viruses in HD patients in the Iranian population, most of these studies have been restricted to specific geographic locations or provinces, and there is a lack of reports from southern Iran, especially in the Hormozgan province. Thus, in order to obtain information about developmental trends and outcomes and patients, initiate effective future planning, and ultimately reduce disease prevalence and recirculation, the present study was conducted to determine the prevalence of hepatitis viruses C and G among HD patients in the Hormozgan province of southern Iran.
3. Methods
3.1. Participants
This single center, cross-sectional study was carried out in the HD center of Shahid Mohammadi hospital in the Hormozgan province of southern Iran in 2015. A total of 149 patients under HD treatment were recruited to the study by census sampling method from January 1, 2015 to March 31, 2015. Enrolled patients provided written informed consent, and those who did not consent to participate were ruled out. The eligibility criteria were consent of subjects and referral to the HD center of Shahid Mohammadi Hospital during the aforementioned period of time.
Demographic characteristics and history of related risk factors including age, sex, history of addiction, duration of HD, history of use of shared HD devices, and blood transfusion were recorded for each participant in a pre-designed data collection sheet. All serum samples were initially tested for anti-HCV and anti-HGV antibodies by specific enzyme linked immunoassay (ELISA) kits (Diapro, Italy), over a period of two months. Thereafter, real-time polymerase chain reaction (RT-PCR) testing was performed on sera obtained from the samples. All procedures were conducted in a single HD center by a limited number of expert technicians to avoid any research bias in laboratory tests and data collection.
3.2. HGV and HCV Serological Assays
Blood samples were obtained from the subjects and sent for anti-HGV E2 and anti-HCV antibody tests. Antibodies against HGV E2 glycoprotein were detected in the serum samples by third generation ELISA using a commercially available kit (DIAPRO, Italy) according to the manufacturer’s instructions. In addition, all sera were screened using anti-HCV antibody assays with commercial ELISA microplate kits (DIAPRO, Italy) according to the manufacturer’s instructions.
3.3. HGV and HCV PCR Protocol
The HCV and HGV genomes were detected from serum samples by RNX plus an extraction procedure, as previously described (Kao et al., 1997). Thereafter, cDNA was sensitized from the genomes by the following protocol: first, 3 μL of extracted RNA was treated with random hexamer and Moloney murine leukemia virus reverse transcriptase (M-MuLV-RT) and then incubated for 60 minutes at 25°C and for 10 minutes at 72°C. The PCR master mix contained 1.5 mmol of MgCl2, 2.5 U of Taq DNA polymerase, 2 μL of cDNA, 2.5 μL of 10X PCR buffer, and 0.1 pmol/μL of primers. According to the highly conserved domains of the 5’ non-coding regions of the HGV genome, the nucleotide sequences were as follows: sense, 150 - 169 nt, 5’-CACTATAGGTGGGTCTTAAG-3’ and antisense, 352 - 333 nt, 5’-GCCTATTGGTCAAGAGAGAC-3’ for the first round and sense, 207–226 nt, 5’- GCGCACGGTCCACAGGTGTT-3’ and antisense, 326 - 307 nt, 5’-GGGCGACGTGGACCGTACGT-3’ for the second round of amplification. On the other side, primers for the detection of HCV were sense, -319 to -297 nt, 5’-TTGGCGGCCGCACTCCACCATRRATCACTCCCC -3’ and antisense, -1 to -21 nt, 5’- GTGCACGGTCTACGAGACCT-3’ for first round and sense, -315 to -289 nt, 5’-GGGGCGGCCGCCACCATRRATCACTCCCCTGTGAGG -3’ and antisense, -66 to -47 5’- CACTCTCGAGCACCCTATCAGGCAGTACC -3’. PCR amplification was conducted for 30 cycles with a first round at 94°C for 30 seconds, a second round at 55°C for 90 seconds, a third round at 72°C for 90 seconds, and finalized with an extension at 72°C for 90 seconds and 35 cycles with the same thermocycling conditions as those used in the second round of PCR. Amplified products were separated with agarose gel (2%) electrophoresis and visualized by ethidium bromide staining under a UV-detector system.
3.4. Statistical Analysis
Statistical analysis was conducted using SPSS version 19 (SPSS Inc., Chicago, IL, USA). A chi-square test was used to compare the categorical variables, and a Student’s t-test was used to compare continuous variables between the two groups. A confidence interval was described for reporting prevalence values among the study population. A two sided α = 0.05 was considered statistically significant.
4. Results
4.1. Demographic Characteristics
A total of 188 HD patients were eligible to participate in the present survey, and 149 cases were recruited, confirmed, and analyzed in accordance with the eligibility criteria. No participants were excluded from the study during the investigation period. The mean age of subjects was 56.23 ± 12.35 years (minimum age 18, maximum age 85). Ninety-two (61.74%) of the patients were male and 57 (38.26%) were female. Complete demographic characteristics of the investigated patients are shown in Table 1.
Table 1. Demographic Characteristics and Statistical Analysis of Study Variablesa.
| Variables | HCV Positive | HCV Negative | Total | P Value |
|---|---|---|---|---|
| Age b , Mean ± SD | 53.80 ± 11.26 | 54.07 ± 12.72 | - | > 0.05 |
| Duration of hemodialysis c , Mean ± SD | 13.15 ± 9.62 | 11.28 ± 11.81 | - | ≤ 0.008d |
| Gender | > 0.05 | |||
| Male | 4 (2.69) | 88 (59.06) | 92 (61.74) | |
| Female | 1 (0.67) | 56 (37.58) | 57 (38.26) | |
| Alanine aminotransferase | > 0.05 | |||
| Abnormal | 1 (0.67) | 16 (10.74) | 17 (11.41) | |
| Normal | 4 (2.69) | 128 (85.9) | 132 (88.59) | |
| Aspartate aminotransferase | > 0.05 | |||
| Abnormal | 1 (0.67) | 4 (2.69) | 5 (3.36) | |
| Normal | 4 (2.69) | 140 (93.95) | 144 (96.64) | |
| Shared hemodialysis devices | > 0.05 | |||
| Yes | 4 (2.61) | 127 (85.31) | 131 (87.92) | |
| No | 1 (0.67) | 17 (11.41) | 18 (12.08) | |
| Addiction | ≤ 0.035d | |||
| Yes | 2 (1.34) | 8 (5.37) | 10 (6.71) | |
| No | 3 (2.01) | 136 (91.28) | 139 (93.29) | |
| Blood transfusion | ≤ 0.037d | |||
| Yes | 5 (3.36) | 124 (83.22) | 129 (86.58) | |
| No | 0 (0) | 20 (13.42) | 20 (13.42) |
Abbreviation: HCV, Hepatitis C virus.
aValues are expressed as No. (%) unless otherwise indicated.
bUnits of measurement: year.
cUnits of measurement: month.
4.2. Clinical Assay Findings
Serological tests determined that five (3.36%) patients were HCV positive (3.36; 95% CI, 1.44 -7.62), whereas no HGV positive patients were identified. In addition, the prevalences of elevated serum aminotransferase levels including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were 11.41% (17 subjects) and 3.39% (five subjects), respectively. (The normal ranges of values for AST and ALT are about 5 - 40 units per liter and 7 - 56 units per liter of serum, respectively). More details regarding the measurements of serum aminotransferase levels can be found in Table 2.
Table 2. Serum Aminotransferase Levels in the Study Population.
| Variables | No. (%) | P Value |
|---|---|---|
| AST | > 0.05 | |
| Female | ||
| Normal | 54 (36.24) | |
| Abnormal | 3 (2.01) | |
| Male | ||
| Normal | 90 (60.41) | |
| Abnormal | 2 (1.34) | |
| ALT | > 0.05 | |
| Female | ||
| Normal | 52 (34.9) | |
| Abnormal | 5 (3.36) | |
| Male | ||
| Normal | 80 (53.69) | |
| Abnormal | 12 (8.05) |
Abbreviations: AST, Aspartate aminotransferase; ALT, Alanine aminotransferase.
All serum samples were evaluated using PCR analysis and electrophoresis to detect positive specimens. As shown in Figure 1, only a 173-bp fragment was found by electrophoresis in five serum samples. Thus, the molecular assays confirmed that the prevalence rate of HCV infection was 3.39% in this HD population, whereas no HGV-positive cases were identified in our investigation.
Figure 1. HCV PCR Products on Agarose Gel (2%) Electrophoresis Visualized by Ethidium Bromide Staining Under a UV-Detector System.
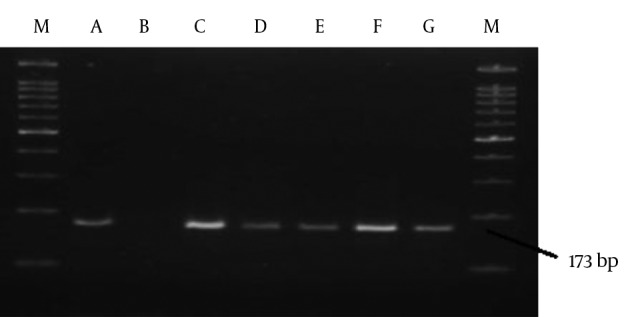
M, marker 100 bp; A, positive control; B, negative control; C-G, replicated regions in serum samples.
4.3. Social Characteristics and Prevalence of HCV in the Study Population
In accordance with Table 1, there were statistically significant differences between the groups with respect to drug addiction (P value = 0.035), blood transfusion (P value = 0.037), and duration of HD (P value = 0.008). The aforementioned variables were considerably higher in HCV positive patients than in HCV-negative cases. However, there were no statistically significant differences between the groups in terms of other variables, including age, gender, serum aminotransferase levels, and shared HD devices (P value > 0.05).
5. Discussion
The present survey represents the first local report on the prevalence of HCV infection in the Iranian HD population of the Hormozgan Province in southern Iran. In the present study, an HCV infection prevalence of 3.36% in HD patients was observed. This value was almost 13 times lower than that found in Hosseini-Moghaddam et al.’s multicenter study in Tehran, Iran in 2006 (23). Taziki and Espahbodi also assessed the prevalence of HCV infection in HD patients in 1,006 individuals in the province of Mazandaran, northern Iran from January, 2001 to December, 2006. They discovered that the prevalence of anti-HCV antibodies was 18% in 2001, whereas by December, 2006, it had decreased to 12% (24). Another study in the Guilan province (2011) in northern Iran also indicated found an HCV infection prevalence of 11.9% among HD patients (25). In addition, the results of another study (2012) in the Kerman Province of southeastern Iran demonstrated an HCV prevalence of 7% in HD patients (26). Therefore, the prevalences of HCV in HD populations are significantly higher in northern areas and in older studies. The probable reasons for these differences are the higher frequency of transmission risk factors for HCV in northern regions and the better management and sufficiency of screening methods in recent years, respectively (27).
In addition, the ELISA test was assumed as the reference standard for HCV infection in older studies, which may underestimate HCV prevalence in HD populations, mainly due to its inaccuracy, at least in the early stages of infection (28, 29). However, in accordance with the present study, molecular-based tests (sensitive diagnostic assays that identify HCV genomes, including transcription-mediated amplification and PCR), used in later studies may have reduced these underestimations (30).
Similar investigations in Sudan, British Columbia, France, and Jordan have found HCV infection prevalence rates of 23.7%, 5.4%, 7.7%, and 28%, respectively (18, 31-33). Therefore, the prevalence of HCV infection is higher in developing countries than in developed countries. The literature suggests that these differences are mainly due to lower levels of knowledge and poor management and screening strategies in developing countries (34).
Another aim of the present study was to determine the main risk factors for HCV and HGV transmission in this region of southern Iran. In agreement with previous studies, three variables were markedly associated with the prevalence of HCV infection in the present study: drug addiction, positive history of blood transfusion, and longer time under HD treatment (35, 36).
The correlation between drug addiction and HCV infection is not surprising given that drug addiction facilitates infection by impacting several mechanisms of transmission, such as sharing contaminated needles and syringes, imprisonment and poor hygiene (37, 38). However, the association between HCV infection and longer duration of HD is less clear. In fact, the longer an individual underwent HD, the higher their risk of HCV infection. The relationship between HCV infection and the length of time on HD reinforces the probability of nosocomial transmission in the investigated center (39). In this regard, some studies have provided evidence of nosocomial transmission in HD centers using sequence analysis of HCV isolates (40).
Previous studies conducted at Shahid Mohammadi hospital regarding the association between the prevalence of HIV and other hepatitis viruses in the HD center and history of blood transfusion have described a low rates of infection and no statistically significant relationship between HD and blood transfusion and infection rates (11, 41). Unfortunately, a significant association between positive history of blood transfusion in HD patients and viral infection suggests that the screening methods used for HCV detection in the transfusion centers of this region are insufficient in comparison to the screening methods used for HIV and other hepatitis infections. Indeed, these results indicate the vital role of efficient screening for anti-HCV antibodies in blood donors (42).
The findings of this survey should be used with caution due to the study objectives, limitations, multiplicity of analyses, and results from similar studies. The main limitation of this study was occult HCV infection (OCI), which is defined as the presence of HCV-RNA in hepatocytes and the absence of HCV-RNA in the serum according to routine tests. Indeed, the gold standard method for OCI diagnosis is a liver biopsy to obtain hepatocyte specimens, which is an invasive technique. Several previous studies have revealed that considering OCI in high-risk groups (such as HD patients) may be necessary (43, 44).
There were also other limitations in our investigation. Many risk factors were not considered in the present study, such as the socioeconomic status of patients, history of treatment in multiple dialysis centers, and imprisonment. In addition, this is a single-center study with a small study population. Finally, despite the preventive strategies mentioned in the methods section, sources of potential biases or imprecision could adversely affect the findings of this study. Therefore, it is suggested that the aforementioned factors be considered in future investigations.
Finally, we can conclude that the prevalence of HCV among HD patients in the Hormozgan province is relatively low, which probably reflects the effective management strategies imposed by healthcare authorities. In addition, drug addiction, positive history of blood transfusion, and longer duration of HD treatment are associated with the HCV infection prevalence in the Hormozgan province in 2015.
Acknowledgments
The authors would like to thank the student research committee of the Hormozgan University of Medical Sciences for their help and support.
Footnotes
Authors’ Contribution:Iman Ghasemzadeh and Mohammad Kargar were responsible for the study design; Ali Kargar Kheirabad and Fahime Bahri were responsible for patient recruitment and clinical and laboratory assessment. Finally, all authors contributed to the final version of the manuscript.
Conflicts of Interest:The authors declare that they have no conflicts of interest.
References
- 1.Ramia S, Koussa S, Taher A, Haraki S, Klayme S, Sarkis D, et al. Hepatitis-C-virus genotypes and hepatitis-G-virus infection in Lebanese thalassaemics. Ann Trop Med Parasitol. 2002;96(2):197–202. doi: 10.1179/000349802125000439. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald M, Crofts N, Kaldor J. Transmission of hepatitis C virus: rates, routes, and cofactors. Epidemiol Rev. 1996;18(2):137–48. doi: 10.1093/oxfordjournals.epirev.a017921. [DOI] [PubMed] [Google Scholar]
- 3.Taherkhani R, Farshadpour F. Epidemiology of hepatitis C virus in Iran. World J Gastroenterol. 2015;21(38):10790–810. doi: 10.3748/wjg.v21.i38.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DB, Cuceanu N, Davidson F, Jarvis LM, Mokili JL, Hamid S, et al. Discrimination of hepatitis G virus/GBV-C geographical variants by analysis of the 5' non-coding region. J Gen Virol. 1997;78 ( Pt 7):1533–42. doi: 10.1099/0022-1317-78-7-1533. [DOI] [PubMed] [Google Scholar]
- 5.Abdulkarim AS, Zein NN, Germer JJ, Kolbert CP, Kabbani L, Krajnik KL, et al. Hepatitis C virus genotypes and hepatitis G virus in hemodialysis patients from Syria: identification of two novel hepatitis C virus subtypes. Am J Trop Med Hyg. 1998;59(4):571–6. doi: 10.4269/ajtmh.1998.59.571. [DOI] [PubMed] [Google Scholar]
- 6.Alavian SM, Ahmadzad-Asl M, Lankarani K, Shahbabaie M, Bahrami Ahmadi A, Kabir A. Hepatitis C infection in the general population of Iran: A systematic review. Hepat Mon. 2009;9(3):211–23. [Google Scholar]
- 7.Schwarze-Zander C, Blackard JT, Zheng H, Addo MM, Lin W, Robbins GK, et al. GB virus C (GBV-C) infection in hepatitis C virus (HCV)/HIV-coinfected patients receiving HCV treatment: importance of the GBV-C genotype. J Infect Dis. 2006;194(4):410–9. doi: 10.1086/505713. [DOI] [PubMed] [Google Scholar]
- 8.Kafi-Abad S, Samiei S, Talebian A, Maghsudloo M, Gharehbaghian A. Hepatitis G virus infection in Iranian blood donors and high-risk groups. Hepat Mon. 2009;9(4):282–6. [Google Scholar]
- 9.Eslamifar A, Hamkar R, Ramezani A, Ahmadi F, Gachkar L, Jalilvand S, et al. Hepatitis G virus exposure in dialysis patients. Int Urol Nephrol. 2007;39(4):1257–63. doi: 10.1007/s11255-007-9267-x. [DOI] [PubMed] [Google Scholar]
- 10.Giret MT, Miraglia JL, Sucupira MC, Nishiya A, Levi JE, Diaz RS, et al. Prevalence, incidence density, and genotype distribution of GB virus C infection in a cohort of recently HIV-1-infected subjects in Sao Paulo, Brazil. PLoS One. 2011;6(4):18407. doi: 10.1371/journal.pone.0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahri F, Kargar Kheirabad A, Ghasemzadeh I, Shoja S, Gouklani H. Hepatitis Viruses B and D and Human Immunodeficiency Virus Infections in Hemodialysis Patients in the South of Iran: Prevalence and Genotypes. Hepat Mon. 2016;16(1):32971. doi: 10.5812/hepatmon.32971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alavian SM, Kabir A, Ahmadi AB, Lankarani KB, Shahbabaie MA, Ahmadzad-Asl M. Hepatitis C infection in hemodialysis patients in Iran: a systematic review. Hemodial Int. 2010;14(3):253–62. doi: 10.1111/j.1542-4758.2010.00437.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang JF, Dai CY, Chuang WL, Lin WY, Lin ZY, Chen SC, et al. Prevalence and clinical significance of HGV/GBV-C infection in patients with chronic hepatitis B or C. Jpn J Infect Dis. 2006;59(1):25–30. [PubMed] [Google Scholar]
- 14.Hofer H, Aydin I, Neumueller-Guber S, Mueller C, Scherzer TM, Staufer K, et al. Prevalence and clinical significance of GB virus type C/hepatitis G virus coinfection in patients with chronic hepatitis C undergoing antiviral therapy. J Viral Hepat. 2011;18(7):513–7. doi: 10.1111/j.1365-2893.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 15.Samadi M, Keyvani H, Moghaddam SMH. Prevalence and risk factors of the hepatitis G (HGV) infection in hemodialysis patients. Arch Clin Infect Dis. 2008;3(1) [Google Scholar]
- 16.Fallahian F, Alavian SM, Rasoulinejad M. Epidemiology and transmission of hepatitis G virus infection in dialysis patients. Saudi J Kidney Dis Transpl. 2010;21(5):831–4. [PubMed] [Google Scholar]
- 17.Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: systematic review and meta-analysis. Public Health. 2008;122(10):990–1003. doi: 10.1016/j.puhe.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Su Y, Norris JL, Zang C, Peng Z, Wang N. Incidence of hepatitis C virus infection in patients on hemodialysis: a systematic review and meta-analysis. Hemodial Int. 2013;17(4):532–41. doi: 10.1111/j.1542-4758.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 19.Makkar V, Gupta D, Bansal K, Khaira NS. Prevalence, seroconversion and risk factors of hepatitis b and c infection in patients on maintenance hemodialysis. JEMDS. 2014;3(11790-7):50. [Google Scholar]
- 20.Hassanshahi G, Arababadi MK, Assar S, Hakimi H, Karimabad MN, Abedinzadeh M, et al. Post-transfusion-transmitted hepatitis C virus infection: a study on thalassemia and hemodialysis patients in southeastern Iran. Arch Virol. 2011;156(7):1111–5. doi: 10.1007/s00705-011-0950-y. [DOI] [PubMed] [Google Scholar]
- 21.Mousavi SF, Moosavy SH, Alavian SM, Eghbali H, Mahboobi H. Distribution of hepatitis C virus genotypes among patients with hepatitis C virus infection in hormozgan, iran. Hepat Mon. 2013;13(12):14324. doi: 10.5812/hepatmon.14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makiani MJ, Davoodian P, Abedi F, Hossini M, Zare S, Rahimi S, et al. AIDS and hepatitis B and C high risk behaviors among 15 to 45 years old individuals in Bandar Abbas (Iran) in 2012. Electron Physician. 2014;6(3):884–9. doi: 10.14661/2014.883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosseini-Moghaddam SM, Keyvani H, Kasiri H, Kazemeyni SM, Basiri A, Aghel N, et al. Distribution of hepatitis C virus genotypes among hemodialysis patients in Tehran--a multicenter study. J Med Virol. 2006;78(5):569–73. doi: 10.1002/jmv.20577. [DOI] [PubMed] [Google Scholar]
- 24.Taziki O, Espahbodi F. Prevalence of hepatitis C virus infection in hemodialysis patients. Saudi J Kidney Dis Transpl. 2008;19(3):475–8. [PubMed] [Google Scholar]
- 25.Joukar F, Besharati S, Mirpour H, Mansour-Ghanaei F. Hepatitis C and hepatitis B seroprevalence and associated risk factors in hemodialysis patients in Guilan province, north of Iran: HCV and HBV seroprevalence in hemodialysis patients. Hepat Mon. 2011;11(3):178–81. [PMC free article] [PubMed] [Google Scholar]
- 26.Zahedi MJ, Darvish Moghaddam S, Alavian SM, Dalili M. Seroprevalence of Hepatitis Viruses B, C, D and HIV Infection Among Hemodialysis Patients in Kerman Province, South-East Iran. Hepat Mon. 2012;12(5):339–43. doi: 10.5812/hepatmon.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amiri ZM, Shakib AJ, Toorchi M. Seroprevalence of hepatitis C and risk factors in haemodialysis patients in Guilan, Islamic Republic of Iran. East Mediterr Health J. 2005;11(3):372–6. [PubMed] [Google Scholar]
- 28.Huraib SO. Hepatitis C in dialysis patients. Saudi J Kidney Dis Transpl. 2003;14(4):442–50. [PubMed] [Google Scholar]
- 29.Zeldis JB, Depner TA, Kuramoto IK, Gish RG, Holland PV. The prevalence of hepatitis C virus antibodies among hemodialysis patients. Ann Intern Med. 1990;112(12):958–60. doi: 10.7326/0003-4819-112-12-958. [DOI] [PubMed] [Google Scholar]
- 30.Marinaki S, Boletis JN, Sakellariou S, Delladetsima IK. Hepatitis C in hemodialysis patients. World J Hepatol. 2015;7(3):548–58. doi: 10.4254/wjh.v7.i3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Amin HH, Osman EM, Mekki MO, Abdelraheem MB, Ismail MO, Yousif ME, et al. Hepatitis C virus infection in hemodialysis patients in Sudan: two centers' report. Saudi J Kidney Dis Transpl. 2007;18(1):101–6. [PubMed] [Google Scholar]
- 32.Tu AW, Buxton JA, Whitlock M, Djurdjev O, Chong M, Krajden M, et al. Prevalence and incidence of hepatitis C virus in hemodialysis patients in British Columbia: Follow-up after a possible breach in hemodialysis machines. Can J Infect Dis Med Microbiol. 2009;20(2):19–23. doi: 10.1155/2009/641941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Jamal M, Al-Qudah A, Al-Shishi KF, Al-Sarayreh A, Al-Quraan L. Hepatitis C virus (HCV) infection in hemodialysis patients in the south of Jordan. Saudi J Kidney Dis Transpl. 2009;20(3):488–92. [PubMed] [Google Scholar]
- 34.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Albuquerque AC, Coelho MR, Lopes EP, Lemos MF, Moreira RC. Prevalence and risk factors of hepatitis C virus infection in hemodialysis patients from one center in Recife, Brazil. Mem Inst Oswaldo Cruz. 2005;100(5):467–70. doi: 10.1590/s0074-02762005000500003. [DOI] [PubMed] [Google Scholar]
- 36.Bdour S. Hepatitis C virus infection in Jordanian haemodialysis units: serological diagnosis and genotyping. J Med Microbiol. 2002;51(8):700–4. doi: 10.1099/0022-1317-51-8-700. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer M, Mauss S. Hepatitis C treatment in patients with drug addiction: clinical management of interferon-alpha-associated psychiatric side effects. Curr Drug Abuse Rev. 2008;1(2):177–87. doi: 10.2174/1874473710801020177. [DOI] [PubMed] [Google Scholar]
- 38.Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction. 2005;100(6):820–8. doi: 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 39.Olmer M, Bouchouareb D, Zandotti C, de Micco P, de Lamballerie X. Transmission of the hepatitis C virus in an hemodialysis unit: evidence for nosocomial infection. Clin Nephrol. 1997;47(4):263–70. [PubMed] [Google Scholar]
- 40.Alfurayh O, Sabeel A, Al Ahdal MN, Almeshari K, Kessie G, Hamid M, et al. Hand contamination with hepatitis C virus in staff looking after hepatitis C-positive hemodialysis patients. Am J Nephrol. 2000;20(2):103–6. doi: 10.1159/000013565. [DOI] [PubMed] [Google Scholar]
- 41.Abedian F, Yavarian M, Shakibzadeh A, Khalvati B, Asadi AH. A pilot Seroepidemiologic study of HTLV in thalassemia, hemophilia, and hemodialysed patients in Hormozgan. HMJ. 2009;13(2):75–80. [Google Scholar]
- 42.Hassan AA, Khalil R. Hepatitis C in dialysis patients in egypt: relationship to dialysis duration, blood transfusion, and liver disease. Saudi J Kidney Dis Transpl. 2000;11(1):72–3. [PubMed] [Google Scholar]
- 43.Rezaee-Zavareh MS, Hadi R, Karimi-Sari H, Hossein Khosravi M, Ajudani R, Dolatimehr F, et al. Occult HCV Infection: The Current State of Knowledge. Iran Red Crescent Med J. 2015;17(11):34181. doi: 10.5812/ircmj.34181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezaee-Zavareh MS, Ramezani-Binabaj M, Moayed Alavian S. Screening for occult hepatitis C virus infection: Does it need special attention? Hepatology. 2015;62(1):321–2. doi: 10.1002/hep.27626. [DOI] [PubMed] [Google Scholar]


