Abstract
A new kind of ‘para-effect’ under electron ionization (EI) conditions has been discovered for a series of bis(perfluoroacyl) derivatives of o-, m- and p-phenylenediamines, -hydroxybenzeneamines and -mercaptobenzeneamines of a common structure RCOX–C6H4–NHCOR (X = NH, S, O; R = CF3, C2F5, C3F7). Only the para-isomers showed successive loss of a radical RCO• and a molecule RCN, leading to very intense peaks in the EI spectra. The composition and the origin of the [M–COR–NCR]+ ions were confirmed by exact mass measurements and linked scan experiments. The proposed mechanism of their formation takes into account likely para-quinoid structures of the precursor ions. A similar rearrangement has not been observed for para-isomers in the series of bis(perfluoroacyl) derivatives of benzenediols, mercaptophenols and dimercaptobenzenes.
Commonly, electron ionization mass spectra cannot be reliably predicted, although a large number of mass spectrometric rules describing the behavior of organic molecules under electron ionization (EI) are known. As a result the spectra of compounds to be identified are often treated simply as molecular fingerprints. This is particularly true for compounds undergoing fragmentation through hydrogen migration or skeletal rearrangements. Our long-term project on the quality improvement and the extension of the National Institute of Standards and Technology/National Institute of Health/Environmental Protection Agency (NIST/NIH/EPA) Mass Spectral Library includes computer assisted ‘manual’ evaluation of every spectrum.1 This requires a knowledge of many fragmentation pathways, and the recognition of new unexpected reactions may be particularly helpful in this mission.
The EI spectra of the derivatives under investigation were obtained to extend the NIST/EPA/NIH Mass Spectral Library. During the course of this work a ‘para-effect’ for EI-induced dissociation of diacyl derivatives of bifunctional aminobenzenes was found. This is a novel process, not predictable with the known mass spectrometry rules. Unlike ‘ortho-effects’ (see2,3, for example), far fewer ‘para-effects’ are known in organic mass spectrometry (see4, for example).
The main objects of our investigation were the bis-acyl derivatives (acyl = perfluoro-acetyl, -propionyl and -butyryl) of o-, m- and p-phenylendimaines, o-, m- and p-hydroxyanilines and o-, m- and p-mercaptoanilines presented as structures 1a–c to 3a–c:
EXPERIMENTAL
Initial o-, m- and p- amino-, hydroxy-, -mercaptobenzeneamines as well as o-, m- and p- benzenediols, -benzenedithiols and -hydroxybenzenethiols and derivatizing agents (trifluoroacetyl, pentafluoropropionyl and heptafluorobutyryl anhydrides or chlorides) were commercially available (Sigma-Aldrich, St. Louis, MO, USA)a.
Derivatization of the bifunctional compounds was accomplished by reaction with trifluoroacetyl, pentafluoropropionyl and heptafluorobutyryl anhydrides or chlorides in the presence of pyridine.5
The EI mass spectra were measured using gas chromatography/mass spectrometry (GC/MS) systems with quadrupole (Agilent 6890/5973: Agilent Technologies, Santa Clara, CA, USA; ionization energy 70eV; ion source temperature 230°C) and magnetic sector (Finnigan MAT 95XL: Thermo Scientific, Bremen, Germany; ionization energy 70eV; temperature of ionization chamber 220°C) analyzers. Derivatized analytes were introduced into the mass spectrometer via GC. In all cases a fused quartz capillary column (stationary phase polydimethylsiloxane +5% phenyl groups; 30 m × 0.25 mm i.d.) was used (injection port temperature was 250 to 270°C; temperature of the oven increased from 60°C to 200–270°C at a rate of 5°C/min).
Exact mass determinations and linked-scan (collision-induced dissociation gas: helium) experiments were performed on a GCmate II magnetic sector mass spectrometer (JEOL, Tokyo, Japan).
RESULTS AND DISCUSSION
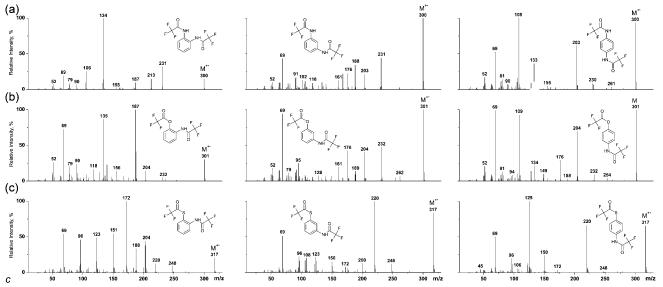
The structure of the bis(trifluoroacetyl), bis(pentafluoropropionyl) and bis(heptafluorobutyryl) derivatives of the o-, m- and p-phenylenediamines, -hydroxybenzeneamines and -mercaptobenzeneamines under investigation may be represented as RCOX–C6H4–NHCOR (X = NH, S, O; R = CF3, C2F5, C3F7). Complete EI mass spectra of the trifluoroacetyl derivatives of all the compounds are depicted in Fig. 1 and partial spectra, revealing the most characteristic ions for all the derivatives under study, are given in Table 1. As can be seen, all the spectra contain rather abundant M+• ions and the intensities of these ions are especially high for the meta- and para-isomers. As expected, the primary decomposition of the molecular ions was due to the loss of the •R and •COR radicals. For the ortho-isomers, the loss of RCOXH and RCONH2 was also expected. Some other characteristic ions are given in Table 1. It should be noted that the elimination of the first acyl radical occurs from the RCOX group, and the resulting ions are particularly characteristic for para-isomers. The spectrum of a mixed derivative prepared from para-mercaptoaniline by successive N- and S-acylation with different derivatizing agents (Fig. 2) unambiguously confirms the above statement:
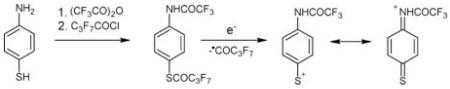
Figure 1.
EI mass spectra (70 eV) of bis(trifluoroacetyl) derivatives of o-, m- and p-phenylendiamines (a), o-, m- and p-hydroxyanilines (b) and o-, m- and p-mercaptoanilines (c) (Agilent 6890/5973).
Table 1.
Partial EI mass spectra (70 eV) of bis(trifluoroacetyl) derivatives of isomeric phenylenediamines, hydroxyanilines and mercaptoanilines [m/z (relative intensity, %)]
| Compounds 1–3 |
ortho |
meta |
para |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M+• | [M–R]+ | [M–RCO]+• | [M–RCOXH]+• | [M–RCONH2]+• | [M–R–RCO]+• | M+• | [M–R]+ | [M–RCO]+ | [M–RCOX]+ | M+• | [M–R]+ | [M–RCO]+ | XC6H4OH+ | |
| X = NH, R = CF3 | 300 (14.6) | 231 (31.8) | 203 (0.5) | 187 (9.6) | 187 (9.6) | 134 (100) | 300 (100) | 231 (44.1) | 203 (21.0) | 188 (34.4) | 300 (93.8) | 231 (6.9) | 203 (61.5) | 108 (100) |
| X = NH, R = C2F5 | 400 (11.6) | 281 (24.6) | 253 (0.1) | 237 (3.2) | 237 (3.2) | 134 (100) | 400 (100) | 281 (68.9) | 253 (26.5) | 238 (42.4) | 400 (84.8) | 281 (11.9) | 253 (66.1) | 108 (100) |
| X = NH, R = C3F7 | 500 (8.2) | 331 (24.1) | 303 (0.1) | 287 (3.2) | 287 (3.2) | 134 (100) | 500 (100) | 331 (98.5) | 303 (48.4) | 288 (73.4) | 500 (63.3) | 331 (8.4) | 303 (89.5) | 108 (100) |
| X = O, R = CF3 | 301 (30.3) | 232 (5.4) | 204 (14.2) | 187 (100) | 188 (41.6) | 135 (86.5) | 301 (100) | 232 (47.9) | 204 (40.3) | 188 (9.4) | 301 (100) | 232 (9.2) | 204 (69.8) | 109 (92.8) |
| X = O, R = C2F5 | 401 (20.6) | 282 (5.0) | 254 (6.5) | 237 (20.4) | 238 (16.8) | 135 (100) | 401 (100) | 282 (67.6) | 254 (43.1) | 238 (10.0) | 401 (67.4) | 282 (11.2) | 254 (47.7) | 109 (74.2) |
| X = O, R = C3F7 | 501 (21.8) | 332 (6.1) | 304 (9.9) | 287 (16.6) | 288 (23.6) | 135 (100) | 501 (100) | 332 (94.0) | 304 (61.0) | 288 (23.7) | 501 (74.8) | 332 (17.4) | 304 (93.2) | 109 (100) |
| X = S, R = CF3 | 317 (20.5) | 248 (9.2) | 220 (12.5) | 187 (2.6) | 204 (44.2) | 151 (54.9) | 317 (83.8) | 248 (12.4) | 220 (100) | 188 (1.5) | 317 (65.6) | 248 (1.8) | 220 (66.1) | 125 (100) |
| X = S, R = C2F5 | 417 (29.3) | 298 (20.1) | 270 (28.3) | 237 (0) | 254 (41.8) | 151 (100) | 417 (54.4) | 298 (10.0) | 270 (100) | 238 (1.6) | 417 (53.1) | 298 (4.8) | 270 (100) | 125 (86.8) |
| X = S, R = C3F7 | 517 (23.4) | 348 (20.2) | 320 (28.5) | 287 (0) | 304 (39.8) | 151 (100) | 517 (32.0) | 348 (4.5) | 320 (100) | 288 (1.8) | 517 (31.6) | 348 (1.5) | 320 (100) | 125 (67.1) |
Figure 2.
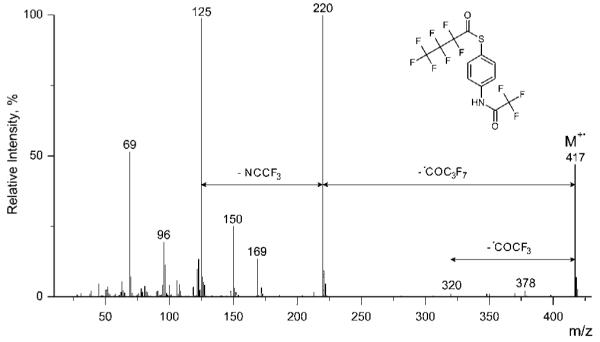
EI mass spectrum (70 eV) of N-trifluoroacetyl-S-heptafluorobutyryl-p-mercaptoaniline (Agilent 6890/5973).
Further fragmentation of the primary ions differed for the different isomeric compounds. For the ortho-isomers the successive loss of the •R and •COR radicals was especially prominent as the expected consequence of the ortho-effect:
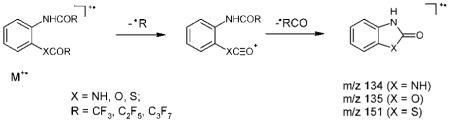
The possible driving force for the process is the formation of stable cation radicals and this fragmentation pattern is another example of exceptions to the ‘even-electron’ rule since the resulting ions can be formed, at least partly, through the step-wise loss of the noted radicals.
For the meta- and ortho-isomers the elimination of the •COR radical proceeds more easily than for the para-isomers. The [M–COR]+ ions become predominant for the para-isomers. The most interesting fragmentation of these ions includes a rearrangement process accompanied by the loss of a RCN molecule. The resulting [M–COR–NCR]+ ions appear as very intense peaks and in some cases they are the base peak. The compositions and the origins of the ions are confirmed by exact mass measurements (Table 2) and linked scan experiments. The analysis of precursor ion scan spectra clearly demonstrates that the [M–COR]+ ion was the only source of the [M–COR–NCR]+ ion. Without doubt, this fragmentation mode is a manifestation of a ‘para-effect’, and the suggested mechanism of the reaction takes into account the likely para-quinoid structures of the precursor ions:
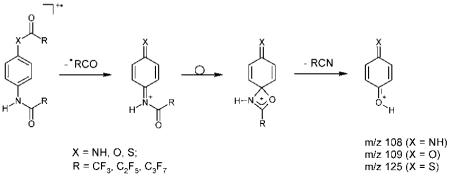
Table 2.
High-resolution data for characteristic ions derived from various bis(perfluoroacyl) derivatives of p-phenylenediamines, p-hydroxyanilines and p-mercaptoanilines
| Compounds 3a–c | Ion | Elemental composition | Calculated mass | Measured mass | Error (ppm) |
|---|---|---|---|---|---|
| X = NH, R = CF3 | m/z 108 | C6H6NO | 108.0449 | 108.0450 | 0.9 |
| X = NH, R = C2F5 | m/z 108 | C6H6NO | 108.0449 | 108.0453 | 3.7 |
| X = NH, R = C3F7 | m/z 108 | C6H6NO | 108.0449 | 108.0442 | −6.5 |
| X = O, R = CF3 | m/z 109 | C6H5O2 | 109.0289 | 109.0286 | −2.7 |
| X = O, R = C2F5 | m/z 109 | C6H5O2 | 109.0289 | 109.0295 | 5.5 |
| X = O, R = C3F7 | m/z 109 | C6H5O2 | 109.0289 | 109.0281 | −7.3 |
| X = S, R = CF3 | m/z 125 | C6H5OS | 125.0061 | 125.0060 | −0.8 |
| X = S, R = C2F5 | m/z 125 | C6H5OS | 125.0061 | 125.0066 | 4.0 |
| X = S, R = C3F7 | m/z 125 | C6H5OS | 125.0061 | 125.0072 | 8.8 |
In principle, a similar rearrangement could be expected for the ortho-isomers, where ortho-quinoid ion structures may also be formed after elimination of an RCO radical from M+•, but the spectra demonstrate the almost complete absence of such a reaction. It is likely that the competitive ortho-effect, noted above, suppresses the fragmentation route characteristic of para-isomers.
The possible elimination of the stable perfluoroalkanenitriles from a para-quinoid cation [M–COR]+ is likely to be the driving force of the described para-effect. This can lead to the ‘para-effect’ only in derivatives of bifunctional anilines. In fact, no similar process was observed for the para-isomers in the series of bis(perfluoroacyl) derivatives of benzenediols, mercaptophenols and dimercaptobenzenes. Mass spectrometric investigation of the complete series of homologous compounds will be published elsewhere.
CONCLUSIONS
The study of a series of bis(perfluoroacyl) derivatives of o-, m- and p-phenylenediamines, hydroxyanilines and mercaptoanilines allowed the discovery of a very prominent para-effect consisting of successive losses of an acyl radical and a molecule of perfluoroalkanenitrile. The driving force of the two-stage process is the formation of a para-quinoid cation, its rearrangement and decomposition to form protonated para-benzoquinone, -thiobenzoquinone or -iminobenzoquinone, and a very stable molecule of perfluoroalkanenitrile. This process was only characteristic of perfluoroacyl derivatives of bifunctional aminobenzenes and no similar reaction was observed for the same derivatives of para-benzenediols, -mercaptophenols and -dimercaptobenzenes.
Footnotes
This article is a U.S. Government work and is in the public domain in the U.S.A.
Certain commercial materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the identified materials are necessarily the best available for the purpose.
REFERENCES
- 1.Ausloos P, Clifton CL, Lias SG, Mikaya AI, Stein SE, Tchekhovskoi DV, Sparkman OD, Zaikin V, Zhu D. J. Am. Soc. Mass Spectrom. 1999;10:287. doi: 10.1016/S1044-0305(98)00159-7. [DOI] [PubMed] [Google Scholar]
- 2.Glagovich NM. In: The Encyclopedia of Mass Spectrometry: Molecular Ionization Methods. Gross ML, Caprioli RM, editors. Vol. 6. Elsevier; Amsterdam: 2007. p. 136. [Google Scholar]
- 3.Watson JT, Sparkman OD. Introduction to Mass Spectrometry. 4th edn John Wiley; Chichester: 2007. p. 819. [Google Scholar]
- 4.Zampronio CG, Moraes LAB, Eberlin MN, Poppi RJ. Talanta. 2003;60:37. doi: 10.1016/S0039-9140(03)00045-6. [DOI] [PubMed] [Google Scholar]
- 5.Zaikin V, Halket J. A Handbook of Derivatives for Mass Spectrometry. IM Publications; Chichester: 2009. p. 517. [Google Scholar]