Abstract
Background: Automated insulin management features of the MiniMed® 640G sensor-augmented pump system include suspension in response to predicted low sensor glucose (SG) values (“suspend before low”), suspension in response to existing low SG values (“suspend on low”), and automatic restarting of basal insulin delivery upon SG recovery. The effectiveness of these features was evaluated using CareLink® software data.
Methods: Anonymized data from MiniMed 640G system users (n = 4818), MiniMed 530G system users (n = 39,219), and MiniMed Paradigm® Veo™ system users (n = 43,193) who voluntarily uploaded pump and sensor data were retrospectively analyzed. Comparisons were made between days in which system features were enabled at any time and those in which they were not. Comparisons were also made between pump suspension events for which insulin delivery was automatically or manually resumed and between glycemic parameters of users who switched from the MiniMed Paradigm Veo system to the MiniMed 640G system.
Results: Days in which the MiniMed 640G “suspend before low” feature was enabled had lower percentages of SG readings ≤70 mg/dL (3.9 mmol/L) or ≥240 mg/dL (13.3 mmol/L) than days when it was not enabled (P < 0.001 for each). Users who switched from the MiniMed Paradigm Veo system to the MiniMed 640G system had fewer excursions below ≤70 mg/dL (P < 0.001) and ≥240 mg/dL (P < 0.001). SG values following automatically resumed pump suspension events recovered more rapidly and had a more stabilized endpoint than following manually resumed events.
Conclusions: Automated insulin management features of the MiniMed 640G system can reduce the frequency of both high and low SG values and help stabilize SG after resumption of insulin delivery.
Introduction
Sensor-augmented pump systems provide insulin delivery and continuous glucose monitoring therapies that can improve the management of diabetes. The MiniMed Paradigm Veo and MiniMed 530G systems (Medtronic, Northridge, CA) are capable of suspending insulin delivery in response to sensor glucose (SG) values at or below a prespecified threshold (Low Glucose Suspend [LGS] and Threshold Suspend [TS], respectively) (Table 1). The suspension automatically resumes (“auto-resume”) in 2 hours unless basal insulin delivery is manually resumed. Four hours after automatic insulin delivery resumption, the threshold suspend feature of both systems can allow resuspension of insulin delivery if the sensor glucose is still at or below prespecified threshold. This feature significantly reduces the severity and duration of hypoglycemic events once they occur, and has a salutary effect on the frequency of such events.1–7
Table 1.
Sensor-Augmented Pump System Features
| Sensor-augmented pump system | |||
|---|---|---|---|
| MiniMed Paradigm Veo | MiniMed 530G | MiniMed 640G | |
| Suspend on lowa | Yes (LGS) | Yes (TS) | Yes (LGS) |
| Suspend before low | No | No | Yes |
| Suspend threshold range | 40–110 mg/dL (2.2–6.1 mmol/L) | 60–90 mg/dL (3.3–5.0 mmol/L) | 50–90 mg/dL (2.8–5.0 mmol/L) |
| Auto-resume based on SG | No | No | Yesb |
| Maximum suspension duration, hours | 2 | 2 | 2 |
| Minimum interval between suspension, hours | 4 | 4 | 4 |
The difference between LGS and TS is the suspend threshold range.
Automatic resumption can occur sooner if, at 30 min since the start of pump suspension, the SG value is above the low limit and predicted to remain above the low limit within 30 min.
LGS, Low Glucose Suspend; SG, sensor glucose; TS, Threshold Suspend.
In addition to a threshold suspend feature (“suspend on low”), the MiniMed 640G system (Medtronic) can suspend insulin delivery based on predicted hypoglycemia (“suspend before low”) (Table 1), which can reduce the frequency of hypoglycemic events to a greater extent.8,9 When “suspend before low” is enabled, basal insulin delivery is stopped if the SG value is predicted to reach or fall below a preset low limit within 30 min. Similar to the MiniMed Paradigm Veo and MiniMed 530G systems, basal insulin delivery can be manually resumed at any time during a suspend event and automatically resumes after 2 hours. In contrast, “auto-resume” can occur sooner if, at 30 min since the start of pump suspension, the SG value is above the low limit and predicted to remain above the low limit within 30 min.
CareLink® Personal Therapy Management Software for diabetes provides remote storage and retrospective analysis capabilities of data from the pump, sensor, and blood glucose meter.10 CareLink software data from individual users can be reviewed in collaboration with healthcare providers to guide therapy adjustments, or can be aggregated and analyzed to detect large-scale patterns and outcomes in an anonymized manner. The effectiveness and usage patterns of the automated insulin management features of the MiniMed 640G system, in addition to specific MiniMed Paradigm Veo and MiniMed 530G comparator data, were evaluated.
Methods
Anonymized CareLink data, voluntarily uploaded from 4818 individuals using the MiniMed 640G system between January 13, 2015, and January 14, 2016 (286,149 user-days of data); 39,219 individuals using MiniMed 530G system between October 15, 2013, and June 25, 2015 (2,777,117 user-days of data); and 43,193 individuals using the MiniMed Paradigm Veo system between October 1, 2011, and July 14, 2015 (4,101,706 user-days of data); were analyzed. Users of each system uploaded, at least, 5 days of CareLink data during the aforementioned time periods. The aforementioned data included information on insulin delivery from the pumps and information from CGM sensors (Enlite® sensor [MiniMed 530G system] and Enhanced Enlite® sensor [MiniMed Paradigm Veo and MiniMed 640G systems]; Medtronic).
For this retrospective analysis, users were not provided specific instruction from healthcare professionals on when or how long to use system features or how often to calibrate systems with self-monitored blood glucose measurements. Voluntarily uploaded and anonymized data were captured from CareLink software. To compare SG trajectories associated with different types of pump suspension, user-days were assigned to the feature (i.e., predictive “suspend before low” or reactive “suspend on low”) in use during that day. The threshold range for the MiniMed 640G “suspend before low” and “suspend on low” was 50–90 mg/dL (3.3–5.0 mmol/L). The threshold range for the MiniMed Paradigm Veo system was 40–110 mg/dL (2.2–6.1 mmol/L) and that for the MiniMed 530G was 60–90 mg/dL (2.8–5.0 mmol/L). While the MiniMed Paradigm Veo LGS and the MiniMed 530G TS features differ in suspend threshold range, they are considered functionally equivalent.2 For simplicity, the threshold suspend features in all three systems have been referred to as “suspend on low”. Pump suspension events were characterized according to whether insulin delivery was resumed automatically or manually. Hypoglycemia and hyperglycemia were defined as SG values ≤70 mg/dL (≤3.9 mmol/L) and 240–300 mg/dL (13.3–16.7 mmol/L), respectively, and were compared across systems using a Kruskal–Wallis analysis. Severe hyperglycemia was defined as ≥300 mg/dL (16.7 mmol/L). Hypoglycemic and hyperglycemic excursions were defined as ≥2 consecutive hypoglycemic or hyperglycemic SG values per day or night (8:00 PM–8:00 AM) and, in addition to area under the glucose concentration–time curve (AUC), were compared between the MiniMed Paradigm Veo and MiniMed 640G systems using a Wilcoxon signed-rank analysis.
Glycemic variability between manual and automatic resumptions, as measured by a coefficient of variability (CV, standard deviation/mean), was calculated using the last SG value collected from each resumption and analyzed using a paired t-test. To characterize the postsuspend behavior of SG values, “recovery time” was defined as the interval from pump suspend to the start of the first 20-min interval with SG values 68–119 mg/dL (3.8–6.6 mmol/L).
For additional glycemic comparisons between the “suspend before low” and the “suspend on low” features, we identified 851 people who switched from the MiniMed Paradigm Veo system to the MiniMed 640G system and uploaded ≥7 days of data from both systems between February 28, 2010, and September 29, 2015.
Results
The MiniMed 640G “suspend before low” feature was used on 83% of the user-days, “suspend on low” on 11% of the user-days, and neither suspend feature on 6% of the user-days. More than 99% of all users used one or both suspend features at least once; most (59%) used “suspend before low” exclusively. Among all SG values from MiniMed 640G users when “suspend before low” was enabled, 0.39% were in the ≤50 mg/dL range, 2.11% were in the 50–70 mg/dL range, 63.53% were in the 70–180 mg/dL range, 30.42% were in the 180–300 mg/dL range, and 3.55% were in the >300 mg/dL range. In comparison, the “suspend on low” feature was used on 72% of the MiniMed Paradigm Veo and 82% of MiniMed 530G user-days. Among all SG values from MiniMed Paradigm Veo users, when “suspend on low” was enabled, 1.0% were in the ≤50 mg/dL range, 4.1% were in the 50–70 mg/dL range, 61.2% were in the 70–180 mg/dL range, 29.6% were in the 180–300 mg/dL range, and 4.1% were in the >300 mg/dL range. Among all SG values from MiniMed 530G users, when “suspend on low” was enabled, 0.7% were in the ≤50 mg/dL range, 3.4% were in the 50–70 mg/dL range, 60.6% were in the 70–180 mg/dL range, 30.7% were in the 180–300 mg/dL range, and 4.6% were in the >300 mg/dL range.
Basal insulin delivery was automatically suspended as a result of enabled MiniMed 640G “suspend before low” on 693,626 occasions, at an average rate of 2.9 per user-day. Over half of the suspend events (55%, 381,803) ended due to “auto-resume” based on SG, 11.5% (79,525) due to the 2-h “auto-resume,” and the remainder due to manual resumption (33.5%, 232,298). The mean duration of MiniMed 640G “auto-resume” based on SG events was 58 ± 25 min and that of the manually resumed events was 33 ± 23 min. SG values associated with automatically resumed events showed less glycemic variability than those associated with manually resumed events, as measured by the coefficient of variation (CV) (P < 0.001, 0.18 vs. 0.24, respectively), and faster recovery times (P < 0.001, 29.4 vs. 35.1 min, respectively).
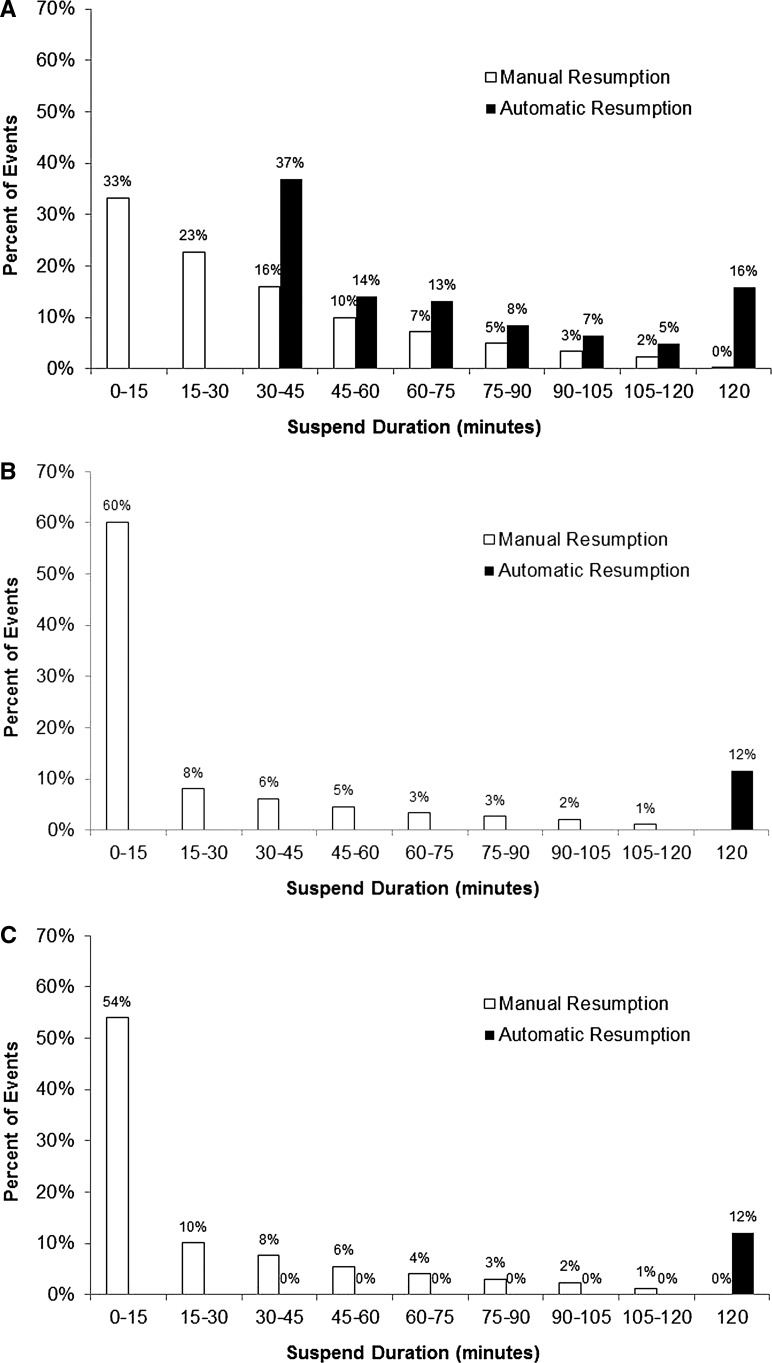
The distribution of pump suspension events across duration for each system is shown in Figure 1. About one-third of manually resumed events for the MiniMed 640G system (Fig. 1A) and more than half for the MiniMed 530G (Fig. 1B) and MiniMed Paradigm Veo (Fig. 1C) systems occurred within the first 15 min of suspension. The distribution in automatically resumed events for the MiniMed 640G system was partly bimodal. Specifically, many “auto resume” events occurred soon after the minimum 30-min interval (inherent to MiniMed 640G technology) and continued, although at a decreased percent, until the maximum suspend duration of 2 hours.
FIG. 1.
The distribution of manual and automatic pump resumption events across bins of suspend duration time (min) is shown for MiniMed 640G (A), MiniMed 530G (B), and MiniMed Paradigm Veo (C) systems. For all three, a majority of pump resumption events were triggered manually within the first 15 min of pump suspension.
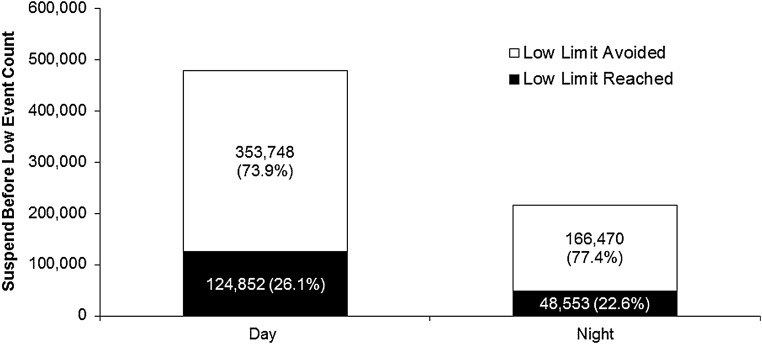
Figure 2 shows the number and percent of “suspend before low” events during the day and night associated with the set low limit SG value (Mean ± SD = 66.7 ± 9.9 mg/dL [3.7 ± 0.5 mmol/L]). Overall, 75% (353,748 [Day] + 166,470 [Night] = 520,218) of the 693,626 predicted hypoglycemic events were not followed by an SG value at or below the low limit. Most suspend events occurred during the daytime hours, and the hypoglycemia prevention rate was slightly higher for suspend events occurring at night.
FIG. 2.
The MiniMed 640G-enabled “suspend before low” feature resulted in the avoidance of 73.9% of predicted hypoglycemic events during the day and 77.4% of predicted hypoglycemic events during the night (white bars). The proportion of events that reached or fell below the preset limit (black bars) was 26.1% and 22.6%, respectively.
The benefits of automated pump suspension with predictive versus reactive pump suspension features during nocturnal hypoglycemia are shown in Table 2. The duration of hypoglycemia between 8:00 PM and 8:00 AM was calculated for users of the MiniMed Paradigm Veo, MiniMed 530G, and MiniMed 640G systems on nights when the pumps' suspend features were not in use and compared to nights when they were in use. Statistically significant reductions were seen when either pump suspension feature was used (P < 0.001), but the reductions associated with the predictive feature of the MiniMed 640G system were greater.
Table 2.
Duration of Nighttime Hypoglycemia
| MiniMed Paradigm Veo | MiniMed 530G | MiniMed 640G | ||||
|---|---|---|---|---|---|---|
| Setting | Suspend on low OFF | Suspend on low ON | Suspend on low OFF | Suspend on low ON | Suspend before low OFF | Suspend before low ON |
| Hypoglycemia, hours/night, Mean ± SD | 0.4 ± 0.8 | 0.2 ± 0.5 | 0.4 ± 1.0 | 0.2 ± 0.4 | 0.4 ± 1.0 | 0.1 ± 0.3 |
| P < 0.001 | P < 0.001 | P < 0.001 | ||||
| Users, N | 24,715 | 30,785 | 13,166 | 34,402 | 1230 | 4480 |
Night time = 8:00 PM–8:00 AM. Hypoglycemia was defined as ≤70 mg/dL (3.9 mmol/L).
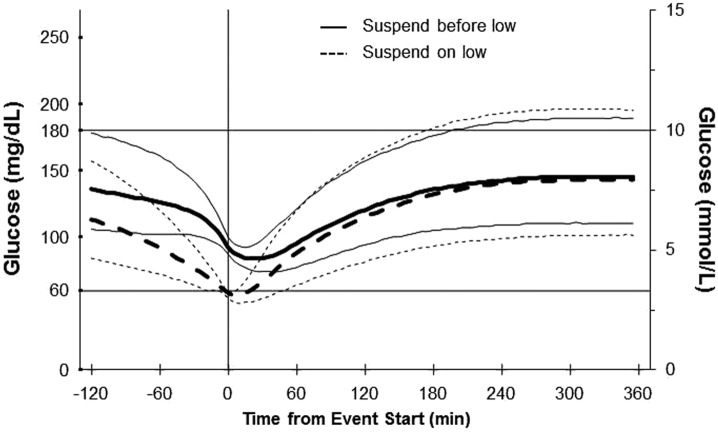
The “suspend before low” and “suspend on low” pump suspension features are compared in Figure 3, in which SG trajectories surrounding 59,823 “suspend before low” events of any duration were compared with trajectories surrounding 603,592 “suspend on low” events of any duration. In all cases, the low limit was set to 60 mg/dL. At the outset, the median SG value for “suspend on low” events was 58 mg/dL, whereas the median value at the time the “suspend before low” events began was 92 mg/dL. For both types of suspend events, the median SG value stabilized to around 144 mg/dL by 4 hours. SG values associated with “suspend before low” events followed a more predictable trajectory during the recovery phase, as indicated by the narrower interquartile range.
FIG. 3.
SG trajectories associated with enabled event start time (min) for “suspend before low” and “suspend on low” features. The thick solid line shows median SG values for 59,823 MiniMed 640G “suspend before low” events of any duration for which the low limit was set to 60 mg/dL. The thick dashed line shows median SG values for 603,592 MiniMed Paradigm Veo “suspend on low” events of any duration for which the low limit was set to 60 mg/dL. Thin solid and dashed lines show the 25th and 75th percentile values. SG, sensor glucose.
A separate analysis of data from 851 users who switched from the MiniMed Paradigm Veo system to the MiniMed 640G system is presented in Table 3. After switching, users experienced less time in hypoglycemia and their hypoglycemic exposure was significantly less in duration and severity as measured by excursions and the AUC. There was also a significant improvement in severe hyperglycemic parameters after switching to the 640G system.
Table 3.
Glycemic Parameters Associated with Suspend on Low and Suspend Before Low
| Suspend on Low | Suspend before Low | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Median | 25th | 75th | Median | 25th | 75th | P |
| Hypoglycemia | |||||||
| Excursions per day, n | 0.949 | 0.606 | 1.508 | 0.738 | 0.398 | 1.214 | <0.001 |
| Duration, min/day | 25.796 | 14.731 | 46.520 | 15.721 | 7.831 | 28.889 | <0.001 |
| AUC, mg/dL × day | 0.393 | 0.271 | 0.536 | 0.280 | 0.197 | 0.396 | <0.001 |
| Severe Hyperglycemia | |||||||
| Excursions per day, n | 0.420 | 0.185 | 0.752 | 0.333 | 0.130 | 0.723 | <0.001 |
| Duration, min/day | 15.975 | 6.410 | 32.186 | 12.778 | 4.163 | 31.820 | <0.001 |
| AUC, mg/dL × day | 2.208 | 1.547 | 3.069 | 1.737 | 1.050 | 2.589 | <0.001 |
Hypoglycemia was defined as SG ≤70 mg/dL (3.9 mmol/L). Severe Hyperglycemia was defined as SG ≥300 mg/dL (16.7 mmol/L). The “suspend on low” comprised data from the 851 MiniMed Paradigm Veo users who transitioned to MiniMed 640G.
AUC, area under the glucose concentration–time curve.
Discussion
Hypoglycemia remains an important concern for people with diabetes. The rate of hypoglycemia, whether severe or nonsevere, during the day or at night, may be underestimated in clinical trial settings,11 stressing the need for retrospective real-world analyses such as this one. In 2015, Dexcom reported data from 300 users of their Platinum G4® CGM system,12 in which users experienced a mean of 1.2 ± 1.9 (Mean ± SD) hours per day with sensor readings ≤70 mg/dL, half of which were at night. This rate is in agreement with data from users of Medtronic CGM systems on nights when system suspend functions were not used. As shown in Table 2, the duration of nocturnal hypoglycemia was reduced by ∼50% with use of the MiniMed Paradigm Veo and MiniMed 530G “suspend on low” features, and was reduced by ∼75% with use of the MiniMed 640G “suspend before low” feature. This reduction in hypoglycemia exposure (i.e., number of events) attributable to the predictive insulin pump suspension technology in the MiniMed 640G system agrees with that reported in a recent study of children and adolescents that found slight elevations in overnight mean glucose levels and no increase in morning blood ketosis on nights that pump suspensions occurred.13
The hypoglycemia prevention attributable to the MiniMed 640G system was also measured in a recent Predictive Low Glucose Management (PLGM) pivotal study,14 in which standardized increases in basal insulin delivery were used to trigger insulin pump suspension. A 60% reduction in hypoglycemia was reported based on 68 automatic pump-suspension events, among which the hypoglycemic criterion (two or more reference plasma glucose levels of <65 mg/dL) did not occur in 41 cases. This is less than the 75% prevention rate reported here and the 83% prevention rate reported in a PLGM user evaluation study.8 The smaller reduction observed in the pivotal study was likely due to the basal insulin delivery escalation protocol known to result in hypoglycemia 93% of the time,15 relative to the day-to-day environment settings of the later studies.
In the present study data, the MiniMed 640G algorithm governing automatic resumption of insulin delivery based on SG appeared to work well and resumed insulin delivery primarily within 30–45 min after pump suspension. While more than half of manually resumed events occurred within the first 15 min after pump suspension for the MiniMed Paradigm Veo or MiniMed 530G systems, only one-third occurred within the same period for MiniMed 640G. It is not known what user characteristics or physiologic and metabolic factors may have influenced the different extent of manual resumption between the systems. The “auto resume” based on SG feature, not available in MiniMed Paradigm Veo or MiniMed 530G systems, appeared to allow a more immediate real-time solution for maintaining target glucose levels. Specifically, there was a faster hypoglycemia recovery and less glycemic variability (as measured by the CV of SG values), compared to users who restart the pump manually.
This study benefits from the large number of users and user-days available for analyses, although data from system users who did not upload to CareLink software were unavailable. A limitation of the study is that the MiniMed 640G “suspend on low” versus MiniMed 640G “suspend before low” data could not be analyzed, due to an insufficient number of events during use of the former feature. The lack of detailed user demographic information is another limitation, as is a lack of detailed information regarding user-system interface (e.g., calibrations, sensor changes, and CareLink software upload frequency), physician-to-user instructions, and dietary and exercise behaviors. The false-positive rate of predictive pump suspensions, as well as sensor accuracy, could not be assessed because of the study's retrospective nature. The effect of sensor life on the rate of pump suspension events was also not possible to assess. Within-user comparisons of MiniMed Paradigm Veo and MiniMed 640G system use may have been influenced by the unidirectional nature of the MiniMed Paradigm Veo system use preceding MiniMed 640G system use, and by uncharacterized or unknown behaviors related to becoming familiar with a new pump system.
Several challenges remain on the path to a fully closed-loop insulin delivery system. Although no such systems are commercially available, a recent Hybrid-Closed Loop (HCL) pivotal trial, completed in April 2016 (NCT02463097), allowed subjects to wear investigational systems in an unsupervised outpatient setting for an extended period of time (i.e., 3 months). In other outpatient studies, HCL systems outperformed sensor-augmented pumps in terms of glucose control, time in hypoglycemia, and glycated hemoglobin levels.16,17
The MiniMed 640G system is the first commercially available system to offer predictive suspension and automatic resumption, based on SG, of basal insulin delivery. The benefits attributable to these features were previously reported in the context of a user evaluation study.8 Analysis of real-world usage patterns of MiniMed 640G use and the predictive pump suspension feature reported here supports earlier results and establishes the validity of this integrative technology for routine use in insulin-requiring individuals with diabetes.
Author Disclosure Statement
A.Z., C.M., P.A., J.B.W., T.L.C., and F.R.K. are or were employees of Medtronic during development of this manuscript. P.C. has received research support from Medtronic.
Product Disclosure Statement
The MiniMed 530G system is available for use only in the U.S. The MiniMed Paradigm Veo and MiniMed 640G systems are not commercially available for use in the U.S.
References
- 1.Agrawal P, Welsh JB, Kannard B, et al. : Usage and effectiveness of the low glucose suspend feature of the Medtronic Paradigm Veo insulin pump. J Diabetes Sci Technol 2011;5:1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal P, Zhong A, Welsh JB, et al. : Retrospective analysis of the real-world use of the threshold suspend feature of sensor-augmented insulin pumps. Diabetes Technol Ther 2015;17:316–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhary P, Shin J, Wang Y, et al. : Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care 2011;34:2023–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danne T, Kordonouri O, Holder M, et al. : Prevention of hypoglycemia by using low glucose suspend function in sensor-augmented pump therapy. Diabetes Technol Ther 2011;13:1129–1134 [DOI] [PubMed] [Google Scholar]
- 5.Elbarbary NS: Effectiveness of the low glucose suspend feature of insulin pump during fasting in Ramadan in type 1 diabetes mellitus. Diabetes Metab Res Rev 2016; doi: 10.1002/dmrr.2781 [DOI] [PubMed] [Google Scholar]
- 6.Garg S, Brazg RL, Bailey TS, et al. : Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technol Ther 2012;14:205–209 [DOI] [PubMed] [Google Scholar]
- 7.Pickup JC: Semi-closed-loop insulin delivery systems: early experience with low-glucose insulin suspend pumps. Diabetes Technol Ther 2011;13:695–698 [DOI] [PubMed] [Google Scholar]
- 8.Choudhary P, Olsen BS, Conget I, et al. : Hypoglycemia prevention and user acceptance of an insulin pump system with predictive low glucose management. Diabetes Technol Ther 2016;18:288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bock MI, Roy A, Cooper MN, et al. : Feasibility of outpatient 24-hour closed-loop insulin delivery. Diabetes Care 2015;38:e186–e187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corriveau EA, Durso PJ, Kaufman ED, et al. : Effect of Carelink, an internet-based insulin pump monitoring system, on glycemic control in rural and urban children with type 1 diabetes mellitus. Pediatr Diabetes 2008;9:360–366 [DOI] [PubMed] [Google Scholar]
- 11.Elliott L, Fidler C, Ditchfield A, et al. : Hypoglycemia event rates: a comparison between real-world data and randomized controlled trial populations in insulin-treated diabetes. Diabetes Ther 2016;7:45–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Kent T, Hall T, et al. : Regular-life use of patient real-time CGM data. Diabetes 2015;64:A243 [Google Scholar]
- 13.Buckingham BA, Raghinaru D, Cameron F, et al. : Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care 2015;38:1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey T, Buckingham B, Christiansen M, et al. : In-clinic evaluation of a predictive low glucose management system (PLGM) for hypoglycemia prevention. Diabetes Technol Ther 2016;18(suppl 1):A24–A25 [DOI] [PubMed] [Google Scholar]
- 15.Buckingham B, Chase HP, Dassau E, et al. : Prevention of nocturnal hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Care 2010;33:1013–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tauschmann M, Allen JM, Wilinska ME, et al. : Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016;39:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thabit H, Tauschmann M, Allen JM, et al. : Home Use of an Artificial Beta Cell in Type 1 Diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]